Summary
Relaxin is an approximately 6-kilodalton peptide hormone secreted by the corpus luteum, and circulates in the maternal blood during pregnancy. Relaxin administration to awake, chronically instrumented, nonpregnant rats mimics the vasodilatory phenomena of pregnancy. Furthermore, immunoneutralization of relaxin or its elimination from the circulation during midterm pregnancy in awake rats prevents maternal systemic and renal vasodilation, and the increase in global arterial compliance. Human investigation, albeit limited through 2009, also reveals vasodilatory effects of relaxin in the nonpregnant condition and observations consistent with a role for relaxin in gestational hyperfiltration. Recent evidence suggests that the vasodilatory responses of relaxin are mediated by its major receptor, the relaxin/insulin-like family peptide 1 receptor, RFXP1. The molecular mechanisms of relaxin vasodilation depend on the duration of hormone exposure (ie, there are rapid and sustained vasodilatory responses). Newly emerging data support the role of Gai/o protein coupling to phosphatidylinositol-3 kinase/Akt (protein kinase B)-dependent phosphorylation and activation of endothelial nitric oxide synthase in the rapid vasodilatory responses of relaxin. Sustained vasodilatory responses critically depend on vascular endothelial and placental growth factors, and increases in arterial gelatinase(s) activity. Gelatinases hydrolyze big endothelin (ET) at a gly-leu bond to form ET1–32, which activates the endothelial ETB/nitric oxide vasodilatory pathway. Although the relevance of relaxin biology to preeclampsia is largely speculative at this point in time, there are several potential tantalizing links that are discussed in the context of our current understanding of the etiology and pathophysiology of the disease.
Keywords: Preeclampsia, intrauterine growth restriction, assisted reproductive technology, placenta, endometrium, decidualization, trophoblast, hypoxia, cardiovascular, arterial compliance, renal circulation, nitric oxide, endothelin, matrix metalloproteinases, angiogenic growth factors
Relaxin, an approximately 6-kilodalton peptide hormone secreted by the corpus luteum, circulates in the blood during pregnancy in human beings, nonhuman primates, rats, and mice1 (Fig. 1, and see Appendix for relaxin ligand and receptor nomenclature). The hormone also is detectable in the circulation during the luteal phase of the menstrual cycle in both women and nonhuman primates.1 Besides the ovary, there is local expression of relaxin and its major receptor, relaxin/insulin-like family peptide receptor 1 (RXFP1), by various cell types in the female reproductive tract including the nonpregnant endometrium, decidual cells in the pregnant endometrium, and in cytotrophoblasts and syncytiotrophoblasts.1 Local expression of relaxin and its receptor also has been described in blood vessels. 2,3 Thus, in addition to its endocrine actions, there are autocrine and/or paracrine roles for relaxin.
Figure 1.
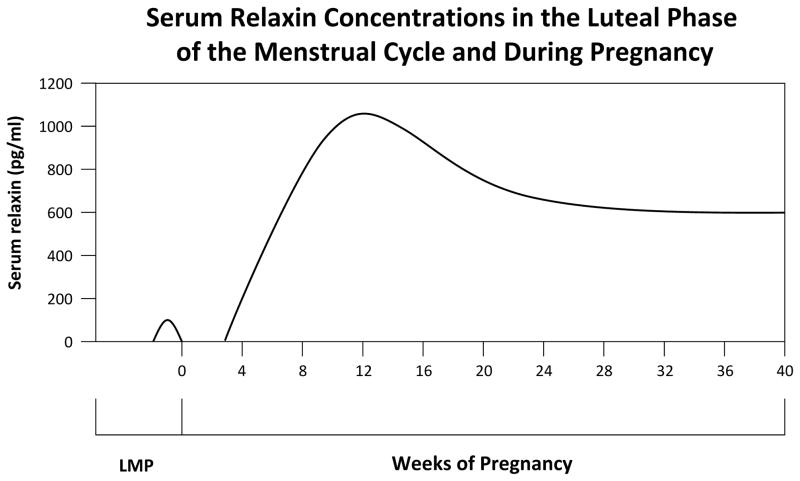
Serum concentrations of relaxin in the luteal phase of the menstrual cycle and during pregnancy in human beings. Figure 1 is based on data from Szlachter et al, 126 Stewart et al,135 and O’Byrne et al.136
Traditionally, relaxin has been investigated in the context of the reproductive tract.1 However, Hisaw4 and Ziel et al,5 the hormone’s founders, appear also as the first to provide evidence (albeit structural in nature) that the vasculature, too, is a relaxin target tissue. They administered relaxin to ovariectomized monkeys noting marked changes consistent with hypertrophy and hyperplasia in endothelial cells of endometrial vessels, as well as enlargement of arterioles and capillaries.6,7 These findings also suggested an angiogenic role for relaxin and this subsequently was shown (reviewed by Conrad and Novak8 and Jeyabalan et al9). Evidence for a vasodilatory role of the hormone was reported by St-Louis and Massicotte,10 who showed that chronic infusion of purified rat or porcine relaxin decreased systolic blood pressure in female SHR, but not WKY rats. This group subsequently showed that short-term administration of purified rat relaxin decreased mean arterial pressure in female SHR rats as early as 8 hours after starting the infusion, and that vasoconstrictor responses to norepinephrine and arginine vasopressin were attenuated in the animals’ mesenteric circulation perfused in situ.11 However a report by Ahokas et al12 that the decline in systolic blood pressure and decrease in vascular reactivity to angiotensin II were comparable in gravid SHR rats with (circulating relaxin present) and without (relaxin absent) ovaries created doubt for an important physiological vascular role of circulating relaxin. However, evidence for such vascular effects subsequently were provided by Bani-Sacchi et al13 who used Langendorff preparations to show that relaxin acutely increased coronary blood flow in rat and guinea pig hearts. Searching mechanisms they noted that the vasodilatory action of relaxin in the coronary circulation was prevented by NG-monomethyl-L-arginine, a nitric oxide synthase (NOS) inhibitor.13
More recent data pertinent to relaxin’s role as a vasodilatory hormone relate to studies from investigations of renal and cardiovascular adaptations to pregnancy, and form the objectives of this review: (1) to highlight the vasodilatory actions of relaxin, particularly in pregnancy; (2) to outline perspectives on the mechanisms underlying the hormone’s vasodilatory role, and (3) to consider the implications of relaxin biology to preeclampsia.
MATERNAL ADAPTATIONS IN NORMAL PREGNANCY
Systemic Hemodynamics and Arterial Mechanical Properties
The maternal circulation is markedly vasodilated throughout gestation. In human beings, systemic vascular resistance (SVR) decreases and cardiac output increases reciprocally by approximately 50%, reaching nadir and peak, respectively, by the end of the first or the beginning of the second trimester (reviewed by Jeyabalan and Conrad14). Similar vasodilation occurs during pregnancy in chronically instrumented, awake rats.15,16 In both species, the increase in cardiac output during early gestation is in apparent anticipation of the high oxygen and nutrient demands of the rapidly growing placenta and fetus that occur mainly in the second half of pregnancy.15,17 Consistent with this concept is the narrowing of the difference in oxygen content between arterial and mixed venous blood, consequent to oxygen delivery exceeding demand at this early stage of gestation.15,18 Interestingly, hemodynamic changes similar to human gestation, but of a lesser degree, occur in the luteal phase of the menstrual cycle when SVR decreases and cardiac output increases (relative to the follicular stage).14,19 Coincidentally, relaxin emanates from the corpus luteum during the luteal phase of the menstrual cycle, producing low (compared with pregnancy) but readily detectable concentrations of the hormone.1
Global arterial compliance (AC) increases during human pregnancy in parallel with the systemic hemodynamic changes.20 In keeping with this, other measures indicative of increasing AC—augmentation index, carotid-radial and carotid-femoral pulse wave velocities—all significantly decline during early pregnancy.21 Similarly, the gestational decline in augmentation index is anticipated in the menstrual cycle’s luteal phase,21 and increases in global AC also occur in chronically instrumented, awake pregnant rats.22 In the face of large increases in stroke volume and cardiac output and decrease in SVR, the simultaneous increase in global AC is pivotal to cardiovascular homeostasis during pregnancy by maintaining efficient ventricular-arterial coupling and diastolic perfusion pressure.20,23
Renal Hemodynamics
Renal vascular resistance decreases in early pregnancy and is a major contributor to the overall reduction in SVR. Consequently, renal plasma flow (RPF) and glomerular filtration rate (GFR) both increase by approximately 50% compared with nonpregnant levels (reviewed by Jeyabalan and Conrad,14 Conrad et al,24 and Conrad25). Comparable changes, but of lesser magnitude, occur in the luteal phase of the menstrual cycle (relative to the follicular stage),14,24,25 when serum relaxin becomes detectable.1 Increases in RPF and GFR also transpire during pregnancy in chronically instrumented, conscious rats, peaking at midterm.14,25,26
RELAXIN ADMINISTRATION MIMICS VASODILATORY CHANGES OF PREGNANCY
Systemic Hemodynamics and Arterial Mechanical Properties
Administration of recombinant human relaxin by subcutaneous osmotic pump to chronically instrumented, awake, virgin female rats significantly decreased SVR and increased cardiac output by approximately 20%.23 A comparable magnitude of change in systemic hemodynamics was reported in midterm pregnant rats.15,16 Similar changes in systemic hemodynamics in response to chronic relaxin administration also were observed in male and female, normotensive control and hypertensive rats.27,28 In contrast, short-term intravenous infusion of relaxin for several hours was only vasodilatory in the angiotensin II model of hypertension, but not in SHR or normotensive rats.28 Of note, in all of these studies of chronically instrumented, awake rats, the vasodilatory action of relaxin failed to decrease mean arterial pressure because the SVR decrement was compensated by an increase in cardiac output.23,27,28 Indeed, in the face of the marked decrease in SVR during pregnancy, there is but a modest decline in mean and diastolic arterial pressures owing to the reciprocal increases in cardiac output and global arterial compliance, respectively.20,23
There are recent data revealing the effects of relaxin on systemic hemodynamics in human beings. Based on the potentially therapeutic profile of relaxin on systemic hemodynamics and arterial mechanical properties, as well as renal hemodynamics (presented later), relaxin was proposed as a novel means to reduce ventricular after load in congestive heart failure.23 Indeed, in a phase I trial of relaxin in stable congestive heart failure, Dschietzig et al29 showed that the hormone decreased SVR and increased cardiac output, and improved renal function. They further showed that relaxin decreased pulmonary capillary wedge pressure and N-terminal pro brain natriuretic peptide, findings not predicted from the studies in normal healthy rats. The differences in the circulating concentration of relaxin attained or the pathologic setting of congestive heart failure may explain these additional findings in human beings.29 Thus, the systemic vasodilatory action of relaxin initially described in conscious rats may translate to human beings.
By using methodology developed for its use in chronically instrumented, conscious rats,23 global AC was assessed using two independent approaches: (1) diastolic decay of aortic pressure, and (2) ratio of stroke volume-to-pulse pressure. The results were comparable for the two methods. In the same experiments in which systemic hemodynamics were measured, administration of relaxin by subcutaneous osmotic pump significantly augmented global AC by approximately 20% in chronically instrumented, awake female rats.23 Similar increases of global AC in response to chronic relaxin administration were observed in male and female, normotensive control and hypertensive rats.27,28 In contrast, short-term intravenous infusion of relaxin over several hours only increased global AC in the angiotensin II model of hypertension, and not in SHR or normotensive rats.28
In addition to large arteries, small arteries contribute to global AC.30 Small renal arteries dissected from female rats after 5 days of relaxin administration showed increases in passive compliance relative to controls.23 Moreover, small renal arteries from relaxin knock-out mice were stiffer than those from wild-type animals.3 These results implicate alterations in vascular structure (ie, cellular components or extracellular matrix), in addition to decreased vascular smooth muscle tone in the relaxin-induced increase in global AC.23,31
Renal Hemodynamics
Chronic administration of recombinant human or porcine relaxin by subcutaneous osmotic pump to chronically instrumented, awake, virgin female rats reduced renal vascular resistance, and increased RPF and GFR to levels observed during midterm pregnancy, when renal function peaks in this species.26,32 This vasodilatory effect of relaxin was observed in ovariectomized female32 and male rats.33 Also, chronic relaxin treatment attenuated the renal vasoconstrictor response to intravenous angiotensin II,32 and a similar attenuated renal pressor response to angiotensin II also occurred during rat gestation.34–36 In anesthetized male rats, chronic subcutaneous osmotic pump administration of relaxin increased RPF, but not GFR,37 and small renal arteries isolated from rats chronically administered relaxin showed inhibited myogenic reactivity.38 This latter finding is identical to the inhibition of myogenic reactivity in small renal arteries isolated from midterm pregnant rats.39
Short-term administration by infusion (1–6 hours intravenously) or osmotic pump (subcutaneously) of relaxin to conscious rats also produced significant renal vasodilation and hyperfiltration.40 In anesthetized male rats, 2-hour intravenous infusion of relaxin increased RPF, but not GFR.37 In normal human subjects, short-term intravenous infusion of relaxin for 6 hours increased RPF by 60%, but surprisingly did not increase GFR.41 This renal vasodilatory effect occurred in both men and women as early as 30 minutes after starting the infusion, and with no significant changes in blood pressure.41 Finally, during 26 weeks of subcutaneous relaxin infusion in patients with mild scleroderma, the creatinine clearance (calculated as estimated GFR) increased by approximately 20% and diastolic blood pressure declined slightly, but significantly.42,43 Thus, the renal vasodilatory effects of relaxin first described in awake rats is likely to translate to human beings, although GFR was increased inconsistently among the various studies in rats and human beings.
RELAXIN IMMUNONEUTRALIZATION OR ELIMINATION FROM THE CIRCULATION INHIBITS MATERNAL VASODILATION OF PREGNANCY
Systemic Hemodynamics and Arterial Mechanical Properties
Relaxin is essential to the alterations in systemic hemodynamics and arterial mechanical properties during midterm pregnancy in conscious rats.22 The increase in cardiac output and global AC and decrease in SVR during midterm pregnancy were prevented by daily administration of rat relaxin-neutralizing antibodies when started on day 8.22 However, in preliminary studies, relaxin immunoneutralization during late pregnancy only partly prevented the approximately 45% gestational increase in cardiac output and global AC and decrease in SVR, suggesting that other hormones (possibly of placental origin) may contribute at this stage of gestation (unpublished data). Whether relaxin might contribute to the gestational changes in systemic hemodynamics and global AC in pregnant women is currently under investigation.
Renal Hemodynamics
Relaxin is also critical for the changes in renal hemodynamics during midterm pregnancy in conscious rats.44 The gestational increases in RPF and GFR and decrease in renal vascular resistance observed during midterm pregnancy were completely prevented either by ovariectomy (while maintaining pregnancy with exogenous estrogen and progesterone), or daily administration of rat relaxin-neutralizing antibodies beginning on day 8.44 These interventions also precluded the gestational inhibition of myogenic reactivity in small renal arteries isolated from the same rats.44
In women with ovarian failure made pregnant through egg donation, in vitro fertilization, and embryo transfer, the first trimester increase in GFR was blunted significantly.45 These women lacked ovarian function and a corpus luteum, and circulating relaxin was undetectable,1 implicating relaxin in the initiation of gestational hyperfiltration. However, unlike gravid rats in which the gestational increase in GFR was completely dependent on circulating relaxin, a partial increase in GFR may have persisted in these pregnant women despite the absence of circulating relaxin.
MOLECULAR MECHANISMS OF RENAL VASODILATION AND HYPERFILTRATION DURING PREGNANCY OR RELAXIN ADMINISTRATION
The vasodilatory mechanisms of relaxin may differ depending on the duration of hormone exposure (ie, there are sustained and rapid vasodilatory responses to relaxin).
SUSTAINED VASODILATORY RESPONSES
Nitric Oxide
When a major role for prostaglandins in the vasodilatory phenomena of pregnancy proved unlikely,24,25 attention turned to the more recently discovered and attractive candidate, endothelium-derived relaxing factor (Fig. 2). First guanosine 3′, 5′-cyclic monophosphate (cGMP), a major second messenger of endothelium-derived relaxing factor, or NO was studied because its extracellular levels reflect intracellular production. Plasma levels, 24-hour urinary excretion, and the “metabolic production rate” of cGMP were measured in conscious rats, with increases in all observed during pregnancy and pseudopregnancy.46,47 Comparable increments in plasma levels and urinary excretion were reported for human pregnancy.48–50 These studies were consistent with the potential role for NO in the vasodilation of pregnancy.
Figure 2.
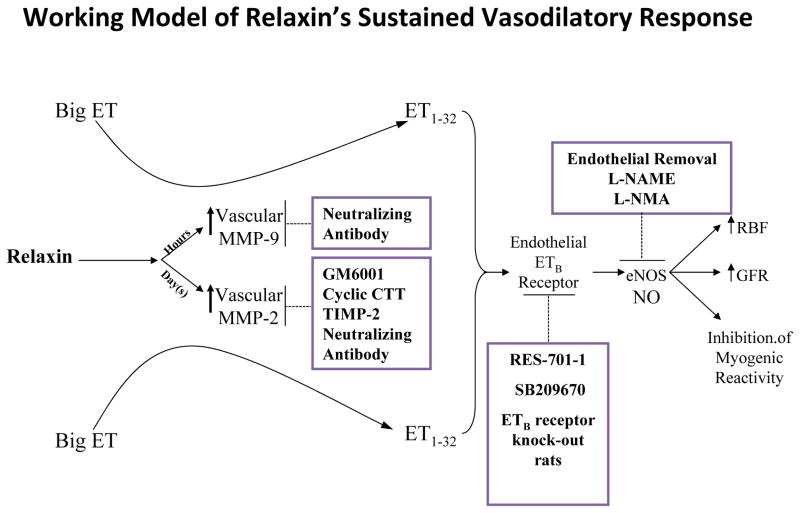
Working model for the sustained vasodilatory responses of relaxin. Inhibitors of relaxin vasodilation are shown in the boxes. GM6001, general MMP inhibitor; RES-701–1, specific ETB receptor antagonist; SB209670, mixed ETA and ETB receptor antagonist; L-NAME, nitro-L-arginine methyl ester; L-NMMA, NG-monomethyl-L-arginine. Note that phosphoramidon (an inhibitor of the classic ET-converting enzyme), STT (control peptide for cyclic CTT), heat-inactivated TIMP-2, BQ-123 (a specific ETA-receptor antagonist), D-NAME, and IgGs (control antibodies for MMP neutralizing antibodies) did not affect the slow vasodilatory responses of relaxin. Not depicted in this schema are the roles of the Lgr7 (RXFP1) receptor, and VEGFs and PGFs in mediating the vasodilatory actions of relaxin as published in preliminary reports. See text for further details.
Upon identifying endothelial-derived re laxing factor as NO,51 the stable metabolites, nitrate and nitrite (NOx), were explored. Twenty-four–hour urinary NOx increased in pregnant and pseudopregnant rats consuming low-NOx diets, paralleling an increase in cGMP.52 This increase was inhibited by nitro-L-arginine methyl ester administration, which provides strong evidence that the increment derived from NO, and ultimately its substrate L-arginine.52 Plasma levels of NOx also were increased during rat gestation, and NO-hemoglobin was detected in red blood cells from pregnant, but not nonpregnant, rats.52 These findings have been confirmed (eg, by Lubarsky et al53 and Deng et al54), and support the concept that NO production is augmented during pregnancy. Although the tissue source(s) for the gestational increases in NO and cGMP were not identified, the arterial wall was an attractive candidate, thus placing these molecules in the vasodilatory pathway of pregnancy. Interestingly, similar increases in NOx were reported in gravid ewes.55 However, the status of NO biosynthesis during normal pregnancy in women (and in preeclampsia) is not clear.48,56–58
Discovery that some arginine analogs inhibit NOS and NO production51 produced tools to explore the functional role of NO in gestational renal vasodilation and hyperfiltration. Short-term, low-dose administration of arginine analogs to chronically instrumented, conscious virgin control and midterm pregnant rats led to a convergence of GFR, RPF, and renal vascular resistance in both groups, with total blockade of gestational renal vasodilation and hyperfiltration.35 That is, gravid rats responded more robustly to NOS inhibition with greater declines in GFR and RPF, and increases in renal vascular resistances. Some,59,60 but not all,61 reports have suggested that the gestational renal vasodilation and hyperfiltration are also prevented by chronic administration of NOS inhibitors. In line with these in vivo investigations, loss of myogenic reactivity in small renal arteries from midterm pregnant rats was restored to the robust levels of arteries from virgin control rats after addition of NOS inhibitors to the bath or removal of the endothelium.39
Basal hand and forearm blood flows, assessed by venous occlusion plethysmography,62,63 were increased during late pregnancy, and the vasoconstrictor response to brachial artery infusion of NG-monomethyl-L-arginine was enhanced significantly relative to nonpregnant control subjects. These studies suggested that NO contributes to reduced arterial tone during pregnancy.
An essential role for NO in the renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small renal arteries also was established for nonpregnant rats administered relaxin, again by using NOS inhibitors.32,38 Unexpectedly, however, the 24-hour urinary excretion of cGMP and NOx did not increase in relaxin-treated rats despite the proven functional role of NO in relaxin-mediated vasodilation of the renal circulation.32 Thus, ironically, the increases in urinary and plasma cGMP and NOx that initially were observed during pregnancy, and motivated further investigation of NO in pregnancy vasodilation, may not be of vascular origin or of hemodynamic relevance.
Endothelial EndothelinB Receptor
The mechanism for the NO-dependent vasodilatory changes in the renal circulation during rat gestation or relaxin administration in nonpregnant rats did not appear to be a consequence of increases in endothelial NOS protein.64,65 Although a role for other renal NOS isoforms cannot be excluded, the possibility was explored that NOS, presumably endothelial NOS, might be activated by endothelin (ET), thereby mediating the NO-dependent renal vasodilatory changes of pregnancy or by relaxin administration.66 This hypothesis was based on previous investigations that established a role for the endothelial ETB receptor in the maintenance of low renal vascular tone in the nonpregnant condition, most likely by tonic stimulation of nitric oxide (reviewed by Conrad and Novak,8 Conrad et al,24 and Conrad25). It was postulated that during pregnancy, relaxin accentuates this vasodilatory pathway in the renal circulation.66
Analogous to NOS inhibition, short-term, low-dose administration of the endothelial ETB-receptor antagonist RES-701–1 inhibited renal vasodilation and hyperfiltration leading to a convergence of GFR, RPF, and renal vascular resistance in chronically instrumented, conscious midterm pregnant and virgin rats.66 Consistent with these in vivo studies, loss of myogenic reactivity in small renal arteries from midterm rats was restored by introducing RES-701–1 or the mixed ETA/B antagonist, SB209670, but not the ETA antagonist, BQ123, into the bath.39 In additional investigations, the NO-cGMP signaling pathway was implicated in transducing the vasodilatory action of endogenous ET via the ETB receptor in the renal circulation during pregnancy.39,66 In complementary studies, a critical role for the endothelial ETB receptor in mediating renal vasodilation, hyperfiltration, and inhibiting small renal artery myogenic reactivity also was established for relaxin administration in virgin female rats.33,38
Arterial Gelatinases
Given the essential, albeit perhaps paradoxic, role for endothelin (better known as a vasoconstrictor) in the relaxin vasodilatory pathway, a logical hypothesis was that relaxin up-regulates the endothelial ETB receptor. This idea needs further study because although one group noted evidence in favor of the hypothesis, we failed to find any supportive evidence.67,68 In view of this impasse, an alternative hypothesis was formulated, founded on the confluence of several findings: first, the essential role of relaxin, the endothelial ETB receptor, and NO in pregnancy-mediated renal vasodilation; second, the ability of relaxin to up-regulate matrix metalloproteinase (MMP)-2 and -9 activities (also known as gelatinases A and B, respectively), at least in several nonvascular cell types; and, third, the potential for MMPs (such as −2 and −9) to process big ET at a gly-leu bond to ET1–32, the latter fully capable of activating ET receptors.69–72 It was reasoned that relaxin might up-regulate MMP-2 or -9 activity in the renal vasculature during pregnancy, thereby mediating renal vasodilation, hyperfiltration, and inhibition of myogenic reactivity in an ET- and NO-dependent manner. This alternative pathway for ET formation was particularly compelling because phosphoramidon, which blocks the traditional endothelin-converting enzyme and ET1–21 formation, failed to impact the renal vasodilatory responses of relaxin even though the dose used completely inhibited the slow pressor response to big ET-1.70
To study this new hypothesis, a specific gelatinase inhibitor was used, cyclic CTTHWGFTLC (cyclic CTT), which preferentially inhibits the activity of MMP-2 relative to MMP-9.73 Because cyclic CTT is approximately 10 times more potent than STTHWGFTLS (STT), the latter served as a control peptide.73 Short-term infusion of low-dose cyclic CTT (but not of STT), which did not significantly increase blood pressure, abrogated renal vasodilation and hyperfiltration induced by long-term administration of relaxin in chronically instrumented animals.70 To corroborate these findings, a general inhibitor was used, GM6001, that is structurally distinct from cyclic CTT.74 GM6001 inhibited relaxin-mediated renal vasodilation and hyperfiltration.70
The myogenic reactivity bioassay also was used. Small renal arteries from midterm pregnant or relaxin-treated nonpregnant rats showed loss of myogenic reactivity that was restored by cyclic CTT, but not STT; GM6001, but not dilute vehicle; tissue inhibitor of metalloproteinases (TIMP-2), but not heat-inactivated TIMP-2; and MMP-2 neutralizing antibody, but not control IgG antibody introduced into the bath or arterial lumen.70 In contrast, phosphoramidon failed to restore myogenic reactivity.70 These in vitro results were consistent with those observed in vivo, and taken together suggested a pivotal role for gelatinase (likely MMP-2) in the renal vasodilatory responses to relaxin and pregnancy. Moreover, the in vitro studies implicated local arterial gelatinase activity, rather than circulating enzyme.
Although these studies established a critical role for arterial MMP-2 in the renal vasodilatory pathway of relaxin and pregnancy, they did not determine whether MMP-2 activity itself was being regulated. To address this question, gelatinase activity, protein, and messenger RNA were quantitated in small renal and mesenteric arteries, and aortae isolated from midterm pregnant or relaxin-treated nonpregnant rats, and an approximately 40% increase in both pro- and active MMP-2 activity, pro–MMP-2 protein, and messenger RNA was shown.70,72 Interestingly, pro–MMP-9 activity was increased consistently in small renal arteries from midterm pregnant rats too, but markedly less than MMP-2.70 The increase in maternal systemic arterial gelatinase activity during pregnancy or relaxin administration has been confirmed.75–77 Although there were no significant differences in arterial TIMP-1 or TIMP-2 activities, there was considerable variability in the reverse zymography assay.72 MMP-2 was localized to both the endothelium and vascular smooth muscle of the small renal arteries by immunohistochemistry,72 but further inquiry is needed to determine in which of these compartment(s) it is up-regulated by either pregnancy or relaxin.
Unexpectedly, MMP-9 rather than MMP-2 activity was increased in small renal and mesenteric arteries harvested from rats after short-term subcutaneous administration of relaxin by osmotic pump for 4 to 6 hours.71 Consistent with this finding, isolated small renal arteries showed loss of myogenic reactivity that was restored by a specific MMP-9– rather than MMP-2–neutralizing antibody. MMP-9 was immunolocalized to the vascular smooth muscle. It should be noted that MMP-9 also can hydrolyze big ET to ET1–32 at a gly-leu bond.69 Of note, cyclic CTT failed to restore myogenic reactivity in this setting, suggesting that, in this tissue and at the dosage used, the peptide specifically inhibits MMP-2. Finally, similar to long-term administration of relaxin, the inhibition of myogenic reactivity in arteries isolated only after 4 to 6 hours of administration also was mediated by the endothelial ETB receptor and NO.71
Evidence was gathered showing arterial MMP-2 was in series with, and upstream of, the endothelial ETB receptor and NO rather than as part of a separate and parallel vasodilatory pathway. Inhibition of myogenic reactivity was not observed in small renal arteries isolated from relaxin-treated or midterm pregnant ETB-receptor–deficient rats, corroborating the studies using pharmacologic inhibitors of the ETB receptor.70,78 Nevertheless, arterial MMP-2 activity was increased.70 This dissociation of increased arterial MMP-2 activity from the functional end point of inhibited myogenic reactivity, the latter which was not observed due to genetic disruption of the ETB receptor, strongly suggested that MMP-2 was in series with, and upstream of, the endothelial ETB receptor and NO (Fig. 2).
Emerging Role of Angiogenic Growth Factors
By using knockout mice, it was suggested in a preliminary report that the major relaxin receptor, RXFP1, mediates the arterial responses to relaxin, and not the lower-affinity receptor, RXFP279 (see Appendix for relaxin ligand and receptor nomenclature). Moreover, RXFP1-receptor messenger RNA and protein expression in vascular smooth muscle greatly exceeds that of endothelium (unpublished data). This last finding suggested the possibility that other factors (eg, angiogenic growth factors) may be secreted by the vascular smooth muscle upon RXFP1 activation, which then diffuse to the endothelium, where they stimulate expression of gelatinase(s), activating the endothelial ETB receptor–NO vasodilatory pathway. However, this hypothetical chain of events may be incorrect: it is possible that endothelial RXFP1 is the receptor of physiological relevance to the relaxin vasodilatory pathway, despite its vastly lower expression compared with vascular smooth muscle. Nevertheless, given that relaxin has been shown to increase vascular endothelial growth factor (VEGF) expression at least in some nonvascular cell types and is angiogenic (reviewed by Conrad and Novak8 and Jeyabalan et al9), whether VEGF or placental growth factor (PGF) might be the factor(s) linking the vascular smooth muscle and endothelium was considered. To date, functional approaches have been taken to test this hypothesis, and the results showed that the VEGF-receptor tyrosine kinase inhibitor, SU5416, and specific VEGF- and PGF-neutralizing antibodies each prevent the inhibition of myogenic reactivity by relaxin in mouse and rat small renal arteries, and in human subcutaneous arteries.80 Moreover, SU5416 prevented relaxin-mediated decreases in renal vascular resistance, and increases in RPF and GFR in chronically instrumented, conscious rats.81 Thus, emerging evidence suggests that angiogenic growth factors may play a role in the relaxin vasodilatory pathway, but the molecular details await elucidation.
RAPID RELAXATION RESPONSES
Recently, Fisher et al82 showed that relaxin also elicited a rapid relaxation response (ie, within minutes), in isolated human arteries, and in an endothelium-dependent fashion. Interestingly, this effect was observed in vessels obtained from gluteal biopsy specimens, but not pulmonary tissues. In a preliminary report,83 the molecular underpinnings of this rapid vasodilatory action of relaxin were studied. The phenomenon was observed in small renal arteries from rats and mice, but not in mesenteric or coronary septal arteries. Rapid relaxation to relaxin also was shown in isolated human subcutaneous arteries. Brief incubation of cultured human endothelial, but not vascular smooth muscle, cells with relaxin increased NO production within minutes as detected by the fluorescent probe, 4-amino-5-methylamino-2′7′-difluorofluorescein. These rapid responses to relaxin in the isolated arteries and cultured endothelial cells were abrogated by L-arginine analogs, PI3 kinase inhibitors, and pertussis toxin, but not by the VEGF-receptor tyrosine kinase inhibitor, SU5416. In support of these functional studies, there was increased phosphorylation of Akt and endothelial NOS in cultured endothelial cells. These studies suggested that relaxin rapidly dilates arteries from select arterial beds across a range of animal species. The mechanism apparently involves Gαi/o protein coupling to PI3K, Akt, and endothelial NOS, but not VEGF-receptor transactivation.
IMPLICATIONS OF RELAXIN BIOLOGY FOR PREECLAMPSIA
Currently, the relevance of relaxin biology to preeclampsia is largely speculative because its role in the maternal adaptations of normal pregnancy is only now being unveiled. Nevertheless, there are several intriguing implications of relaxin biology for preeclampsia that are highlighted in relation to our current understanding of the etiology and pathophysiology of the disease.
PATHOPHYSIOLOGY OF PREECLAMPSIA: STAGE I
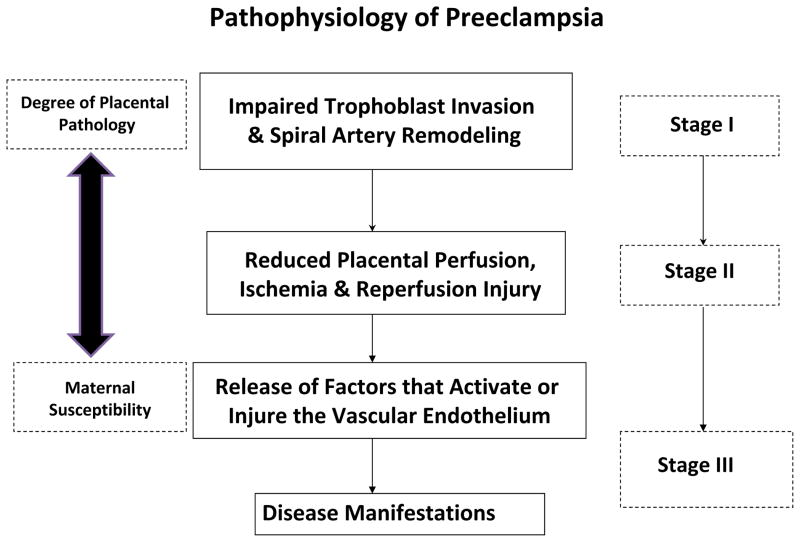
Briefly, preeclampsia can be considered a three-stage disease (Fig. 3). Stage I occurs in early pregnancy and especially involves impaired trophoblast invasion of uterine spiral arteries. Consequently, these arteries fail to be remodeled adequately from small-caliber, high-resistance to large-caliber, low-resistance vessels, thus impeding uteroplacental blood flow.84 Failure of spiral arteries to convert to conduit-size vessels also may confer higher blood flow velocities entering the intervillous space, further damaging placental villi by mechanical forces.85 The structural remodeling of spiral arteries in normal pregnancy, owing primarily to their interaction with invading trophoblasts, occurs by apoptosis of spiral artery smooth muscle and endothelial cells, as well as dissolution of extracellular matrix components such as collagen, and the deposition of fibrinoid material in the vascular wall.84,86–89 However, precursor changes occur in spiral arteries of the endometrium and inner myometrium during decidualization, which may be permissive for the subsequent wave of trophoblast invasion (see later).
Figure 3.
Three-stage model of preeclampsia. See text for details.
Trophoblast Invasion
The underlying cause(s) of successful and failed trophoblast invasion in stage I is an active area of investigation.90 The possible hormones, growth factors, chemokines, and cytokines involved are discussed elsewhere,91 but no reports of the influence of relaxin on trophoblast invasion could be located in PubMed through March 2010. Nevertheless, trophoblasts likely express H1 and H2 relaxins, as well as the RXFP1 receptor.1 They also express VEGF and PGF, as well as the cognate receptors.92 Invading trophoblasts express gelatinase activity facilitating migration and invasion of the uterus.93,94 Analogous to endothelial cell migration,95 there are data, albeit more limited, suggesting that the ETB-receptor–endothelial NOS system promotes trophoblast migration and invasion.96–98 Thus, all of the signaling components in the relaxin vasodilatory pathway described in the Sustained Vasodilatory Responses section may be present in invading trophoblasts. It is tempting to consider that relaxin may harness partor all of this molecular pathway to promote trophoblast invasion. Thus, deficient relaxin signaling may contribute to defective trophoblast invasion, a hypothesis that remains to be tested.
Decidualization
The impairment of trophoblast invasion in preeclampsia is logically caused by intrinsic defect(s) within the invading trophoblasts themselves, in the maternal endometrium, and inner-third of the myometrium, particularly the spiral arteries in which they invade, or both. Thus, abnormalities of maturation in the endometrium, inner-myometrium, and spiral arteries therein, before and after conception (called predecidualization and decidualization, respectively), may be etiologic.99 Decidual changes in the endometrial and inner-myometrial spiral arteries normally include “intimal swelling, an edematous media, and separation of the musculoelastic layers.”84 Alternatively, the optimal complement of uterine immune cells and associated cytokines, and their interaction with resident cells of the endometrium and inner-myometrium, as well as invading trophoblasts, may be perturbed. Indeed, global gene expression profiling of chorionic villous and decidual tissue obtained at 10 to 12 weeks’ gestation from women who developed preeclampsia 6 months later prominently featured abnormal expression of genes related to decidualization and immune function.100 By extrapolation, it is not inconceivable that in some women the antecedents of preeclampsia may lie with defective predecidualization in the secretory phase of the menstrual cycle.84,100 Because the secretory phase of the menstrual cycle and early pregnancy are primarily under the endocrine control of the corpus luteum (eg, progesterone and relaxin), the etiology of preeclampsia may reside with abnormal ovarian function or end-organ resistance to ovarian hormones in some women who develop the disease.
In this regard, relaxin’s contribution to the process of decidualization is especially well characterized in vitro.101,102 The role of relaxin in modifying endometrial structure and function in vivo was elegantly shown by Goldsmith et al101,103 in a nonhuman primate model. They showed that circulating relaxin markedly stimulated endometrial angiogenesis and increased endometrial lymphocyte numbers, consistent with the changes observed in early human pregnancy. This finding, enhanced endometrial angiogenesis in response to relaxin, is consistent with the earlier work of Hisaw et al104 (see Introduction), and with the menometrorrhagia noted in many patients participating in a clinical trial designed to test the efficacy of relaxin in treating scleroderma. Such angiogenic attributes of relaxin also have been established in other organs and pathologic settings.8,9
Circulating Relaxin
One possibility is that circulating relaxin may be decreased during stage I in women destined to develop preeclampsia. Consistent with this hypothesis is a preliminary report in which spontaneously conceiving women with first-trimesterserum relaxin levels that were approximately half of normal levels in the first trimester have an adjusted odds ratio of 7.5 for developing preeclampsia.105 This study raised the possibility that these women may experience defective decidualization and trophoblast invasion or fail to adequately vasodilate in early pregnancy owing to low levels of circulating relaxin, thereby predisposing them to develop preeclampsia.
Infertile women made pregnant by egg donation, in vitro fertilization, and embryo transfer are completely devoid of circulating relaxin throughout pregnancy.1 Surprisingly, their cardiovascular and renal adaptations to pregnancy appear not to have been studied with the exception of one limited study, in which markedly blunted increases in GFR (and decreases in plasma osmolality) were observed in the first trimester.45 Perhaps not coincidentally, there is growing evidence suggesting that these women may be at increased risk for hypertensive disorders of pregnancy including preeclampsia, as well as having small for gestational age babies.106
PATHOPHYSIOLOGY OF PREECLAMPSIA: STAGE II
Stage II, a consequence of stage I, entails inadequate uteroplacental perfusion leading to placental ischemia-hypoxia.107 As well, the persistence of uterine spiral artery smooth muscle should render these blood vessels susceptible to local and circulating vasoconstrictors resulting in vasospasm and hypoxia-reperfusion injury to the placenta. Another major consequence of the disturbed uteroplacental blood flow is up-regulation of placental hypoxia inducible transcription factors (HIF-1α and -2α) via hypoxia, although reactive oxygen species, various cytokines, and growth factors also can increase HIF-α.108–110 Placental villi also undergo apoptosis and necrosis.111,112
Whether relaxin increases uterine blood flow is not clear and requires more study. However, reports that support the hypothesis113 raise the possibility that relaxin deficiency during pregnancy may result in reduced uterine blood flow, and relaxin administration could prevent or treat preeclampsia by enhancing perfusion. In contrast are reports of a direct relationship between serum relaxin levels and the uterine artery Doppler resistance index,114,115 results suggesting relaxin is associated with increased, not reduced, uterine vascular resistance.
It may be that circulating or locally expressed relaxin counteracts placental HIF-1α and -2α independent of an increase in uterine blood flow. If so, relaxin may serve to dampen the production and release of the HIF-α regulated gene products that circulate and damage the maternal endothelium in preeclampsia. The hypothesis remains to be explored.
In other cell types, relaxin has been found to be anti-apoptotic,116 but such action on trophoblast or other placental cells has not been investigated. Accelerated trophoblast apoptosis is deleterious in preeclampsia,111,112,117 and relaxin conceivably might oppose this process.
PATHOPHYSIOLOGY OF PREECLAMPSIA: STAGE III
Stage III begins when factors from the damaged placenta enter the maternal circulation, cause endothelial activation and injury, and produce disease manifestations including hypertension, proteinuria, and glomerular endotheliosis. Identification of these factors has been hotly pursued for years, and are likely to be a multitude of injurious agents, the complement of which may vary from patient to patient, and with no one factor alone being responsible. Currently, the leading candidates include inflammatory cytokines, agonistic angiotensin type I receptor autoantibodies, syncytiotrophoblast microparticles, soluble endoglin, and soluble fms-like tyrosine kinase-1 (sFlt-1 or soluble VEGF receptor 1), the latter thought to act by binding and sequestering VEGF and PGF.112,118–120 Levels of some of these factors increase in the maternal circulation before clinical onset, suggesting they cause rather than result from the disease.
Susceptibility to these circulating factors may vary among gravidas, thereby necessitating a little or a lot of placental pathology, respectively, to precipitate clinical disease (Fig. 3), and this may explain why women with pre-existing endothelial dysfunction (eg, renal disease, chronic hypertension, or diabetes mellitus) may be predisposed to preeclampsia. In these women, pre-existing endothelial dysfunction also may affect the uterine vasculature, contributing to impaired trophoblast invasion in stage I and exacerbating stage II. It also should be noted that circulating deleterious factors in preeclampsia may derive from other sources besides, or in addition to, the placenta.120–122
Evidence for the pathologic role of increased sFlt1 levels in preeclampsia represents an important breakthrough in our understanding of disease pathophysiology (detailed in the article by Maynard et al in this issue). Placental HIF-α proteins and HIF-α regulated genes including membrane-bound Flt1 and sFlt1 are increased in preeclampsia.108,110,119,123 Thus, sFlt1 is likely to be an important (but certainly not the only) factor emanating from the placenta that contributes to endothelial dysfunction in preeclampsia. The additional findings that sFlt1 is not increased in the maternal circulation during the first half of pregnancy in women destined to develop preeclampsia,124,125 and the lack of evidence for up-regulation of hypoxia or oxidative stress regulated genes including Flt1 in chorionic villous and decidual tissues at 10 to 12 weeks of gestation, suggest that these may be later events in the disease.100
Circulating Relaxin
Given that relaxin contributes to the vasodilatory phenomena of normal pregnancy, an obvious consideration is whether circulating levels are decreased during the clinical manifestations of preeclampsia. However, serum immunoreactive H2 relaxin was reported to be similar in women with preeclampsia and normal pregnancy at comparable gestational ages.126 One caveat to this study is that strict definition of preeclampsia was not used, insofar as women both with and without proteinuria were included. In addition, immunoreactivity may not equate to bioactivity, which could be compromised (eg, by increased circulating levels of a putative soluble RXFP1 receptor).127,128 Alternatively, and by analogy to other ligand-receptor systems, fewer RXFP1 receptors or increased expression of truncated RXFP1 receptors on blood vessels could undermine relaxin signaling in arteries, thereby leading to relative vasoconstriction.3,127,128
Maternal Adaptations in Preeclampsia
These are diametrically opposed to normal pregnancy, at least during disease. That is, relative to normal pregnancy, there is systemic and renal vasoconstriction leading to reduced organ perfusion (reviewed by Jeyabalan and Conrad14). On the one hand, this may be part and parcel of the generalized “endothelial dysfunction” that contributes to the pathogenesis of preeclampsia. Similar to other cardiovascular diseases associated with endothelial dysfunction, disruption of the caveolar microenvironment could contribute to the endothelial abnormalities of preeclampsia.129 Endothelial and caveolar homeostasis may be perturbed by any number of circulating factors in the disease including syncytiotrophoblast microparticles, oxidized low-density lipoprotein, tumor necrosis factor-α, agonist angiotensin type I receptor autoantibodies, sFlt1, and soluble endoglin. Insofar as the relaxin signaling pathway may wholly or partly reside in endothelial cell caveolae (see the study by Jeyabalan70), disruption of this caveolar microenvironment conceivably could compromise relaxin vasodilation. For example, oxidized low-density lipoprotein modifies the lipid milieu of caveolae by depleting cholesterol that, in turn, compromises endothelial NOS activation.129
On the other hand, interference with specific mechanisms of the relaxin vasodilatory pathway, independent of general disruption of the caveolar microenvironment, also may predispose or contribute to preeclampsia. For example, one possibility is that increased circulating levels of sFlt1 neutralize the activities of VEGF and PGF in the arterial wall, which, as described earlier, are crucial for the sustained vasodilatory action of relaxin. Another possibility is that excessive production of arterial MMP-2 or -9 or circulating enzymes130 leads to excessive formation of ET1–32, which “spills over,” interacting with ETA and ETB receptors on vascular smooth muscle that are vasoconstricting, thus overwhelming relaxin-induced vasodilation.
POTENTIAL THERAPEUTIC ROLE OF RELAXIN IN PREECLAMPSIA
As reviewed earlier, increased circulating sFlt1 or soluble VEGF-receptor 1 was identified as an important mediator of endothelial dysfunction in preeclampsia.119 This soluble receptor sequesters the essential angiogenic growth factors, VEGF and PGF, and prevents their activity in endothelial cells. Furthermore, VEGF and PGF are emerging as essential mediators in relaxin-induced vasodilation.81,131 Thus, convergence of these observations provides mechanistic support for the therapeutic application of relaxin in preeclampsia, in addition to the more obvious salutary effects the hormone may be expected to have on blood flow and organ perfusion. That is, by locally up-regulating arterial VEGF and PGF activity, relaxin administration could neutralize the deleterious effects of circulating sFlt1, thus improving the pro-angiogenic/anti-angiogenic balance, leading to improved endothelial function, increased NO, systemic and uterine vasodilation, as well as attenuation of disease manifestations.
In light of the serious ramifications of preeclampsia on maternal and perinatal health (particularly in geographic regions with poor antenatal care); the possibility it may predispose to remote cardiovascular disease; the lack of a cure for preeclampsia other than delivery; the emerging role of relaxin in cardiovascular adaptations to normal pregnancy; and growing evidence for relaxin’s efficacy in cardiovascular disease in the nonpregnant population, preclinical studies of the hormone in preeclampsia animal models would seem warranted.
Acknowledgments
The author thanks Marshall D. Lindeimer, MD, and Jonathan T. McGuane, PhD, for their critical review of the manuscript. The work in the author’s laboratory would not have been possible without the invaluable contributions of many outstanding colleagues over the years, particularly collaborators: Sanjeev G. Shroff, PhD, Lee A. Danielson, PhD, Laura J. Parry, PhD, Jacqueline Novak, PhD, Arun Jeyabalan, MD, and John M. Davison, MD.
The author also gratefully acknowledges the financial support of the National Institutes of Health (RO1 HD030325, RO1 DK063321, RO1 HL067937, and R21 HL093334), and a Grant-in-Aid from the American Heart Association (0855090E). The author discloses use patents related to relaxin.
APPENDIX
Relaxin Ligand and Receptor Nomenclature [132]
Human beings have three relaxin genes designated relaxin-1, -2, and -3. Rats and mice each have two relaxin genes designated relaxin-1 and -3. Human relaxin-2, as well as rat and mouse relaxin-1 gene products, are true orthologs insofar as they are secreted by the corpus luteum during pregnancy and circulate. Human beings, rats, and mice have one receptor, the so-called leucine rich repeat-containing G-protein–coupled receptor (recently renamed relaxin/insulin-like family peptide 1 receptor [RXFP1]) that binds relaxin. Although human relaxin-2 also may bind to the Lgr8 receptor (RFXP2), albeit with reduced affinity,132,133 the preferred ligand for Lgr8 is Insl-3. Recently, two new receptors have been described for relaxin-3: GPCR135 and 142134 (although GPCR142 is a pseudogene in rats).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sherwood OD. Relaxin. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 861–1008. [Google Scholar]
- 2.Kohsaka T, Min G, Lukas G, Trupin S, Campbell ET, Sherwood OD. Identification of specific relaxin-binding cells in the human female. Biol Reprod. 1998;59:991–9. doi: 10.1095/biolreprod59.4.991. [DOI] [PubMed] [Google Scholar]
- 3.Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, et al. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J. 2006;20:2352–62. doi: 10.1096/fj.06-6263com. [DOI] [PubMed] [Google Scholar]
- 4.Hisaw FL. Experimental relaxation of the public ligament of the guinea pig. Proc Exp Biol Med. 1926;23:661–3. [Google Scholar]
- 5.Ziel HK, Swain CT, Frederick L. Hisaw and the discovery of relaxin. Endocrinologist. 2000;10:215–8. [Google Scholar]
- 6.Dallenbach-Hellweg G, Dawson AB, Hisaw FL. The effect of relaxin on the endometrium of monkeys. Histological and histochemical studies. Am J Anat. 1966;119:61–78. [Google Scholar]
- 7.Hisaw FL, Hisaw FL, Jr, Dawson AB. Effects of relaxin on the endothelium of endometrial blood vessels in monkeys (Macaca mulatta) Endocrinology. 1967;81:375–85. doi: 10.1210/endo-81-2-375. [DOI] [PubMed] [Google Scholar]
- 8.Conrad KP, Novak J. The emerging role of relaxin in renal and cardiovascular function. Am J Physiol Regul Integr Comp Physiol. 2004;287:R250–61. doi: 10.1152/ajpregu.00672.2003. [DOI] [PubMed] [Google Scholar]
- 9.Jeyabalan A, Shroff SG, Novak J, Conrad KP. The vascular actions of relaxin. Adv Exp Med Biol. 2007;612:65–87. doi: 10.1007/978-0-387-74672-2_6. [DOI] [PubMed] [Google Scholar]
- 10.St-Louis J, Massicotte G. Chronic decrease of blood pressure by rat relaxin in spontaneously hypertensive rats. Life Sci. 1985;37:1351–7. doi: 10.1016/0024-3205(85)90251-6. [DOI] [PubMed] [Google Scholar]
- 11.Massicotte G, Parent A, St-Louis J. Blunted responses to vasoconstrictors in mesenteric vasculature but not in portal vein of spontaneously hypertensive rats treated with relaxin. Proc Soc Exp Biol Med. 1989;190:254–9. doi: 10.3181/00379727-190-42857. [DOI] [PubMed] [Google Scholar]
- 12.Ahokas RA, Sabai BM, Anderson GD. Lack of evidence of a vasodepressor role for relaxin in spontaneously hypertensive and normotensive pregnant rats. Am J Obstet Gynecol. 1989;161:618–22. doi: 10.1016/0002-9378(89)90365-7. [DOI] [PubMed] [Google Scholar]
- 13.Bani-Sacchi T, Bigazzi M, Bani D, Mannaioni PF, Masini E. Relaxin-induced increased coronary flow through stimulation of nitric oxide production. Br J Pharmacol. 1995;116:1589–94. doi: 10.1111/j.1476-5381.1995.tb16377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyabalan A, Conrad KP. Renal physiology and pathophysiology in pregnancy. In: Schrier RW, editor. Renal and electrolyte disorders. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 462–518. [Google Scholar]
- 15.Gilson GJ, Mosher MD, Conrad KP. Systemic hemodynamics and oxygen transport during pregnancy in chronically instrumented, conscious rats. Am J Physiol. 1992;263:H1911–8. doi: 10.1152/ajpheart.1992.263.6.H1911. [DOI] [PubMed] [Google Scholar]
- 16.Slangen BF, Out IC, Verkeste CM, Peeters LL. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am J Physiol. 1996;270:H1779–84. doi: 10.1152/ajpheart.1996.270.5.H1779. [DOI] [PubMed] [Google Scholar]
- 17.Spaanderman ME, Meertens M, van Bussel M, Ekhart TH, Peeters LL. Cardiac output increases independently of basal metabolic rate in early human pregnancy. Am J Physiol. 2000;278:H1585–8. doi: 10.1152/ajpheart.2000.278.5.H1585. [DOI] [PubMed] [Google Scholar]
- 18.Bader NW, Bader ME, Rose DJ, Braunwald E. Hemodynamics at rest and during exercise in normal pregnancy as studied by cardiac catheterization. J Clin Invest. 1955;34:1524–36. doi: 10.1172/JCI103205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273:F777–82. doi: 10.1152/ajprenal.1997.273.5.F777. [DOI] [PubMed] [Google Scholar]
- 20.Poppas A, Shroff SG, Korcarz CE, Hibbard JU, Berger DS, Lindheimer MD, et al. Serial assessment of the cardiovascular system in normal pregnancy. Role of arterial compliance and pulsatile arterial load. Circulation. 1997;95:2407–15. doi: 10.1161/01.cir.95.10.2407. [DOI] [PubMed] [Google Scholar]
- 21.Robb AO, Mills NL, Din JN, Smith IB, Paterson F, Newby DE, et al. Influence of the menstrual cycle, pregnancy, and preeclampsia on arterial stiffness. Hypertension. 2009;53:952–8. doi: 10.1161/HYPERTENSIONAHA.109.130898. [DOI] [PubMed] [Google Scholar]
- 22.Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006;147:5126–31. doi: 10.1210/en.2006-0567. [DOI] [PubMed] [Google Scholar]
- 23.Conrad KP, Debrah DO, Novak J, Danielson LA, Shroff SG. Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology. 2004;145:3289–96. doi: 10.1210/en.2003-1612. [DOI] [PubMed] [Google Scholar]
- 24.Conrad KP, Gaber LW, Lindheimer M. The kidney in normal pregnancy and preeclampsia. In: Lindheimer M, Roberts JM, Cunningham FG, editors. Chesley’s hypertensive disorders in pregnancy. San Diego: Elsevier (Academic Press); 2009. pp. 297–334. [Google Scholar]
- 25.Conrad KP. Mechanisms of renal vasodilation and hyperfiltration during pregnancy. J Soc Gynecol Invest. 2004;11:438–48. doi: 10.1016/j.jsgi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Conrad KP. Renal hemodynamics during pregnancy in chronically catheterized, conscious rats. Kidney Int. 1984;26:24–9. doi: 10.1038/ki.1984.129. [DOI] [PubMed] [Google Scholar]
- 27.Debrah DO, Conrad KP, Danielson LA, Shroff SG. Effects of relaxin on systemic arterial hemodynamics and mechanical properties in conscious rats: sex dependency and dose response. J Appl Physiol. 2005;98:1013–20. doi: 10.1152/japplphysiol.01083.2004. [DOI] [PubMed] [Google Scholar]
- 28.Debrah DO, Conrad KP, Jeyabalan A, Danielson LA, Shroff SG. Relaxin increases cardiac output and reduces systemic arterial load in hypertensive rats. Hypertension. 2005;46:745–50. doi: 10.1161/01.HYP.0000184230.52059.33. [DOI] [PubMed] [Google Scholar]
- 29.Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, et al. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail. 2009;15:182–90. doi: 10.1016/j.cardfail.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Shroff SG, Berger DS, Korcarz C, Lang RM, Marcus RH, Miller DE. Physiological relevance of T-tube model parameters with emphasis on arterial compliances. Am J Physiol. 1995;269:H365–74. doi: 10.1152/ajpheart.1995.269.1.H365. [DOI] [PubMed] [Google Scholar]
- 31.McGuane JT, Debrah JE, Debrah DO, Rubin JP, Segal MS, Shroff SG, et al. Role of relaxin in maternal systemic and renal vascular adapations during gestation. Ann N Y Acad Sci. 2009;1160:304–12. doi: 10.1111/j.1749-6632.2009.03829.x. [DOI] [PubMed] [Google Scholar]
- 32.Danielson LA, Sherwood OD, Conrad KP. Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest. 1999;103:525–33. doi: 10.1172/JCI5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danielson LA, Kercher LJ, Conrad KP. Impact of gender and endothelin on renal vasodilation and hyperfiltration induced by relaxin in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1298–304. doi: 10.1152/ajpregu.2000.279.4.R1298. [DOI] [PubMed] [Google Scholar]
- 34.Conrad KP, Colpoys MC. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J Clin Invest. 1986;77:236–45. doi: 10.1172/JCI112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest. 1995;96:482–90. doi: 10.1172/JCI118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak J, Reckelhoff J, Bumgarner L, Cockrell K, Kassab S, Granger JP. Reduced sensitivity of the renal circulation to angiotensin II in pregnant rats. Hypertension. 1997;30:580–4. doi: 10.1161/01.hyp.30.3.580. [DOI] [PubMed] [Google Scholar]
- 37.Bogzil AH, Eardley R, Ashton N. Relaxin-induced changes in renal sodium excretion in the anesthetized male rat. Am J Physiol. 2005;288:R322–8. doi: 10.1152/ajpregu.00509.2004. [DOI] [PubMed] [Google Scholar]
- 38.Novak J, Ramirez RJ, Gandley RE, Sherwood OD, Conrad KP. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R349–55. doi: 10.1152/ajpregu.00635.2001. [DOI] [PubMed] [Google Scholar]
- 39.Gandley RE, Conrad KP, McLaughlin MK. Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1–7. doi: 10.1152/ajpregu.2001.280.1.R1. [DOI] [PubMed] [Google Scholar]
- 40.Danielson LA, Conrad KP. Time course and dose response of relaxin-mediated renal vasodilation, hyperfiltration, and changes in plasma osmolality in conscious rats. J Appl Physiol. 2003;95:1509–14. doi: 10.1152/japplphysiol.00545.2003. [DOI] [PubMed] [Google Scholar]
- 41.Smith MC, Danielson LA, Conrad KP, Davison JM. Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol. 2006;17:3192–7. doi: 10.1681/ASN.2005090950. [DOI] [PubMed] [Google Scholar]
- 42.Erikson MS, Unemori EN. Relaxin clinical trials in systemic sclerosis. Relaxin 2000: proceedings of the third international conference on relaxin and related peptides; Dordrecht, The Netherlands: Kulwer Academic Publishers; 2000. [Google Scholar]
- 43.Khanna D, Clements PJ, Furst DE, Korn JH, Ellman M, Rothfield N, et al. Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:1102–11. doi: 10.1002/art.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, et al. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107:1469–75. doi: 10.1172/JCI11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril. 2006;86:253–5. doi: 10.1016/j.fertnstert.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 46.Conrad K. Possible mechanisms for changes in renal hemodynamics during pregnancy: studies from animal models. Am J Kidney Dis. 1987;9:253–9. doi: 10.1016/s0272-6386(87)80118-x. [DOI] [PubMed] [Google Scholar]
- 47.Conrad KP, Vernier KA. Plasma level, urinary excretion, and metabolic production of cGMP during gestation in rats. Am J Physiol. 1989;257:R847–53. doi: 10.1152/ajpregu.1989.257.4.R847. [DOI] [PubMed] [Google Scholar]
- 48.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NO(x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO(x) diet. Am J Physiol. 1999;277:F48–57. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 49.Kopp L, Paradiz G, Tucci J. Urinary excretion of cyclic 3′, 5′-adenosine monophosphate and cyclic 3′, 5′-guanosine monophosphate during and after pregnancy. J Clin Endocrinol Metab. 1977;44:590–4. doi: 10.1210/jcem-44-3-590. [DOI] [PubMed] [Google Scholar]
- 50.Sala C, Campise M, Ambroso G, Motta T, Zanchetti A, Morganti A. Atrial natriuretic peptide and hemodynamics changes during normal human pregnancy. Hypertension. 1995;25:631–6. doi: 10.1161/01.hyp.25.4.631. [DOI] [PubMed] [Google Scholar]
- 51.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 52.Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, et al. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993;7:566–71. [PubMed] [Google Scholar]
- 53.Lubarsky SL, Ahokas RA, Friedman SA, Sibai BM. The effect of chronic nitric oxide synthesis inhibition on blood pressure and angiotensin II responsiveness in the pregnant rat. Am J Obstet Gynecol. 1997;176:1069–76. doi: 10.1016/s0002-9378(97)70404-6. [DOI] [PubMed] [Google Scholar]
- 54.Deng A, Engels K, Baylis C. Impact of nitric oxide deficiency on blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int. 1996;50:1132–8. doi: 10.1038/ki.1996.420. [DOI] [PubMed] [Google Scholar]
- 55.Yang D, Lang U, Greenberg SG, Myatt L, Clark KE. Elevation of nitrate levels in pregnant ewes and their fetuses. Am J Obstet Gynecol. 1996;174:573–7. doi: 10.1016/s0002-9378(96)70430-1. [DOI] [PubMed] [Google Scholar]
- 56.Baylis C, Suto T, Conrad K. Importance of nitric oxide in control of systemic and renal hemodynamics during normal pregnancy: studies in the rat implications for preeclampsia. Hypertens Pregnancy. 1996;15:147–69. [Google Scholar]
- 57.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–63. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Jaramillo P, Arenas WD, Garcia RG, Rincon MY, Lopez M. The role of the L-arginine-nitric oxide pathway in preeclampsia. Ther Adv Cardiovasc Dis. 2008;2:261–75. doi: 10.1177/1753944708092277. [DOI] [PubMed] [Google Scholar]
- 59.Cadnapaphornchai MA, Ohara M, Morris KG, Jr, Knotek M, Rogachev B, Ladtkow T, et al. Chronic NOS inhibition reverses systemic vasodilation and glomerular hyperfiltration in pregnancy. Am J Physiol. 2001;280:F592–8. doi: 10.1152/ajprenal.2001.280.4.F592. [DOI] [PubMed] [Google Scholar]
- 60.Baylis C, Engels K. Adverse interactions between pregnancy and a new model of systemic hypertension produced by chronic blockade of endothelial derived relaxing factor (EDRF) in the rat. Clin Exp Hypertens Pregnancy. 1992;B11:117–29. [Google Scholar]
- 61.Danielson LA, Conrad KP. Prostaglandins maintain renal vasodilation and hyperfiltration during chronic nitric oxide synthase blockade in conscious pregnant rats. Circ Res. 1996;79:1161–6. doi: 10.1161/01.res.79.6.1161. [DOI] [PubMed] [Google Scholar]
- 62.Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol. 1997;272:H748–52. doi: 10.1152/ajpheart.1997.272.2.H748. [DOI] [PubMed] [Google Scholar]
- 63.Anumba DO, Robson SC, Boys RJ, Ford GA. Nitric oxide activity in the peripheral vasculature during normotensive and preeclamptic pregnancy. Am J Physiol. 1999;277:H848–54. doi: 10.1152/ajpheart.1999.277.2.H848. [DOI] [PubMed] [Google Scholar]
- 64.Novak J, Rajakumar A, Miles TM, Conrad KP. Nitric oxide synthase isoforms in the rat kidney during pregnancy. J Soc Gynecol Investig. 2004;11:280–8. doi: 10.1016/j.jsgi.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, et al. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension. 1999;33:435–9. doi: 10.1161/01.hyp.33.1.435. [DOI] [PubMed] [Google Scholar]
- 66.Conrad KP, Gandley RE, Ogawa T, Nakanishi S, Danielson LA. Endothelin mediates renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. Am J Physiol. 1999;276:F767–76. doi: 10.1152/ajprenal.1999.276.5.F767. [DOI] [PubMed] [Google Scholar]
- 67.Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circ Res. 2003;92:32–40. doi: 10.1161/01.res.0000051884.27117.7e. [DOI] [PubMed] [Google Scholar]
- 68.Kerchner LJ, Novak J, Hanley-Yanez K, Doty KD, Danielson LA, Conrad KP. Evidence against the hypothesis that endothelial endothelin B receptor expression is regulated by relaxin and pregnancy. Endocrinology. 2005;146:2791–7. doi: 10.1210/en.2004-1602. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–11. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 70.Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003;93:1249–57. doi: 10.1161/01.RES.0000104086.43830.6C. [DOI] [PubMed] [Google Scholar]
- 71.Jeyabalan A, Novak J, Doty KD, Matthews J, Fisher MC, Kerchner LJ, et al. Vascular matrix metalloproteinase-9 mediates the inhibition of myogenic reactivity in small arteries isolated from rats after short-term administration of relaxin. Endocrinology. 2007;148:189–97. doi: 10.1210/en.2006-0989. [DOI] [PubMed] [Google Scholar]
- 72.Jeyabalan A, Kerchner LJ, Fisher MC, McGuane JT, Doty KD, Conrad KP. Matrix metalloproteinase-2 activity, protein, mRNA, and tissue inhibitors in small arteries from pregnant and relaxin-treated nonpregnant rats. J Appl Physiol. 2006;100:1955–63. doi: 10.1152/japplphysiol.01330.2005. [DOI] [PubMed] [Google Scholar]
- 73.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17:768–74. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 74.Galardy RE, Cassabonne ME, Giese C, Gilbert JH, Lapierre F, Lopez H, et al. Low molecular weight inhibitors in corneal ulceration. Ann N Y Acad Sci. 1994;732:315–23. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 75.Kelly BA, Bond BC, Poston L. Aortic adaptation to pregnancy: elevated expression of matrix metalloproteinases-2 and -3 in rat gestation. Mol Hum Reprod. 2004;10:331–7. doi: 10.1093/humrep/gah045. [DOI] [PubMed] [Google Scholar]
- 76.Danielson LA, Welford A, Harris A. Relaxin improves renal function and histology in aging Munich Wistar rats. J Am Soc Nephrol. 2006;17:1325–33. doi: 10.1681/ASN.2005121307. [DOI] [PubMed] [Google Scholar]
- 77.van Eijndhoven HW, Janssen GM, Aardenburg R, Spaanderman ME, Peeters LL, De Mey JG. Mechanisms leading to increased vasodilator responses to calcitonin-gene-related peptide in mesenteric resistance arteries of early pregnant rats. J Vasc Res. 2008;45:350–6. doi: 10.1159/000119754. [DOI] [PubMed] [Google Scholar]
- 78.Novak J, Conrad KP. Small renal arteries isolated from ETB receptor deficient rats fail to exhibit the normal maternal adaptation to pregnancy [abstract] FASEB J. 2004;18:32. [Google Scholar]
- 79.Debrah JE, Agoulnik A, Conrad KP. Changes in arterial function by chronic relaxin infusion are mediated by the leucine rich repeat G coupled Lgr7 receptor [abstract] Reprod Sci. 2008;15 (Suppl):217A. [Google Scholar]
- 80.Debrah JE, McGuane JT, Novak J, Rubin JP, Conrad KP. Vascular endothelial and placental growth factors: new players in the slow relaxin vasodilatory pathway. Ann N Y Acad Sci. 2009;1160:304–12. [Google Scholar]
- 81.Danielson LA, McGuane JT, Debrah JE, Conrad KP. Relaxin-induced renal vasodilation and hyperfiltration are mediated by vascular endothelial growth factor receptor(s) in chronically-instrumented, conscious rats [abstract] Reprod Sci. 2010 In press. [Google Scholar]
- 82.Fisher C, MacLean M, Morecroft I, Seed A, Johnston F, Hillier C, et al. Is the pregnancy hormone relaxin also a vasodilator peptide secreted by the heart? Circulation. 2002;106:292–5. doi: 10.1161/01.cir.0000025630.05387.45. [DOI] [PubMed] [Google Scholar]
- 83.McGuane JT, Sautina L, Debrah JE, Segal MS, Conrad KP. Mechanisms of relaxin-induced rapid arterial relaxation [abstract] Reprod Sci. 2009;16:106A. [Google Scholar]
- 84.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–23. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 85.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–82. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–52. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitley GS, Cartwright JE. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat. 2009;215:21–6. doi: 10.1111/j.1469-7580.2008.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harris LK. Review: trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010;31 (Suppl):S93–8. doi: 10.1016/j.placenta.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 89.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fisher SJ, McMaster M, Roberts JM. The placenta in normal pregnancy and preeclampsia. In: Lindheimer MD, Roberts JM, Cunningham FG, editors. Chesley’s hypertensive disorders in pregnancy. San Diego: Academic Press; 2009. pp. 73–86. [Google Scholar]
- 91.Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–80. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18:657–65. doi: 10.1016/s0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- 93.Fisher SJ, Damsky CH. Human cytotrophoblast invasion. Semin Cell Biol. 1993;4:183–8. doi: 10.1006/scel.1993.1022. [DOI] [PubMed] [Google Scholar]
- 94.Maruo N, Nakabayashi K, Wakahashi S, Yata A, Maruo T. Effects of recombinant H2 relaxin on the expression of matrix metalloproteinases and tissue inhibitor metalloproteinase in cultured early placental extravillous trophoblasts. Endocrine. 2007;32:303–10. doi: 10.1007/s12020-008-9034-5. [DOI] [PubMed] [Google Scholar]
- 95.Goligorsky MS, Budzikowski AS, Tsukahara H, Noiri E. Co-operation between endothelin and nitric oxide in promoting endothelial cell migration and angiogenesis. Clin Exp Pharmacol Physiol. 1999;26:269–71. doi: 10.1046/j.1440-1681.1999.03029.x. [DOI] [PubMed] [Google Scholar]
- 96.Martin D, Conrad KP. Expression of endothelial nitric oxide synthase by extravillous trophoblast cells in the human placenta. Placenta. 2000;21:23–31. doi: 10.1053/plac.1999.0428. [DOI] [PubMed] [Google Scholar]
- 97.Cervar M, Puerstner P, Kainer F, Desoye G. Endothelin-1 stimulates the proliferation and invasion of first trimester trophoblastic cells in vitro—a possible role in the etiology of pre-eclampsia? J Investig Med. 1996;44:447–53. [PubMed] [Google Scholar]
- 98.Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, Lala PK. Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAP kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol. 2003;201:63–73. doi: 10.1016/s0303-7207(02)00431-8. [DOI] [PubMed] [Google Scholar]
- 99.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–53. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 100.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldsmith LT, Weiss G, Palejwala S, Plant TM, Wojtczuk A, Lambert WC, et al. Relaxin regulation of endometrial structure and function in the rhesus monkey. Proc Natl Acad Sci U S A. 2004;101:4685–9. doi: 10.1073/pnas.0400776101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hayes ES. Biology of primate relaxin: a paracrine signal in early pregnancy? Reprod Biol Endocrinol. 2004;2:36. doi: 10.1186/1477-7827-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goldsmith LT, Weiss G. Relaxin in human pregnancy. Ann N Y Acad Sci. 2009;1160:130–5. doi: 10.1111/j.1749-6632.2008.03800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14:800–6. doi: 10.1093/humrep/14.3.800. [DOI] [PubMed] [Google Scholar]
- 105.Jeyabalan A, Stewart DR, McGonigal SC, Powers RW, Conrad KP. Low relaxin concentrations in the first trimester are associated with increased risk of developing preeclampsia [abstract] Reproductive Sci. 2009;16:101A. [Google Scholar]
- 106.Kalra SK, Molinaro TA. The association of in vitro fertilization and perinatal morbidity. Semin Reprod Med. 2008;26:423–35. doi: 10.1055/s-0028-1087108. [DOI] [PubMed] [Google Scholar]
- 107.Page EW. The relation between hydatid moles, relative ischemia of the gravid uterus, and the placental origin of eclampsia. Am J Obstet Gynecol. 1939;37:291–3. [Google Scholar]
- 108.Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am J Physiol. 2007;293:R766–74. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- 109.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30 (Suppl A):S38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 110.Rajakumar A, Whitelock KA, Weissfeld LA, Daftary AR, Markovic N, Conrad KP. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia (erratum: Biol Reprod. 2001;64:1019–20) Biol Reprod. 2001;64:499–506. doi: 10.1093/biolreprod/64.2.499. [DOI] [PubMed] [Google Scholar]
- 111.Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007;76:61–7. doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 113.Parry LJ, Vodstrcil LA. Relaxin physiology in the female reproductive tract during pregnancy. Adv Exp Med Biol. 2007;612:34–48. doi: 10.1007/978-0-387-74672-2_4. [DOI] [PubMed] [Google Scholar]
- 114.Jauniaux E, Johnson MR, Jurkovic D, Ramsay B, Campbell S, Meuris S. The role of relaxin in the development of the uteroplacental circulation in early pregnancy. Obstet Gynecol. 1994;84:338–42. [PubMed] [Google Scholar]
- 115.Anumba DO, El Gelany S, Elliott SL, Li TC. Serum relaxin levels are reduced in pregnant women with a history of recurrent miscarriage, and correlate with maternal uterine artery Doppler indices in first trimester. Eur J Obstet Gynecol Reprod Biol. 2009;147:41–5. doi: 10.1016/j.ejogrb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Yao L, Agoulnik AI, Cooke PS, Meling DD, Sherwood OD. Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina. Endocrinology. 2008;149:2072–9. doi: 10.1210/en.2007-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gammill HS, Roberts JM. Emerging concepts in preeclampsia investigation. Front Biosci. 2007;12:2403–11. doi: 10.2741/2242. [DOI] [PubMed] [Google Scholar]
- 119.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–73. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–12. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 123.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–9. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 124.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 125.Powers RW, Roberts JM, Cooper KM, Gallaher MJ, Frank MP, Harger GF, et al. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. Am J Obstet Gynecol. 2005;193:185–91. doi: 10.1016/j.ajog.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 126.Szlachter BN, Quagliarello J, Jewelewicz R, Osathanondh R, Spellacy WN, Weiss G. Relaxin in normal and pathogenic pregnancies. Obstet Gynecol. 1982;59:167–70. [PubMed] [Google Scholar]
- 127.Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJ, Tregear GW, et al. Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem. 2006;281:34942–54. doi: 10.1074/jbc.M602728200. [DOI] [PubMed] [Google Scholar]
- 128.Kern A, Bryant-Greenwood GD. Mechanisms of relaxin receptor (LGR7/RXFP1) expression and function. Ann N Y Acad Sci. 2009;1160:60–6. doi: 10.1111/j.1749-6632.2008.03826.x. [DOI] [PubMed] [Google Scholar]
- 129.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 130.Narumiya H, Zhang Y, Fernandez-Patron C, Guilbert LJ, Davidge ST. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens Pregnancy. 2001;20:185–94. doi: 10.1081/PRG-100106968. [DOI] [PubMed] [Google Scholar]
- 131.Debrah JE, McGuane JT, Novak J, Rubin JP, Conrad KP. Vascular endothelial and placental growth factors: new players in the slow relaxin vasodilatory pathway. 5th International Converence on Relaxin and Related Peptides; 2008; Hawaii. [Google Scholar]
- 132.Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25:205–34. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 133.Bathgate RA, Samuel CS, Burazin TC, Gundlach AL, Tregear GW. Relaxin: new peptides, receptors and novel actions. Trends Endocrinol Metab. 2003;14:207–13. doi: 10.1016/s1043-2760(03)00081-x. [DOI] [PubMed] [Google Scholar]
- 134.Chen J, Kuei C, Sutton SW, Bonaventure P, Nepomuceno D, Eriste E, et al. Pharmacological characterization of relaxin-3/INSL7 receptors GPCR135 and GPCR142 from different mammalian species. J Pharmacol Exp Ther. 2005;312:83–95. doi: 10.1124/jpet.104.073486. [DOI] [PubMed] [Google Scholar]
- 135.Stewart DR, Celniker AC, Taylor CA, Jr, Cragun JR, Overstreet JW, Lasley BL. Relaxin in the peri-implantation period. J Clin Endocrinol Metab. 1990;70:1771–3. doi: 10.1210/jcem-70-6-1771. [DOI] [PubMed] [Google Scholar]
- 136.O’Byrne EM, Carriere BT, Sorensen L, Segaloff A, Schwabe C, Steinetz BG. Plasma immunoreactive relaxin levels in pregnant and nonpregnant women. J Clin Endocrinol Metab. 1978;47:1106–10. doi: 10.1210/jcem-47-5-1106. [DOI] [PubMed] [Google Scholar]