Abstract
Purpose
Antiestrogen therapy has been used successfully to prolong disease-free and overall survival of ER positive breast cancer patients. However, 50% of patients with ER+ tumors fail to respond to such therapy or eventually acquire resistance to endocrine therapy, resulting in tumor progression and mortality. It is imperative, therefore, to understand the mechanisms that lead to hormone refractory breast cancer in order to develop therapeutics that can modulate the resistance to antiestrogen therapy. The protease, ADAM12, can be detected in the urine of breast cancer patients and its levels correlate with disease status, stage and cancer risk. Within the context of the current study, we have investigated the role of the two distinct isoforms of ADAM12 in breast tumor cell proliferation and as potential mediators of endocrine resistance.
Methods
Using stable clones of ADAM12-overexpressing MCF-7 cells, we analyzed proliferation rates of these ER+ breast tumor cells both in estrogen-depleted medium and in the presence of the antiestrogens, tamoxifen and ICI 182,780. Acquired estrogen resistance in these cells was analyzed using phosphoRTK analysis. Upregulation and phosphorylation of proteins were detected via immunoprecipitation and immunoblotting. EGFR and MAPK inhibitors were used to explore the mechanism of acquired estrogen resistance in breast tumor cells.
Results
We observed that overexpression of the two isoforms, transmembrane ADAM12-L and secreted ADAM12-S, in breast tumor cells promoted estrogen-independent proliferation. In ADAM12-L-expressing cells, estrogen-independence was a direct result of increased EGFR expression and MAPK activation, whereas, the mechanism in ADAM12-S-expressing cells may be enhanced IGF-1R signaling. The importance of the EGFR signaling pathway in the estrogen-independent growth of ADAM12-L expressing cells was highlighted by the effect of EGFR inhibitors AG1478 and PD15035 or MAPK inhibitor U0126, each of which abolished the antiestrogen resistance in these cells.
Conclusions
Taken together, these results demonstrate that ADAM12 isoforms confer a proliferative advantage to MCF-7 cells in the absence of estrogen stimulation, and suggest that downregulation of ADAM12 in combination with endocrine therapy may represent a useful pharmacological approach to breast cancer therapy.
Keywords: ADAM, metalloprotease, breast tumor proliferation, antiestrogen resistance, EGFR, amphiregulin
Introduction
Breast cancer is one of the most prevalent diseases in women worldwide. Estrogen receptor (ER) positive breast cancers are the most common [1]. Antiestrogens such as tamoxifen have proven very effective as first-line endocrine therapy of early and advanced breast cancers to prolong disease-free and overall survival in these patients. However, 50% of patients with ER+ tumors fail to respond to these drugs and even patients who initially respond eventually acquire resistance to endocrine therapy, resulting in tumor progression and mortality [2]. This resistance does not necessarily require a loss of expression of, or mutations, in ER in these tumors. In fact, more than 60% of tamoxifen resistant tumors continue to express ER [2]. The mechanisms of innate or acquired antiestrogen tumor resistance are complex and range from loss of, phosphorylation of, or mutations in, the ER, dysfunctional GATA-3/FOXA1 transcriptional networks [3], modulation of TGFβ signaling by hormone-responsive miR-128a [4] and activation of other growth promoting pathways [5-7]. There is increasing evidence that in endocrine resistant breast tumors and cell lines aberrant activation of growth factor signaling cascades can provide proliferative and survival advantages.
Human ADAM12 is expressed as two alternatively spliced forms, a transmembrane form (ADAM12-L) and a secreted form (ADAM12-S) [8]. ADAM12-L and –S share high overall sequence homology differing only in the transmembrane domain (which ADAM12-S lacks) and the C-terminus. The catalytic domain of ADAM12 contains the consensus HExGHxxGxxHD zinc-binding motif and both isoforms are active proteases. ADAM12-L sheds several membrane-bound ligands including HB-EGF [9], EGF [10], betacellulin [10], Notch ligand Delta-like 1 [11] and placental leucine aminopeptidase [12]. ADAM12-S can cleave IGFBP-3 and IGFBP-5 [13,14] and degrade ECM substrates [15]. ADAM12 mRNA and protein are highly expressed in a variety of malignant tumor tissues and tumor cell lines including breast, brain, bladder, gastric, colon, lung, laryngeal and hepatocellular carcinomas (For review see: [16-24].
ADAM12 can be detected in the urine of breast [15] and bladder [19] cancer patients and its levels have been shown to correlate with disease status, stage and cancer risk [15,25]. The discovery of ADAM12 as a potential biomarker for breast cancer raised the question of its relevance in human breast tumorigenesis. Recently, we have demonstrated that overexpression of both ADAM12-L and ADAM12-S isoforms in breast tumor cells results in a significantly higher rate of tumor take and increased tumor size in an orthotopic breast tumor model [26]. In the current study we observed that the overexpression of the two isoforms, transmembrane ADAM12-L and secreted ADAM12-S, in breast tumor cells promotes estrogen-independent breast tumor cell proliferation. Within the context of the current study, we have investigated the role of the two distinct isoforms of ADAM12 in breast tumor cell proliferation and as potential mediators of endocrine resistance.
Materials and Methods
Reagents, antibodies and cell lines
ADAM12 antibody rb122 and plasmids for ADAM12 isoforms were a kind gift from Dr. Ulla Wewer (Institute of Molecular Pathology, Copenhagen, Denmark). Other antibodies used in the study include: EGFR, phosphoEGFR, ErbB2, ErbB3, ErbB4, total and phosphop44/42 MAPK from Cell Signaling Technology Inc. (Danvers, MA), ERα from Santa Cruz Biotechnology (Santa Cruz, CA), phosphotyrosine (clone 4G10, Upstate, Waltham, MA), GAPDH (Millipore, Temecula, CA), HB-EGF, amphiregulin, epiregulin (R & D Systems, Minneapolis, MN), TGFα (US Biologicals, Swampscott, MA) and EGF (Abcam, Cambridge, MA). HRP-conjugated anti-rabbit and anti-mouse antibodies were from Vector Biolabs (Burlingame, CA). MCF-7 and T47-D cells were obtained from American type culture collection (ATCC, Manassas, VA) and cultured as per ATCC protocols. Tamoxifen-sensitive and tamoxifen-resistant MCF-7 clones were the kind gift of Dr. Toshi Shioda and cultured as described previously [27].
Transfection of breast cancer cells with human ADAM12-L and ADAM12-S
MCF-7 and T47-D cells were stably transfected with pcDNA3 plasmid encoding human full-length ADAM12-L, ADAM12-S and ADAM12-Scatmut (with a E351Q point mutation) using Amaxa Nucleofector Kit (Lonza, Walkersville, MD). Stable clones were selected based on neomycin resistant growth (G418, 0.5 mg/ml; Life Technologies, CA). ADAM12-expressing MCF-7 stable clones were selected based on significantly higher ADAM12 mRNA and protein expression as described previously [26]. Pools of ADAM12 stable clones were used for analysis of T47-D cells (Suppl. Fig. 1).
Immunoblotting
Cell lysates were prepared using 1X lysis buffer (Cell Signaling Technology). Serum-free CM was used for analysis of ADAM12-S and shed EGF ligands. For the analysis of EGF ligand shedding serum-free CM was concentrated 100-fold using 3 kDa cutoff filters (YM-3 Microcon, Millipore Corporation). Protein concentration of the lysates and CM was determined using the Bradford method (Biorad Laboratories, Hercules, CA). Immunoblotting and densitometric analysis was conducted as described previously [15].
Proliferation assay
Cells (5000/well) were plated in triplicate in 24-well plates and were maintained in standard culture medium. Cells were harvested at 24, 48 and 72 h time points and counted using a coulter-counter or hemocytometer. For estrogen-depleted growth conditions, cells were cultured until ~90% confluent, washed 4-6 times with phenol red free DMEM and maintained in 10% charcoal-stripped FBS containing medium. Subsequently, the cells were plated for proliferation as described above. For anti-estrogen growth studies ER antagonist tamoxifen (3 μM) and ER inhibitor ICI 182,780 (100nM) were added at 0 h. For EGFR inhibition studies the EGFR inhibitor AG1478 (10 μM), PD15035 (1 μM) and MAPK inhibitor U0126 (10 μM) were added in combination with ICI 182,780.
RT-PCR analysis
Total RNA was extracted from cells using the RNAeasy Qiagen Kit according to the manufacturer's protocol (Qiagen, Germantown, MD). cDNA was prepared by reverse transcription from 1μg of total RNA using the Superscript III Reverse Transcriptase Kit (Invitrogen). Forward and reverse primers were designed as follows: ERα (forward 5’-ccaccaaccagtgcaccatt-3’, reverse 5’-ggtcttttcgtatcccacctttc-3’); GAPDH (forward 5’-cagcctca agatcatcagca-3’, reverse 5’-gtcttctgggtggcagtgat-3’); EGFR (forward 5’-ccacctgtccatccaaact-3’, reverse 5 ’-gccgatggacgggatctt-3’); ADAM 9 (forward 5 ’-gcatttgtggcaacagtgtg-3”, reverse 5’-gctctttgc tccacaggaac-3’); ADAM10 (forward 5’-tccccttgcaacgattttag-3’, reverse 5’-aatactgcccaccaatgagc-3’); ADAM15 (forward 5’-tgtcaccctcga aaacttcc-3’, reverse 5’-tccatgttcacacctcctga-3’); ADAM17 (forward 5’-ggtggtggatggtaaaaacg-3’, reverse 5’-gccccatctgtgttgattct-3’); Amphiregulin (forward 5’ccacagtgctgatggatttg-3’, reverse 5’-agccaggtatttgtggttcg-3’); HB-EGF (forward 5’-agaagag gttgggcttccat-3’, reverse 5’-ctgcatggagcaccaga-3’); EGF (forward 5’-gccaagcagtctgtgattga-3’, reverse 5’-ctgatggcatagcccaatct-3’). GAPDH expression was used to normalize cDNA levels. Real time RT-PCR was conducted on a MJ Research DNA Engine Opticon 2 (Biorad Laboratories) using iQ™ SYBR® Supermix (Biorad Laboratories, Hercules, CA).
Receptor Tyrosine Kinase (RTK) phosphorylation analysis
Phosphorylated growth factor receptor tyrosine kinases (RTKs) were detected using the Proteome Profiler Array Kit (R & D Systems). WT MCF-7 cells and ADAM12-L and ADAM12-S clones were cultured until ~70% confluent. Cells were starved overnight in serum-free medium and activated for 15 min with 10% serum containing medium. RTK arrays were then incubated with cell lysates (300 μg total protein) and subsequently dot blots were developed according to manufacturer's protocol. Densitometric measurement of dot blots was conducted as described previously [15].
Statistical analysis
Results for proliferation and inhibition assays are reported as mean ± SD of at least 3 independent experiments. Differences between experimental groups were statistically analyzed using the Mann-Whitney test. p≤ 0.05 were considered to be statistically significant.
Results
ADAM12 expression promotes higher proliferation rates in breast tumor cells
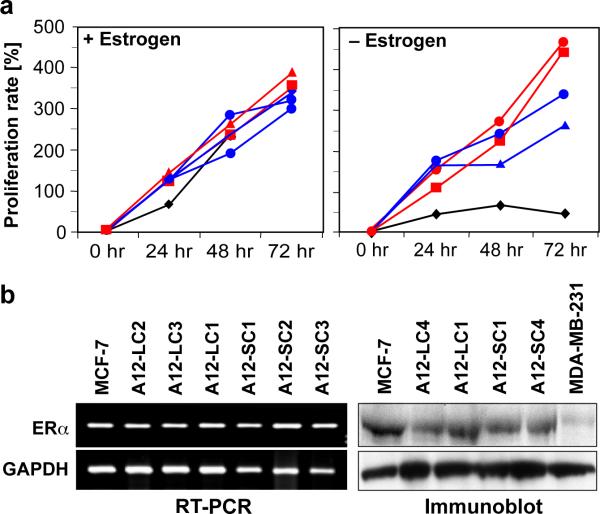
To explore the role of the protease ADAM12 in breast tumorigenesis, we selected a breast tumor cell line that express low levels of this metalloprotease, MCF-7 and engineered stable clones for overexpression studies [26]. Both WT MCF-7 and stable ADAM12 overexpressing MCF-7 clones demonstrated comparable proliferation rates (Fig. 1a, left panel) in the presence of estrogen. In contrast, when estrogen was depleted from the medium (using charcoal-stripped FBS and phenol red free DMEM), the ADAM12-S and ADAM12-L stable clones appeared to proliferate at a significantly faster rate than did the WT MCF-7 cells (Fig. 1a, right panel). DNA synthesis, as measured by [3H]thymidine uptake assay, indicated that both ADAM12-L and ADAM12-S clones had slightly and moderately higher rates of DNA synthesis respectively, as compared to the WT MCF-7 cells, however, the differences did not approach significance (data not shown). Transcript and protein levels of ERα remained unchanged, indicating that the acquired estrogen-independent growth of the ADAM12-expressing cells was not accompanied by a loss of ERα expression (Fig. 1b).
Fig. 1. ADAM12 expression promotes estrogen-independent growth.
Proliferation rates of WT MCF-7 (black) and representative ADAM12 clones (ADAM12-S; red, ADAM12-L; blue) grown in the presence of estrogen are comparable (a, left panel). In the absence of exogenous estrogen, ADAM12 clones proliferated significantly faster (a, right panel). ADAM12-expressing cells retain ERα mRNA (left panel) and protein expression (right panel) (b). MDA-MB-231 lysate was used as a negative control for ERα protein expression. Individual ADAM12-L and ADAM12-S clones are indicated as C1, C2, C3 respectively.
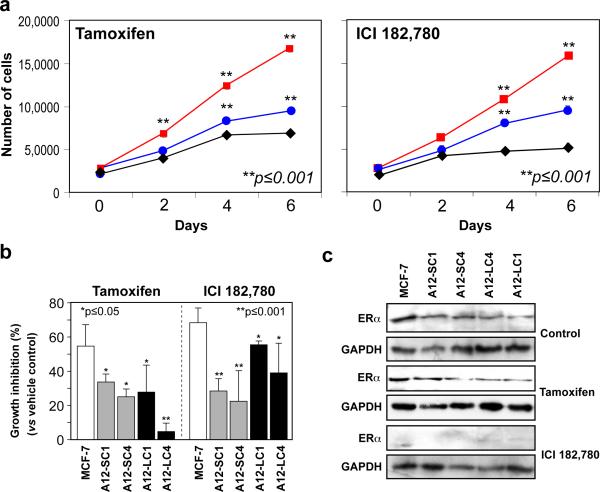
Since overexpression of the ADAM12 isoforms appeared to promote estrogen-independent growth in MCF-7, we next asked whether ADAM12-expressing cells were resistant to antiestrogen treatment. ADAM12-L and ADAM12-S-expressing clones proliferate at significantly faster rates than WT MCF-7 in the presence of the antiestrogen compounds tamoxifen (3 μM), or ER inhibitor ICI 182,780 (100 nM) (Fig. 2a). Whereas, WT MCF-7 cells displayed ≥ 50% growth inhibition when cultured in the presence of tamoxifen as previously reported [28], ADAM12-expressing MCF-7 clones displayed significantly lower growth inhibition under the same conditions (Fig. 2b), although ERα expression was maintained in these cells (Fig. 2c). Similarly, when cultured in the presence of ICI 182,780, a compound that completely ablates ERα expression (Fig. 2c), MCF-7 cells display ≥ 75% growth inhibition whereas ADAM12-L and ADAM12-S-expressing cells were inhibited significantly less (Fig. 2b). Taken together, these data indicate that ADAM12 expression appears to protect MCF-7 cells from antiestrogen treatment and allows these cells to proliferate in the absence of ERα signaling.
Fig. 2. Effect of antiestrogen treatment on ADAM12-expressing cells.
WT MCF-7 and ADAM12 clones were treated with tamoxifen (3μM) or ICI 182,780 (100nM). In the presence of tamoxifen (a, left panel) and ICI 182,780 (a, right panel) ADAM12-L (blue circle; A12-LC1) and ADAM12-S-expressing (red square; A12-SC4) cells proliferated significantly faster than WT MCF-7 (black diamond) cells. Tamoxifen and ICI 182,780 treatment resulted in significantly greater growth inhibition (versus vehicle control) in WT MCF-7 cells as compared to ADAM12-S-expressing (ADAM12-S clones 1 and 4) and ADAM12-L-expressing (ADAM12-L clones 1 and 4) respectively. These results are expressed as the mean (SD) of three independent experiments. ERα protein expression remained unchanged in vehicle control and tamoxifen treated cells, however, there was complete loss of ERα expression in ICI 182,780 treated cells (c).
ADAM12 isoforms induce estrogen-independent growth of breast tumor cells via upregulation of alternative pathways
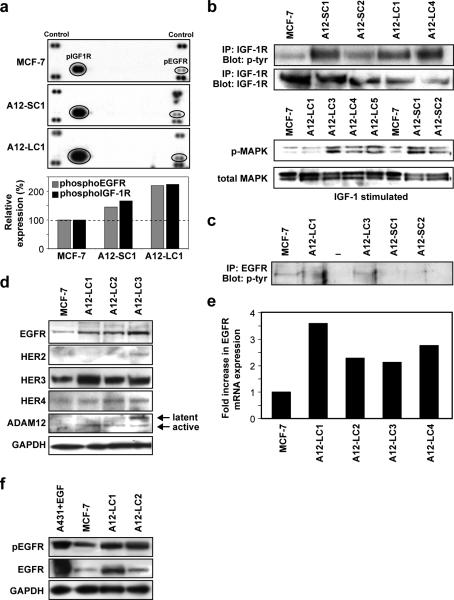
To define the mechanism by which ADAM12-overexpression provides a proliferative advantage to breast tumor cells, we asked whether switching to alternate growth pathways might facilitate estrogen-independent growth in these cells. We determined the phospho-receptor tyrosine kinase (pRTK) profile of serum-activated WT MCF-7 and ADAM12-overexpressing clones. PhosphoEGFR and pIGF-1R levels increased 1.5-2.0-fold in ADAM12-S and ADAM12-L clones, respectively, compared to WT MCF-7 cells (Fig. 3a). Phosphorylation of other receptors of the ErbB family, such as ErbB2, ErbB3 or ErbB4 was not detected. To confirm these findings, we tested the effect of IGF-1 or EGF treatment on cultured cells. Cell lysates were immunoprecipitated with anti-IGF-1R or anti-EGFR antibodies and probed with a pan-phosphotyrosine antibody. We observed increased phosphorylation of IGF-1R in both ADAM12-S and ADAM12-L expressing cells as compared to WT MCF-7 cells (Fig. 3b, upper panel), whereas increased pEGFR levels were detected only in the ADAM12-L-expressing cells (Fig. 3c). Interestingly, levels of pMAPK, a downstream mediator of IGF-1R, were also preferentially elevated in ADAM12-S-expressing clones as compared to WT MCF-7 (Fig. 3b, lower panel). These data, combined with the fact that pIGF-1R levels are higher in ADAM12-S-expressing breast tumor cells, suggest that ADAM12-S expression may stimulate signaling via the IGF-1/IGF-1R pathway and thereby increase proliferation.
Fig. 3. ADAM12-L expression confers estrogen independent growth capability to breast tumor cells via upregulation of EGFR expression.
Receptor tyrosine kinase (RTK) profile of WT MCF-7 and ADAM12 clones (a, upper panel). Densitometric analysis indicated increased levels of pEGFR and pIGF-1R in ADAM12-L (~2-fold) and ADAM12-S clones (~1.3-fold) (a, lower panel). Individual ADAM12-L and ADAM12-S clones are indicated as C1, C2, C3 respectively. Effect of IGF-1 or EGF treatment of ADAM12-expressing clones. Increased pIGF-1R levels were detected in both ADAM12-S and ADAM12-L expressing cells as compared to WT MCF-7 cells (b), whereas increased pEGFR levels were only detected in the ADAM12-L-expressing cells (c). pMAPK levels were elevated in IGF-1 treated ADAM12-S clones as compared to WT MCF-7 (b). Comparative expression of EGF receptor family members in ADAM12-L clones (d). ADAM12-L-expressing clones had increased protein expression of EGFR and HER3 (ErbB3), whereas HER2 (ErbB2) and HER4 (ErbB4) levels remained unchanged (d). EGFR transcript levels were also upregulated in ADAM12-L clones as compared to WT MCF-7 (e). pEGFR levels were higher in ADAM12-L-expressing clones as compared to WT MCF-7, GAPDH is used as a loading control (f).
Since pEGFR was selectively upregulated in response to ADAM12-L expression, we analyzed the expression of EGFR family members in these cells. ADAM12-L expression resulted in significantly higher levels of EGFR protein and transcript as compared to WT MCF-7 (Fig. 3d, e). Though Her3 expression was slightly elevated in the ADAM12-L clones, Her2 and Her4 expression remained unchanged. Upregulation of EGFR expression also correlated with ADAM12 overexpression in T47-D cells, another ERα positive breast cancer cell line. Analysis of WT T47-D and stable clone pools of ADAM12-expressing cells indicated that EGFR protein was significantly upregulated in response to ADAM12 expression whereas ERα levels remained unchanged (Suppl. Fig. 1d). EGF stimulation resulted in higher pEGFR levels in ADAM12-L-expressing clones as compared to WT MCF-7 cells (Fig. 3f). A similar increase in EGFR mRNA and/or protein expression or activation was not observed in the ADAM12-S-expressing clones, where levels remained comparable to WT MCF-7 cells (data not shown). Taken together, the upregulation in EGFR and MAPK activation in ADAM12-L-expressing MCF-7 cells suggests that, in contrast to ADAM12-S expression, ADAM12-L expression may stimulate signaling via the EGFR/MAPK pathway.
ADAM12-L expression stimulates amphiregulin shedding in breast tumor cells
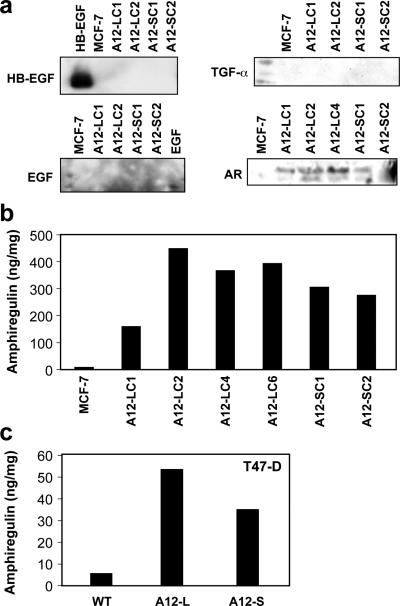
Based on the previously reported sheddase function of ADAM12, we asked whether the increase in EGFR expression in our model could be a consequence of increased EGF ligand shedding. We analyzed serum-free conditioned media (CM) for the presence of a panel of EGFR ligands, HB-EGF, EGF, TGFα and amphiregulin (AR) (Fig. 4a). Conditioned media from ADAM12-L- and ADAM12-S-expressing clones had significantly (10-40-fold) higher levels of free AR as compared to WT MCF-7 (Fig. 4a, b). This increased shedding of AR in response to ADAM12 expression was not limited to MCF-7 cells. In fact, CM from T47-D cells expressing ADAM12 isoforms also had higher AR levels as compared to control cells (Fig. 4c). To our knowledge, this is the first report of ADAM12-mediated AR shedding in breast tumor cells. The increase in AR shedding was a function of ADAM12 expression and not due to a compensatory expression of other proteases such as ADAM9, ADAM10, ADAM15 or ADAM17, since the expression of these remained unchanged (Suppl. Fig. 2a). We did not observe an increase in AR or EGF mRNA expression levels in ADAM12-expressing MCF-7 clones (Suppl. Fig. 2b). Although HB-EGF expression appeared to be upregulated in these cells we did not detect a concomitant increase in HB-EGF shedding in these cells (Fig. 4a).
Fig. 4. ADAM12 expression stimulates amphiregulin shedding in breast tumor cells.
ADAM12 expression stimulated shedding of amphiregulin (AR) but not for HB-EGF, EGF, TGFα or epiregulin. Immunoblot of conditioned medium (a) or amphiregulin elisa (b,c) indicated increased shedding of AR in response to ADAM12 expression in MCF-7 and T47-D cells respectively. The results are representative of three independent experiments.
EGFR and MAPK inhibition results in loss of estrogen-independent growth in ADAM12-expressing breast tumor cells
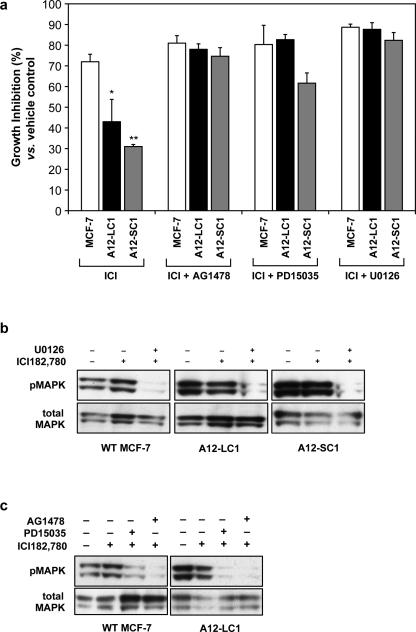
Finally, to test the hypothesis that ADAM12 expression may stimulate EGFR and MAPK signaling and thereby increase cell proliferation and estrogen-independent growth, we analyzed the effects of EGF receptor kinase or MAPK inhibition on proliferation of ADAM12-expressing clones and WT MCF-7. MCF-7 cells displayed ≥75% growth inhibition when treated with ICI 182,780, whereas ADAM12-L and ADAM12-S-expressing cells were inhibited significantly less (~43%; p≤ 0.01 and ~31%; p ≤ 0.001) despite the fact that ICI 182,780 treatment completely abrogated ERα protein expression (Fig. 2c). Treatment of ADAM12-L-expressing cells with the EGFR inhibitor AG1478 (10μM) or the selective EGFR inhibitor, PD15035 (1μM) or the MAPK inhibitor U0126 (10μM) in combination with the ER inhibitor ICI 182,780, resulted in 78-90% growth inhibition in the ADAM12-L-expressing clones and, therefore, a complete loss of resistance to the estrogen inhibitor ICI 182,780 (Fig. 5a). Similar results were observed for ADAM12-S-expressing clones, where AG1478 and U0126 treatment led to 61-82% growth inhibition in response to ICI 182,780 treatment. Interestingly, PD15035 treatment resulted in only 61±5% inhibition in these cells (Fig. 5a), whereas, in ADAM12-L-expressing cells PD15035 treatment resulted in 83±3% growth inhibition (Fig. 5a). Since PD15035 used at lower concentrations is a specific inhibitor of EGFR [29], these data suggest that ADAM12-S-expressing cells may not be as susceptible to EGFR inhibition as are ADAM12-L-expressing cells. Treatment of WT MCF-7 cells with AG1478 or U0126 alone resulted in 30±5% and 45±5% inhibition, whereas treatment of ADAM12-L-expressing cells with AG1478 or U0126 alone resulted in 10±5% and 35±5% inhibition respectively (data not shown). To determine the effectiveness of the inhibitors used in the cell proliferation assay, we analyzed MAPK levels. As expected, U0126 treatment resulted in significantly lower levels of pMAPK in ADAM12-expressing and WT MCF-7 cells (Fig. 5b). In addition, pMAPK levels were also downregulated when ADAM12-expressing clones and WT-MCF-7 cells were treated with the EGFR inhibitors PD15035 and AG1478 (Fig. 5c). Taken together, these results indicate that the increased proliferation observed in ADAM12-L and ADAM12-S clones is likely due to the activation of alternate growth pathways that allows the cells to survive and proliferate even in the absence of estrogen signaling.
Fig. 5. EGFR and MAPK inhibition results in loss of estrogen independent growth in ADAM12-expressing MCF-7 cells.
MCF-7 cells displayed ≥ 75% growth inhibition (vs vehicle control) when treated with ICI 182,780, whereas ADAM12-L and ADAM12-S-expressing cells were inhibited significantly less (~43%; p≤ 0.01 and ~31%; p ≤ 0.001) compared to WT MCF-7 cells. Treatment of ADAM12-L-expressing cells with the EGFR inhibitor AG1478 (10μM) or the selective EGFR inhibitor, PD15035 (1μM) or the MAPK inhibitor U0126 (10μM) in combination with the ER inhibitor ICI 182,780, resulted in a complete loss of resistance to the estrogen inhibitor ICI 182,780. Similarly, AG1478 and U0126 treatment of ADAM12-S-expressing clones led to 61-82% growth inhibition (a). These results are expressed as the mean (SD) of three independent experiments conducted with WT MCF-7 and two individual clones of ADAM12-L- and ADAM12-S-expressing cells respectively. Treatment with the MAPK inhibitor U0126, resulted in significant downregulation of pMAPK levels while total MAPK remained unchanged (b). Similarly, treatment of ADAM12-L-expressing clone LC1 or WT MCF-7 with EGFR inhibitors PD15035 or AG1478 also resulted in downregulation of pMAPK levels (c).
ADAM12 expression is upregulated in tamoxifen-resistant breast tumor cells
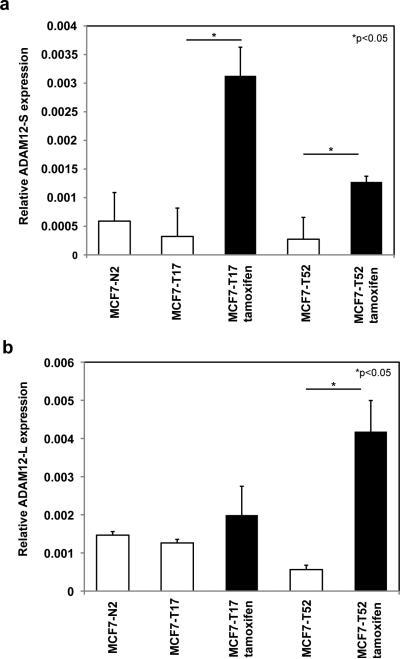
Having demonstrated that ADAM12-expressing MCF-7 cells are resistant to growth inhibition in the presence of antiestrogens such as tamoxifen and ICI 182,780, we analyzed expression of the two isoforms of ADAM12 in tamoxifen-resistant breast tumor cells. Since the proliferation of MCF-7 cells is dependent on estrogen, this cell line has been widely used to explore mechanisms of clinical endocrine therapy resistance. Using tamoxifen-resistant and tamoxifen-sensitive clones of MCF-7 previously established by Coser et. al. [27], we found that basal levels of ADAM12 expression are not significantly different between tamoxifen-resistant and tamoxifen-sensitive MCF-7 clones. However, in the presence of tamoxifen (1μm), ADAM12-S expression was significantly higher in tamoxifen-resistant clones MCFT-17 (15-fold; p≤0.05) and MCF-T52 (7.5-fold; p≤0.05) (Fig. 6a). Similarly, in the presence of tamoxifen (1μm), ADAM12-L levels were also significantly higher in tamoxifen-resistant clones MCF-T17 (2-fold increase) and MCF-T52 (9-fold; p≤0.05) (Fig. 6b). Taken together, our data suggest that the upregulation of ADAM12 expression may be the mechanism by which antiestrogen-resistant breast tumor cells bypass the growth inhibitory effects of estrogen inhibitors.
Fig. 6. ADAM12 expression is upregulated in tamoxifen-resistant breast tumor cells.
ADAM12 expression remains unchanged for tamoxifen-sensitive (MCF-N2) and tamoxifen-resistant (MCF-T17, MCF-T52) cells under basal conditions (vehicle control; white bars). When tamoxifen (1μM) was added to the medium ADAM12-S expression was significantly higher in tamoxifen-resistant clones MCF-T17 (15-fold; *p≤0.05) and MCF-T52 (7.5-fold; *p≤0.05) (a). Similarly, ADAM12-L expression was also upregulated in tamoxifen-resistant clones MCF-T17 (2-fold increase) and MCF-T52 (9-fold; p≤0.05) (b).
Discussion
We have previously reported that elevated levels of urinary ADAM12 are predictive of disease status and stage in breast cancer patients and that ADAM12 protein levels in urine increase with progression of disease [15]. These data suggested an active role for ADAM12 in tumor progression, however, the mechanism(s) by which either ADAM12 isoform might be influencing tumor growth or metastasis remained unknown. In the course of addressing this question, we observed that ADAM12 expression appears to confer a proliferative advantage to MCF-7 cells in the absence of estrogen. In vitro proliferation rates of ADAM12-L and ADAM12-S clones were significantly faster than WT MCF-7 in the absence of estrogen. In addition, while WT MCF-7 cells displayed significant growth inhibition when treated with either the ER antagonist tamoxifen or the ER inhibitor ICI 182,780, ADAM12-L and ADAM12-S clones were significantly less inhibited under the same growth conditions.
Our finding that ADAM12 expression confers estrogen-independence to breast tumor cells is novel and potentially important given that hormone independence is a hallmark of aggressive breast cancer. Acquired resistance to endocrine therapy in breast cancer is a clinical challenge. In the current study, RTK phosphoproteome analysis indicated an increase in pEGFR and pIGF-1R levels in response to ADAM12 expression. In ADAM12-L-expressing cells, AR shedding was enhanced, and EGFR transcript and protein levels were higher, compared to WT MCF-7 suggesting a positive feed-back loop that may be driving the upregulation of EGFR expression. Importantly, ADAM10 and ADAM17, two known AR sheddases, were not involved in this increased shedding of AR. We did not observe any difference in their expression levels in the ADAM12-overexpressing clones. To our knowledge, this is the first report to describe ADAM12-mediated shedding of AR and a concomitant increase in EGFR expression and activation which ultimately leads to increased proliferation of breast tumor cells. The importance of the EGFR signaling pathway in the estrogen-independent growth of ADAM12-L expressing cells was highlighted by the effect of EGFR inhibitors AG1478 and PD15035 or MAPK inhibitor U0126, each of which abrogated the antiestrogen resistance in these cells. Upregulation of EGFR and heregulin 2 expression has recently been attributed to acquired resistance of breast cancer cells to the antiestrogen, fulvestrant [5]. Similarly, upregulation of autocrine AR signaling via the EGFR pathway has previously been correlated with cisplatin resistance in breast cancer cells [30]. Androgen-sensitive prostate cancer cells, when continuously exposed to HB-EGF, another EGF ligand, proliferate independently of androgen signaling [31]. Therefore, the increased shedding of AR by ADAM12-L could be the potential mechanism by which this protease confers estrogen-independent proliferation in breast tumor cells.
Interestingly, we detected an upregulation of ADAM12-L and ADAM12-S expression in tamoxifen-resistant MCF-7 cells when these cells were treated with the antiestrogen. This finding is consistent with our in vitro data and suggests that the upregulation of ADAM12 expression and increased AR shedding leads to a switch to EGFR signaling that may rescue estrogen dependent MCF-7 cells from the growth inhibitory effects of estrogen inhibitors such as tamoxifen.
Endogenous EGFR expression has been reported to be low in WT MCF-7 cells [28,32,33]. In ADAM12-L-expressing MCF-7 cells however, both EGFR protein and mRNA levels were significantly elevated. This effect on EGFR expression was also recapitulated in ADAM12-expressing T47-D cells, another ERα positive breast tumor cell line. Consistent with our results for MCF-7, pooled T47-D clones expressing ADAM12-S displayed less growth inhibition in the presence of ICI 182,780 as compared to WT T47-D (Suppl. Fig. 1e). ADAM12 expression has not previously been correlated with enhanced cancer cell proliferation although its effect on proliferation of normal cells has been reported. For example, ADAM12 was reported to regulate bronchial epithelial cell proliferation and apoptosis in a HB-EGF and EGFR-dependent manner [34]. In osteoarthritis, ADAM12-L has been reported to enhance chondrocyte proliferation via the degradation of IGFBP-5 and increased bioavailability of IGF [35]. Our data represent the first demonstration that ADAM12-L overexpression in tumor cells can be directly linked to upregulation of EGFR mRNA and protein expression and downstream MAPK activation and cellular proliferation.
Interestingly, we found that although the ADAM12-S-expressing cells display increased AR shedding, there does not appear to be a concomitant upregulation of EGFR protein or mRNA expression or activation. Instead, our data suggest that ADAM12-S expression in breast tumor cells may lead to activation of IGF-1R and MAPK resulting in increased proliferation. Mammary stromal and breast cancer cells are known to express IGFs and IGFBPs [36]. ADAM12-S cleaves IGFBP-3 and -5 [14] such that one might speculate that increased bioavailability of free IGF could result in increased activation of IGF-1R and increased proliferation of breast tumor cells in our model.
Taken together, our data suggests that overexpression of either transmembrane or secreted isoforms of ADAM12 results in estrogen-independent growth in breast tumor cells. Wild type MCF-7 proliferation primarily depends on ERα signaling, expression of ADAM12 isoforms appears to allow a switch to EGFR and/or IGF-1R signaling in these cells, thereby rescuing MCF-7 cells from growth inhibition in the absence of estrogen or presence of antiestrogens in vitro ultimately resulting in increased tumor growth in vivo.
Our study suggests a potentially novel role for ADAM12 in breast tumor resistance to antiestrogen therapy regimens via the upregulation of alternate growth pathways. Although further studies are required to establish a correlation between ADAM12 function and antiestrogen resistance, targeting ADAM12 and EGFR and/or IGF-1R pathways in combination with hormone therapy may represent a novel approach to address antiestrogen resistance in human breast cancer.
Conclusions
In conclusion, we have shown that expression of ADAM12 transmembrane and secreted isoforms confer estrogen-independent growth capabilities in breast tumor cells via distinct mechanisms. In ADAM12-L-expressing cells, this estrogen-independence was a direct result of increased EGFR expression and MAPK activation, whereas, the mechanism in ADAM12-S-expressing cells may be enhanced IGF-1R signaling. Taken together, our data suggest a novel role of ADAM12 in mediating antiestrogen-resistance in breast tumor cells and that targeting ADAM12 in combination with hormone therapy, alone or along with EGFR and/or IGF-1R pathways, may represent a novel approach to address antiestrogen resistance in human breast cancer.
Acknowledgements
This work is dedicated to the memory of our mentor, the late M. Judah Folkman, M.D. The authors are grateful for his enthusiastic interest in this work and for many helpful discussions and suggestions. Tamoxifen-resistant MCF-7 cells were the kind gift of Dr. Toshi Shioda (Massachusetts General Hospital). This work was supported by The Breast Cancer Research Foundation, NIH PO1 CA45548, NIH R01 CA118764, The JoAnn Webb Fund for Angiogenesis Research and The Fortin Foundation. We thank Kristin Johnson for assistance with the figures.
Abbreviations
- ADAM
a disintegrin and metalloprotease
- ER
estrogen receptor
- EGFR/her1
epidermal growth factor receptor
- Her2
epidermal growth factor receptor 2
- Her3
epidermal growth factor receptor 3
- MAPK
mitogen-activated protein kinase
- IGF-1R
insulin-like growth factor receptor 1
- IGFBP
insulin-like growth factor binding protein
- EGF
epidermal growth factor
- HB-EGF
heparin binding-epidermal growth factor
- AR
amphiregulin
References
- 1.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast-cancer incidence in 2003 in the united states. N Engl J Med. 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105. doi:356/16/1670 [pii]10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Fuqua SA. Mechanisms of tamoxifen resistance. Breast Cancer Res Treat. 1994;32(1):49–55. doi: 10.1007/BF00666205. [DOI] [PubMed] [Google Scholar]
- 3.McCune K, Bhat-Nakshatri P, Thorat MA, Nephew KP, Badve S, Nakshatri H. Prognosis of hormone-dependent breast cancers: Implications of the presence of dysfunctional transcriptional networks activated by insulin via the immune transcription factor t-bet. Cancer Res. 70(2):685–696. doi: 10.1158/0008-5472.CAN-09-1530. doi:0008-5472.CAN-09-1530 [pii]10.1158/0008-5472.CAN-09-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masri S, Liu Z, Phung S, Wang E, Yuan YC, Chen S. The role of microrna-128a in regulating tgfbeta signaling in letrozole-resistant breast cancer cells. Breast Cancer Res Treat. doi: 10.1007/s10549-009-0716-3. doi:10.1007/s10549-009-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, Rasmussen LM, Riese DJ, 2nd, de Cremoux P, Stenvang J, Lykkesfeldt AE. Activation of erbb3, egfr and erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat. 2009;114(2):263–275. doi: 10.1007/s10549-008-0011-8. doi:10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonne-Hansen K, Norrie IC, Emdal KB, Benjaminsen RV, Frogne T, Christiansen IJ, Kirkegaard T, Lykkesfeldt AE. Breast cancer cells can switch between estrogen receptor alpha and erbb signaling and combined treatment against both signaling pathways postpones development of resistance. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0506-y. doi:10.1007/s10549-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 7.Ghayad SE, Vendrell JA, Larbi SB, Dumontet C, Bieche I, Cohen PA. Endocrine resistance associated with activated erbb system in breast cancer cells is reversed by inhibiting mapk or pi3k/akt signaling pathways. Int J Cancer. 126(2):545–562. doi: 10.1002/ijc.24750. doi:10.1002/ijc.24750. [DOI] [PubMed] [Google Scholar]
- 8.Iba K, Albrechtsen R, Gilpin BJ, Loechel F, Wewer UM. Cysteine-rich domain of human adam 12 (meltrin alpha) supports tumor cell adhesion. Am J Pathol. 1999;154(5):1489–1501. doi: 10.1016/s0002-9440(10)65403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, Asanuma H, Sanada S, Matsumura Y, Takeda H, Beppu S, Tada M, Hori M, Higashiyama S. Cardiac hypertrophy is inhibited by antagonism of adam12 processing of hb-egf: Metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8(1):35–40. doi: 10.1038/nm0102-35. doi:10.1038/nm0102-35nm0102-35 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi K, Zhou HM, Kelly K, Manova K, Blobel CP. Evaluation of the contributions of adams 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins beta1 and beta2. Dev Biol. 2005;283(2):459–471. doi: 10.1016/j.ydbio.2005.05.004. doi:S0012-1606(05)00298-8 [pii]10.1016/j.ydbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Dyczynska E, Sun D, Yi H, Sehara-Fujisawa A, Blobel CP, Zolkiewska A. Proteolytic processing of delta-like 1 by adam proteases. J Biol Chem. 2007;282(1):436–444. doi: 10.1074/jbc.M605451200. doi:M605451200 [pii]10.1074/jbc.M605451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito N, Nomura S, Iwase A, Ito T, Kikkawa F, Tsujimoto M, Ishiura S, Mizutani S. Adams, a disintegrin and metalloproteinases, mediate shedding of oxytocinase. Biochem Biophys Res Commun. 2004;314(4):1008–1013. doi: 10.1016/j.bbrc.2003.12.183. doi:S0006291X03028079 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Loechel F, Gilpin BJ, Engvall E, Albrechtsen R, Wewer UM. Human adam 12 (meltrin alpha) is an active metalloprotease. J Biol Chem. 1998;273(27):16993–16997. doi: 10.1074/jbc.273.27.16993. [DOI] [PubMed] [Google Scholar]
- 14.Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM. Adam 12-s cleaves igfbp-3 and igfbp-5 and is inhibited by timp-3. Biochem Biophys Res Commun. 2000;278(3):511515. doi: 10.1006/bbrc.2000.3835. doi:10.1006/bbrc.2000.3835S0006-291X(00)93835-X [pii] [DOI] [PubMed] [Google Scholar]
- 15.Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA. Adam 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem. 2004;279(49):51323–51330. doi: 10.1074/jbc.M409565200. [DOI] [PubMed] [Google Scholar]
- 16.Kveiborg M, Frohlich C, Albrechtsen R, Tischler V, Dietrich N, Holck P, Kronqvist P, Rank F, Mercurio AM, Wewer UM. A role for adam12 in breast tumor progression and stromal cell apoptosis. Cancer Res. 2005;65(11):4754–4761. doi: 10.1158/0008-5472.CAN-05-0262. doi:65/11/4754 [pii]10.1158/0008-5472.CAN-05-0262. [DOI] [PubMed] [Google Scholar]
- 17.Kveiborg M, Albrechtsen R, Couchman JR, Wewer UM. Cellular roles of adam12 in health and disease. Int J Biochem Cell Biol. 2008;40(9):1685–1702. doi: 10.1016/j.biocel.2008.01.025. doi:S1357-2725(08)00047-2 [pii]10.1016/j.biocel.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Lendeckel U, Kohl J, Arndt M, Carl-McGrath S, Donat H, Rocken C. Increased expression of adam family members in human breast cancer and breast cancer cell lines. J Cancer Res Clin Oncol. 2005;131(1):41–48. doi: 10.1007/s00432-004-0619-y. doi:10.1007/s00432-004-0619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frohlich C, Albrechtsen R, Dyrskjot L, Rudkjaer L, Orntoft TF, Wewer UM. Molecular profiling of adam12 in human bladder cancer. Clin Cancer Res. 2006;12(24):7359–7368. doi: 10.1158/1078-0432.CCR-06-1066. doi:12/24/7359 [pii] 10.1158/1078-0432.CCR-06-1066. [DOI] [PubMed] [Google Scholar]
- 20.Kodama T, Ikeda E, Okada A, Ohtsuka T, Shimoda M, Shiomi T, Yoshida K, Nakada M, Ohuchi E, Okada Y. Adam12 is selectively overexpressed in human glioblastomas and is associated with glioblastoma cell proliferation and shedding of heparin-binding epidermal growth factor. Am J Pathol. 2004;165(5):1743–1753. doi: 10.1016/S0002-9440(10)63429-3. doi:165/5/1743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carl-McGrath S, Lendeckel U, Ebert M, Roessner A, Rocken C. The disintegrin-metalloproteinases adam9, adam12, and adam15 are upregulated in gastric cancer. Int J Oncol. 2005;26(1):17–24. [PubMed] [Google Scholar]
- 22.Markowski J, Oczko-Wojciechowska M, Gierek T, Jarzab M, Paluch J, Kowalska M, Wygoda Z, Pfeifer A, Tyszkiewicz T, Jarzab B, Niedzielska I, Borgiel-Marek H. Gene expression profile analysis in laryngeal cancer by high-density oligonucleotide microarrays. J Physiol Pharmacol. 2009;60(Suppl 1):57–63. [PubMed] [Google Scholar]
- 23.Mino N, Miyahara R, Nakayama E, Takahashi T, Takahashi A, Iwakiri S, Sonobe M, Okubo K, Hirata T, Sehara A, Date H. A disintegrin and metalloprotease 12 (adam12) is a prognostic factor in resected pathological stage i lung adenocarcinoma. J Surg Oncol. 2009;100(3):267–272. doi: 10.1002/jso.21313. doi:10.1002/jso.21313. [DOI] [PubMed] [Google Scholar]
- 24.Le Pabic H, Bonnier D, Wewer UM, Coutand A, Musso O, Baffet G, Clement B, Theret N. Adam12 in human liver cancers: Tgf-beta-regulated expression in stellate cells is associated with matrix remodeling. Hepatology. 2003;37(5):1056–1066. doi: 10.1053/jhep.2003.50205. doi:10.1053/jhep.2003.50205S0270913903002544 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Pories SE, Zurakowski D, Roy R, Lamb CC, Raza S, Exarhopoulos A, Scheib RG, Schumer S, Lenahan C, Borges V, Louis GW, Anand A, Isakovich N, Hirshfield-Bartek J, Wewer U, Lotz MM, Moses MA. Urinary metalloproteinases: Noninvasive biomarkers for breast cancer risk assessment. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1034–1042. doi: 10.1158/1055-9965.EPI-07-0365. doi:17/5/1034 [pii]10.1158/1055-9965.EPI-07-0365. [DOI] [PubMed] [Google Scholar]
- 26.Roy R BD, Rodig S, Moses M. Adam12 long and short isoforms promote breast tumor growth and metastasis via distinct mechanisms [abstract].. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Washington, DC Philadelphia (PA). Apr 17-21; 2010. Abstract nr 416. [Google Scholar]
- 27.Coser KR, Wittner BS, Rosenthal NF, Collins SC, Melas A, Smith SL, Mahoney CJ, Shioda K, Isselbacher KJ, Ramaswamy S, Shioda T. Antiestrogen-resistant subclones of mcf-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor. Proc Natl Acad Sci U S A. 2009;106(34):14536–14541. doi: 10.1073/pnas.0907560106. doi:106/34/14536 [pii] 10.1073/pnas.0907560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbb2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant mcf-7 cells. Endocrinology. 2003;144(3):1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 29.Bos M, Mendelsohn J, Kim YM, Albanell J, Fry DW, Baselga J. Pd153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clin Cancer Res. 1997;3(11):2099–2106. [PubMed] [Google Scholar]
- 30.Eckstein N, Servan K, Girard L, Cai D, von Jonquieres G, Jaehde U, Kassack MU, Gazdar AF, Minna JD, Royer HD. Epidermal growth factor receptor pathway analysis identifies amphiregulin as a key factor for cisplatin resistance of human breast cancer cells. J Biol Chem. 2008;283(2):739–750. doi: 10.1074/jbc.M706287200. doi:M706287200 [pii]10.1074/jbc.M706287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adam RM, Kim J, Lin J, Orsola A, Zhuang L, Rice DC, Freeman MR. Heparin-binding epidermal growth factor-like growth factor stimulates androgen-independent prostate tumor growth and antagonizes androgen receptor function. Endocrinology. 2002;143(12):4599–4608. doi: 10.1210/en.2002-220561. [DOI] [PubMed] [Google Scholar]
- 32.Larsen SS, Egeblad M, Jaattela M, Lykkesfeldt AE. Acquired antiestrogen resistance in mcf-7 human breast cancer sublines is not accomplished by altered expression of receptors in the erbb-family. Breast Cancer Res Treat. 1999;58(1):41–56. doi: 10.1023/a:1006232830161. [DOI] [PubMed] [Google Scholar]
- 33.deFazio A, Chiew YE, Sini RL, Janes PW, Sutherland RL. Expression of c-erbb receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer. 2000;87(4):487–498. doi:10.1002/1097-0215(20000815)87:4<487::AID-IJC5>3.0.CO;2-J [pii] [PubMed] [Google Scholar]
- 34.Rocks N, Estrella C, Paulissen G, Quesada-Calvo F, Gilles C, Gueders MM, Crahay C, Foidart JM, Gosset P, Noel A, Cataldo DD. The metalloproteinase adam-12 regulates bronchial epithelial cell proliferation and apoptosis. Cell Prolif. 2008;41(6):988–1001. doi: 10.1111/j.1365-2184.2008.00557.x. doi:CPR557 [pii] 10.1111/j.1365-2184.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada A, Mochizuki S, Yatabe T, Kimura T, Shiomi T, Fujita Y, Matsumoto H, Sehara-Fujisawa A, Iwamoto Y, Okada Y. Adam-12 (meltrin alpha) is involved in chondrocyte proliferation via cleavage of insulin-like growth factor binding protein 5 in osteoarthritic cartilage. Arthritis Rheum. 2008;58(3):778–789. doi: 10.1002/art.23262. doi:10.1002/art.23262. [DOI] [PubMed] [Google Scholar]
- 36.Wood TL, Richert MM, Stull MA, Allar MA. The insulin-like growth factors (igfs) and igf binding proteins in postnatal development of murine mammary glands. J Mammary Gland Biol Neoplasia. 2000;5(1):31–42. doi: 10.1023/a:1009511131541. [DOI] [PubMed] [Google Scholar]