Abstract
The effect of low temperature thermal treatment on soils from a former Superfund wood-treating site contaminated with pentachlorophenol (PCP) and the environmentally persistent free radical (EPFR), pentachlorophenoxyl, was determined. The pentachlorophenoxyl EPFRs’ and the PCP molecules’ chemical behavior were simultaneously monitored at temperatures ranging from 25 °C to 300 °C via electron paramagnetic resonance (EPR) spectroscopy and GC-MS analysis, respectively. Two types of thermal treatment were employed: a closed heating (oxygen-starved condition) where the soil was heated under vacuum and an open heating system (oxygen-rich conditions), where the soil was heated in ambient air. EPR analyses for closed heating indicated the EPFR concentration was 2–12 × 1018 spins/g of soil, with a g-factor and linewidth (ΔHp-p) of 2.00311 – 2.00323 and 4.190 – 5.472 Gauss, respectively. EPR analyses for the open heating soils revealed a slightly broader and weaker radical signal, with a concentration of 1–10 × 1018 spins/g of soil, g-factor of 2.00327 – 2.00341, and ΔHp-p of 5.209 – 6.721 Gauss. This suggested the open heating resulted in the formation of a more oxygen-centered structure of the pentachlorophenoxyl radical or additional, similar radicals. The EPFR concentration peaked at 10 × 1018 spins/g of soil at 100 °C for open heating and 12 × 1018 spins/g at 75 °C for closed heating. The half-lives of the EPFRs were 2 – 24 days at room temperature in ambient air. These results suggest low temperature treatment of soils contaminated with PCP can convert the PCP to potentially more toxic pentachlorophenoxyl EPFRs, which may persist in the environment long enough for human exposure.
Introduction
Thermal treatment of contaminated soils continues to be used as a remediation option at Superfund sites [1, 2]. Thermal treatment options include: low or moderate temperature desorption (~100–350 °C) and high temperature thermal desorption (~350–600 °C). Both are processes in which the contaminants are separated from the soil, collected, and transported off-site for destruction (usually by incineration). On-site thermal destruction (600–1000°C) is another treatment option, which is in effect a low grade incineration process, and thermal desorption coupled with high-temperature incineration (>1000 °C) is also employed [1].
However, incineration is not favored because it can produce chronically toxic products of incomplete combustion (PICs), e.g. benzene, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated dibenzo-pdioxins/dibenzofurans (PCDD/F) [2]. As a result, low or moderate temperature desorption has been used more frequently. All of these processes result in gas-phase species condensing on fine and ultra-fine particulate matter as the gases cool [1]. Research has clearly shown inhalation of combustion-generated fine and ultrafine particles can induce significant health impacts [1, 3, 4]. Unfortunately, using lower temperatures may result in less destruction of toxic components and increased adsorption on particulate matter [1] — for example, a kinetic study of PCP-contaminated soil heated at temperatures ranging 30 °C to 90 °C showed that desorption of PCP decelerates, indicating increased adsorption of PCP on the soil matrix [5]. Consequently, human exposure results if the particles are not completely captured.
Our research has shown substituted aromatic molecules (e.g. chlorophenols and chlorobenzenes) can form environmentally persistent free radicals (EPFRs) on the surfaces of transition metal-containing particles at temperatures of 100–600°C [3, 4, 6–8]. Research has also shown EPFRs can induce oxidative stress, which is a progenitor of cardiopulmonary dysfunction and other diseases [2, 3, 6].
We recently reported the presence of the pentachlorophenoxyl EPFR of pentachlorophenol (PCP) in contaminated soils from a former wood treatment facility site [9]. This suggested formation of EPFRs is not confined to combustion but can also occur under ambient environmental conditions where a chemical pollutant and metal-containing particles are in contact for years. This suggests the destruction of EPFRs should also be addressed in the remediation of contaminated soils. Since EPFRs are reported to form by the interaction of molecular precursors and particulate matter at temperatures below 600 °C, additional EPFRs may be formed during incineration and, even more so, during low and moderate temperature thermal treatment. Additionally, increased adsorption of PCP on soil has been reported in the low temperature regime [5], suggesting availability for further reaction.
In this manuscript, we report the effects of low temperature thermal treatment of soils contaminated with PCP and pentachlorophenoxyl EPFR. Two types of low temperature thermal treatment (25 °C to 300 °C) were conducted: open system heating (oxygen-rich condition) wherein soils were heated in ambient air and closed system heating (oxygen starved condition) wherein soils were heated under vacuum. Conversion of molecular PCP to pentachlorophenoxyl EPFR, as well as persistency of the EPFRs, was monitored using GC-MS and electron paramagnetic resonance (EPR) analyses.
Materials and Methods
Site Description
The site was a 4 acre wood treatment facility from 1946–1991, formerly used for treating railroad ties and poles [10]. Until the 1970’s, the facility utilized creosote in the preservation process. Pentachlorophenol (PCP) was added to the process and used until the 1980’s, when PCP was used exclusively until the facility closed [10]. In 1994, Environmental Protection Agency (EPA) removed left over tanks on site containing 30,723 gallons of PCP and creosote as part of the remediation [10].
Soil Sampling and Preparation
A detailed discussion of sample collection was presented previously [9]. At each location, soil samples were collected at three different depths: top (0–10 cm), middle (>10–20 cm), and bottom (>20–30 cm). The background, non-contaminated soil samples were collected approximately 500 feet outside of the contaminated area. The soil samples were dried in an oven for 12 hrs at 55 °C to remove water prior to chemical analyses. They were then ground to a homogeneous powder and sieved through a USA Standard Testing Sieve No. 120 (125 μm opening) to eliminate any coarse-sized mineral and vegetative matter. The soil samples prepared in this way are referred to as the whole soil (WS).
Humic Substances (HS) Extraction Method
The HS extraction method was adapted from our first reported study [9] in accordance with the procedure recommended by the International Humic Substances Society (IHSS) [11–17]. Four replicates of each extraction were performed. Briefly, 2.0 g of soil sample were extracted with 20 mL of 0.1 M HCl. The pH of the solution was adjusted between 1.0 and 2.0 with 1.0 M HCl. The soil/HCl mixture was shaken for 1 hr, and the suspension was allowed to settle. The mixture was then centrifuged at 1478 × g for 10 min, and the acid soluble supernatant was separated from the acid insoluble precipitate. The acid insoluble precipitate was neutralized with 1.0 M NaOH to a pH of 7.0, and a 20 mL of 0.1 M NaOH was added under a nitrogen atmosphere. The mixture was shaken for 24 hrs and allowed to settle overnight. The acid-base insoluble precipitate, which we termed as clays/minerals/humins fraction (the main sample investigated in this study) was air dried and evaporated in a vacuum system with controlled circulating air at at room temperature prior to EPR analysis.
Electron Paramagnetic Resonance (EPR) Spectroscopic Analysis
The clays/minerals/humins fraction obtained from the HS extraction method was placed in high purity quartz EPR tubes and analyzed at room temperature in a Bruker EMX – 10/2.7 EPR Spectrometer with X-band microwave frequency of 9.72 GHz, microwave power of 2.02 mW, spectral window of 1000 Gauss, and modulation amplitude of 4.00 Gauss.
The g-factor values were calculated using Bruker’s WINEPR program. The calculated g-factors were checked using a standard of known radical concentration, 2,2-Diphenyl-1-picrylhydrazyl (DPPH; free radical 98%), obtained from Aldrich Chemical Co [18, 19]. The concentrations of free radicals in the clays/minerals/humins fraction were calculated using double integration of the first derivative signal and comparison with the DPPH standard [18,19].
GC-MS Analysis of Pentachlorophenol (PCP)
The PCP GC-MS analysis was adapted from our previous [9]. 200–250 mg of the clays/minerals/humins fraction were placed in scintillation vials, and 4-methyl-2-pentanone was added as the extracting solvent. 250 μL aliquots were placed in an amber vial, to which 250 μL of derivatizing agent, N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA), and 500 μL of extracting solvent, tert-butylmethyl ether (TBME), were added, making up a total volume of 1000 μL. The vial was capped using Teflon/Silicone, 11 mm crimp caps and inverted to mix. The vial was then placed in a pre-heated heating block for 30 minutes at 76 °C (±5 °C) and subsequently cooled to room temperature for GC-MS analysis. These sample solutions were verified to contain pentachlorophenol concentrations falling within the range of our calibration curve.
An Agilent 6890 Gas Chromatograph (GC), fitted with a 5973 Mass Selective Detector (MSD) in the manual injection mode, was used with the following parameters: column type - J&W DB5 MS 60 m × 0.25 mm i.d. × 0.25 μm, preceded by 5 m of 0.25 mm deactivated retention gap; injection type and temperature - splitless / 250 °C; column temperature program - initial 60 °C hold for 6 minutes, ramp 10 °C/min to 180 °C, 15 °C/min to 300 °C, hold for 2 minutes; total run time was 28.0 minutes; carrier gas - Helium; transfer line temperature – 280 °C; injection volume - 1 μL; column flow - 1 μL/min (constant flow): solvent Delay - 14 minutes; MS source temperature – 230 °C; MS quadrupole temperature – 150 °C; MS mode - SIM; and ion dwell time - 100 ms.
Low Temperature Thermal Treatment
The low temperature thermal treatment conducted in our study indirectly heated the soil sample via heat transfer from the preset temperature of a thermoelectrically-controlled furnace. Controlled quantities of oxygen were added using our custom-made vacuum exposure apparatus, previously utilized in our reported study [9]. Briefly, ~100 mg of the clays/minerals/humins fractions were thermally treated (4 trials for each temperature.). The set up consisted of a vacuum gauge, dosing vial port, equilibration chamber, and two reactors. A vacuum valve controlled the vacuum of the two outlets from the equilibration chamber. The equilibration chamber was thermocouple controlled to maintain a preset heating temperature. A detachable two-bulb-shaped pyrex reactor, with a protruding suprasil quartz EPR tube as a side arm for EPR spectral measurements, was attached to the system. The bulb-shaped reactors, containing the clays/minerals/humins fractions, were placed in a vertically oriented small tube furnace. Prior to heating, the fractions were evacuated to 10−2 torr to remove other interfering organic contaminants. For the closed heating scenario (oxygen starved), each fraction was then heated at different temperatures ranging from 25 °C to 300 °C for 5 minutes under vacuum condition. For the open heating scenario (oxygen rich), the fractions were heated on the same system, open to the room atmosphere for 30 minutes. The samples were cooled for 10–15 minutes to room temperature prior to EPR measurements.
Results and Discussion
For the purpose of discussion, the term “soil/s” pertains to the clays/minerals/humins fraction. This clay/minerals/humins fraction represents 90% (mass recovery) of the original un-extracted Whole Soil (WS) sample, and this fraction of the soil was also determined to contain 90% of the EPR signal detected from the WS [9]. Considering the percentage mass and EPFR recovery, this fraction represented the major characteristics of the WS in terms of EPFRs, was a good representative of the characteristic of the WS, and, most importantly, provided increased sensitivity for EPFR detection.
EPR Spectra
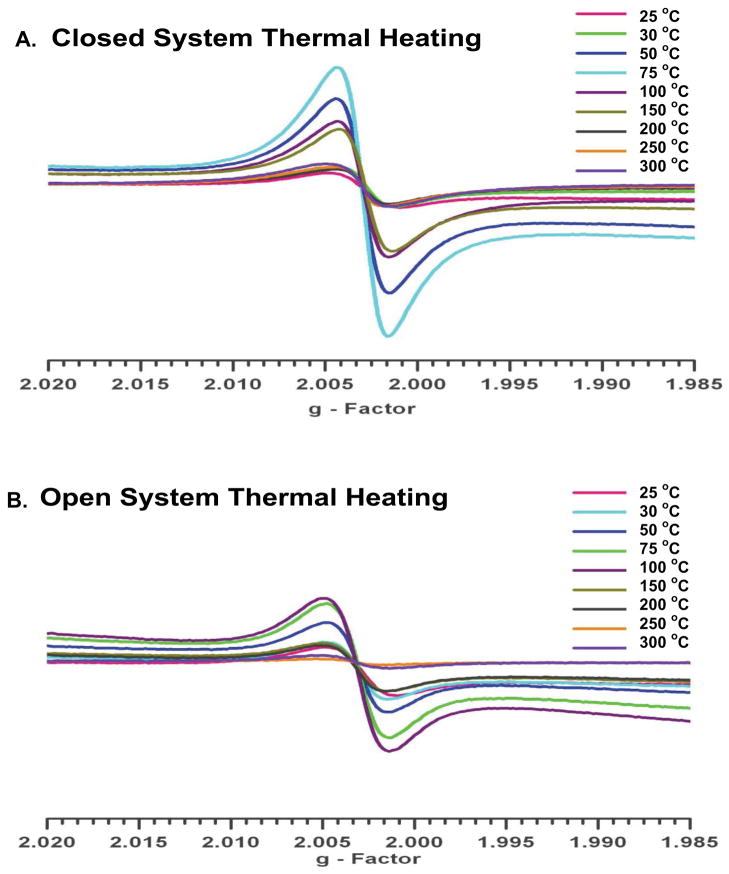
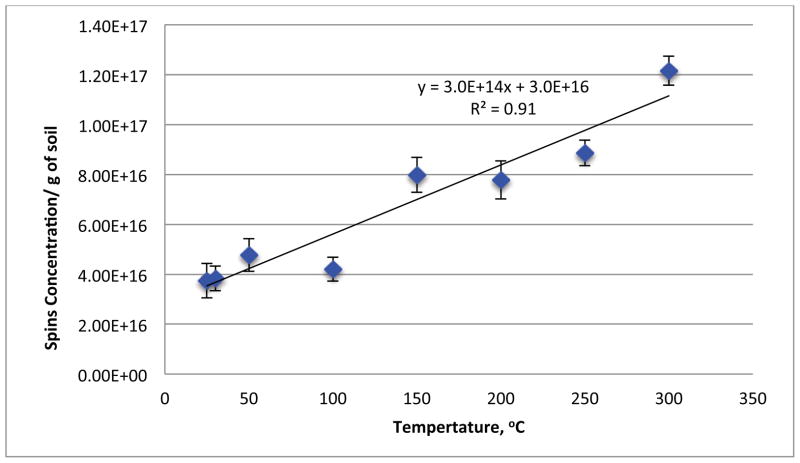
Figure 1 depicts the EPR spectra of the soils subjected to closed and open heating from 25 to 300 °C. Both methods resulted in a singlet signal devoid of hyperfine structure. For the closed system heating, the EPFR concentration range was 2–12 × 1018 spins/g of soil with an EPR g-factor of 2.00311 – 2.00323 and linewidth (ΔHp-p) of 4.190 – 5.472 Gauss. The open heating resulted in a slightly shifted, broader, and weaker signal, with an EPFR concentration of 1–10 × 1018 spins/g of soil, g-factor of 2.00327 – 2.00341, and ΔHp-p of 5.209 – 6.721 Gauss. Both sets of spectra are consistent with pentachlorophenoxyl radical, previously reported to be present in this soil [9, 20–26]. The singlet spectrum remained with slight alteration of EPR parameters as a function of heating temperature except for a significant increase in signal intensity, indicating formation of additional radicals. Varying the EPR microwave power to saturate the signal of some of the potentially multiple radicals resulted in no change in the spectra. This is consistent with our previous assertion that conversion of PCP to its EPFR is the principal source of the observed signal or any newly formed radicals are very similar to pentachlorophenoxyl, e.g. partially dechlorinated. Thermal treatment of the non-contaminated soil (Figure 2) resulted in a slight an increase in the EPFR concentration with increasing temperature. However, the maximum observed concentration at any temperature was only 1.2 × 1017 spins/g, which was only 6% of the EPFR concentration of the contaminated soil (20 × 1017 spins/g). This suggests the naturally occurring radicals in the clays/minerals/humins fraction had a very minimal impact on the increase of EPFR concentrations observed for the thermally heated contaminated soils.
Figure 1.
EPR spectra of soils heated from 25–300 °C. A) closed system – under vacuum for 5 min. B) open system - ambient air for 30 min.
Figure 2.
Thermal treatment of the non-contaminated soil from 25–300 °C and the corresponding spin concentration for each temperature.
Low Temperature Heating in a Closed System
This type of heating emulates thermal treatment under pyrolytic or oxidative pyrolysis condition in which the amount of oxygen present during the process is minimized or controlled, e.g. “thermal screw desorption systems”. This type of thermal treatment is used for soils containing a high percentage of total carbon [27].
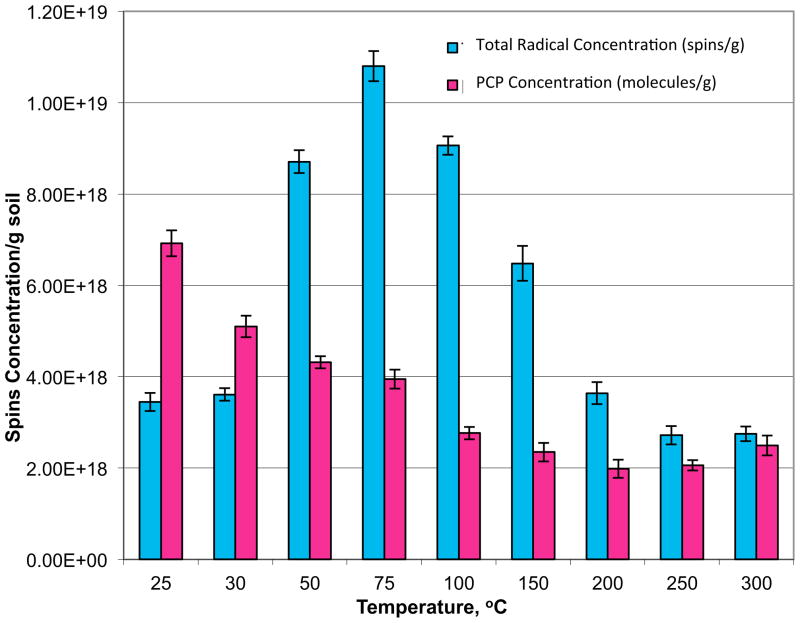
EPFR concentration profiles were obtained for temperatures ranging from 25 °C to 300 °C (Figure 3 and Table 1). The total radical concentration initially increased gradually from 25 °C, achieving a maximum at 75 °C. Concomitantly, the PCP concentrations expressed in molecules/gram decreased gradually as the temperature was increased. These data indicate PCP was converted to its EPFR upon mild heating above 30 °C. Below this temperature, the presence of moisture in the soil hindered this conversion, possibly due to hydrolysis of the chemisorbed EPFR to reform the PCP molecule [18, 19, 28, 29]. At 50 °C, with the continuous removal of moisture and increased thermal activation, an abrupt increase in the total EPFR concentration resulted, which increased to a maximum at 75 °C. The increase from 25 °C up to 75 °C corresponded to a decrease in PCP concentration. Above 75 °C, both the EPFR and PCP concentration decreased. At 300 °C, the PCP concentration increased slightly, possibly due to catalytic formation of PCP in the soil [18, 19].
Figure 3.
EPFR and PCP concentrations in contaminated soil samples from closed system thermal heating for 5 min at temperatures of 25–300 °C.
Table 1.
EPR parameters for 5 minutes closed system thermal heating.
| Temp, °C | g- Factor | ΔHp-p |
|---|---|---|
| 25 | 2.00323 ±0.0000889 | 5.472 ±0.1134 |
| 30 | 2.00315 ±0.0000404 | 5.155 ±0.0302 |
| 50 | 2.00317 ±0.0000173 | 5.019 ±0.0679 |
| 75 | 2.00317 ±0.0000152 | 4.788 ±0.0771 |
| 100 | 2.00318 ±0.0000404 | 4.728 ±0.0605 |
| 150 | 2.00313 ±0.0000100 | 4.291 ±0.1408 |
| 200 | 2.00317 ±0.0000306 | 4.333 ±0.1137 |
| 250 | 2.00318 ±0.0000200 | 4.602 ±0.1789 |
| 300 | 2.00322 ±0.0000252 | 4.190 ±0.1563 |
The most likely explanation for the increase in EPFR concentration is conversion of PCP to its pentachlorophenoxyl EPFR. Other phenomena reported in the literature may also make slight contributions. The carbonization of soil components cannot be responsible for EPFR formation at these relatively low temperatures [28]. However, the soil is contaminated with other chlorinated aromatics, PAHs, and aliphatic hydrocarbons, which can be catalytically chlorinated and react to form PCP and its EPFR [18. 19]. In addition, a degradation process wherein large macromolecular or polyaromatic structures undergo decomposition to aromatic subunits and radical formation may contribute to the increased EPR signal [30]. The sample soils under study do contain significant humin, which is composed of weakly associated molecular assemblies that could form more carbon-centered radicals. This is consistent with the slightly lower g-value observed upon heating. Mild heating will also desorb paramagnetic oxygen which can also shift the g-value slightly lower [28, 31]. Minimization of water in the sample matrix also enhances the EPR spectra; this is, in part, due to the high dielectric constant of water, which interacts with the electric component of the EPR microwave field causing dissipation of microwave energy [31–33].
To elucidate the concomitant increase in EPFR signal and decrease in PCP concentration, the quantities of PCP destroyed and EPFRs formed for each temperature, relative to the untreated initial concentrations at 20 °C were calculated (Table 2). For the GC-MS analyses performed, it was presumed, as previously reported by our group, that the PCP concentration is the combination of the extracted molecular and radical forms of PCP from the soil sample. The data indicated the maximum conversion of PCP to its EPFR was 81% at 75 °C. From 75 °C to 250 °C, the percent conversion gradually decreases, probably by annihilation via radical-radical recombination or thermal decomposition. This can result in formation of new, secondary, molecular pollutants [18, 19].
Table 2.
Calculated concentrations of PCP in molecular and EPFR form from the closed system thermal heating.
| Temp (°C) | Total PCP (molecule & radical) Destroyed (molecules/gram) | PCP EPFR formed (spins/gram) | PCP Molecule Destroyed (molecules/gram) | % PCP Converted to EPFR |
|---|---|---|---|---|
| 25 | 7.29E+18 ±7.43E+17 | 1.18E+18 ±2.13E+17 | 6.11E+18 ±7.37E+17 | 16.19 ±3.36 |
| 30 | 9.27E+18 ±7.12E+17 | 1.20E+18 ±1.71E+17 | 8.07E+18 ±7.32E+17 | 12.95 ±2.10 |
| 50 | 1.01E+19 ±6.85E+17 | 6.39E+18 ±2.62E+17 | 3.66E+18 ±7.33E+17 | 63.60 ±5.06 |
| 75 | 1.04E+19 ±7.04E+17 | 8.49E+18 ±3.38E+17 | 1.93E+18 ±7.81E+17 | 81.48 ±6.39 |
| 100 | 1.16E+19 ±6.85E+17 | 6.75E+18 ±2.13E+17 | 4.85E+18 ±7.17E+17 | 58.16 ±3.89 |
| 150 | 1.20E+19 ±7.03E+17 | 4.17E+18 ±3.87E+17 | 7.85E+18 ±8.02E+17 | 34.68 ±3.81 |
| 200 | 1.24E+19 ±7.01E+17 | 1.33E+18 ±2.51E+17 | 1.11E+19 ±7.45E+17 | 10.72 ±2.12 |
| 250 | 1.23E+19 ±6.82E+17 | 4.07E+17 ±2.15E+17 | 1.19E+19 ±7.15E+17 | 3.31 ±1.76 |
| 300 | 1.19E+19 ±7.06E+17 | 4.37E+17 ±1.77E+17 | 1.14E+19 ±7.28E+17 | 3.68 ±1.51 |
Low Temperature Heating in an Open System
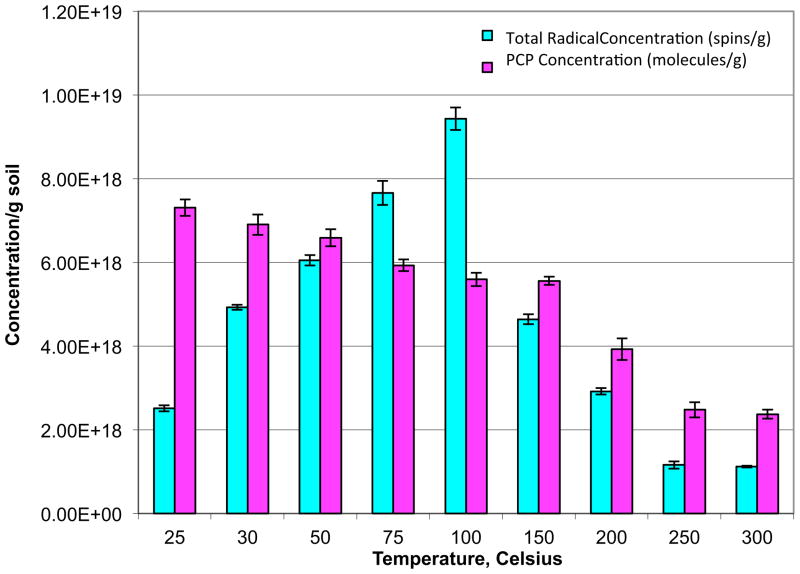
This type of heating emulates low temperature, thermal oxidation and is typically used for soils containing < 5% carbon [27]. The total EPFR concentration gradually increased from 25 °C until achieving a maximum at 100 °C (Figure 4). The PCP concentration exhibited a slow decrease with increasing temperature, again suggesting conversion of PCP to its EPFR. Above 150 °C, both the PCP and EPFR concentrations decreased with increasing temperature, the decrease being more precipitous for the EPFR.
Figure 4.
EPFR and PCP concentrations in contaminated soil samples from open system thermal heating for 30 min at temperatures 25–300 °C.
The conversion of PCP to its pentachlorophenoxl EPFR was calculated for open heating in same manner used for closed heating (Table 4). Below 200 °C, the results were similar to closed heating, with 81% of PCP molecule being converted to EPFR at 100 °C. At 250 °C and 300 °C, a negative percentage yield of EPFR was calculated, which suggests additional EPFRs were not forming and PCP EPFRs originally detected on soils were also destroyed.
Table 4.
Calculated concentrations of PCP in molecular and EPFR form from the open system thermal heating.
| Temp (°C) | Total PCP (molecule & radical) Destroyed (molecules/gram) | PCP EPFR formed (spins/gram) | PCP Molecule Destroyed (molecules/gram) | % PCP Converted to EPFR |
|---|---|---|---|---|
| 25 | 7.05E+18 ±7.01E+17 | 1.20E+18 ±1.03E+17 | 5.85E+18 ±7.09E+17 | 17.01 ±2.23 |
| 30 | 7.46E+18 ±7.12E+17 | 2.62E+18 ±9.62E+16 | 4.84E+18 ±7.18E+17 | 35.08 ±3.59 |
| 50 | 7.78E+18 ±7.02E+17 | 3.74E+18 ±1.43E+17 | 4.04E+18 ±7.16E+17 | 48.06 ±4.71 |
| 75 | 8.44E+18 ±6.86E+17 | 5.35E+18 ±2.96E+17 | 3.09E+18 ±7.47E+17 | 63.39 ±6.24 |
| 100 | 8.77E+18 ±6.91E+17 | 7.12E+18 ±2.79E+17 | 1.65E+18 ±7.45E+17 | 81.15 ±7.14 |
| 150 | 8.80E+18 ±6.79E+17 | 2.33E+18 ±1.39E+17 | 6.48E+18 ±6.93E+17 | 26.43 ±2.58 |
| 200 | 1.04E+19 ±7.21E+17 | 6.07E+17 ±1.10E+17 | 9.83E+18 ±7.29E+17 | 5.82 ±1.13 |
| 250 | 1.19E+19 ±6.96E+17 | −1.15E+18 ±1.15E+17 | 1.19E+19 ±6.96E+17 | −9.70 ±1.12 |
| 300 | 1.20E+19 ±6.80E+17 | −1.19E+18 ±7.75E+16 | 1.20E+19 ±6.80E+17 | −9.95 ±0.86 |
For open system heating, the formation of the EPFRs may be affected by atmospheric oxygen, which may oxidize various functional groups [18, 19, 34, 35] present in soil, the molecular contaminant, or the unpaired electron in the EPFR. The reaction with the chemisorbed EPFR is apparently slow below 100 °C because the total spin concentration increases. However, the oxidation reaction of the physisorbed molecular PCP created new pentachlorophenoxyl EPFRs. Trapped PCP or other contaminant impurities, released from the lattice imperfections [33] by heating, were also available for oxidation reactions leading to additional increases in EPFR concentration. Above 100 °C, the EPFRs formed from oxidation of the molecular species and the originally present EPFRs, may have reacted via Eley-Rideal or Langmuir-Hinshelwood surface reactions [36] to form new pollutants, such as PCDD/Fs, as the EPFR concentration decreases. In addition, since the soil is continuously exposed to the atmosphere, continuous adsorption of water at active metal sites can shift equilibrium away from chemisorption [18, 19], leading to a decrease in EPFR concentration.
The EPR g-values (Table 3) did not change significantly upon heating (0.6 – 5.0% increase). This suggests only a slight rearrangement to a more oxygen-centered radical. This is in agreement with observations for EPFRs in cigarette smoke, where the increase of the g-factor with increased oxygen concentration was attributed to either the oxidation of some carbon-centered radicals to oxygen centered radicals or the carbon centered radicals being destroyed [18, 19]. However, formation of new oxy- or peroxy- radicals was not observed. As the temperature increased, the ΔHp-p broadened measurably by 1.5 – 20.0%. This is typical for radicals oxidized by molecular oxygen or forming complexes with molecular oxygen.
Table 3.
EPR parameters for 30 minutes open system thermal heating.
| Temp, °C | g - Factor | ΔAHp-p |
|---|---|---|
| 25 | 2.00335 ±0.0000500 | 5.209 ±0.0571 |
| 30 | 2.00328 ±0.0000251 | 5.457 ±0.0425 |
| 50 | 2.00328 ±0.0000252 | 6.189 ±0.0769 |
| 75 | 2.00329 ±0.0000115 | 6.578 ±0.1048 |
| 100 | 2.00327 ±0.0000416 | 6.297 ±0.0931 |
| 150 | 2.00333 ±0.0000208 | 6.265 ±0.0943 |
| 200 | 2.00332 ±0.0000586 | 6.129 ±0.0891 |
| 250 | 2.00341 ±0.0000200 | 6.721 ±0.1234 |
| 300 | 2.00338 ±0.0000351 | 6.238 ±0.0998 |
EPFR Half-lives
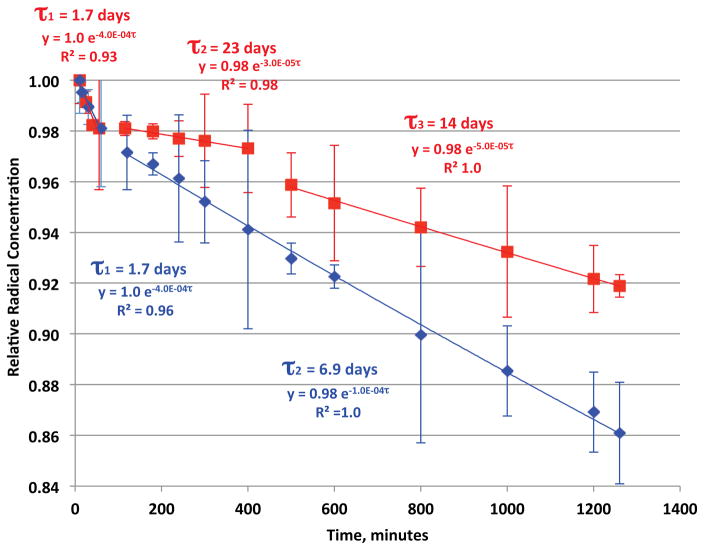
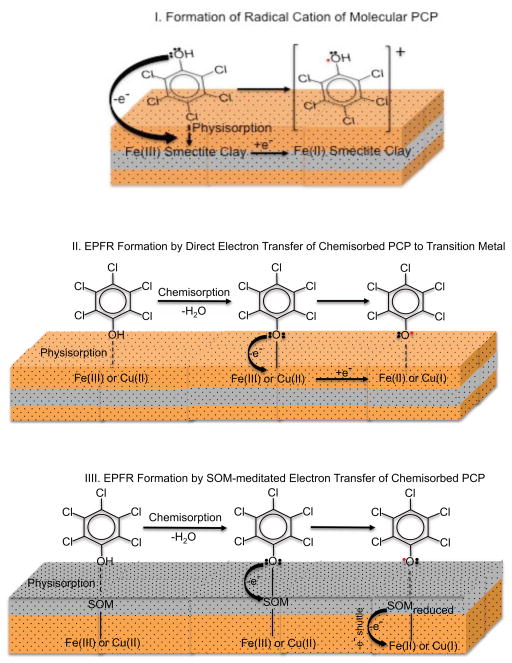
The decays and 1/e half-lives (τ) of EPFRs in air at room temperature formed at 75 °C and 100 °C from open and closed heating, respectively, are depicted in Figure 5. Both systems exhibited multiple decays over their observable lifetimes. The decays for both systems are similar, suggesting similarity of the source of the EPFRs signal observed. However, three distinct decays were observed for the open system (τ = 1.7, 23, and 14 days) and two (τ = 1.7 and 6.9 days) for the closed system. This suggests the EPFR decays are combinations of multiple forms of radicals derived from the PCP molecular precursor. A mechanism of formation of PCP radical on a metal surface adapted from our previous report illustrated a number of possible mesomeric radical structures (oxygen centered, carbon centered, bidentate), all of which could contribute to the observed EPR signal [8, 9]. A direct pollutant to metal center electron transfer process is a plausible for EPFR formation involving soils and sediment with low organic content. However, given the high organic content of the Georgia soil a bare (not coated by soil organic matter) mineral surface may not be available. Soil organic matter (SOM) has been reported to act as an electron conduit between the pollutant and the metal center; thus a direct contact of the pollutant to the metal center is not necessarily needed to facilitate the electron transfer process [37]. Therefore, even when the mineral/clay surface is completely covered by organic matter, as can be assumed from the high organic content of the Georgia soil, the metal center can serve as the final electron sink. Partially catalyzed formation of radicals from PCP can also be facilitated by the metal center donating the acquired electron to the SOM. The idea of SOM stabilizing the formed radical by local effects, such as π-stacking and hydrophobic associations, supports both mechanisms [38]. Thus, the inorganic, organic, and biological components of the soil, as well as their combined or synergistic interactions, must be considered in developing a mechanism of EPFR formation. The mechanisms of radical-cation formation [39, 40], EPFR formations via direct electron transfer to a transition metal and soil-mediated electron transfer are depicted in Figure 6. However, further investigation is warranted to determine if the structure of the radical may be changing with time.
Figure 5.
First order decay (normalized) and 1/e half lives (τ) of EPFRs at their maximum temperature of formation: A) for 5 minutes thermal heating at 75 °C under vacuum (blue): B) for 30 minutes thermal heating at 100 °C in ambient air (red).
Figure 6.
Three proposed mechanisms of radical formation, mechanism I involves molecular PCP while mechanisms II and III involves chemisorbed pentachlorophenolate.
Environmental Implications
The data suggest remediation of soils contaminated with hazardous materials by the use of low temperature thermal desorption or oxidation greatly influences the formation and stabilization of EPFRs. While heating reduces the PCP concentration, the quantitative total spin concentration of EPFRs of PCP increased up to temperatures of 75 °C and 100 °C under oxidative or pyrolytic conditions, respectively. The long half-lives of the EPFRs associated with particulate matter escaping the treatment processes can result in human exposure. They may also be transported in the atmosphere, eventually participating in atmospheric chemistry. Simple recognition of the existence of these potentially toxic EPFRs should be taken into consideration when designing thermal treatment systems for remediation of soils contaminated with hazardous materials capable of generating EPFRs. Even when systems have a mean temperature exceeding 100 °C, some particles will follow flow paths with reduced time at temperature. Thus, a suitable safety factor should be included in the design.
Acknowledgments
We gratefully acknowledge the support for this research provided by the NIEHS Superfund Research Program through grants, 2P42ES013648-03, 3P42ES013648-02S1, 5P42ES013648-02, and the LSU Patrick F. Taylor Chair. We also thank Mr. Scott Miller of EPA for providing access to the Superfund site.
References
- 1.Falciglia PP, Giustra MG, Vagliasindi FGA. Low-temperature thermal desorption of diesel polluted soil: Influence of temperature and soil texture on contaminat removal kinetics. J Hazard Mater. 2011;185:392–400. doi: 10.1016/j.jhazmat.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Cormier SA, Lomnicki S, Backes W, Dellinger D. Origin and health impacts of emissions of toxic by-products and fine particles from combustion and thermal treatment of hazardous wastes and materials. Environ Health Perspect. 2006;114(6):810–817. doi: 10.1289/ehp.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellinger B, Lomnicki S, Pryor W, Cueto R, Squadrito GL, Deutsch WA. The role of combustion-generated radicals in the toxicity of PM2.5. Proc Combust Inst. 2000;28:2675–2681. [Google Scholar]
- 4.Dellinger B, Lomnicki S, Khatchatryan L, Maskos Z, Hall RW, Adounkpe J, McFerrin C, Truong H. Formation and stabilization of persistent free radicals. Proc Combust Inst. 2007;31:521–528. doi: 10.1016/j.proci.2006.07.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse KKC, Lo S. Desorption kinetics of PCP-contaminated soil: effect of temperature. Wat Res. 2002;36:284–290. doi: 10.1016/s0043-1354(01)00191-9. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger B, Pryor W, Cueto R, Squadrito GL, Hedge V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14:1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 7.Truong H, Lomnicki S, Dellinger B. Potential for misidentification of environmentally persistent free radicals as molecular pollutants in particulate matter. Environ Sci Technol. 2010;44(6):1933–1939. doi: 10.1021/es902648t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lomnicki S, Truong H, Vejerano E, Dellinger B. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ Sci Technol. 2008;42:4982–4988. doi: 10.1021/es071708h. [DOI] [PubMed] [Google Scholar]
- 9.dela Cruz ALN, Gehling W, Lomnicki S, Cook R, Dellinger B. Detection of Environmentally Persistent Free Radicals at a Superfund Wood Treating Site. Environ Sci Technol. 2011;45(15):6356–6365. doi: 10.1021/es2012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Superfund, United States Environmental Protection Agencty (EPA) – Official Website. [Accesed June 18, 2010]; Available from: http://www.epa.gov/superfund/
- 11.International Humic Substances Society (IHSS) – Official Website. [Accesed June 18, 2010]; Available from: http://ihss.gatech.edu/ihss2/
- 12.Rosa AH, Simoes ML, de Oliveira LC, Rocha JC, Martin-Neto L, Milori DMBP. Multimethod study of the degree of humification of humic substances extracted from different tropical soil profiles in Brazil’s Amazonian region. Geoderma. 2005;127:1–10. [Google Scholar]
- 13.Rosa AH, Vicente A, Rocha JC, Trevisan HC. A new application of humic substances: activation supports for invertase immobilization. Fres J Anal Chem. 2000;368:730–733. doi: 10.1007/s002160000535. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Perez M, Martin-Neto L, Colnago LA, Milori DMBP, de Camargo OA, Berton R, Bettiol W. Characterization of humic acids extracted from sewage sludge-amended oxisols by electron paramagnetic resonance. Soil Tillage Res. 2006;91:95–100. [Google Scholar]
- 15.Novotny EH, Martin-Neto L. Effects of humidity and metal ions on the free radicals analysis of peat humus. Geoderma. 2002;106:305–317. [Google Scholar]
- 16.Saab SC, Martin-Neto L. Studies of semiquinone free radicals by ESR in the whole soil, HA, FA, and humin substances. J Braz Chem Soc. 2004;15(1):34–37. [Google Scholar]
- 17.Saab SC, Martin-Neto L. Characterization by electron paramagnetic resonance of organic matter in whole soil (Gleysoil) and organic-mineral fractions. J Braz Chem Soc. 2008;19(3):413–417. [Google Scholar]
- 18.Maskos Z, Dellinger B. Radicals form Oxidative Pyrolysis of Tobacco. Energy Fuels. 2008;22:1675–1679. [Google Scholar]
- 19.Maskos Z, Dellinger B. Formation of Secondary Radicals from the Aging of Tobacco Smoke. Energy Fuels. 2008;22:382–388. [Google Scholar]
- 20.Senesi N, Loffredo E. The chemistry of soil organic matter. In: Sparks DL, editor. In Soil Physical Chemistry. CRC Press; Boca Raton: 1999. pp. 242–345. [Google Scholar]
- 21.Delhaes P, Marchand A. Analyse de la formeet de la position de signaixrpe observes sur des carbonesgraphitiquespulverulents. Carbon. 1968;6(2):257–266. [Google Scholar]
- 22.Szent-Gyorgi A, Isenberg I, Baird SL., Jr On the electron donating properties of carcinogens. Proc Natl Acad Sci USA. 1960;46:1444–1449. doi: 10.1073/pnas.46.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barclay LRC, Vinqvist MR. Membrane peroxidation: Inhibiting effects of water-soluble antioxidants on phospholipids of different charge types. Free Radic Biol Med. 1994;16:779–788. doi: 10.1016/0891-5849(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 24.Hales B. Immobilized radicals. I. Principal electron spin resonance parameters of the benzosemiquinone radical. J Am Chem Soc. 1975;97(21):5993–5997. [Google Scholar]
- 25.Graf F, Loth K, Gunthard H. Chlorine hyperfine splittings and spin density distributions of phenoxy radicals. An ESR and quantum chemical study. Helv Chim Acta. 1977;60(3):710–721. [Google Scholar]
- 26.Soma Y, Soma M, Harada I. Reactions of aromatic molecules in the interlayer of transition-metal ion-exchanged montmorillonite studies by resonance Raman spectroscopy. 2. Monosubstituted benzenes and 4,4’-disubstituted biphenyls. J Phys Chem. 1985;89:738–742. [Google Scholar]
- 27.Chapter VI: Low temperature thermal desorption: How to evaluate alternative cleanup technologies for underground storate tank sites. Office of Underground Storage Tanks (OUST), United States Environmental Protection Agency (EPA); [Accesed February 20, 2011]. Official Website. Available from: http://www.epa.gov/swerust1/pubs/tums.htm. [Google Scholar]
- 28.Lagercrantz C, Yhland M. Electron spin resonance in solid state samples of phenothiazine and oxazine dyes. Acta Chem Scand. 1961;15(5):1204–1205. [Google Scholar]
- 29.Saito H, Bucala V, Howard JB, Peters WA. Thermal Removal of Pyrene Contamination from Soil: Basic Studies and Environmental Health Implications. Environ Health Perpect. 1998;106(4):1097–1106. doi: 10.1289/ehp.98106s41097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czechowski F. EPR studies on petrographic constituents of bituminous coals,chars of brown coals group components, and humic acids 600 °C char upon oxygen and solvent action. Energy Fuels. 1997;11:951–964. [Google Scholar]
- 31.Wertz JE, Bolton JR. Electron Spin Resonance: Elementary Theory and Practical Applocations. New York: McGraw Hill; 1972. [Google Scholar]
- 32.Mohanty JG, Rifkind JM. The effect of temperature on ESR signal intensities in aqueous solutions. J Magn Res. 1984;57:178–184. [Google Scholar]
- 33.Eastman JW, Androes GM, Calvin M. Electron spin resonance absorption and other properties of some solid hydrocarbon-quinone complexes. J Chem Phys. 1962;36(5):1197–1208. [Google Scholar]
- 34.Adounkpe J, Khatchatryan L, Dellinger B, Ghosh M. Radicals from the Atmospheric Pressure Pyrolysis and Oxidative Pyrolysis of Hydroquinone, Catechol, and Phenol. Energy Fuels. 2009;23:1551–1554. [Google Scholar]
- 35.Grinberg OY, Williams BB, Ruuge AE, Grinberg SA, Wilcox DE, Swartz HM, Freed JH. Oxygen Effects on the EPR Signals from Wood Charcoals: Experimental Results and the Development of a Model. J Phys Chem B. 2007;111:13316–13324. doi: 10.1021/jp072656l. [DOI] [PubMed] [Google Scholar]
- 36.Lomnicki S, Dellinger B. A detailed mechanism of the surface mediated formation of PCDD/F from the oxidation of 2-monochlorophenol on a CuO/silica surface. J Phys Chem A. 2003;107:4387–4395. [Google Scholar]
- 37.Aeschbacher M, Sanders M, Schwarzenbach RP. Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol. 2010;44:87–93. doi: 10.1021/es902627p. [DOI] [PubMed] [Google Scholar]
- 38.Coates JD, Cole KA, Chakraborty R, O’Connor SM, Achenbach LA. Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Appl Environ Microbiol. 2002;68:2445–2452. doi: 10.1128/AEM.68.5.2445-2452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd SA, Mortland MM. Dioxin radical formation and polymerization on Cu(II)-smectite. Nature. 1985;316:532–535. [Google Scholar]
- 40.Boyd SA, Mortland MM. Radical formation and polymerization of chlorophenols and chloroanisole on Copper(II)-Smectite. Eviron Sci Technol. 1986;20:1056–1058. doi: 10.1021/es00152a017. [DOI] [PubMed] [Google Scholar]