Abstract
Background
Mast cells derive from hematopoietic cell precursors and participate in tissue allergic, immune, and inflammatory processes. They secrete many mediators, including preformed TNF, in response to allergic, neuropeptide, and environmental triggers. However, regulation of mast cell degranulation is not well understood.
Objective
We investigated the role of mitochondrial dynamics in degranulation of human cultured mast cells.
Methods
Human umbilical cord blood–derived mast cells (hCBMCs) and Laboratory of Allergic Diseases 2 (LAD2) mast cells were examined by confocal and differential interference contrast microscopy during activation by IgE/antigen and substance P (SP). Mast cells in control and atopic dermatitis (AD) skin were evaluated by transmission electron microscopy. LAD2 cells were pretreated with mitochondrial division inhibitor, a dynamin-related protein 1 (Drp1) inhibitor, and small interfering RNA for Drp1, which is necessary for mitochondrial fission and translocation. Calcineurin and Drp1 gene expression was analyzed in stimulated LAD2 cells and AD skin biopsies.
Results
Stimulation of hCBMCs with IgE/antigen or LAD2 cells with SP leads to rapid (30 minutes) secretion of preformed TNF. Degranulation is accompanied by mitochondrial translocation from a perinuclear location to exocytosis sites. Extracellular calcium depletion prevents these effects, indicating calcium requirement. The calcium-dependent calcineurin and Drp1 are activated 30 minutes after SP stimulation. Reduction of Drp1 activity by mitochondrial division inhibitor and decrease of Drp1 expression using small interfering RNA inhibit mitochondrial translocation, degranulation, and TNF secretion. Mitochondrial translocation is also evident by transmission electron microscopy in skin mast cells from AD biopsies, in which gene expression of calcineurin, Drp1, and SP is higher than in normal skin.
Conclusion
Human mast cell degranulation requires mitochondrial dynamics, also implicated in AD. (J Allergy Clin Immunol 2011;127:1522-31.)
Keywords: Atopic dermatitis, degranulation, inflammation, mast cells, mitochondria, substance P, TNF
Mast cells are tissue immune cells deriving from hematopoietic precursors that can secrete prestored mediators such as histamine, tryptase, and IL-4 through degranulation in response to allergic or neuropeptide triggers.1,2 In addition, mast cells uniquely store preformed TNF in their secretory granules3,4 and constitute a major source of rapid (1–30 minutes) TNF secretion.5 Stimulation of human mast cells by the proinflammatory peptide substance P (SP) induces degranulation and secretion of preformed TNF.6 The pathophysiology of many inflammatory diseases also involves mast cells,2 SP,7 and TNF.8,9
Regulation of mast cell stimulation after aggregation of the high-affinity surface IgE receptor (FceRI) has been studied extensively.10–12 Activation by SP, however, is less well understood and may involve the full neurokinin 1 receptor,13 its truncated form,14 or the Mas-related G protein-coupled member X receptor.15 However, the regulation of the steps involved in degranulation after stimulation regardless of triggers is still largely unknown. Mast cell degranulation requires intracellular calcium and metabolic energy.16 Mitochondria are the primary energy-generating organelles in eukaryotic cells17 and also regulate intracellular calcium.18 Mitochondria can accumulate in subcellular regions requiring high metabolic activity, such as growth cones of developing neurons19 or dendritic protrusions in spines and synapses.20 Mitochondrial dynamics enable them to participate in various complex cell behaviors, such as neuronal development 20 and insulin secretion.21 In particular, translocation of mitochondria is required for T-cell chemotaxis22 and T-cell activation by antigens.23
The intracellular pathways that participate in mitochondrial dynamics have been studied in several different biological systems. 17 In human cells, mitochondrial shape results from a regulated balance between fusion and fission events, tightly controlled by a growing family of proteins that include the dynamin-like guanosine triphosphatases (GTPases) Optic Atrophy 1 (Opa1) and mitofusin 1 and 2, which promote fusion; the cytosolic GTPase dynamin-related protein 1 (Drp1); and the mitochondrial fission protein 1 (hFis1), an outer mitochondrial membrane adaptor.
Drp1 is one of the most important regulators of both mitochondrial fission and translocation.22,23 Drp1 is found in the cytoplasm in an inactive form.24 Multiple modifications contribute to localization and proper function of Drp1. Dephosphorylation at Ser-637 by calcium-activated calcineurin is critical to recruit Drp1 from the cytoplasm to the mitochondrial outer membrane.25 Then phosphorylation at Ser-616 controls its enzyme activity responsible for mitochondrial fission.26 Mitochondrial dynamics and the function of Drp1 have not previously been studied in mast cell degranulation or in inflammatory diseases.
Here we show that human mast cell degranulation and secretion of preformed TNF require mitochondrial translocation to sites of exocytosis. This process is regulated by calcium-dependent calcineurin and Drp1 activation. We also show that calcineurin and Drp1 gene expression is increased in activated mast cells and in affected skin from patients with atopic dermatitis (AD).
METHODS
Culture of human mast cells
Human umbilical cord blood was collected after normal uncomplicated deliveries under the approval of the human subjects institutional review board of Tufts Medical Center, Boston, Mass. Hematopoietic stem cells (CD34+) were isolated by positive selection of CD34+/AC133+ cells with magnetic cell sorting using an AC133+ cell isolation kit (Miltenyi Biotec, Auburn, Calif) as previously reported.27 CD34+ cells were suspended in AIM-V Medium (Gibco-BRL, Carlsbad, Calif), supplemented with 100 ng/mL recombinant human stem cell factor (rhSCF; kindly supplied by Biovitrum, Stockholm, Sweden) and 50 ng/mL IL-6 (Millipore, Billerica, Mass) for 12 to 16 weeks. The purity of human umbilical cord blood–derived mast cells (hCBMCs) was evaluated by immunocytochemical staining for tryptase as previously described.27 Mast cells (100% purity) cultured over 12 weeks were used for the experiments. Laboratory of Allergic Diseases 2 (LAD2) cells (kindly supplied by Dr A. S. Kirshenbaum, National Institutes of Health, Bethesda, Md) deriving from a human mast cell leukemia28 were cultured in StemPro-34 serum-free medium (Invitrogen, Carlsbad, Calif) supplemented with 100 U/mL penicillin/streptomycin 100 U/mL glutamine and 100 ng/mL rhSCF.
Confocal microscopy
hCBMCs and LAD2 mast cells were incubated with 20 nmol/L Mito-Tracker deep red probe (Invitrogen) for 20 minutes and 50 nmol/L LysoTracker DND (Invitrogen) for 30 minutes. Cells were washed, moved to glass-bottom culture dishes (MatTek, Ashland, Mass), and observed using a Leica TCS SP2 Confocal microscope (Leica, Tokyo, Japan). Stimulation of the hCBMCs was achieved as follows: hCBMCs 12 to 14 weeks old were washed once in both Dulbecco PBS and human Tyrode buffer and were resuspended in fresh medium containing rhSCF (100 ng/mL) alone, which was included in the stimulation medium in all experiments for optimal mast cell viability. HCBMCs were resuspended (106 cells/mL) and passively sensitized by incubation with biotinylated human myeloma IgE (1 and 2 µg/mL/106 cells; Chemicon, Billerica, Mass) for 48 hours at 37°C. Cells were then washed, resuspended in medium containing rhSCF alone, and distributed to 96-well plates (1 × 105 cells per 200 µL) for stimulation with streptavidin (125 ng/mL; DAKO, Carpinteria, Calif) at 37°C in 5% CO2 for 30 minutes. LAD2 cells were stimulated with SP (2 µmol/L for 30 minutes at 37°C; Sigma, St Louis, Mo). The percentage of cells undergoing mitochondrial translocation was calculated after examination of 100 randomly selected mast cells in each experiment. Confocal digital images were processed using the ImageJ 1.32 mitochondria calculator plug-in (Wayne Rasband, National Institutes of Health, Bethesda, Md; http://rsb.info.nih.gov/ij/)29 and Adobe Photoshop 7.0 (Adobe Systems Inc, San Jose, Calif) programs. The Z-stack mitochondrial density projection was performed by using ImageJ 1.32 from 30 different layers of the same cell.
β-Hexosaminidase assay
β-Hexosaminidase (β-hex) release, as an index of mast cell degranulation, was assayed by using a fluorometric assay. Briefly, hCBMCs and LAD2 cells (0.5 × 105/tube) were stimulated, supernatant fluids were collected, and cell pellets were lysed with 1% Triton X-100 (Sigma). Supernatants and cell lysates were incubated in reaction buffer (p-nitrophenyl-N-acetyl-β-D-glucosaminide from Sigma) for 1 hour, and then 0.2 mol/L glycine was added to stop the reaction. Absorbance was measured at 405 nm in an ELISA reader, and the results are expressed as the percentage of β-hex released over the total (n = 3; *P <.05 compared with control). Mitochondrial division inhibitor-1 (mdivi-1; kindly supplied by Dr O. Shirihai, Boston University Medical School, Boston, Mass) was dissolved in dimethyl sulfoxide (Sigma), which had no effect on mast cell activation or viability at the final concentration of 0.1%.
TNF release assay
LAD2 cells were treated with SP (1 or 2 µmol/L for 30 minutes) or pretreated with mdivi-1 for 30 minutes before stimulation with SP. TNF release was measured by ELISA (R&D Systems, Minneapolis, Minn) in the supernatant fluid or in lysates of unstimulated LAD2 cells.
Calcineurin activity assay
See this article’s Methods in the Online Repository at www.jacionline.org.
Western blot analysis of total and phosphorylated Drp1
LAD2 cells were stimulated with SP (2 µmol/L) for 30 minutes. The reaction was stopped by addition of ice-cold PBS. Cells were washed once with PBS and then lysed in cell lysis buffer (Cell Signaling, Danvers, Mass). Equal amounts of protein were electrophoresed on 4% to 12% polyacrylamide gels and then transferred to a 0.44-µm Polyvinylidene fluoride membrane (Invitrogen). After blocking with 5% BSA, membranes were probed with antibody against Drp1 or the Ser-616 phosphorylated form of Drp1 (Cell Signaling, Boston, Mass) at 1:1000 dilution. B-actin was used as an internal control (Cell Signaling). For detection, the membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Cell Signaling) at 1:1000 dilution, and the blots were visualized with enhanced chemiluminescence. Scanned densitometry and protein density calculation was performed by ImageJ.
Cytosolic calcium measurements
Cytosolic calcium was measured in LAD2 cells at 37°C by using Fura-2-acetoxymethyl ester (Fura-2 AM; Invitrogen) as the indicator. LAD2 cells suspended in Tyrode buffer were loaded with 30 nmol/L Fura-2 AM for 20 minutes to allow Fura-2 to enter the cells. After centrifugation to remove excess dye, cells were resuspended in Tyrode buffer at a concentration of 106/mL and incubated for another 20 minutes. Cells were then transferred to 96-well plates (100 µL/well), and SP (2 µmol/L) was added. Real-time Fura-2 fluorescence was read by MDC FlexStation II (Molecular Devices, Sunnyvale, Calif) at an excitation wavelength of 340 nm/380 nm and emission wavelength of 510 nm. Results were analyzed according to the Invitrogen Fura-2 protocol and reported as the relative value of OD340/380 nm as described previously.30
Drp1 siRNA transfection
LAD2 cells were transfected by specific small interfering RNA (siRNA) for Drp1 by using the siRNA transfection kit (Santa Cruz, Santa Cruz, Calif). LAD2 cells were incubated with either predesigned specific (sc-43732; Santa Cruz) or scrambled (control) siRNA (sc-37007; Santa Cruz) in transfection medium for 5 hours according to the protocol. Then cells were resuspended in complete medium for 48 hours. Drp1 knockdown efficacy was tested by real-time PCR and Western blot analysis.
PCR and quantitative PCR
Total RNA from cultured mast cells and human skin biopsies was isolated by using the RNeasy Mini Kit (Qiagen, Valencia, Calif) and Trizol reagent (Invitrogen), respectively, according to the manufacturers’ instructions. Reverse transcription was performed with 300 ng total RNA by using the iScript cDNA synthesis kit (BIO-RAD, Hercules, Calif). To measure calcineurin, Drp1, and SP expression, quantitative real-time PCR was performed by using Taqman gene expression assays (Applied Biosystems, Carlsbad, Calif). The following probes obtained from Applied Biosystems were used: Hs 00174223_m1 PPP3CA for calcineurin, Hs 00247152_m1 DNM1L for Drp1, and Hs 00243225_m1 TAC1 for SP. TAC1 is an abbreviation for protachykinin 1, a protein that is encoded by the TAC1 gene, which is responsible for 4 products of the tachykinin peptide hormone family: substance P, neurokinin A, and the related peptides, neuropeptide K and neuropeptide γ. We used TAC1 because there were no primers available for SP alone. Samples were run at 45 cycles by using the Applied Biosystems 7300 Real-Time PCR System. Relative mRNA abundance was determined from standard curves run with each experiment; calcineurin, Drp1, and TAC1 (used to identify SP) gene expressions were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Hu, VIC TAMRA) endogenous control.
Patients and biopsies
Two full-depth punch skin biopsies (4 mm3) were collected from patients (n = 10; 6 females and 4 males; mean age ± SD, 32.9 ± 21.1 years) and controls (n = 10; 6 females and 4 males; mean age ± SD, 39.8 ± 17.2 years) who had not received any medication for 15 days before the biopsy and were seen at the 1st and 2nd Departments of Dermatology of the University of Athens Medical School, Athens, Greece. The Scientific Affairs Committee and the institutional review board approved this protocol. All participants gave their written informed consent according to the Declaration of Helsinki principles. One set of biopsies was immediately placed in RNA later solution (Ambion, Inc, Austin, Tex) and stored at −20°C, whereas the other was preserved for transmission electron microscopy (TEM) as described.
TEM
Human skin biopsies from control subjects (n = 3) and patients with AD (n = 5) were fixed in modified Karnovsky fixative containing 0.2% paraformaldehyde, 3% glutaraldehyde, and 0.5% tannic acid in 0.1 mmol/L Nacacodylate buffer. Sections (5 µm) were cut by using a microtome and observed by using a Philips-300 TEM (FEI, Hillsboro, Ore). High-quality glossy photographs of individual mast cells were evaluated by 3 independent operators for the number of mitochondria and their distances from the cell surface. All mast cells from patients with AD showed evidence of degranulation to different extents, defined as 5 or more altered secretory granules.
Data analysis
Image analysis was performed blind to the treatment conditions. For each experimental condition, confocal cell images were randomly taken from different wells of the microscope plate, and ImageJ software was used for confocal image processing. TEM photomicrographs of mast cells were evaluated for mitochondria by 3 independent operators. All data are expressed as mean ± SD. Statistical significance was determined by the Student t test or the nonparametric Mann-Whitney U test for the patient samples using the Sigma-Plot 9.0 (SPSS, Chicago, Ill). Significant differences were considered if P <.05.
RESULTS
Mitochondrial translocation in degranulating mast cells
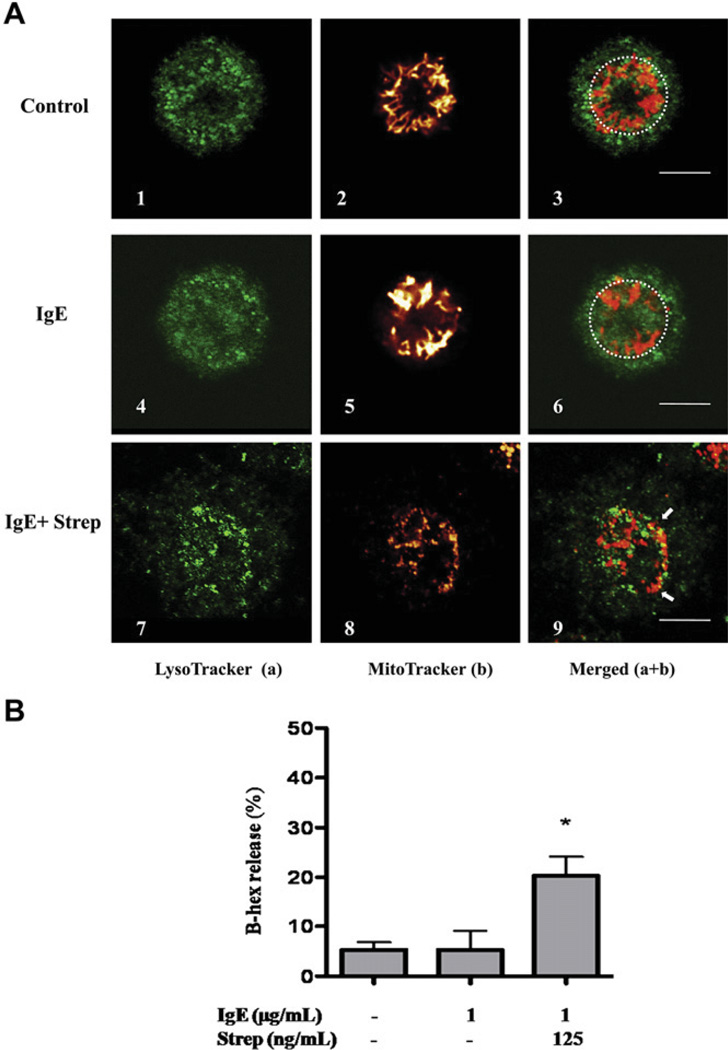
Observation with confocal microscopy of unstimulated hCBMCs (Fig 1, A, upper panels), as well as those treated only with IgE (1 µg/mL) for 30 minutes (Fig 1, A, middle panels), after staining with LysoTracker green (to identify secretory granules) and MitoTracker red probe (to identify the mitochondria), reveals that the overwhelming majority of mitochondria are connected in a net located in the perinuclear region (within the dash circles). LysoTracker identifies secretory granules because their pH is 5.5, similar to that of lysosomes.31 In contrast, hCBMCs stimulated by IgE (1 µg/mL) and streptavidin (125 ng/mL) show rapid (30 minutes) degranulation (Fig 1, A, 7) and mitochondrial translocation (Fig 1, A, 8). The merged images (Fig 1, A, 9) clearly show the presence of mitochondrial-associated fluorescence near the cell surface of a degranulating mast cell (arrows). Degranulation was also confirmed by β-hex release (Fig 1, B). Similar results were obtained with human LAD2 mast cells stimulated by SP (2 µmol/L for 30 minutes; Fig 2, A and B).
FIG 1.
Mitochondrial translocation and degranulation in hCBMCs observed by confocal microscopy. A, Mitochondrial distribution in resting (panels 1–3), IgE-incubated (1 µg/mL) (panels 4–6), and IgE (1 µg/mL) + streptavidin (125 ng/mL)–stimulated (panels 7–9) cells. Cells were stained with LysoTracker (a, green) and MitoTracker (b, red). B, β-hex release from hCBMCs treated with IgE or IgE + streptavidin (Strep) for 30 minutes. n = 3; *P < .05 compared with control. Bars equal to 5 µm.
FIG 2.
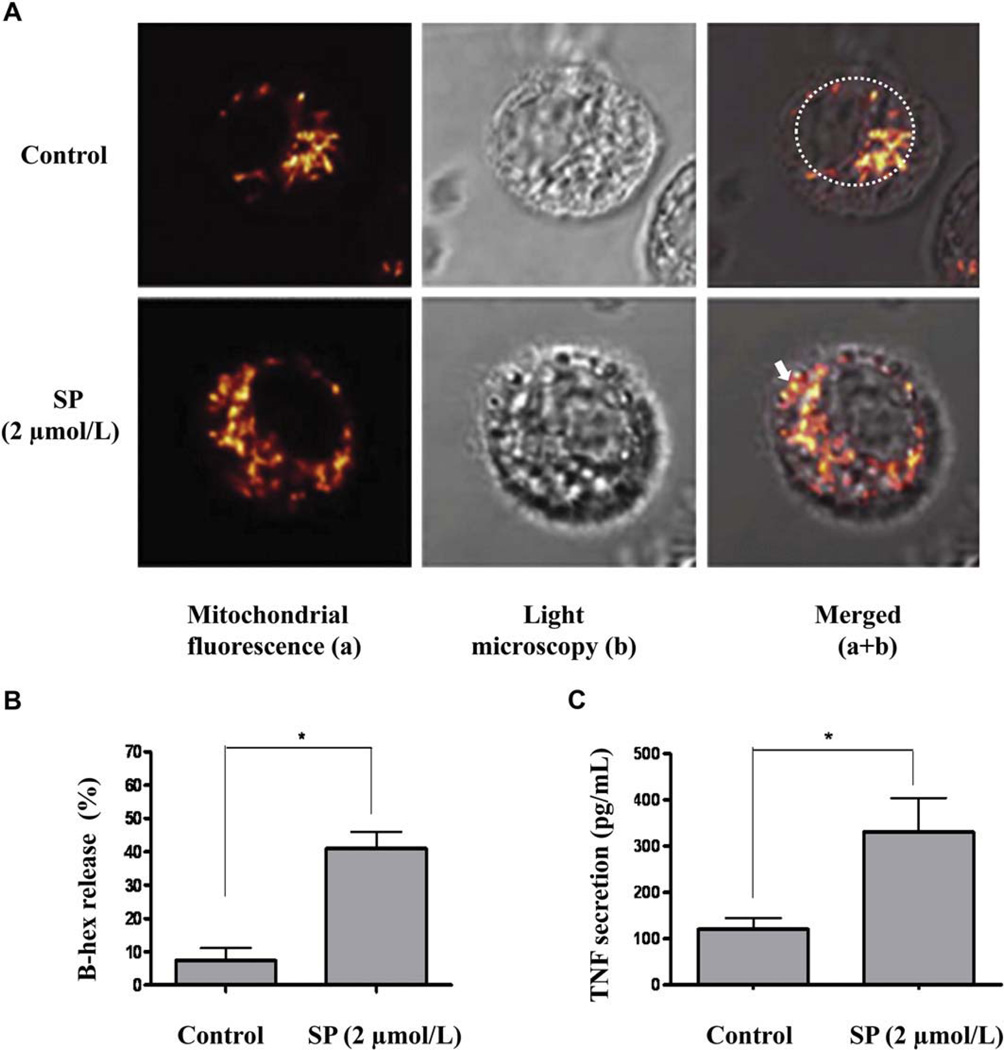
Mitochondrial translocation observed by differential interference contrast, and degranulation and TNF secretion in LAD2 mast cells. A, Mitochondrial distribution of control (upper panels) and degranulated (bottom panels) mast cells stimulated with SP (2 µmol/L) for 30 minutes. Cells were stained with Mito-Tracker (red). Mast cell degranulation by SP was confirmed by β-hex release (B) and TNF secretion (C). n = 3; *P < .05 compared with control.
This time, we also used light microscopy to visualize degranulation: we alternated objectives at the confocal microscope and also obtained images of mast cells using differential interference contrast, clearly showing degranulation (Fig 2, A, middle lower panel). Like stimulation with IgE and streptavidin, mast cell stimulation by SP (2 µmol/L) at 37°C results in rapid cell degranulation (noticeable at 30 minutes), whereas mast cells stained with LysoTracker after stimulation with SP (2 µmol/L) show extensive degranulation with numerous granule core heparin particles outside the domain of the cells that stained with LysoTracker (Fig 3, A, 5). There are also concomitant mitochondrial morphologic changes, consistent with translocation throughout the cells (Fig 3, A, 6). Merging of LysoTracker and MitoTracker images shows mitochondria close to the cell surface in the mast cell undergoing degranulation (Fig 3, A, 7). Secretion is also confirmed with β-hex (Fig 2, B) and TNF (Fig 2, C) release.
FIG 3.
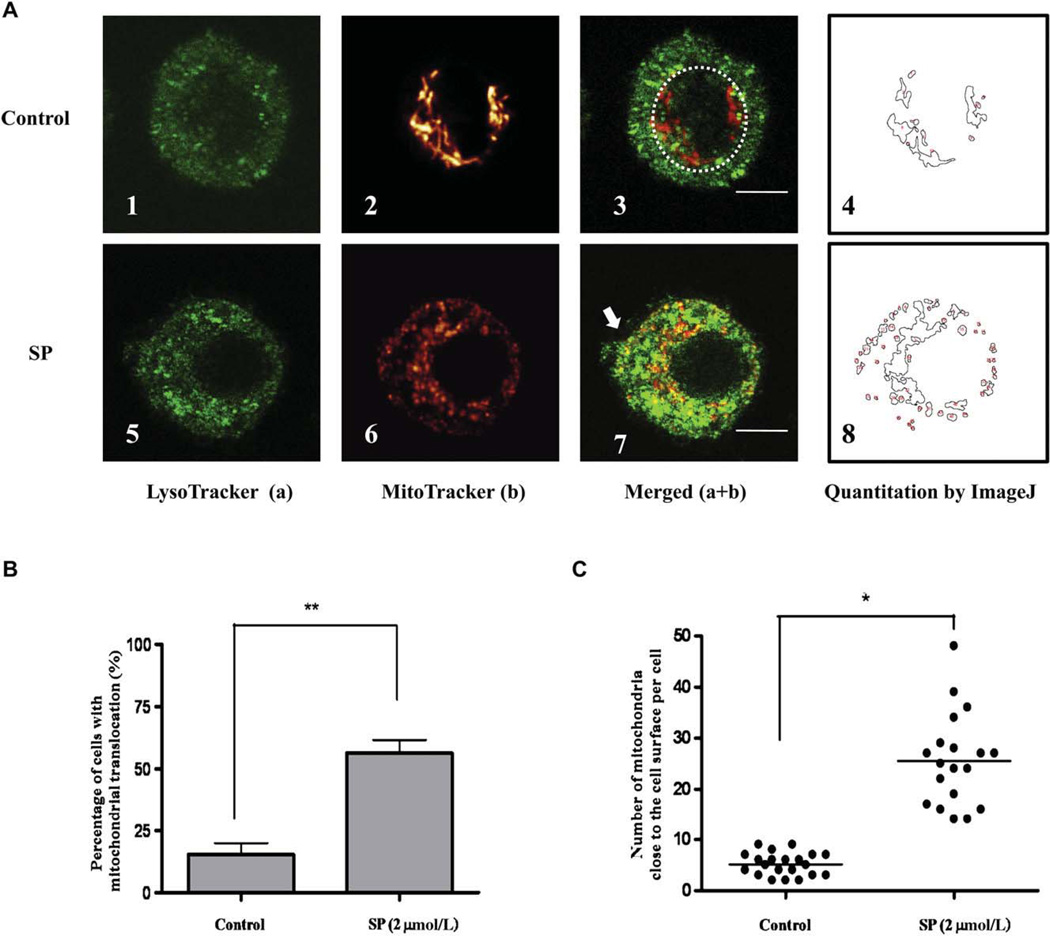
Quantification of mitochondrial translocation during degranulation in human LAD2 cells. A, Control (n = 19; panels 1–4) and degranulated (n = 20; panels 5–8) mast cells (SP 2 µmol/L, 30 minutes). B, Percentage of translocated mitochondria in resting and stimulated cells (n = 3). C, Number of mitochondria per cell within 1 µm from the cell surface. Cells were randomly selected for analysis (*P < .05; **P < .01; horizontal bars indicate the means). Bars equal to 5 µm.
FIG 5.
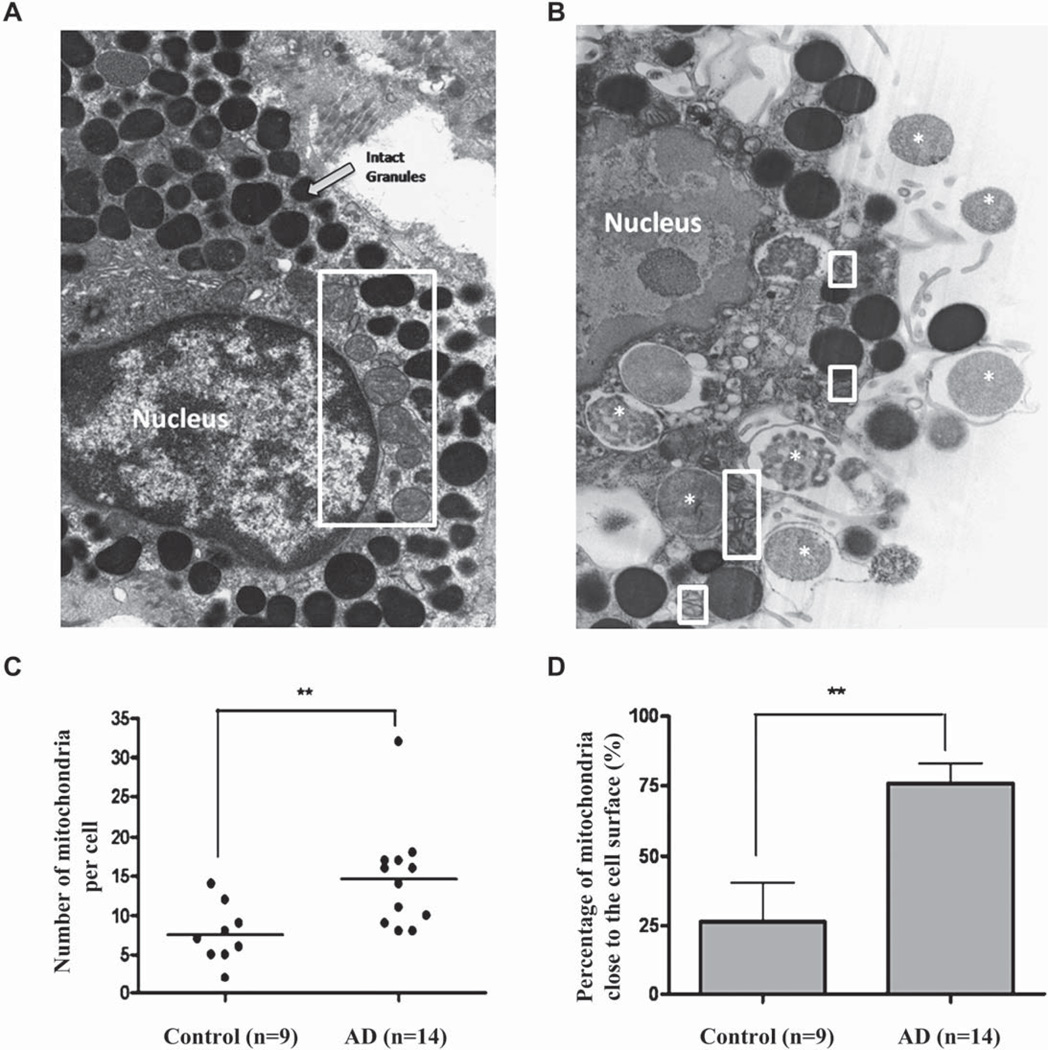
Electron photomicrographs showing mitochondrial translocation in human skin mast cells from patients with AD. Human skin mast cells from control (3 subjects, 9 mast cells; A) and lesional skin from patients with AD (5 subjects, 14 mast cells; magnification × 13,800; B). Mitochondria are shown within white rectangles. White asterisks represent secreted granular material. C, Number of mitochondria per cell. D, Percentage of mitochondria close to the cell surface in each cell (**P < .01; horizontal bars indicate the means).
FIG 6.
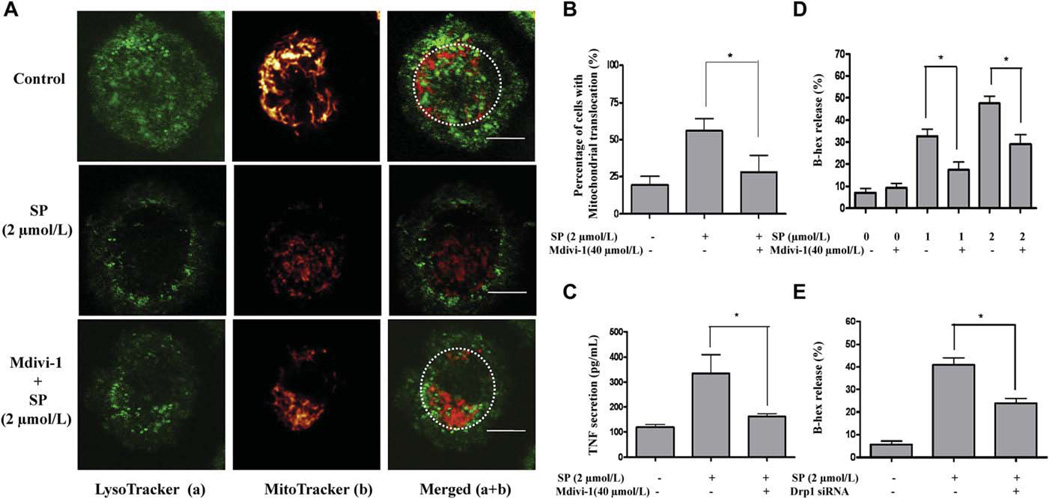
Mdivi-1 and Drp1 siRNA inhibit mast cell mitochondrial translocation, degranulation, and preformed TNF secretion. A, Mitochondrial morphology before or after stimulation with SP and pretreatment with mdivi-1. Cells were stained with LysoTracker (green) and MitoTracker (red). B, Percentage of translocated mitochondria in resting, stimulated, and mdivi-1-treated cells. C, TNF secretion. D, β-hex release. E, β-hex release after treatment with Drp1 siRNA before SP stimulation (n = 3; *P < .05). Bars equal to 5 µm.
FIG 7.
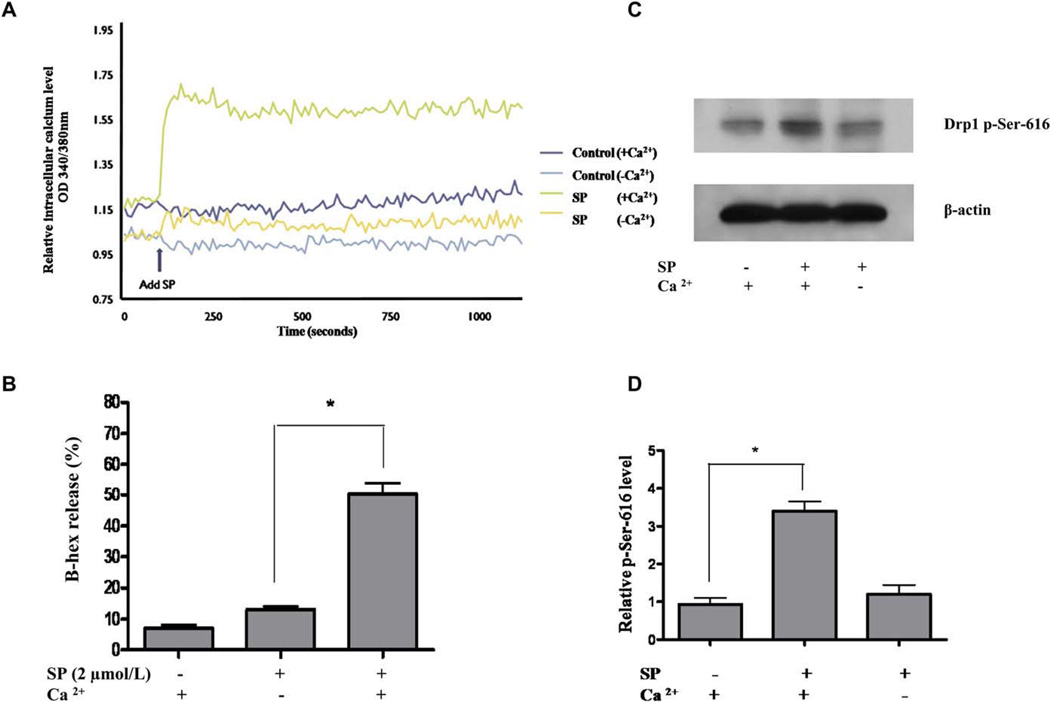
Intracellular calcium increase and Drp1 phosphorylation at Ser-616 during LAD2 mast cell degranulation. A, Intracellular calciumlevel was measured after SP stimulation. B, LAD2 cell degranulation with or without extracellular calcium, as measured by β-hex release. C, Western blot of Drp1 phosphorylation at Ser-616 after SP (2 µmol/L) stimulation with or without extracellular calcium. D, Drp1 protein density was normalized against β-actin (n = 3; *P < .05). There was no statistical difference in Drp1 phosphorylation at Ser-616 between control and SP stimulation in the absence of calcium.
Cell viability measured in LAD2 cells stimulated by SP (1, 5, or 10 µmol/L) for 30 minutes is >90% (see this article’s Fig E1 in the Online Repository at www.jacionline.org), indicating that mitochondrial translocation is not the result of apoptosis or necrosis. TNF secretion during 30 minutes after stimulation with SP is preformed and derives from secretory granules because it is well known that de novo TNF synthesis requires 6 to 24 hours. Moreover, lysed, unstimulated LAD2 cells contain substantial amount of preformed TNF as shown by ELISA (see this article’s Fig E2 in the Online Repository at www.jacionline.org).
The number of mitochondria located close to the cell surface area is further calculated by using the ImageJ Software mitochondria calculator plug-in as reported previously (Fig 3, A, 4 and 8).29 Mitochondrial translocation was observed in approximately 60% of LAD2 cells stimulated with SP (2 µmol/L) compared with 20% of control cells (Fig 3, B). The cells undergoing degranulation are the ones presenting with mitochondrial translocation according to our observations in the experiments described. The number of mitochondria close to the cell surface is significantly increased after SP stimulation (2 µmol/L) compared with the control cells (Fig 3, C). Human cultured mast cells do not attach to the culture .ask; to minimize the effects of multiple layers, images shown were chosen to represent the layer with the maximum nuclear diameter.
FIG 4.
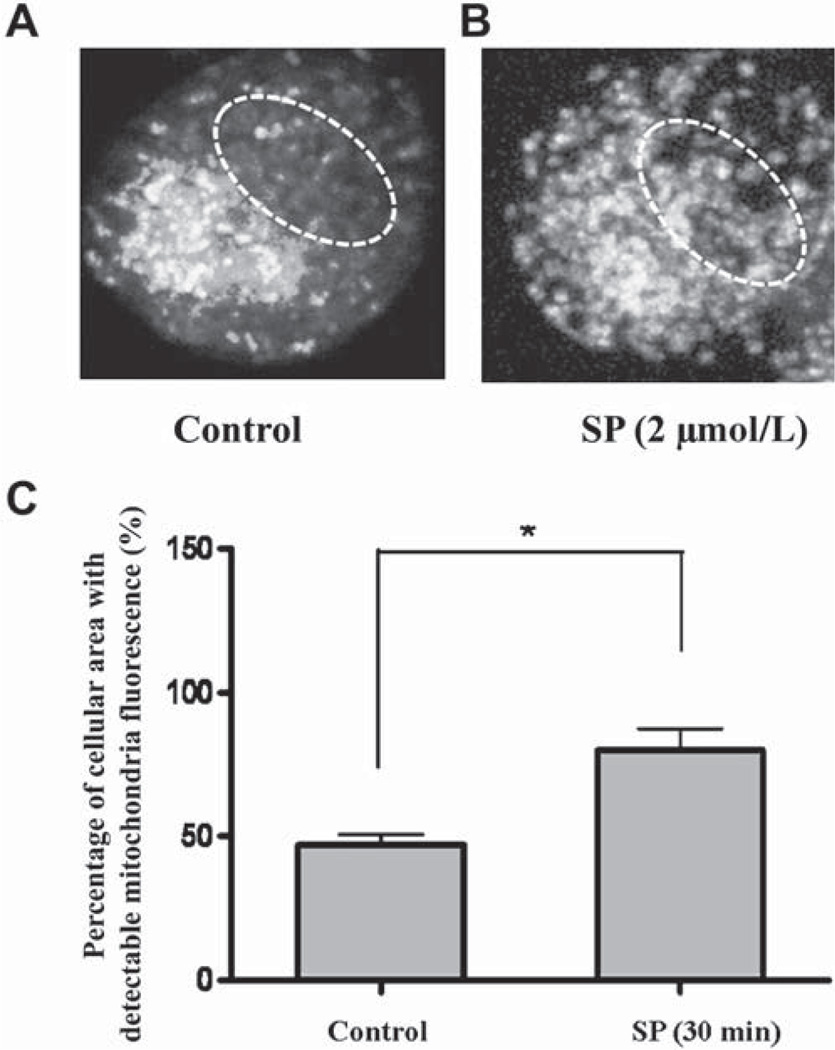
Z-stack mitochondrial fluorescence projection in control and stimulated human LAD2 mast cells. Control (A) and degranulated (B) LAD2 cell. White dashed lines represent the nuclear region. C, The percentage of the cellular area with detectable mitochondrial fluorescence indicating mitochondrial distribution was calculated from 20 cells (*P < .05).
FIG 8.
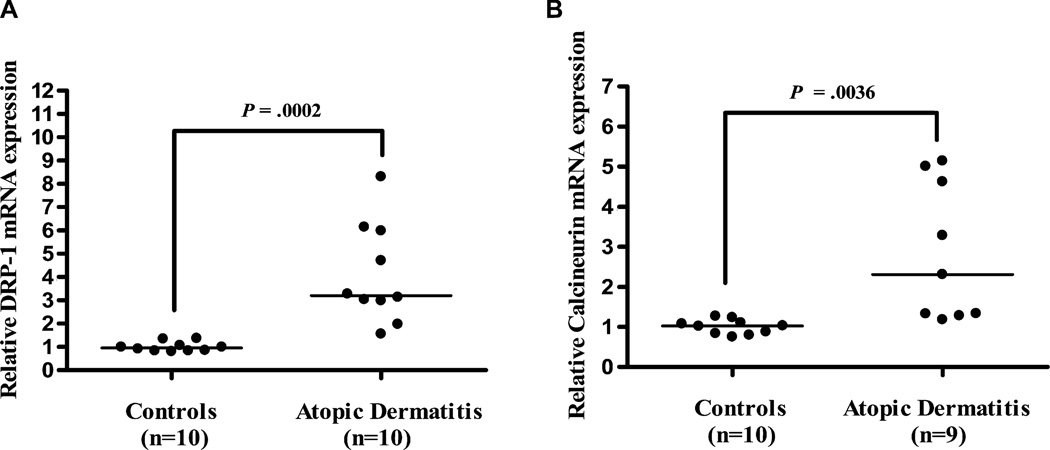
Drp1 and calcineurin gene expression in skin from patients with AD compared with healthy controls. Gene expression of Drp1 (controls, n = 10; patients, n = 10; A) and calcineurin (controls, n = 10; patients, n = 9; B). The calcineurin patient samples were 1 fewer because the cDNA was exhausted. Relative quantities of mRNA expression were measured by quantitative real-time-PCR and normalized to GAPDH (horizontal bars indicate the means).
To confirm further that our results are not distorted by multilayer effects, we used stacked images (30 per cell) from a single mast cell taken at 0.3 µm spacing. Mitochondrial fluorescence density from all images is then projected onto the Z-stack for distribution analysis. Before stimulation, high mitochondrial density is visualized only around the nuclear region (Fig 4, A, dashed circle). After SP stimulation (2 µmol/L), mitochondrial fluorescence is detected throughout the cell (Fig 4, B), and especially close to the cell surface, occupying 80% of the total cell area (Fig 4, C).
Mitochondrial translocation is present in degranulated human skin mast cells from AD skin biopsies
Images of skin mast cells from controls and patients with AD were obtained and examined by using TEM as described previously.32 All mast cells from patients with AD showed evidence of degranulation to different extent, defined as 5 or more altered secretory granules. Mitochondria of control human skin mast cells are clustered around the cell nucleus and appear large and intact (Fig 5, A). In contrast, mitochondria of degranulated mast cells from patients with AD are much smaller and are located close to secretory granules undergoing exocytosis (Fig 5, B). Analysis of the number of mitochondria and their distance from the cell surface shows that the average number of mitochondria (Fig 5, C) and the percentage of those close to the cell surface (Fig 5, D) are both significantly higher in degranulated mast cells than in controls.
Mast cell degranulation and preformed TNF secretion require Drp1-dependent mitochondrial translocation
To address the possibility that mitochondrial translocation to the cell surface may simply be an effect secondary to cell shape changes during degranulation, we investigated the role of Drp1, a protein absolutely necessary for mitochondrial fission, in mast cell degranulation. Pretreatment of LAD2 mast cells for 30 minutes with the Drp1 inhibitor mdivi-1 (40 µmol/L at 37°C)33 inhibits mitochondrial translocation in response to SP (2 µmol/L for 30 minutes; Fig 6, A) by 60% (Fig 6, B). Moreover, pretreatment of LAD2 cells with mdivi-1 significantly inhibits SP-triggered preformed TNF secretion by 75% (Fig 6, C) and β-hex release by 55% (Fig 6, D) compared with cells only stimulated by SP. To minimize the off-target effects of the small molecule inhibitor mdivi-1, Drp1 mRNA was also reduced in LAD2 cells by transfecting cells with specific Drp1 siRNA. After siRNA treatment for 6 hours, Drp1 mRNA levels in LAD2 cells drop to 30% of normal as measured by real-time PCR (see this article’s Fig E3, A, in the Online Repository at www.jacionline.org), whereas Drp1 protein levels measured by Western blot analysis are reduced to 20% of normal, 48 hours after siRNA treatment (Fig E3, B). At 48 hours after siRNA treatment, LAD2 cells were stimulated by SP (2 µmol/L) and β-hex secretion was 60% lower compared with siRNA-untreated, SP-stimulated cells (Fig 6, E). The lack of complete inhibition in these experiments may be a result of the fact that Drp1 expression and function were not blocked 100%.
Mitochondrial translocation and Drp1 function are dependent on extracellular calcium influx
The absence of extracellular calcium prevents SP-triggered intracellular calcium increase (Fig 7, A) and consequent degranulation (Fig 7, B). Instead, LAD2 cells stimulated by the calcium ionophore A23187 show mitochondrial translocation (see this article’s Fig E4 in the Online Repository at www.jacionline.org), suggesting that an increase in calcium alone is sufficient to induce mitochondrial translocation. Intracellular calcium increase is, therefore, critical for mast cell degranulation and mitochondrial translocation. Consequently, we investigated the effect of intracellular calcium on Drp1 activation and Drp1-dependent mitochondrial translocation. We show that LAD2 cells stimulated by SP (2 µmol/L) for 6 hours also produce a significant Drp1 mRNA increase (see this article’s Fig E5, A, in the Online Repository at www.jacionline.org). Drp1 dephosphorylation at Ser-637 is regulated by calcineurin34 and is required for Drp1 translocation to the mitochondrial outer membrane. We also show that 6 hours after SP stimulation, there is a significant increase of calcineurin activity in LAD2 cells (Fig E5, B), an effect diminished in the absence of extracellular calcium (Fig E5, B). Drp1 requires phosphorylation at Ser-616 to be activated and promote mitochondrial fission. We examined Drp1 activation after SP stimulation (2 µmol/L) by using Western blot analysis. On stimulation with SP (2 µmol/L), increased Drp1 phosphorylation is detected within 30 minutes by using Ser-616 phospho-specific antibodies (Fig 7, C) and is statistically significant compared with the control (Fig 7, D). However, this effect is not observed in cells cultured in extracellular calcium-free medium (Fig 7, C), indicating that calcium influx is required for measurable Drp1 activation.
Calcineurin, Drp1, and SP gene expression in AD
Analysis of skin biopsies from AD and normal control subjects reveals that gene expression of Drp1 (Fig 8, A) is significantly increased in patients with AD (n = 10) compared with controls (n = 10). The expression of calcineurin is also significantly increased (Fig 8, B) in patients with AD (n = 9) compared with controls (n = 10).
Given that SP stimulates human mast cells, we investigated whether SP is expressed in lesional skin from patients with AD. Gene expression of SP was also found to be significantly increased in affected skin biopsies from patients with AD (n = 10) compared with controls (n = 10; see this article’s Fig E6 in the Online Repository at www.jacionline.org).
DISCUSSION
Here we show that stimulation of hCBMCs by IgE and antigen, as well as LAD2 cell stimulation by the proinflammatory peptide SP, leads to mitochondrial translocation to the cell surface during degranulation, as documented by confocal microscopy. SP (1 µmol/L) has previously been shown to induce secretion of TNF from LAD2 cells and isolated human skin mast cells.5 This TNF is preformed, which is evidenced by (1) its rapid (30 minutes) secretion compared with de novo synthesized TNF release that occurs much later (6–24 hours), and (2) its presence in unstimulated LAD2 cells confirmed by ELISA. Moreover, SP (1 µmol/L) was able to enhance the rate of oxygen consumption of isolated rat cardiac cell mitochondria.35 The fact that the Drp1 inhibitor mdivi-1 and Drp1 siRNA treatment reduce SP-triggered β-hex and TNF release supports the requirement of Drp1 and mitochondrial translocation in mast cell degranulation. Neither of these treatments completely blocks mitochondrial translocation and mast cell degranulation, possibly because Drp1 function is not completely suppressed by either inhibitory approach.
Previous studies have shown that mitochondria undergo fission during insulin secretion from storage vesicles in activated β-pancreatic cells.21 Also, mitochondria have been reported to accumulate at the uropod of migrating T cells.22 Mitochondrial translocation may, therefore, be necessary to provide energy locally, as has been suggested for ATP-producing organelles and ATP-consuming cellular structures.36 Another possibility would be that translocated mitochondria may buffer calcium ions locally. For instance, our current findings show that increased intracellular calcium in stimulated LAD2 cells is necessary for (1) mitochondrial translocation; (2) calcineurin activation, which leads to dephosphorylation of Drp1 at Ser-637, stimulating recruitment of Drp1 to the mitochondrial surface34; and (3) Drp1 activation through Ser-616 phosphorylation, leading to increased mitochondrial fission37 (see this article’s Fig E7 in the Online Repository at www.jacionline.org). There was no statistical difference in calcineurin and Drp1 activity between control and SP stimulation in the absence of calcium. Some increased β-hex release, even in the absence of extracellular calcium, may be a result of either nominal extracellular calcium bound to the cell surface or intracellular calcium released from endoplasmic reticulum or mitochondria. However, this extent of β-hex release may involve calcineurin and Drp1 activity, which is below the level of detection. Nevertheless, there may still be an unidentified additional mechanism driving β-hex release in the absence of calcium, especially from secretory granules adjacent to the cell surface. Local calcium increase also activates a cytoplasmic GTPase called Miro, which promotes mitochondrial translocation through Drp1.38 It was also recently shown that during T-cell activation, mitochondria translocate to the “immunological synapse,” where they buffer local calcium to permit calcium channels to remain open.39
We do not currently know whether all mitochondria undergo fission and translocation during mast cell degranulation. Some mitochondria may remain perinuclear to provide energy for general cellular needs, whereas others translocate on demand. Previous articles have reported that mitochondria are heterogeneous in terms of their localization, structure, and function.40
Finally, we show that gene expression of calcineurin, Drp1, and SP is increased in skin biopsies from patients with AD. AD is characterized by skin inflammation41 and involves both T cells and mast cells.42 It is of interest that one of the most effective treatments of AD is the calcineurin inhibitor FK506 (tacrolimus),43 which was also reported to prevent pruritus in a mouse model of AD.44 FK506 also inhibits IgE/anti-IgE–induced secretion of histamine from secretory granules in rat mast cells.45 In addition, FK506 depletes SP from peripheral sensory neurons, 44,46 which may be relevant to our current finding of increased SP gene expression in affected AD skin. Mast cells can also serve as antigen-presenting cells47 and can superstimulate activated T cells through TNF and cell-to-cell contact in both mouse47 and human cells.48 Inhibition of preformed TNF secretion from mast cells may help explain why anti-TNF therapy is often useful in severe cases of AD.49–51
The current findings document that human mast cell degranulation and preformed TNF secretion require mitochondrial translocation close to sites of exocytosis. This is the first time that mitochondrial dynamics are shown to regulate mast cell degranulation. Mitochondrial translocation constitutes a novel regulatory process that could be targeted for the development of effective antiallergic and anti-inflammatory drugs.
Supplementary Material
Acknowledgments
Supported in part by NIH grant R01 AR47652 to T.C.T.
We thank Dr A. S. Kirshenbaum and Dr Dean Metcalfe (National Institutes of Health, Bethesda, Md) for the generous supply of LAD2 mast cells. We also thank Dr Orian Shirihai (Boston University Medical School, Boston, Mass) for kindly providing the Drp1 inhibitor mdivi-1 and for useful discussions and encouragement. Thanks are also due to Dr James A. Marchand (Tufts University, Boston, Mass) for his useful comments on this article. We thank Biovitrum AB (Stockholm, Sweden) for their kind gift of rhSCF. B.Z. is supported by a graduate student fellowship from Galenica SA (Athens, Greece). K.-D.A. and A.A. are recipients of postgraduate scholarships from the Hellenic State Scholarships Foundation (Athens, Greece). Rose Fountotos (Montreal, Canada) participated as a high school trainee during summer 2010.
Abbreviations used
- AD
Atopic dermatitis
- β-hex
β-Hexosaminidase
- Drp1
Dynamin-related protein 1
- FcεRI
High-affinity surface IgE receptor
- Fura-2AM
Fura-2-acetoxymethyl ester
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GTPase
Guanosine triphosphatase
- hCBMC
Human umbilical cord blood–derived mast cell
- LAD2
Laboratory of Allergic Diseases 2
- mdivi-1
Mitochondrial division inhibitor-1
- rhSCF
Recombinant human stem cell factor
- siRNA
Small interfering RNA
- SP
Substance P
- TAC1
Protachykinin 1
- TEM
Transmission electron microscopy
Footnotes
Disclosure of potential conflict of interest: T. C. Theoharides and B. Zhang are listed as the inventors of the provisional patent application US 61/405,414. The rest of the authors have declared that they have no conflict of interest.
Clinical implications: Mitochondrial translocation regulates human mast cell degranulation and preformed TNF secretion, providing new insights and possible therapeutic targets, at least for inflammatory skin conditions such as AD.
REFERENCES
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Theoharides TC, Kalogeromitros D. The critical role of mast cell in allergy and inflammation. Ann N Y Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 3.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 4.Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol. 2007;178:5701–5709. doi: 10.4049/jimmunol.178.9.5701. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cell rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- 6.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2007;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 8.Beutler B. TNF, immunity and inflammatory disease: lessons of the past decade. J Investig Med. 1995;43:227–235. [PubMed] [Google Scholar]
- 9.Haas RH. Autism and mitochondrial disease. Dev Disabil Res Rev. 2010;16:144–153. doi: 10.1002/ddrr.112. [DOI] [PubMed] [Google Scholar]
- 10.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Kraft S, Rana S, Jouvin MH, Kinet JP. The role of the FcepsilonRI beta-chain in allergic diseases. Int Arch Allergy Immunol. 2004;135:62–72. doi: 10.1159/000080231. [DOI] [PubMed] [Google Scholar]
- 12.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki R, Furuno T, McKay DM, Wolvers D, Teshima R, Nakanishi M, et al. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol. 1999;163:2410–2415. [PubMed] [Google Scholar]
- 14.Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 16.Douglas WW, Kagayama M. Calcium and stimulus-secretion coupling in the mast cell: stimulant and inhibitory effects of calcium-rich media on exocytosis. J Physiol. 1977;270:691–703. doi: 10.1113/jphysiol.1977.sp011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104(pt 3):917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 25.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 26.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- 28.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 29.Dagda RK, Cherra SJ, III, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RG, Carty SE, Fingerhood BJ, Scarpa A. The internal pH of mast cell granules. FEBS Lett. 1980;120:75–79. doi: 10.1016/0014-5793(80)81050-7. [DOI] [PubMed] [Google Scholar]
- 32.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 33.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cereghetti GM, Stangherlin A, Martins de BO, Chang CR, Blackstone C, Bernardi P, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhakar NR, Runold M, Kumar GK, Cherniack NS, Scarpa A. Substance P and mitochondrial oxygen consumption: evidence for a direct intracellular role for the peptide. Peptides. 1989;10:1003–1006. doi: 10.1016/0196-9781(89)90182-4. [DOI] [PubMed] [Google Scholar]
- 36.Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- 37.Jahani-Asl A, Slack RS. The phosphorylation state of Drp1 determines cell fate. EMBO Rep. 2007;8:912–913. doi: 10.1038/sj.embor.7401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. The in-depth evaluation of suspected mitochondrial disease. Mol Genet Metab. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 42.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson EL. Atopic dermatitis: a review of topical treatment options. Curr Med Res Opin. 2010;26:633–640. doi: 10.1185/03007990903512156. [DOI] [PubMed] [Google Scholar]
- 44.Inagaki N, Shiraishi N, Igeta K, Nagao M, Kim JF, Chikumoto T, et al. Depletion of substance P, a mechanism for inhibition of mouse scratching behavior by tacrolimus. Eur J Pharmacol. 2010;626:283–289. doi: 10.1016/j.ejphar.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 45.Sengoku T, Kishi S, Sakuma S, Ohkubo Y, Goto T. FK506 inhibition of histamine release and cytokine production by mast cells and basophils. Int J Immunopharmacol. 2000;22:189–201. doi: 10.1016/s0192-0561(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 46.Pereira U, Boulais N, Lebonvallet N, Pennec JP, Dorange G, Misery L. Mechanisms of sensory effects of tacrolimus on the skin. Br J Dermatol. 2010;163:70–77. doi: 10.1111/j.1365-2133.2010.09757.x. [DOI] [PubMed] [Google Scholar]
- 47.Gong J, Yang NS, Croft M, Weng IC, Sun L, Liu FT, et al. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC Immunol. 2010;11:34. doi: 10.1186/1471-2172-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, et al. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008;155:1076–1084. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassano N, Loconsole F, Coviello C, Vena GA. Infliximab in recalcitrant severe atopic eczema associated with contact allergy. Int J Immunopathol Pharmacol. 2006;19:237–240. [PubMed] [Google Scholar]
- 50.Rigopoulos D, Korfitis C, Gregoriou S, Katsambas AD. Infliximab in dermatological treatment: beyond psoriasis. Expert Opin Biol Ther. 2008;8:123–133. doi: 10.1517/14712598.8.1.123. [DOI] [PubMed] [Google Scholar]
- 51.Sockolov ME, Alikhan A, Zargari O. Non-psoriatic dermatologic uses of monoclonal antibody therapy. J Dermatolog Treat. 2009;20:319–327. doi: 10.3109/09546630902936778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.