Abstract
Rationale
Spatial heterogeneity in connexin (Cx) expression has been implicated in arrhythmogenesis.
Objective
This study was carried out to quantify the relation between the degree of heterogeneity in Cx43 expression and disturbances in electrical propagation.
Methods and Results
Cell pairs and strands composed of mixtures of Cx43-/- (Cx43KO) or GFP-expressing Cx43+/+ (WTGFP) murine ventricular myocytes were patterned using microlithographic techniques. At the interface between pairs of WTGFP and Cx43KO cells, dual voltage clamp showed a marked decrease in electrical coupling (~5% of wildtype) and voltage gating suggested the presence of mixed Cx43/Cx45 channels. Cx43 and Cx45 immunofluorescence signals were not detectable at this interface, probably because of markedly reduced gap junction size. Macroscopic propagation velocity, measured by multisite high-resolution optical mapping of transmembrane potential in strands of cells of mixed Cx43 genotype, decreased with an increasing proportion of Cx43KO cells in the strand. A marked decrease in conduction velocity was observed in strands composed of <50% wildtype cells. Propagation at the microscopic scale showed a high degree of dissociation between WTGFP and Cx43KO cells, but consistent excitation without development of propagation block.
Conclusions
Heterogeneous ablation of Cx43 leads to a marked decrease in propagation velocity in tissue strands composed of <50% cells with wildtype Cx43 expression and marked dissociation of excitation at the cellular level. However, the small residual electrical conductance between Cx43 and WTGFP myocytes assures excitation of Cx43-/- cells. This explains the previously reported undisturbed contractility in tissues with spatially heterogeneous downregulation of Cx43 expression.
Keywords: Cx43 genotypes, Myocardium, Cellular Coupling, Propagation
INTRODUCTION
Cell-to-cell coupling at gap junctions enables flow of electrical current and diffusion of small molecules between cardiac cells. The low resistance electrical pathways made up by gap junction channels are essential for electrical excitation of the whole heart, and consequently, coordinated contraction. Multiple myocardial connexins (Cx's) have been described. Cx43 is present in atrial and ventricular myocardium, Cx40 in atrial myocardium and in the ventricular conduction system, and Cx45 is found in the sino-atrial and atrio-ventricular nodes 1 Small amounts of Cx45 have also been observed in ventricular 2 and atrial myocardium.3 Recently a fourth cardiac connexin (Cx30.2) has been described in the atrio-ventricular node and in the conduction system of the murine heart.4
Theoretical and experimental studies have demonstrated that homogeneous electrical uncoupling of ventricular myocytes in engineered cell strands reduces conduction velocity by up to 95%. 5-7 In such strands, however, conduction block occurs only if the coupling resistance between cells is increased by >100-fold. This phenomenon is explained by a dual effect of cell-to-cell uncoupling: on one hand cell-to-cell uncoupling slows propagation, on the other hand it is a stabilizing factor that increases propagation safety up to extreme degrees of cell-to-cell uncoupling.
The role of heterogeneity in gap junction expression on propagation has been addressed in clinical, experimental and theoretical studies. Heterogeneous Cx43 distribution is observed in heart failure patients and has been specifically associated with dispersed conduction and ventricular arrhythmias. 8-9 Conditional cardiac-specific knockout of Cx43 in mice results in subtotal ablation of Cx43 in the heart (85-90% of cells express no Cx43 whereas the remaining ~10-15% express normal levels) associated with spontaneous ventricular tachyarrhythmias and sudden death.10-11 However, these hearts exhibit only moderate slowing of conduction velocity with relatively smooth macroscopic propagation and no apparent contractile dysfunction indicating complete ventricular electrical excitation despite the localized lack of Cx43 immunosignal. 11-12 Chimeric mice created from mixtures of wildtype and Cx43-null embryonic stem cells have hearts composed of a macroscopic mosaic of tissue expressing normal or absent Cx43, a pattern associated with highly irregular macroscopic propagation and reduced contractions. These results indicate that the macroscopic vs. microscopic pattern of heterogeneous coupling exerts a powerful influence on electrical and contractile function in the heart In this study, we developed an experimental model to analyze the effect of reproducible, defined heterogeneity in Cx43 expression in ventricular myocardium at a microscopic level of resolution. To this purpose, we co-cultured mixtures of myocytes with germline Cx43 ablation and wild type cells that expressed GFP to definitively identify the Cx43 genotype in both living and fixed preparations. 13 Using fibronectin microprinting,14-15 we engineered pairs of ventricular myocytes and strands of cells of different Cx43 genotype on a patterned growth substrate to measure cell-to-cell coupling and electrical propagation.
METHODS
Cell cultures, fabrication of patterned cell pairs and patterned strands
Hearts were obtained from mice maintained in an inbred C57BL/6J background (Jackson Laboratory, Bar Harbor, ME). Mice expressing GFP (GFP+/-) 13 in the same C57BL/6J background were used as a reporter for Cx43+/+ cells. Cx43-/- fetuses (Cx43KO) were obtained at embryonic day 20 (1 day before birth, D-1), hearts from the Cx43+/+-GFP+/- genotype (WTGFP) were obtained within 24 h after birth (D1). As previously shown, there is no difference in electrical phenotype after 3-4 days of culture in cells obtained at D-1. 16 The genotype of each embryonic heart was determined by PCR using standard protocols.
The techniques to culture neonatal, murine cardiac myocytes on micropatterned strands have been described elsewhere.5, 16 All experiments were approved by the Swiss Federal Veterinary Office and the Swiss National Science Foundation. In brief, suspensions of ventricular myocytes were prepared from each individually genotyped heart. After enzymatic separation, cell types of different genotypes were preplated to eliminate fibroblasts and mixed at a given ratio. Subsequently, the cell mixtures were seeded on fibronectin or collagen patterned coverslips. Patterns of cell pairs were obtained by standard soft microlithography techniques.14-15, 17 Patterns of murine strands (4-5 mm in length and 50, 100 or 200 μm in width) were obtained using photomicrolithography. 5, 16
Whole cell dual voltage clamp and high-resolution optical mapping
The classical dual voltage-clamp (DVC) method used to assess intercellular conductance, gj, has been described previously in detail.5, 17-18 Cell pairs (2 – 5 days in culture) were selected for measurement of transjunctional current, Ij, junctional conductance, gj, and the dependence of steady-state junctional conductance on transjunctional voltage [gj,ss=f(Vj)].
Optical recordings of action potentials and propagation velocities were obtained by high resolution optical mapping at a sampling frequency of 10 kHz using the optical-sensitive dyes RH137 or di-8-ANEPPS (see 5, 16 for details). Measurements were made from a hexagonal array of 128 detectors. The spatial resolution was 25μm (40x objective) or 10μm (100x objective). Details are provided in the on-line supplement.
Immunohistochemistry and confocal microscopy
The amounts of Cx43 and Cx45 at intercellular junctions were quantified by immunohistochemistry in paraformaldehyde-fixed cell preparations using antibodies and protocols described previously.5, 17 Details are provided in the on-line supplement. The amount of immunoreactive signal at intercellular junctions was quantified using laser scanning confocal microscopy, image deconvolution and digital image processing algorithms (IMARIS software; Bitplane Inc, Zuerich, Switzerland) as validated in previous studies. 6, 11 Mouse myocyte cultures were fixed in 4% paraformaldehyde for 5 minutes and then incubated in blocking buffer (HBSS with 10% bovine serum albumin, 0.15% Triton X-100, 3% normal goat serum) for 30 minutes at room temperature. Cultures were incubated with mouse monoclonal anti-Cx43 antibodies (MAB 3068, Chemicon, Billerica, MA) and rabbit polyclonal anti-Cx45 antibodies (kindly provided by Dr. Kathryn Yamada, Washington University, St. Louis) overnight in a humid chamber at 4 °C. Cultures were then incubated with DAPI, tetramethylrhodamine goat anti-mouse antibodies, and Alexa Fluor 633 goat anti-rabbit antibodies (Invitrogen, Carlsbad, CA) for two hours at room temperature. Finally, cultures were incubated with Alexa Fluor 488 conjugate rabbit polyclonal anti-GFP antibodies (A-21311, Invitrogen, Carlsbad, CA) for two hours at room temperature. Coverslips were then mounted on a glass slide with ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA).
Statistics
Results are expressed as mean values ± S.E. ANOVA and non-paired Student's t-tests were used to calculate statistical significances (p< 0.05%).
RESULTS
Junctional Cx43 and Cx45 immunofluorescence signal in pairs of ventricular myocytes with different Cx43 expression
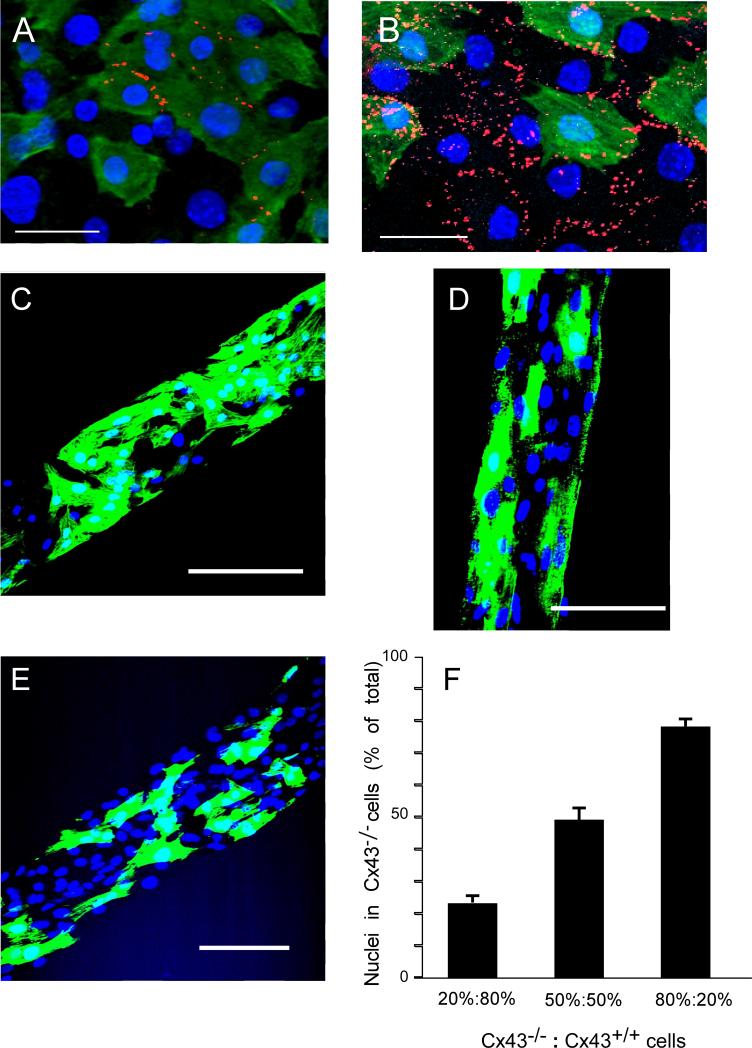
The effects of different levels of Cx43 expression on the amount of Cx43 and Cx45 immunoreactive signal at intercellular junctions was first analyzed in pairs of ventricular myocytes of defined genotype. Since all cultures were made of mixtures of Cx43-null and WTGFP cells, the Cx43 and Cx45 signals present in the junctions of the WTGFP - WTGFP pairs served as controls. As shown in panels A-C of Figure 1, there is considerable but not total co-localization of Cx43 and Cx45 at the junctional interface between WTGFP cells. Similar results were obtained in a total of 17 pairs. In contrast, no Cx43 and Cx45 immunosignals were detected in 15 WTGFP- Cx43KO pairs. In only 1 cell pair a very small Cx43 signal, shown in Figure 1 D-F, could be detected close to the interface between a WTGFP cell and a Cx43KO cell. Thus, only minute amounts if any of Cx43 accumulate at the interface between a WTGFP cell and a Cx43KO cell despite the fact that one member of the pair carries two normal Cx43 alleles. In pairs of Cx43KO cells Cx45 was not detectable in 10 out of 11 pairs analyzed. Panels G–I of Figure 1 show two very small Cx45 immunoreactive signals in the 11th Cx43KO pair. Cx43 signal was absent in all 11 pairs, as expected.
Figure 1.
3-dimensional reconstruction of pairs of neonatal murine ventricular myocytes, engineered from Cx43+/+-GFP+/- (WTGFP) and Cx43 -/- (Cx43 KO) ventricular myocytes. Panels A – C: Top view (Panel A), lateral view (Panel B) and enlarged view of intercellular junction (Panel C) of a WTGFP - WTGFP pair. Green: GFP- immunofluorescence; blue: DAPI; Red: Cx43-immunofluorescence. Cx45-immunofluorescence is labeled in white pseudo-color in Panels A, B and in green on Panel C. Panels D – F: Top view (D), lateral view (E) and enlarged view of intercellular junction (F) of a WTGFP - Cx43 KO cell pair. Panels G – I: Top view (G), lateral view (H) and enlarged view of intercellular junction (I) of cell pair engineered from two Cx KO cells. Calibration bars: Panels A,B,D,E,G,H = 5μm; Panels C,F,I = 2.5μm.
Electrical coupling between pairs of ventricular myocytes with different Cx43 expression
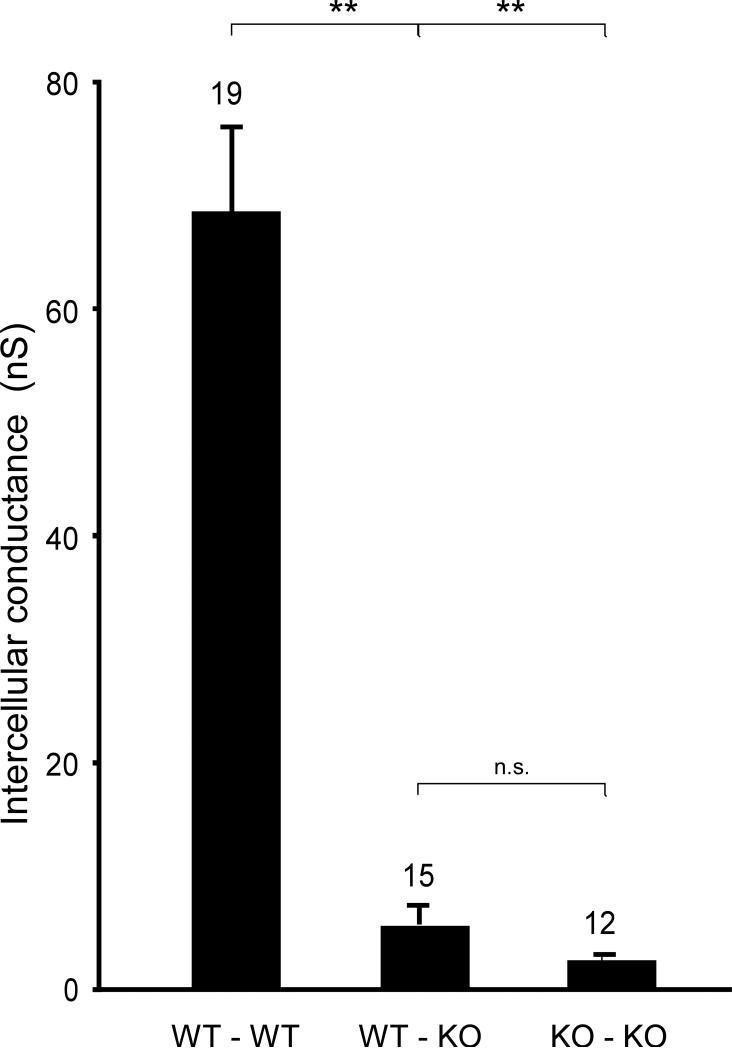
Values of intercellular conductance, gj, in mixed WT-Cx43KO pairs and the comparison to WT-WT and Cx43KO-Cx43KO pairs are illustrated in Figure 2. To control for potential effects of GFP expression, gj was measured in WT/WT (n=8), WTGFP/WTGFP (n=3) and WT/WTGFP (n= 8) cell pairs. The differences between these groups were not significant (74±17 nS vs. 63±12 nS vs. 74±16 nS). The absence of a difference in gj indicated that GFP expression had no effect on intercellular coupling. Ablation of Cx43 in one cell of a pair caused a marked decrease (94%) in mean intercellular conductance, from 68.3±9.6 nS (n=19) to 5.2±1.7 nS (n=15), p<0.001. Ablation of Cx43 in both cells caused a further decrease in gj to 2.0±0.2 nS (n=12). Due to the variability of gj values in the heterogeneous pairs, this difference was not statistically significant. The very low value for intercellular coupling between Cx43KO/Cx43KO cells confirms previous reports.5, 20
Figure 2.
Intercellular conductances, gj, from WT/WT, WTGFP /Cx43 KO and Cx43 KO/Cx43 KO ventricular myocyte pairs. The value of the WT/WT pairs (n=19) was calculated from the lumped measurements of WT/WT (n=8), WT/WTGFP, (n=8) and WTGFP/WTGFP (n=3) pairs (see text). ** denotes statistical significance (p<0.001).
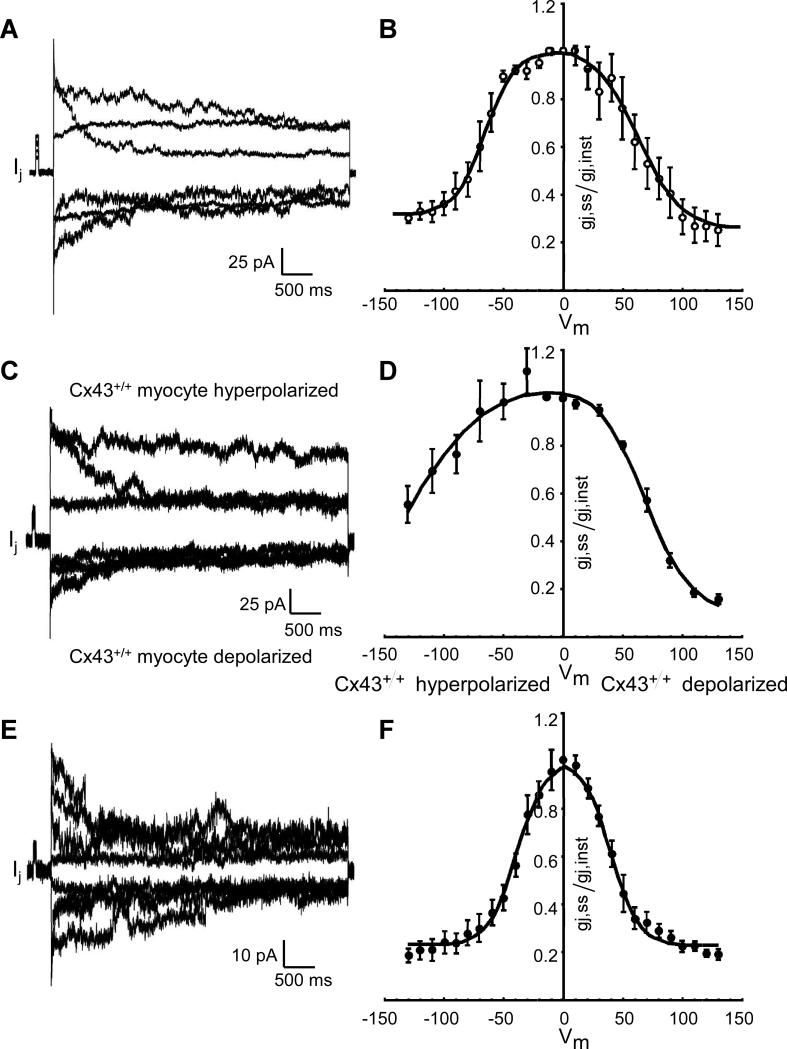
The dependence of steady-state intercellular conductance, gj,ss, on the transjunctional voltage, Vj, is depicted in Figure 3 for mixed WTGFP/Cx43KO pairs. Interestingly, the bell-shaped curve was highly asymmetric with the steep portion corresponding to depolarization of the WTGFP cell. For comparison, curves of the average gj,ss = f(Vj) relationship in WT/WT and Cx43KO/Cx43KO pairs, which as expected show symmetrical Vj dependent gating, are depicted on Panel B, and confirm previous reports.5, 20 The data for the wild type pairs are historical and taken from our previous publication. 5 The highly asymmetrical relationship with a steep voltage-dependence during hyperpolarization of the Cx43KO cell strongly suggests that the Cx43KO cell contributes homomeric Cx45 connexons and the WT cell contributes heteromeric Cx43-Cx45 connexons to create heterotypic gap junction channels.21 The very small overall conductance indicates that the Cx45 expressing cell largely determines the gj value and is in accordance with the absence of Cx45 and Cx43 immunosignals in this type of interface.
Figure 3.
Dependence of relative steady state intercellular conductance gj,ss/gj,inst on voltage across the intercellular junction, Vj, in pairs of murine ventricular myocytes engineered from WTGFP and Cx43 KO (gj,ss = f(Vj) relationship). Original tracings of junctional current, Ij, at different levels of Vj are shown on the left panels, the collected data on the right panels. Panels A and B: WTGFP/WTGFP cell pairs. Panels C and D: mixed WTGFP/Cx43 KO cell pairs. Panels E and F: Cx43 KO/Cx43 KO cell pairs. The data in Panel B represent historical controls and are taken from reference. 5 Note the asymmetrical relationship in the mixed cell pairs, with the steep limb corresponding to the depolarized Cx43 WTGFP cell.
Macroscopic and microscopic propagation in strands engineered from mixtures of WT and Cx43KO cells
To analyze the effect of various degrees of heterogeneous deletion of Cx43 on electrical propagation, we engineered strands from various mixtures of Cx43-null and WT cells as illustrated in Figure 4. Panel A illustrates the effect of ablating Cx43 in 50% of cells in a strand vs. a strand engineered from 50%WT and 50%WTGFP cell suspensions (Panel B, control). As noted in the mixed cell pairs, Cx43 signals were not apparent between WTGFP and Cx43KO cells in strands. Thus, total Cx43 immunoreactive signal in gap junctions in the 50% WT/ 50% Cx43KO strand is markedly reduced and is out of proportion to the percentage of wildtype cells. To verify the average Cx43KO and WTGFP cell content in the mixed strands, we counted nuclei in Cx43-/- cells as a proportion of total nuclei in strands produced from an 80% WTGFP/ 20% Cx43KO cell suspension (Panel C), 50% WTGFP/ 50% Cx43KO suspension (Panel D) and 20% WTGFP/ 80% Cx43KO suspension (Panel E). Panel F shows a close correlation between the cell mixtures in the suspensions before seeding and the respective cell contents in the engineered strands.
Figure 4.
Engineering of strands with heterogeneous Cx43 expression. Panel A: Strand segment from a mixture of 50% Cx43-/- cells not expressing GFP (Cx43KO) and 50% Cx43+/+ cells expressing GFP (WTGFP ). Panel B: Strand segment from a mixture of 50% Cx43+/+ cells not expressing GFP (WT) and 50% WTGFP cells. Panels C-E: GFP fluorescence and DAPI staining of strands engineered from suspensions of varying WTGFP to Cx43 KO cell ratios. WTGFP:Cx43 KO = 80%:20% (C); WTGFP:Cx43 KO = 50%:50% (D); WTGFP:Cx43 KO = 20%:80% (E). Panel F: Percentage nuclei located in Cx43-/- cells as a function of WTGFP:Cx43 KO cell ratios in the cell suspensions. Note close correspondence between respective cell contents in solution and in the engineered strands. Calibration bars: Panels A&B = 10μm; Panels C-E = 50 μm.
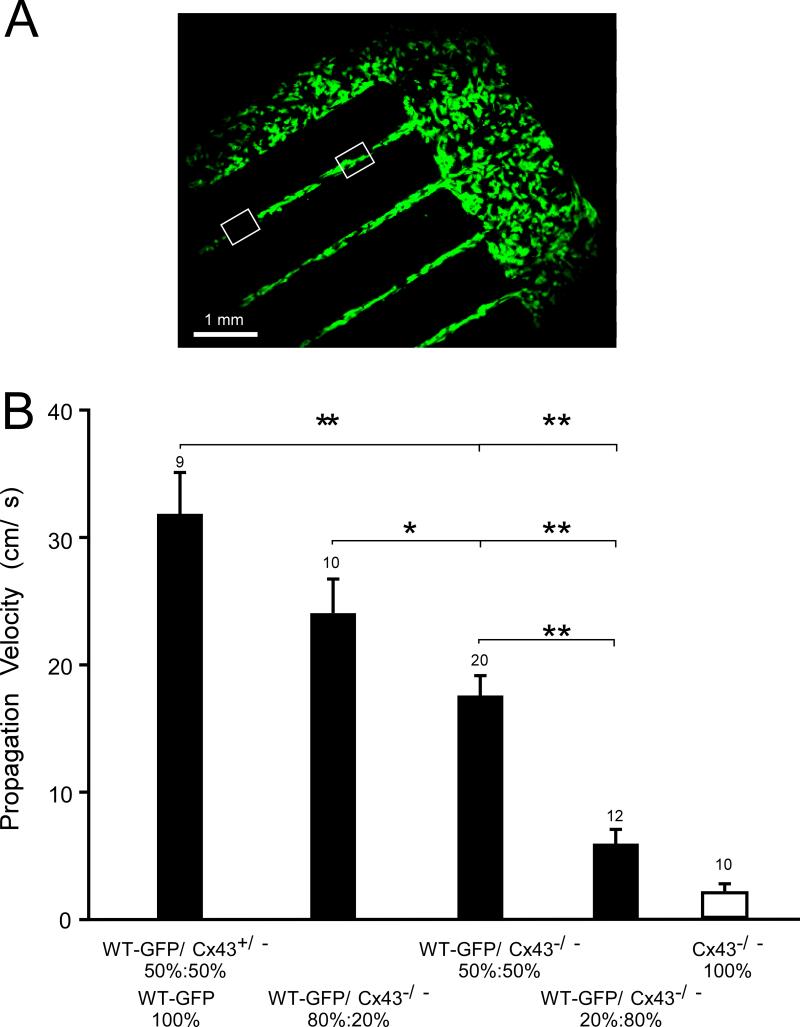
Figure 5 illustrates the pattern used to measure macroscopic propagation velocity, θ, at low magnification. θ was measured from the time difference in average activation and the distance between 2 regions of interest in the various cell mixtures. To control for GFP expression, we measured velocities in strands composed entirely of WTGFP cells (n=6) or a 50% WT - 50% WTGFP mixture (n=3). No significant differences were observed (30.5±2.2 cm/s vs. 31.8±11 cm/s). As illustrated on Panel B, a moderate non-significant decrease in θ was observed in 80% WTGFP/ 20% Cx43KO mixtures. The further decreases seen in 50% WTGFP/ 50% Cx43KO, and 20% WTGFP/ 80% Cx43KO strands were highly significant. Average velocities were 76% of wild type in 80% WTGFP/ 20% Cx43KO mixtures, 55%of wild type in 50% WTGFP/ 50% Cx43KO mixtures, and 19% of wild type in the 20% WTGFP/ 80% Cx43KO mixtures. The value of 5.6±1.3 cm/s in the 20% WTGFP/ 80% Cx43KO mixtures compares to 2.1±0.5 cm/s in strands engineered from 100% Cx43KO cells measured in our previous work (white column in Figure 5B). 5
Figure 5.
Macroscopic velocities along strands engineered from mixed WTGFP:Cx43 KO cell suspensions. Panel A illustrates a mixed patterned cell culture (WTGFP:Cx43 KO = 50%:50%) at low magnification. Two ROI's are marked by white quadrangles. Macroscopic velocity was calculated from the difference in mean activation time within the quadrangles and the distance between the ROI's. The velocity values are depicted on Panel B. Note the relatively moderate increase of propagation velocity in 80%:20% and 50%:50% mixtures and the marked further decrease when the WTGFP cells were reduced to 20%. Statistical significance: **: (p<0.001); *: (p<0.05). The values for propagation in strand engineered from Cx43-/- cells (white column) are taken from our previous work.5
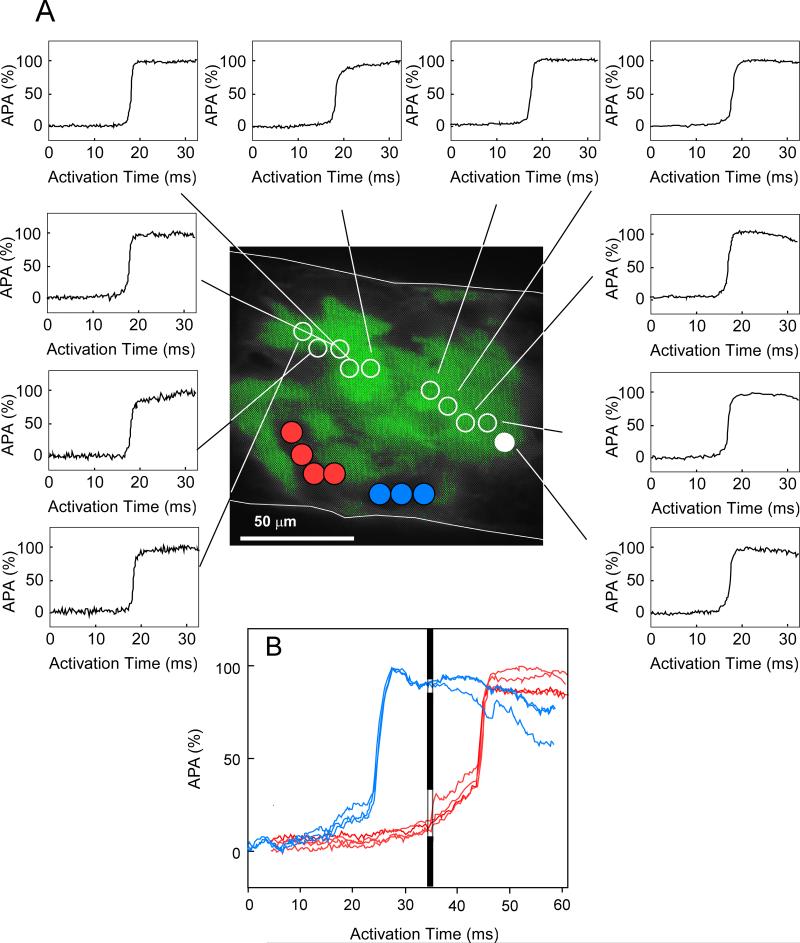
High-resolution multisite optical mapping was used to assess excitation with cellular resolution and to compare impulse spread with the distribution of WT and Cx43KO cells. An example of local propagation in a 100 μm wide strand composed of a 50% WTGFP/ 50% Cx43KO cell mixture is illustrated in Figure 6. The green fluorescence identifies WT cells. Panel A shows sequential action potential upstrokes, measured with 10 μm resolution, from the earliest (filled white circle) to the latest activation of the WT cell cluster. The very small differences between activation times indicate fast propagation through this group of cells. Dividing the meandering pathway of propagation by the activation interval yielded a propagation velocity of 32 cm/s within this cell cluster. Activation of 2 areas where the Cx43KO cells were located indicated a very early activation in one area (blue circles) and a very late activation in an adjacent group of cells (red circles). The transition between the area of early activation and WT cells probably occurred outside the mapping area and was not detected. The action potentials in the area with delayed activation showed a typical foot potential coinciding with the window of fast activation (Panel A) as indicated in Panel B by the black bar. The delayed activation of this area occurred quasi simultaneously. This type excitation is typical for highly uncoupled tissue.5,6,7 Patterns analogous to Figure 6, illustrating meandering fast propagation and dissociated slow propagation, were observed in all recordings and indicated that excitation at a cellular level was highly discontinuous. Importantly however, electrical excitation of Cx43KO clusters was consistently observed, albeit dissociated from neighboring WT cell clusters. It none of the experiments areas were detected, which were not excited by the propagating impulse.
Figure 6.
Microscopic excitation spread within a segment of a strand 100μm in width, recorded at 100x magnification. The green area represents in vivo GFP fluorescence. Dots symbolize localization of measuring diodes (10μm). The upstrokes of the transmembrane action potentials (action potential amplitude in %, APA%) are shown from diode locations in the area occupied by WTGFP (white circles) and two areas occupied by Cx43 null cells (filled red and blue circles). Panel A: Fast electrical propagation along a trajectory within the WTGFP cell cluster within a time window of 950 μs (corresponding to a local velocity of 32 cm/s). The filled white circle indicates the starting point of activation. Note the smooth and rapid upstrokes of action potentials typical for continuous conduction. Panel B: Activation within the Cx43 KO compartment, of sites which are excited significantly earlier (blue circles) and significantly later (red circles). The activation window corresponding to the activation of the WTGFP cell cluster in Panel A is indicated by the black bar. This highly discontinuous excitation, characterized by an initial foot and subsequent delayed quasi simultaneous excitation is typical for highly uncoupled tissue. 5,6,7 However, no areas of excitation failure were observed.
DISCUSSION
The present study was undertaken to define the characteristics of ventricular electrical propagation at a microscopic scale in tissue with heterogeneous expression of Cx43.
The identification of the WT cells by a GFP tag combined with the assessment of intercellular conductance allowed for the characterization of 3 distinct cell-to-cell interfaces. We have previously shown, that ventricular myocyte pairs formed by Cx43-null cells show very low (4% of WT) but consistent electrical coupling, due the presence of Cx45, a finding which is confirmed in this study. As a new and main finding, we show that the electrical conductance, gj, between Cx43 expressing and Cx43 non-expressing myocytes is markedly reduced to levels of 7% of normal. The electrical interface between these hybrid cell pairs most likely consists of homomeric Cx45 connexons, contributed by the Cx43 KO cells and heteromeric Cx43/Cx45 connexons contributed by the WT cells.5, 20 This conclusion is supported by the rectifying gj,ss = (Vj) relationship showing a steep limb with a Vj,0 when the Cx43 KO cell was hyperpolarized, and a flatter limb, i.e. less Vj-dependent gating, when the WT cell was hyperpolarized. An almost identical dependence of gj,ss on Vj has been described in mixed Cx43/Cx45 HeLa and neuroblastoma cell pairs where Cx43 and Cx45 were specifically transfected. 18, 21 Overall, the marked reduction in gj indicates that Cx45 in the Cx43-/- cell is the main determinant of gj of mixed pairs. In the wild type cell pairs, Cx43 and Cx45 immunosignals co-localized in most but not in all junctions.
Despite the presence of two wild type Cx43 alleles in the Cx43+/+ cells, no significant Cx43 immunosignals were detected at the interface between Cx43KO/Cx43KO and WT/Cx43KO cell pairs. Since the WT/WT, Cx43KO/Cx43KO and WT/Cx43KO types of cell pairs were present in all preparations, the Cx43 and Cx45 immunosignals in the WT/WT pairs always served as positive controls. The absence of immunofluorescence in the Cx43KO/Cx43KO and Cx43KO/WT pairs reflects the very small size of the gap junctions beyond the threshold of detection by Cx43 or Cx45 immunofluorescence. We have recently compared Cx43 immunosignal with electrical conductance, gj, in engineered rat ventricular cell pairs and showed that the Cx43 immunosignal is directly related to gj. 17 Importantly, the Cx43 immunosignal is a measure of gap junction size rather than Cx43 protein content, 17, 19 and the relationship between Cx43 signal and gj intersects at about 10nS, i.e. above the levels of measured in Cx43KO/Cx43KO and Cx43KO/WT pairs. 17 Both these studies suggest that the determination of electrical conductance is more sensitive to the presence of connexins that specific immunofluorescence and explain why no Cx43 and Cx45 signals were detected in the Cx43KO/WT pairs and no Cx45 signal in the Cx43KO/Cx43KO pairs, where Cx45 is spread over a much smaller area. We have recently shown that Cx45 is detectable in atrial Cx43-null cells, where is co-localizes with Cx40. 22
Yao et al. 20 studied cell-to-cell coupling in adult myocytes pairs disaggregated from mice hearts with conditional Cx43 knock out. They found that the large majority of interfaces between adult murine ventricular myocytes from conditional Cx43-/- hearts were devoid of Cx43 and Cx45 immunofluorescence and had a very low gj. Similar to our previous 5 and present results (Figure 3B), pairs with very low levels of conductance cells showed voltage-gating characteristics specific for Cx45. However, there are differences between the study of Yao et al. and our study, which remain unexplained. First, Yao et al. did not describe cell junctions with a gj =f(Vj) relationship typical for mixed Cx43KO/WT junctions. Second, a significant part of cell pairs devoid of Cx43 fluorescence showed no evidence of electrical cell-to-cell coupling. The latter observation may be due to cell disaggregation using transient exposure to Ca2+ and Mg2+-free solution. It has been shown in a very early study on cell-to-cell coupling that absence of Ca2+ and Mg2+ can lead to electrical uncoupling preceding mechanical dissociation of myocytes. 23
Although heterogeneity of connexin expression is often implicated as a potential contributor to arrhythmogenesis in atrial fibrillation and heart failure in humans, it has been extremely difficult to prove such a relationship directly. Ventricular tachyarrhythmias associated with heterogeneous Cx43 expression have been observed in patients with chronic heart failure and but the precise role of altered Cx43 expression is unknown.8,9 Mouse models with conditional cardiac deletion of Cx43 11, 24 have been shown to exhibit ventricular tachyarrhythmias and sudden death. Interestingly, the marked reduction in cells expressing Cx43 (up to 90%) and the resultant spatial heterogeneity in Cx43 immunosignal in these mouse models was associated with only a moderate (~50%) decrease in propagation velocity, 11 and no major disturbances of mechanical ventricular function. One factor contributing to the apparent discrepancy between the marked decrease in Cx43 immunosignal and the relatively moderate decrease in propagation velocity relates to the absence of Cx43 immunosignal between Cx43 KO and WT cells. This is expected to lead to an overestimation of Cx43 knock out, if derived from the proportion of overall decrease in immunosignal (Figures 4A and B). The extent of this overestimation depends on the degree of clustering of Cx43+/+ cells, because Cx43 immunosignal is detected only at the interfaces of such cells. Another factor contributing to relatively rapid propagation in strands with a ≥ 50% proportion of Cx43-expressing cells is rapid meandering of the impulse through pathways with maintained Cx43 expression, as shown in Figure 6.
In previous work, 5 we have shown that cardiac strands with ubiquitous germline Cx43 knock out conduct the impulse very slowly (~ 2.1 cm/s). In this condition, propagation is consistently observed, dependent on the presence of Cx45, and blocked by a gap junction uncoupler. Whereas inhibition of depolarizing current leads to propagation block at relatively high velocities, propagation can be maintained at very low levels even with a > 100-fold decrease in electrical cell-to-cell coupling. 6-7 As an alternative mechanism for slow conduction theoretical studies have proposed that the electrical field created by the Na+ inward current in the intercalated disc could depolarize the juxtaposed Na+ channels in the downstream cell to propagate excitation (ephaptic transmission).25-26 Theoretically, ephaptic transmission can occur within a defined range of resistive properties of the intercalated disc space and cellular sodium channel expression. For ephaptic transmission to occur depolarizing current flow has to be confined to the intercalated discs. Both these conditions await experimental verification and remain a matter of debate. Na+ channels have recently been shown be located in both an intercalated disc and a surface compartment, 27-28 but the functionality of the surface fraction has been questioned. As a further intriguing finding, a decrease in Cx43 expression in ventricular and atrial myocardium has been shown to decrease Na+ current and Na+ channels in the intercalated disc. 22, 29 In addition, it has been shown in a theoretical study, that the L-type Ca2+ current and not the inward Na+ current is the main charge carrier driving propagation in markedly uncoupled tissue showing delays in local activation (Figure 6).7 In the context of the present study, it can be concluded that propagation is explained by fast meandering excitation in WT cell clusters and slow excitation at the interface to and within cell clusters expressing Cx45. However, our results do not exclude an additional contribution of ephaptic transmission. Although neonatal tissue has a gap junction expression pattern different from adult tissue, the effect of this difference on propagation is suggested to be small, as shown in a theoretical study. 30 Moreover, neonatal cell-to-cell junctions contain all major electrical and mechanical junction proteins present in adult tissue. 31-33
The complexity inherent to understanding the effect of a heterogeneous decrease in cell-to-cell coupling on propagation is also underlined by the observation that chimeric mice with a patchy ablation of Cx43 showed changes in electrical propagation that were different from the changes observed in conditional knock out (CKO) mice. 12 In contrast to the mice with conditional Cx43 ablation, a marked inhomogeneity of propagation and a decrease of contractility were observed in the chimeric animals. 12 The difference may lay in the fact that Cx43 ablation and partial remaining Cx43 expression occurred at a microscopic scale in the CKO models and in our study, whereas a sharp border between large areas of different Cx43 expression was present in the chimeric model. Such borders would allow formation of pathways for circulating excitation of sufficient length for re-entry to occur. Such re-entry was never observed in our study and in previous studies assessing the effect of genetic or pharmacologic Cx43 ablation, despite the observation of large propagation delays at the microscopic level. Theoretical work has shown that single borders of altered cell-to-cell coupling are much more prone to develop propagation block than gradients in cell-to-cell coupling dispersed at a small scale. 34 In the latter case only, the current sink, which delays and eventually blocks downstream propagation at a border of altered cell-to-cell coupling, 35 is decreased by the presence of further low resistance coupling sites downstream.
In summary, our study, carried out to quantify the degree between heterogeneous Cx43 knock out and propagation, indicates that propagation is relatively well maintained down to a level of ≤ 50% Cx43-null myocytes. This propagation is maintained by fast excitation of WT cell clusters and very slow propagation with areas with Cx43 knock out. The interface between WT and Cx43-null cells is formed by channels composed of Cx45 and mixed Cx43/Cx45 connexons, which exhibit a very low level of coupling. This low level explains the absence of Cx43 immunosignals at the interface. As a consequence, the decrease of Cx43 immunosignals observed in cellular networks (tissue slices, cell cultures) with heterogeneous Cx43 deletion is larger than the proportion of cell with Cx43 ablation. The observation that all cells are electrically excited may explain the lack of a disturbance in ventricular contractility in hearts with conditional Cx43 ablation. 11
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Connexin proteins Cx43 and Cx45 in the ventricular myocardium are responsible for intercellular electrical low resistance pathways and assure normal electrical propagation.
In human cardiac failure, ventricular remodeling of Cx43 leading to heterogeneous Cx43 expression has been associated with arrhythmogenesis.
Mouse models of cardiac-restricted and heterogeneous Cx43 ablation have a high incidence of ventricular arrhythmias if electrical propagation velocity decreases below 50% of normal with average Cx43 immunofluorescence signals as low as 18%.
What New Information Does This Article Contribute?
Cardiac strands engineered with mixtures of Cx43 wild type and Cx43 null cells show a marked decrease of electrical velocity to < 50% of normal velocity if the proportion of Cx43-null cells is increased beyond 50%. The average Cx43 immunosignal underestimates the proportion of Cx43 wild type cells.
At the cellular level, microscopic propagation is characterized by a combination of fast propagation meandering across Cx43 wild type cell clusters and discontinuous, delayed propagation within areas of Cx43 null cells.
The electrical conductance at the interface between cell pairs engineered from Cx43 wild type and Cx43 null ventricular myocytes is very low (< 10% of normal), and determined by the presence of mixed gap junction channels formed from heteromeric Cx43/Cx45 and homomeric Cx45 connexons.
Heterogeneous expression of Cx43 in ventricle has been implicated in arrhythmogenesis in patients and in murine models of cardiac-restricted ablation of Cx43. We used cell pairs and cell strands engineered from 2 populations of myocytes, Cx43 wild type cells expressing GFP and Cx43 null cells, to produce tissue with controlled degrees of heterogeneity in Cx43 expression. Electrical conductance was markedly decreased at the interface between Cx43 wild type and Cx43 expressing cells, suggesting that the homomeric Cx45 hemichannels from the Cx43 null cells, which docked to the normal hemichannels from the Cx43 wild type cells to form gap junction channels, determined the very low intercellular electrical conductance. While electrical cell-to-cell coupling was detectable, neither Cx43 nor Cx45 immunofluorescence signals are present between these heterogeneous pairs, probably due to the very small size of the gap junctions. Macroscopic electrical propagation in engineered strands was significantly decreased if the proportion of Cx43 nulls cells exceeded 50%. At the cellular level, electrical excitation was locally dissociated, with fast meandering excitation through clusters of Cx43 WT cells, and discontinuous delayed excitation of Cx43 null cells. However, all cells were eventually electrically excited, which explains the normal contractility of such tissue previously reported.
Acknowledgments
The authors express their gratitude to Dr. Kathryn A. Yamada, Washington University, St. Louis, for providing the Cx45 antibodies.
Sources of Funding
The study was supported by the Swiss National Science Foundation (310030-120253 to AGK), the National Institutes of Health (R01HL050598 to JES), and the Harvard Materials Research Science and Engineering Center under NSF award number DMR-0213805 and NIH grant 1 R01 HL079126 (KKP).
Non-Standard Abbreviations and Non-Standard Acronyms
- Cx43KO or Cx43-null
null germline connexin43 knock out
- WTGFP
wild type mouse with ubiquitous expression of GFP
- D-1
embryonic day 20, 24 hours before birth
- D1
first day post-partum
- DVC
whole cell dual voltage clamp
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Davis LM, Rodefeld ME, Green K, Beyer EC, Saffitz JE. Gap junction protein phenotypes of the human heart and conduction system. J Cardiovasc Electrophysiol. 1995;6:813–822. doi: 10.1111/j.1540-8167.1995.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CM, Kanter EM, Green KG, Laing JG, Betsuyaku T, Beyer EC, Steinberg TH, Saffitz JE, Yamada KA. Redistribution of connexin45 in gap junctions of connexin43-deficient hearts. Cardiovas Res. 2002;53:921–935. doi: 10.1016/s0008-6363(01)00522-3. [DOI] [PubMed] [Google Scholar]
- 3.Vozzi C, Dupont E, Coppen SR, Yeh HI, Severs NJ. Chamber-related differences in connexin expression in the human heart. J Mol Cell Cardiol. 1999;31:991–1003. doi: 10.1006/jmcc.1999.0937. [DOI] [PubMed] [Google Scholar]
- 4.Bukauskas FF, Kreuzberg MM, Rackauskas M, Bukauskiene A, Bennett MV, Verselis VK, Willecke K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: Implications for atrioventricular conduction in the heart. Proc Natl Acad Sci U S A. 2006;103:9726–9731. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circ Res. 2004;95:170–178. doi: 10.1161/01.RES.0000134923.05174.2f. [DOI] [PubMed] [Google Scholar]
- 6.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, i: Effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res. 1998;83:781–794. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 7.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and l-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 8.Boulaksil M, Winckels SK, Engelen MA, Stein M, van Veen TA, Jansen JA, Linnenbank AC, Bierhuizen MF, Groenewegen WA, van Oosterhout MF, Kirkels JH, de Jonge N, Varro A, Vos MA, de Bakker JM, van Rijen HV. Heterogeneous connexin43 distribution in heart failure is associated with dispersed conduction and enhanced susceptibility to ventricular arrhythmias. Eur J Heart Fail. 2010;12:913–921. doi: 10.1093/eurjhf/hfq092. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano EJ, Kubo S, Hayashi Y, Itoh H, Yokoyama M. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:865–870. doi: 10.1046/j.1540-8167.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- 10.Danik SB, Rosner G, Lader J, Gutstein DE, Fishman GI, Morley GE. Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB J. 2008;22:1204–1212. doi: 10.1096/fj.07-8974com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, Fishman GI. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 13.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 14.Geisse NA, Sheehy SP, Parker KK. Control of myocyte remodeling in vitro with engineered substrates. In Vitro Cell Dev Biol Anim. 2009;45:343–350. doi: 10.1007/s11626-009-9182-9. [DOI] [PubMed] [Google Scholar]
- 15.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 16.Beauchamp P, Yamada KA, Baertschi AJ, Green K, Kanter EM, Saffitz JE, Kleber AG. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99:1216–1224. doi: 10.1161/01.RES.0000250607.34498.b4. [DOI] [PubMed] [Google Scholar]
- 17.McCain ML, Desplantez T, Geisse NA, Rothen-Rutishauser B, Oberer H, Parker KK, Kleber AG. Cell-to-cell coupling in engineered pairs of rat ventricular cardiomyocytes: Relation between cx43 immunofluorescence and intercellular electrical conductance. Am J Physiol - Heart and Circ. 2012;302:H443–450. doi: 10.1152/ajpheart.01218.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desplantez T, Halliday D, Dupont E, Weingart R. Cardiac connexins cx43 and cx45: Formation of diverse gap junction channels with diverse electrical properties. Pflugers Arch. 2004;448:363–375. doi: 10.1007/s00424-004-1250-0. [DOI] [PubMed] [Google Scholar]
- 19.Saffitz JE, Green KG, Kraft WJ, Schechtman KB, Yamada KA. Effects of diminished expression of connexin43 on gap junction number and size in ventricular myocardium. Am J Physiol - Heart and Circ. 2000;278:H1662–1670. doi: 10.1152/ajpheart.2000.278.5.H1662. [DOI] [PubMed] [Google Scholar]
- 20.Yao JA, Gutstein DE, Liu F, Fishman GI, Wit AL. Cell coupling between ventricular myocyte pairs from connexin43-deficient murine hearts. Circ Res. 2003;93:736–743. doi: 10.1161/01.RES.0000095977.66660.86. [DOI] [PubMed] [Google Scholar]
- 21.Elenes S, Martinez AD, Delmar M, Beyer EC, Moreno AP. Heterotypic docking of cx43 and cx45 connexons blocks fast voltage gating of cx43. Biophys J. 2001;81:1406–1418. doi: 10.1016/S0006-3495(01)75796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desplantez T, McCain M, Beauchamp P, Rigoli G, Rothen-Rutishauser B, Parker KK, Kleber AG. Connexin 43 ablation in fetal atrial myocytes decreases electrical coupling, partner connexins and sodium current. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewenstein WR, Socolar SJ, Higashino S, Kanno Y, Davidson N. Intercellular communication: Renal, urinary bladder, sensory, and salivary gland cells. Science. 1965;149:295–298. doi: 10.1126/science.149.3681.295. [DOI] [PubMed] [Google Scholar]
- 24.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res. 2002;91:1176–1182. doi: 10.1161/01.res.0000046237.54156.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3d electrodiffusion. Proc Natl Acad Sci. 2008;105:6463–6468. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. Sap97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels nav1.5 in cardiomyocytes. Circ Res. 2011;108:294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 28.Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL, Fishman GI, Delmar M. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm. 2011;8:1923–1930. doi: 10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV. Reduced heterogeneous expression of cx43 results in decreased nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional cx43 knockout mice. Heart Rhythm. 2011 doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spach MS, Heidlage JF, Dolber PC, Barr RC. Electrophysiological effects of remodeling cardiac gap junctions and cell size: Experimental and model studies of normal cardiac growth. Circ Res. 2000;86:302–311. doi: 10.1161/01.res.86.3.302. [DOI] [PubMed] [Google Scholar]
- 31.Pimentel RC, Yamada KA, Kleber AG, Saffitz JE. Autocrine regulation of myocyte cx43 expression by vegf. Circ Res. 2002;90:671–677. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]
- 32.Shanker AJ, Yamada K, Green KG, Yamada KA, Saffitz JE. Matrix-protein-specific regulation of cx43 expression in cardiac myocytes subjected to mechanical load. Circ Res. 2005;96:558–566. doi: 10.1161/01.RES.0000158964.42008.a2. [DOI] [PubMed] [Google Scholar]
- 33.Yamada K, Green KG, Samarel AM, Saffitz JE. Distinct pathways regulate expression of cardiac electrical and mechanical junction proteins in response to stretch. Circ Res. 2005;97:346–353. doi: 10.1161/01.RES.0000178788.76568.8a. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Rudy Y. Action potential propagation in inhomogeneous cardiac tissue: Safety factor considerations and ionic mechanism. Am J Physiol - Heart and Circ. 2000;278:H1019–1029. doi: 10.1152/ajpheart.2000.278.4.H1019. [DOI] [PubMed] [Google Scholar]
- 35.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.