Abstract
The use of left ventricular assist devices (LVADs) in treating patients with end stage heart failure has increased significantly in recent years, both as a bridge to transplant and as destination therapy in those who are ineligible for cardiac transplantation. This increase is based largely on the results of several recently completed clinical trials with the new second generation continuous flow devices that showed significant improvement in survival, functional capacity, and quality of life. Additional information on the use of the first generation and second generation LVADs has come from a recently released report spanning the years 2006–2009, from The Interagency Registry for Mechanical Circulatory Support (INTERMACS), a National Heart Lung and Blood Institute sponsored collaboration between the United States Food and Drug Administration (FDA), the Center for Medicare and Medicaid (CMS) and the scientific community (1). This paper provides a review of the latest clinical trials and data from the INTERMACS registry with tight integration of the landmark molecular, cellular and genomic research that accompanies the reverse remodeling of the human heart in response to the LVAD and functional recovery that has been reported in a subset of these patients.
Keywords: Ventricular assist device, clinical trials, microarray, miRNA, gene expression, calcium, beta-adrenergic, integrins
Clinical Perspective
Currently the average mortality from the time of diagnosis of heart failure regardless of whether there is preserved or reduced systolic function is greater than 60% at five years and this is much higher when patients are in the most advanced stages (2). Patients typically become progressively less responsive to medical therapy and are left with limited options for meaningful improvement in survival or functional capacity and quality of life (3).
Cardiac transplantation is currently the most established treatment for refractory heart failure (2). However, this option is available to fewer than 2,300 patients per year, with a relatively fixed organ supply (2), and no substantive increase anticipated in the near future. The prevalence of heart failure increases with age and affects an estimated 10% of people over 70 years of age, which is the age that many transplant programs use as a cutoff for accepting candidates. Importantly, the over-65 population is expected to double in the next 20 years, and as a result, the use of left ventricular assist devices (LVADs) in treating patients with end stage heart failure will increase significantly in the coming years, both as a bridge to transplant and as destination therapy in those who are ineligible for cardiac transplantation (1).
In 2009, the Center for Medicaid and Medicare (CMS) set a mandate that patients receiving a ventricular assist device (VAD) be entered into a national database, the Interagency Registry for Mechanical Circulatory Support (INTERMACS) (1). INTERMACS is a National Heart, Lung, and Blood Institute (NHLBI)-funded collaboration between the NHLBI, the United States Food and Drug Administration (FDA), the Center for Medicaid and Medicare (CMS), and the mechanical circulatory support scientific community (1). This registry has shown that patients with a LVAD alone have an overall actuarial survival rate of 74% at one year, and 55% at two years (1). The primary cause of death in the 1092 primary LVAD patients in the INTERMACS registry from June 2006 through March 2009 was cardiac failure, including right ventricular failure and ventricular tachycardia/ventricular fibrillation (1). The secondary cause of death was infection (1).
At the inception of INTERMACS, the HeartMate XVE (pulsatile flow pump) (Thoratec, Pleasanton, CA) was the only approved device for destination therapy in the United States. In April 2008, the HeartMate II, a continuous flow LVAD received FDA approval as a bridge to transplant based on results of the clinical trial (4). Thus the registry began tracking the evolution of continuous flow technology in addition to the original pulsatile flow pumps (1). A recent randomized trial showed that patients receiving treatment with a continuous-flow (Heartmate II) LVAD as destination therapy exhibited significant improvement in the probability of stroke free survival and device failure at 2 years as compared with a pulsatile LVAD (5). Both continuous flow and pulsatile devices significantly improved the quality of life and functional capacity (ClinicalTrials.gov number, NCT00121485) (5). However, the survival outcomes of the pulsatile device were nearly identical to the patients enrolled nearly 10 years earlier in the REMATCH trial for destination therapy patients, indicating little progress with that pump (6).
Clinical heart failure may present with changes referred to as remodeling and include eccentric dilatation of the ventricular chamber, reduction in contractile function, and an increase in cardiac filling pressures and wall stress. These clinical phenotypic changes of heart failure are paralleled by significant histologic, cellular, and molecular changes in most structural and functional components of the myocyte including alterations in myocyte geometry and size, progressive interstitial fibrosis, upregulation of cytokines and inflammation, changes in myosin isoforms, as well as reductions in myocardial energetics, beta receptor density, and calcium handling proteins; this is otherwise described as recapitulation of the fetal gene program. These cellular and molecular changes may arise from environmental modifiers or genetic mutations. There is a progressive body of evidence that has shown a trend toward normalization of these perturbations, or reverse remodeling, with LVAD support. However, response to the LVAD is unique to each individual, suggesting that underlying factors are critical in the response to therapy.
Functional Improvements in Myocytes in response to LVAD unloading, linked to Gene Expression Changes
Translational research from human heart tissue before and after support with a LVAD has taught us that myocytes in end stage failing hearts have the capacity to remodel. These changes are accompanied by alterations in gene expression. We summarize findings in the field that have led to important steps forward in our understanding of the molecular changes and contractile properties of cardiac myocytes pre- and post-unloading with a LVAD.
There is evidence of early mechanical improvements in failing hearts treated with LVADs (7–15). These early findings were important in showing that contractile performance of myocytes was partially recovered in response to therapy with a LVAD. Myocyte contractile performance was significantly greater in isolated myocytes from failing human hearts post LVAD support compared to failing human hearts without LVAD support in patients bridged to transplantation (Table 1) (8). Improvements in the magnitude of shortening were seen in isolated myocytes following LVAD support in response to beta-adrenergic agonists (Table 2) (8). Similar to work in isolated myocytes, developed tension in response to beta-adrenergic stimulation in isolated trabecular muscle preparations was also improved (13) (Figure 1). Basal relaxation was also improved in myocytes following LVAD support (Table 2) (8). However, relaxation in response to isoproterenol was not improved (Table 2) (8). This was confirmed in isolated trabecular preparations in a separate study (Figure 1) (13) Figure 1 also shows the significant magnitude in maximal rates of developed rise and fall in tension in response to the beta agonist isoproterenol. Significant improvements are seen in both rise and fall in tension with the LVAD compared to failing hearts. In sum, these findings suggested that LVAD support improves developed tension and relaxation rates at rest and also improves developed tension in response to beta-agonists. However LVAD support did not improve relaxation rates following stimulation with beta-agonists.
Table 1.
Contractile characteristics are improved in myocytes following support with a left ventricular assist device (RCL = resting cell length) (Taken Adapted from Dipla et al, 1998).
 | ||
|---|---|---|
| Contractile Characteristic | HF-Myocytes (n = 57) |
HF-LVAD-Myocytes (n = 35) |
| Magnitude of shortening, % RCL | 6.9±0.5 | 9.6±0.7* |
| Time to peak contraction, s | 0.75±0.04 | 0.37±0.01* |
| Time to 50% relaxation, s | 1.45±0.11 | 0.55±0.02* |
Significant difference between the HF- and the HF-LVAD-myocytes at P<0.05.
Table 2.
Isoproterenol Response on Contractile Parameters. (Taken from Dipla et al, 1998).
| Contractile Parameter | Disease Group | Baseline | Isoproterenol | Delta Scores |
|---|---|---|---|---|
| Magnitude of shortening, % RCL | HF | 6.43 ±0.73 | 8.92±0.781 | 2.49±0.583 |
| HF-VAD | 7.63 ±0.88 | 12.56±1.011 2 | 4.93±1.42 | |
| Time to 50% relaxation, s | HF | 1.28±0.19 | 0.95±0.16 | 0.32±0.15 |
| HF-VAD | 0.70±0.072 | 0.53±0.03 | 0.17±0.08 |
Significant difference (P<0.0125) between baseline and isoproterenol (paired t test).
Significant difference (P<0.0125) between HF- and HF-VAD-myocytes (t test for independent samples).
Significant difference (P<0.05) between HF- and HF-VAD-myocytes on the delta scores (t test for independent.
Figure 1.
Response of muscles to 1 µmol/L isoproterenol. Muscles from nonfailing hearts (NF), failing hearts without LVAD (Failing), and failing hearts with LVAD (Failing+LVAD). A, TPT (time to peak tension); B, THR (time to half relaxation); C, +dT/dt (maximal rate of tension rise); and D, −dT/dt (maximal rate of tension fall). Data are shown as change from baseline contraction (mean±SEM). *P<0.05 vs NF. n=No. of muscles/No. of hearts. This study shows no change in time to peak tension (A) or time to half relaxation (B) between non-failing, failing hearts and failing + LVAD. A significant fall in +dT/dt (C) and −dT/dt (D) was seen in the failing hearts that was improved with the LVAD. (Adopted from Ogletree-Hughes et al, Circulation, 2001).
Beta-adrenergic Signaling
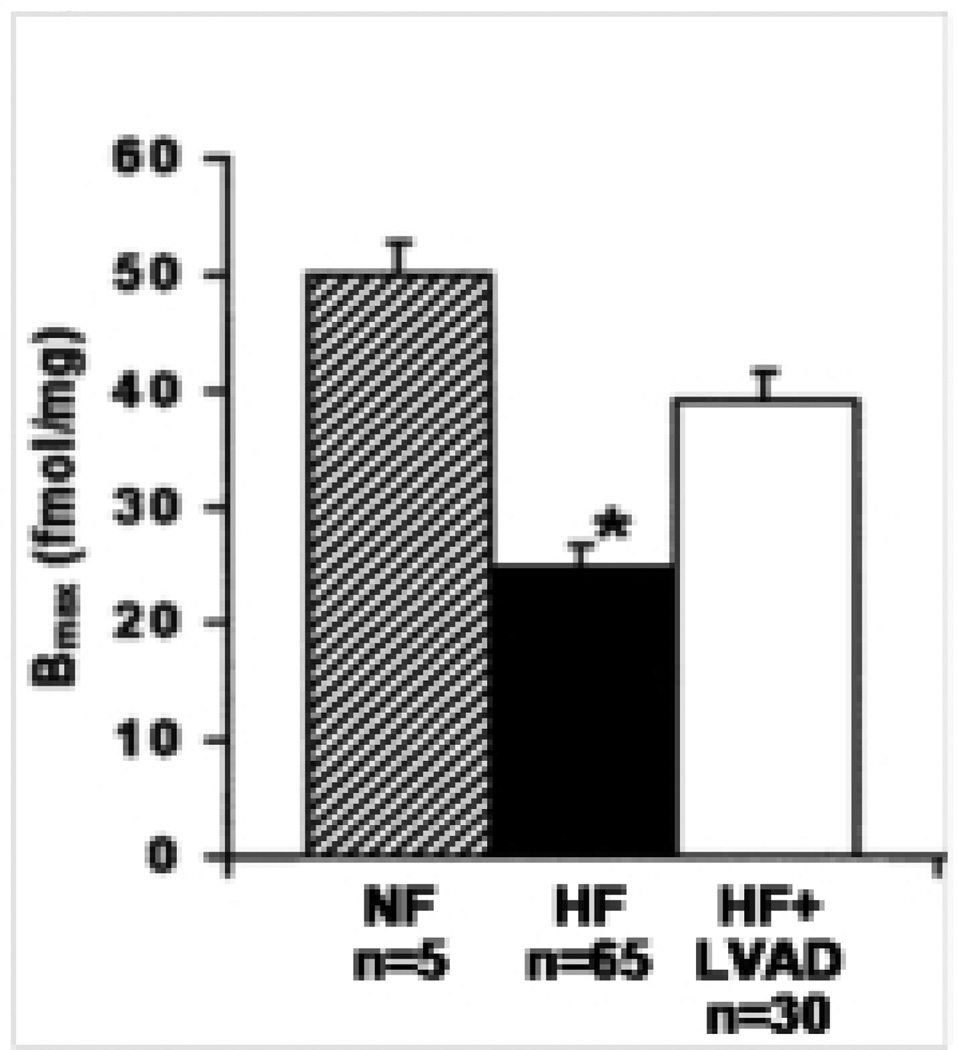
Improvements in developed tension with the LVAD have been shown to be accompanied by increased beta adrenergic receptor density (13,16). Early studies showed that beta adrenergic density was improved following LVAD therapy in 17 patients compared to eight non-failing patients (13). A recent study confirms these findings (15) (Figure 2). Whether this improvement in beta adrenergic density translates to intermediate biochemical improvements in the beta-adrenergic signaling pathway that are associated with improved developed tension are not known. Other molecules in the signaling pathway may still be “desensitized” that control “the brake” on this important pathway that regulates myocyte contractility and remodeling.
Figure 2.
Density of beta-adrenergic receptors in LV and RV myocardial samples of nonfailing (ruled bars), medical (black bars), and LVAD-supported hearts (open bars). Data reported are the means ± SD of maximal binding (Bmax) values determined from Scatchard transformation of saturated binding data. *p < 0.01 vs. nonfailing and LVAD-supported hearts. (Adopted from Klotz et al, 2005).
Beta-adrenergic signaling in Recovery Patients
More recent work assessed beta-adrenergic signaling pathways in an unbiased analysis using a gene chip platform in six paired heart samples from patients that achieved sufficient myocardial recovery to allow explant of the device (17,18). A novel combination therapy consisting of a LVAD combined with pharmacologic therapy including the selective beta-2-agonist, clenbuterol, has shown promise in restoring ventricular function in patients with heart failure (17). The aim of this study was to identify common genes and signaling pathways whose expression was associated with reversal of heart failure and restoration of ventricular function. Microarray analysis was performed on six paired human heart samples harvested at the time of LVAD implant and at the time of LVAD explant for recovery of ventricular function (post). Follow-up data show that the improvements in ventricular function have been maintained for more than 5 years post-explant. This data set represented the first description of signaling pathways associated with the functional recovery of end-stage human heart failure and the identification of new targets in the human heart that are modified by this combination therapy. Analyzing the gene expression profiles from these patients at the time of LVAD explant compared to LVAD implant (heart failure) resulted in the identification of pathways significantly enriched with genes whose expression was statistically different between heart failure and recovery. Instead of a gene-by-gene approach, this study used a network analysis with the goal of identifying pathways that are altered in the process of recovery from heart failure (18). Put simply, gene lists are imported into the Ingenuity database and the program subsequently overlays these genes into several described networks to identify the best overall fit. As seen in Figure 3, significant changes in genes in the beta adrenergic signaling pathway in recovered hearts included Rap guanine nucleotide exchange factor 4, (EPAC2, down-regulated at explant 2.2 fold), protein kinase, regulatory, type I alpha (PKAr, down-regulated at explant 1.5 fold), phosphodiesterase 1A (PDE1A, down-regulated at explant 1.5 fold), phosphodiesterase3B (PDE3B, down-regulated 1.5 fold), and calcineurin A (PPP3CA/PP2B, up-regulated 1.4 fold) (18). The down-regulation in EPAC2 was unique to the recovered group and confirmed in a replication cohort, suggesting that this target may be important for recovery. (This target is discussed again in the calcium handling section below.) It is important to keep in mind that expression changes in mRNA need to translate to functional changes in biochemical signaling to be causally related to functional recovery. Studies of this nature will be critical for moving the field forward.
Figure 3.
Identification of genes in the camp-mediated signaling pathway whose expression was altered in association with recovery. Shaded symbols represent genes whose expression was significantly altered in explanted vs. implanted samples. (Hall et al, 2007).
Calcium Handling
Impaired myocardial relaxation is a physiologic characteristic of heart failure. The reported improvements in basal relaxation rate with the LVAD have not been definitively linked to changes in calcium handling. Elegant functional experiments have been carried out to assess calcium transients and expression of calcium handling proteins (7,19). In a study by Chaudhary et al, LVAD-supported failing myocytes exhibited a faster decay in both early and late stages of the [Ca2+]i transient compared to myocytes from failing hearts not supported with a LVAD (7). These same findings were seen in patients who recovered. An increase in RNA expression of the Na+-Ca2+ exchanger was identified in this study from recovered patients (19). In the study with non-recovered patients, changes were not measured at the RNA level and no changes were detected at the protein level of the Na+-Ca2+ exchanger (NCX1) (7), thus not offering an explanation for the faster decay times in the [Ca2+]i transients (20,21).
Calcium Handling in Recovery Patients
Expanding on these findings, recent work by Terracciano showed the largest improvements in action potential duration and sarcoplasmic reticulum calcium content in individuals that achieved clinical recovery in response to LVAD and pharmacological therapy (Figure 5) (Harefield Recovery Protocol) (22). These findings help distinguish contractile and molecular differences in myocytes from hearts that achieve clinical recovery vs. those that achieve partial recovery (22). Another recent study by Ogletree et al. demonstrated that the benefits of unloading on calcium handling and contractility are time dependent. Patients with a shorter duration of support (< 115 days) achieved a significant increase in SERCA expression levels and a corresponding increase in developed tension (23). These improvements, however, reverted back to failing levels in the patients with longer duration of support (Figure 6) (23). The reason these changes are not persistent is unknown, but it could be an indication of adverse remodeling signals with prolonged unloading.
Figure 5.
Sarcoplasmic reticulum calcium content from myocytes isolated from left ventricular assist device cores and tissue from explanted (recovery) and transplanted hearts without recovery. (Taken from Terracciano et al, 2004).
Figure 6.
Developed tension, expressed as percent change from baseline. (A) Stimulation delay–response curves at delay periods ranging from 2 to 120 seconds (n = number of muscles/number of hearts). (B) Data from the 40-second delay period. Right: separation of F+LVAD by duration of LVAD support. Short duration = 7 to 103 days; long duration = 127 to 334 days. (C) Relationship between duration and PRP recovery (p < 0.05) at 40-second delay in hearts with an LVAD, by heart. F, failing; NF, nonfailing; F+LVAD, LVAD-supported failing heart. *p < 0.05 vs NF. (Adopted from Ogletree et al, 2010).
A recent Phase II clinical trial, the CUPID study, is already testing SERCA2a gene therapy in patients with end stage heart failure with some initial indications of efficacy (24). A second potential target for drug development may be EPAC2. A significant decrease in the calcium regulating gene EPAC2 was identified post LVAD support in 11 recovered hearts (Figure 7) (18). This decrease in EPAC2 was unique to recovered hearts, as the pattern was not found in non-recovered hearts (Figure 7) (18). EPAC2 tethers cAMP to MAPK, calcium-mediated signaling through NFAT, and metabolic signaling pathways. The roles of both EPAC2 and EPAC1 in multiple cell types and tissues including the heart, nerves and pancreas are just beginning to be understood (20,21).
Figure 7.
EPAC2 mRNA levels are significantly reduced in recovered hearts following VAD support (n=11, p< 0.01). No significant differences were seen in non-recovered hearts. (n=5, p ns). (Hall et al, 20
Cytoskeletal Proteins and Integrins in Recovery Patients
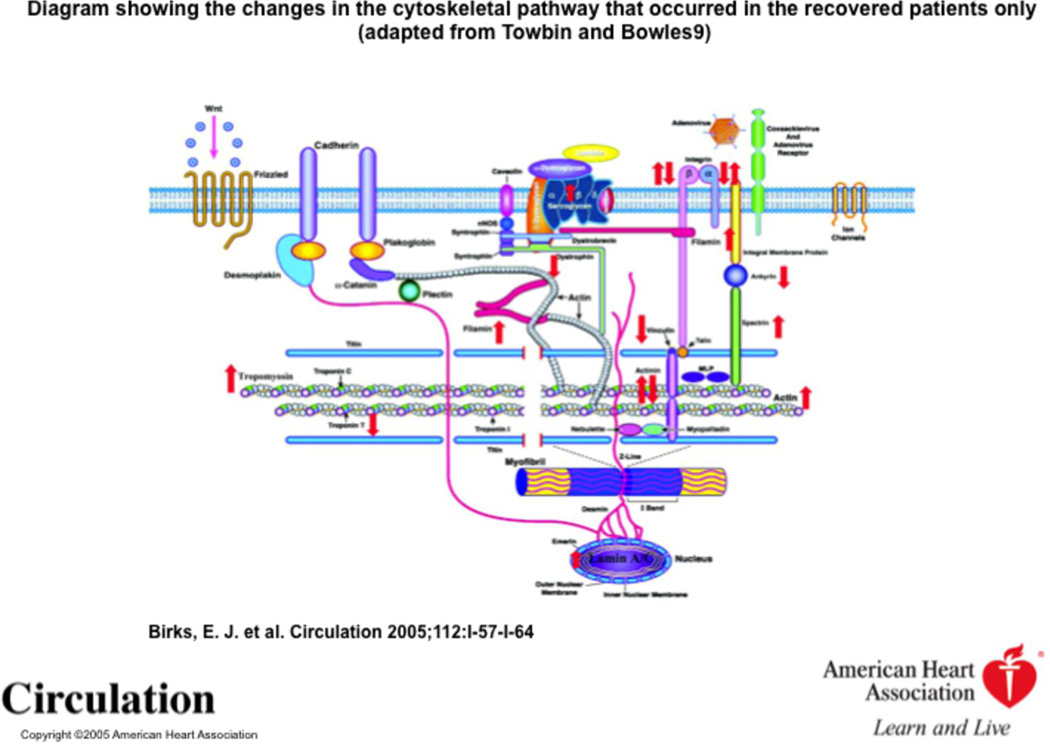
Myocardial recovery has also been associated with a specific pattern of changes in sarcomeric, nonsarcomeric and membrane-associated proteins (25). These changes are highlighted in Figure 8. Affymetrix microarray analysis was performed on paired samples (pre- and post- LVAD) and analyzed with reference to sarcomeric and nonsarcomeric cytoskeletal proteins (25). In the recovery group, of the nonsarcomeric proteins, lamin A/C and spectrin increased from pre-LVAD to post-LVAD (25). The sarcomeric proteins beta-actin, alpha-tropomyosin, alpha-1-actinin, and alpha-filamin A, increased in the recovered group while troponin T3 and alpha2-actinin decreased at explantation (25). Vinculin decreased following LVAD support in the recovered group but increased in the nonrecovered group (25). Further work is needed in this area to define if these changes that occur in expression of cytoskeletal proteins and are uniquely associated with recovery are integral to promoting improvements in contractility that are necessary for recovery.
Figure 8.
Diagram showing the changes in the cytoskeletal pathway that occurred in the recovered patients only (Birks et al, 2005, adapted from Towbin and Bowles).
Integrins are bidirectional signaling molecules and play a role in mechanotransduction by mediating mechanical (stretch) signals from the extracellular matrix, via protein kinase cascades that provoke changes in gene expression, including those involved in the hypertrophic response. Data suggest that the integrin pathway may play a key role at the molecular and cellular level in processes of reverse remodeling and subsequent functional recovery occurring in these patients (25). Work in a pre-clinical rat model has shown similar findings (26). Supplemental Figure 1 summarizes the changes in the integrin signaling pathway along with the cytoskeletal proteins that occurred in the recovery cohort (25).
Metabolism and Growth Factor related Genes and Signaling Pathways in Recovery Patients
In addition to the finding that the process of recovery was associated with cAMP, calcium handling genes, and integrin signaling, several new targets were identified in an unbiased approach, including those that regulate metabolism. Arginine:glycine amidinotransferase (AGAT), a rate-limiting enzyme in the creatine synthesis pathway, was significantly down-regulated following unloading in the recovered hearts, returning to normal levels in direct contrast to the up-regulation of AGAT in patients with heart failure compared with donor hearts (18,27).These changes in AGAT mRNA levels suggest a response to heart failure that involves elevated local creatine synthesis (18,27). The mechanisms leading to induced AGAT expression are unknown but may be a response to the depletion of the local creatine pool, which we and others have shown to be a feature of heart failure.
Insulin Growth Factor (IGF)-I mRNA was elevated at the time of LVAD explant in the patients that received pharmacologic therapy, which included clenbuterol (28). There were 2 groups distinguishable, those with low IGF-I mRNA at implantation who showed significant increase during recovery and those with high IGF-I mRNA at implantation who remained high (28). IGF-I levels correlated with stromal cell-derived factor mRNA measured both in LVAD patients and in a wider cohort of heart failure patients (28). Microarray analysis of implantation and explantation samples of recovery patients further revealed elevated IGF-II and IGF binding proteins IGFBP4 and IGFBP6. IGF-I may act to limit atrophy and apoptosis during reverse remodeling and to promote repair and regeneration in concert with stromal cell derived factor. It may have important effects on fibroblasts (28). A previous analysis in 19 paired patient samples before and after LVAD support with the Heartmate XVE device in a cohort of individuals that were transplanted (did not achieve full recovery) revealed expression changes in the Forkhead transcription factor family, and genes governing angiotensin/insulin signaling axis (29). This analysis also identified a significant correlation in expression between Forkhead box 03A and Ang II type 1 receptor (29). More work will need to be done to determine the role and integration of IGF-1/Forkhead and Ang II in remodeling and recovery and localize these signals to myocytes and /or fibroblasts. A section below will discuss maladaptive fibrotic remodeling in response to the LVAD and the potential role of ACE inhibition in alleviating ventricular stiffness and fibrosis. A recent study from Bhavsar shows that IGF-1 is released preferentially from fibroblasts over cardiac myocytes (30). Thus the interaction between Ang II and IGF-1 both mechanistically and clinically is still not well understood.
miRNA
miRNAs constitute one of the more abundant classes of molecules that regulate genes in animals. miRNAs are small, endogenous noncoding RNAs (31). Many of these miRNAs have been shown to inhibit posttranscriptional processing (31,32). Recent work by Matkovich and colleagues suggests that adding miRNA profiling to mRNA profiling enhances the ability of mRNA profiles to categorize the clinical status of heart failure pre and post biomechanical unloading (33). The results of this study by Matkovich and colleagues confirmed three earlier identified miRNAs associated with heart failure (miR-24, miR-125b and miR-19) (33,34). In addition, Matkovich extended earlier work in mouse models (34,35) showing that miR-21, miR-23a, and miR-199a-3p were also regulated in human heart failure (33). One of the most exciting aspects of the study by Matkovich and colleagues was the reversibility of the miRNAs with LVAD support. A table of miRNAs that normalize following LVAD support are shown in Table 3. It is not yet known which miRNA expression changes are associated with clinical endpoints that correlate with recovery.
Table 3.
miRNAs that normalize after VAD support, n=10 VAD-supported hearts, 80% male, 40%, ischemic, average support time 1.7 months, type of device not disclosed. Fold change and p-values are listed for each miRNA. (Taken from Matkovich et al., 2009).
| Heart failure miRs that normalize after LVAD | ||||||
|---|---|---|---|---|---|---|
| Fold-change | P-value | |||||
| Name | Failing vs nonfailing |
Post-LVAD vs nonfailing |
Post-LVAD vs failing |
Failing vs nonfailing |
Post-LVAD vs nonfailing |
Post-LVAD vs failing |
| miR-27b | 3.15 | 1.14 | −4.75 | 2.60E-04 | 0.270 | 0.0620 |
| miR-30a-5p | 3.06 | 1.07 | −2.85 | 4.76E-04 | 0.566 | 0.0821 |
| miR-30c | 3.31 | 1.32 | −2.51 | 1.24E-04 | 0.105 | 0.0853 |
| miR-30d | 2.96 | 1.23 | −2.40 | 6.47E-04 | 0.211 | 0.116 |
| miR-103 | 3.54 | 1.20 | −2.94 | 1.24E-04 | 0.156 | 0.0834 |
| miR-130a | 3.06 | 1.36 | −2.26 | 6.80E-04 | 0.127 | 0.270 |
| miR-378 | 3.60 | 1.20 | −3.00 | 6.25E-05 | 0.153 | 0.0544 |
| let-7f | 4.84 | 1.18 | −4.11 | 1.50E-06 | 0.211 | 0.00543 |
Natriuretic peptides and chromogranin A are normalized by LVAD support
Despite divergent etiologies, heart failure is characterized by activation of neurohormonal systems: catecholamines, natriuretic peptides and components of the renin-angiotensin axis are increased and have been found to have pathophysiological and prognostic implications. Some of these molecules exert local paracrine activation, but their plasma levels have been demonstrated to be markers of clinical outcome. Increased cardiac ANP and BNP expression in heart failure patients is associated with increased expression of the natriuretic peptides metabolizing NPR-C receptors and blunted responsiveness of GC-A to ANP by reduced cGMP synthesis.
Unloading the failing heart with a LVAD is associated with a decrease in natriuretic peptides and reestablishment of the local responsiveness of GC-A to ANP (36). Cardiac expression of ANP, BNP and NPR-C mRNA also correlated significantly with cardiomyocyte diameters. In contrast to the latter, the levels of the natriuretic peptides are fully reversed to the level of the controls, indicating that their expression is partly independent of cardiac hypertrophy and regulated by heart failure associated factors such as cardiomyocyte stretch (36). Evidence that unloading may be sufficient for a decrease in natriuretic peptides (and that recovery is not necessary) comes from the pre-clinical data in which natriuretic peptides are decreased in heterotopic transplants of failing rat hearts (37,38).
Chromogranin A is an acidic calcium-binding protein and is the major soluble constituent in secretory vesicles throughout the neuroendocrine system. Chromogranin A was found to be significantly up-regulated during heart failure and is co-stored with catecholamines and natriuretic peptides. BNP and chromogranin A are co-stored in the myocardium of patients with dilated cardiomyopathy, whereas this co-localisation was not found in healthy controls (39). Wohlschlaeger and colleagues investigated the expression of natriuretic peptides and chromogranin A by immunohistochemistry and morphometric quantification before and after LVAD (40). In a different set of patients, chromogranin A was evaluated in the plasma (40). We demonstrated that in line with ANP and BNP, chromogranin A is significantly increased in CHF compared to healthy controls and decreased by ventricular support (40). Moreover, sarcoplasmic colocalization of BNP and chromogranin A is diminished after unloading. However, due to its low expression the negative regulation of chromogranin A is not reflected by plasma levels, thus chromogranin A does not appear to be an appropriate biomarker for the monitoring of “reverse cardiac remodeling” after unloading (40) (Supplemental Figure 2).
Summary
In summary, LV unloading in end-stage heart failure patients with LVAD support has been shown to improve myocardial structure and function, including improving β-adrenergic responsiveness and myocyte contractility. The phenotypic changes in the heart associated with the LVAD may arise from changes in mRNA, miRNA or post-translation mechanisms such as ubiquitination or phosphorylation events as discussed by Margulies et al (11). Several working groups, including our own, have defined changes in gene expression that occur with LVAD support that may be important in improving β-adrenergic responsiveness and calcium handling including partial restoration of β-receptor density, and altered regulation of the genes in G-protein coupled signaling and calcium handling. A gene expression profile analysis using gene chips with 199 myocardial samples from failing, LVAD-supported and non-failing human hearts demonstrated that the majority of transcriptional changes accompanying morphological and functional alterations in the heart were modest (41). Changes in miRNA expression in a recent study were more robust (33). Protein expression has been mainly restricted to calcium handling, beta-adrenergic and extra-cellular matrix pathways and much work is yet to be done. Pairing pre-clinical work in model organisms in which these targets (genes or miRNA) are overexpressed or deleted and their function is understood in the context of heart failure will be helpful. Overexpressing genes in which phosphorylation sites are mutated will aid in the identification of how post-translational mechanisms are regulated. However, rodent models do not recapitulate human heart failure and heterotopic transplantation has several limitations in modeling assist devices. The next generation of studies will take advantage of state-of-the-art technology to yield more information from human myocytes from the core biopsies and define how changes in RNA and protein may alter morphology and contractility.
A recent histological study by Drakos and colleagues found that unloading with the LVAD in 15 patients receiving the HeartMate I device (pulsatile) resulted in a 33% increase in microvascular density, although the total lumenal area was decreased, so the functional patency of these vessels is unclear (42).
Some remodeling responses to mechanical unloading may be maladaptive. The most prominent advancement of this hypothesis is the concept of myocardial atrophy and reversal, which is being investigated in the Harefield Recovery studies that were described earlier. Mechanisms regulating cardiac atrophy are unknown, although it is thought that the ubiquitin-proteasome degradation pathway that is active in skeletal muscle and the coronary vascular bed plays a role, and may represent an overcompensation because of the lack of opposing hypertrophic stimuli after chronic unloading (43,44). Another concept in maladaptive remodeling is that of fibrosis and extracellular matrix turnover. One study by Klotz et al. of collagen content and subtypes after LVAD support found a significant increase in total and cross-linked collagen in the myocardium compared to nonfailing and medically managed heart failure subjects, which correlated with increased left ventricular stiffness (45). Interestingly, the majority of LVAD patients after implant were not on ACE inhibitors, which have been demonstrated to improve fibrosis and remodeling (46,47). A subsequent retrospective cohort study by the same group, comparing LVAD patients who did and did not receive ACE inhibitor therapy after implantation, demonstrated a significant decrease in collagen content and myocardial stiffness in the LVAD plus ACE inhibitor cohort (16). On the molecular level, quantitation of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) demonstrated near normalization of the MMP-1/TIMP-1 ratio in LVAD patients receiving ACE inhibitors, suggesting a mechanism for the improvement collagen turnover. These findings support the hypothesis that maximizing optimal medical management after ventricular unloading with LVAD may promote myocardial recovery. In sum, the modest gene expression changes are sufficient to change vascular and fibrotic properties in the heart. However, a more thorough understanding of the unique therapies and underlying genetic and environmental properties will further aid in our understanding of the biological pathways through which unloading with the LVAD leads to remodeling and recovery. A better understanding of the biology may allow us to alter the engineering properties of the device and /or patient selection criteria (see section below) to improve outcome.
Limitations
The LVAD population presents a unique and valuable opportunity to obtain myocardial tissue of end stage heart failure patients at the time of implantation, an often at the time of heart and/or LVAD explant, after a period of unloading. There are, however, some recognized limitations of carrying out molecular studies on the VAD patient population. Study sizes tend to be small, which is a particular challenge when performing large scale transcriptome analysis, although this limitation is being overcome by multi-center collaborations and the overall increase in use of LVADs. Tissue procurement must also be carefully planned, and the tissue received from the apical transmural tissue core may be fibrotic and not representative of the whole myocardium. In addition, the clinical presentation, duration of support and medical management are uncontrollable variables, creating a heterogeneous patient population. Animal models have been created in attempt to study ventricular unloading in a more controlled fashion. These include a thoracic inferior vena cava constriction model described by Lisy et al (48,49), which demonstrates myocardial atrophy with chronic unloading, with significant upregulation of the RAAS axis and decrease in ANP/BNP expression. While the myocardial atrophy findings have important implications for research in human LVAD patients, this model creates a biventricular low cardiac output state from a presumably normal baseline, and thus is not analogous to the physiologic effect of implanting a LVAD. Another established animal model is that of heterotopic cardiac transplantation, described by multiple groups including Tevaerai et al (50). In this model, heart failure is induced in a “donor” cohort of rabbits, after which the hearts are transplanted heterotopically by anastomosing the aorta and pulmonary trunk to the recipient rabbits’ carotid artery and internal jugular vein, respectively. The heart is perfused only through the coronary circulation, through the coronary sinus and returned to the recipient via the right heart, creating a completely unloaded left ventricle (50). While this model is also intriguing, it also involves a transplantation, removing the organ from the systemic physiology of the heart failure “donor” and also subjecting the heart to the immunological and biochemical milieu associated with transplantation. Given the limitations of animal models, it is critical to continue advancing translational research in the LVAD patient population in order to apply the understanding of unloading responses in heart failure to the development of clinical predictors and medical therapy for these patients.
Final Clinical Perspective and Future Directions
Recent clinical trials have shown that both pulsatile and continuous flow LVADs significantly improve the quality of life and functional capacity of patients with heart failure (4,5). Translational research in the area of LVAD support has provided new information on signaling pathways. Tissue procurement, DNA sequencing, RNA sequencing and epigenetic tools will help in our further understanding of the underlying signals regulating the reverse remodeling and recovery potential of the failing human heart. Investigation in the LVAD patient population has been limited by the fact that there is no consensus on medical management toward, and clinical monitoring of, myocardial recovery. For instance, the findings correlating ACE inhibitor use and favorable decrease in myocardial stiffness and collagen subtypes suggest a greater role for continuing optimal medical management after LVAD placement. The elucidation of myocardial atrophy pathways may also illuminate the concept of optimizing or weaning unloading after LVAD placement to minimize atrophy and promote recovery. Our current understanding of the role of extracellular matrix in heart failure and the ability of the fibroblast to remodel in response to the LVAD represents a new focus area. Further elucidating these pathways will allow for development of predictive models as well as novel therapeutic strategies for VAD patients. Perhaps more importantly, using LVADs as a research platform may yield therapies that benefit those in earlier stage heart failure. Identification of the biomarkers and factors that will aid in risk stratification for patients with LVAD therapy will help move this field forward and enhance patient care.
Supplementary Material
Recovered cohort. Gene expression changes in the beta-integrin signaling pathway in the recovered group. Gene expression changes were significantly different at the receptor level (beta-integrins). The differential expression of beta-integrin receptors is associated with the differential expression of genes distal to the receptor, including vinculin (downregulated in the recovered group, where green indicates downregulation after VAD), a gene previously shown to play a role in heart failure, and the Rho family member cdc42. (Birks et al, 2005).
Heart failure and hypertrophy are associated with increased expression of ANP, BNP and Chromogranin A due to tensile stretch during cardiac dilatation in the heart as well as in the blood. After mechanical support ANP and BNP levels decrease in both in the heart and in the blood. In contrast, chromogranin A only decreases in the heart. (modified from Wohlschlaeger et al. 2008)
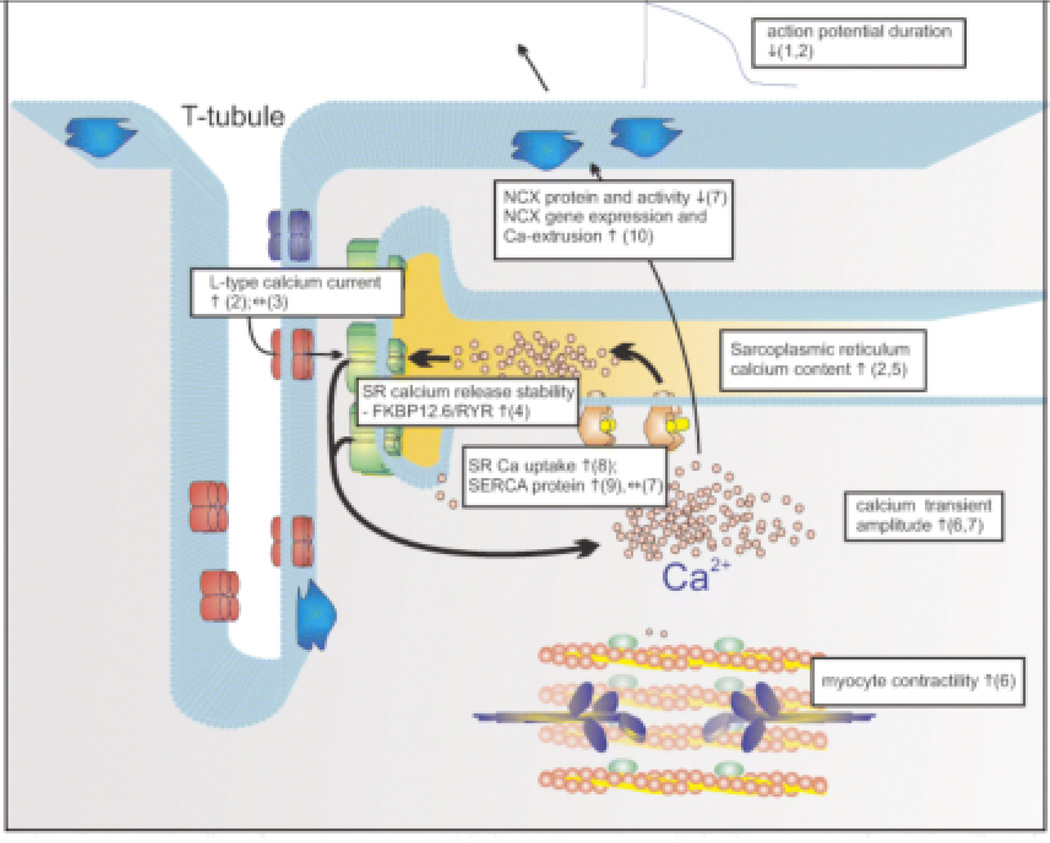
Figure 4.
Calcium handling following LVAD support. This figure summarizes work to date in the literature of VADs. (1) Harding JD et al., Circulation 2001; (2) Terracciano CMet al., Circulation 2004; (3) Chen X et al., Circ. Res. 2002; (4) Marx SO et al., Cell 2000; (5) Terracciano CM et al., Eur. Heart J. 2003; (6) Dipla K et al., Circulation 1998; (7) Chaudhary K et al., J. Am. Coll. Cardiol. 2004; (8) Frazier MD et al., Ann. Thor. Surg. 1999; (9) Heerdt PM et al., Circulation 2000; (10) (Terracciano et al, 2007). (Terracciano, et al, 2007).
Acknowledgments
Funding
This work was funded in part from the National Institutes of Health Research Cardiovascular Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College (PJRB), the American Heart Association (0515584Z) (0655582Z), the National Institute of Health NIH-RO1-HL065462-04 and the Lillehei Scholar Program (lab of JLH).
Abbreviations
- LVAD
left ventricular assist device
- VAD
ventricular assist device
- CHF
congestive heart failure
- ACE
angiotensin convering enzyme
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- SERCA
sarco/endoplasmic reticulum Ca(2+)-ATPase
- EPAC2
exchange protein directly activated by cAMP 2
- mRNA
messenger RNA
- miRNA
micro RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 3.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Rose EA, Moskowitz AJ, Packer M, et al. The REMATCH trial: rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann Thorac Surg. 1999;67:723–730. doi: 10.1016/s0003-4975(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary KW, Rossman EI, Piacentino V, 3rd, et al. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J Am Coll Cardiol. 2004;44:837–845. doi: 10.1016/j.jacc.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 9.Harding JD, Piacentino V, 3rd, Gaughan JP, Houser SR, Margulies KB. Electrophysiological alterations after mechanical circulatory support in patients with advanced cardiac failure. Circulation. 2001;104:1241–1247. doi: 10.1161/hc3601.095718. [DOI] [PubMed] [Google Scholar]
- 10.Heerdt PM, Holmes JW, Cai B, et al. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation. 2000;102:2713–2719. doi: 10.1161/01.cir.102.22.2713. [DOI] [PubMed] [Google Scholar]
- 11.Margulies KB. Reversal mechanisms of left ventricular remodeling: lessons from left ventricular assist device experiments. J Card Fail. 2002;8:S500–S505. doi: 10.1054/jcaf.2002.129264. [DOI] [PubMed] [Google Scholar]
- 12.Maybaum S, Mancini D, Xydas S, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 13.Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation. 2001;104:881–886. doi: 10.1161/hc3301.094911. [DOI] [PubMed] [Google Scholar]
- 14.Zafeiridis A, Jeevanandam V, Houser SR, Margulies KB. Regression of cellular hypertrophy after left ventricular assist device support. Circulation. 1998;98:656–662. doi: 10.1161/01.cir.98.7.656. [DOI] [PubMed] [Google Scholar]
- 15.Klotz S, Barbone A, Reiken S, et al. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol. 2005;45:668–676. doi: 10.1016/j.jacc.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Klotz S, Danser AH, Foronjy RF, et al. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–1174. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 17.Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 18.Hall JL, Birks EJ, Grindle S, et al. Molecular signature of recovery following combination left ventricular assist device (LVAD) support and pharmacologic therapy. Eur Heart J. 2007;28:613–627. doi: 10.1093/eurheartj/ehl365. [DOI] [PubMed] [Google Scholar]
- 19.Terracciano CM, Koban MU, Soppa GK, et al. The role of the cardiac Na+/Ca2+ exchanger in reverse remodeling: relevance for LVAD-recovery. Ann N Y Acad Sci. 2007;1099:349–360. doi: 10.1196/annals.1387.061. [DOI] [PubMed] [Google Scholar]
- 20.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 22.Terracciano CM, Hardy J, Birks EJ, Khaghani A, Banner NR, Yacoub MH. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation. 2004;109:2263–2265. doi: 10.1161/01.CIR.0000129233.51320.92. [DOI] [PubMed] [Google Scholar]
- 23.Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: Effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant. 2010;29:554–561. doi: 10.1016/j.healun.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Jaski BE, Jessup ML, Mancini DM, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birks EJ, Hall JL, Barton PJ, et al. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular-assist device support. Circulation. 2005;112:I57–I64. doi: 10.1161/CIRCULATIONAHA.104.526137. [DOI] [PubMed] [Google Scholar]
- 26.Lara-Pezzi E, Terracciano CM, Soppa GK, et al. A gene expression profile of the myocardial response to clenbuterol. J Cardiovasc Transl Res. 2009;2:191–197. doi: 10.1007/s12265-009-9097-6. [DOI] [PubMed] [Google Scholar]
- 27.Cullen ME, Yuen AH, Felkin LE, et al. Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: potential implications for local creatine synthesis. Circulation. 2006;114:I16–I20. doi: 10.1161/CIRCULATIONAHA.105.000448. [DOI] [PubMed] [Google Scholar]
- 28.Barton PJ, Felkin LE, Birks EJ, et al. Myocardial insulin-like growth factor-I gene expression during recovery from heart failure after combined left ventricular assist device and clenbuterol therapy. Circulation. 2005;112:I46–I50. doi: 10.1161/01.CIRCULATIONAHA.105.525873. [DOI] [PubMed] [Google Scholar]
- 29.Hall JL, Grindle S, Han X, et al. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol Genomics. 2004;17:283–291. doi: 10.1152/physiolgenomics.00004.2004. [DOI] [PubMed] [Google Scholar]
- 30.Bhavsar PK, Brand NJ, Felkin LE, et al. Clenbuterol Induces Cardiac Myocyte Hypertrophy via Paracrine Signalling and Fibroblast-derived IGF-1. J Cardiovasc Transl Res. 2010 doi: 10.1007/s12265-010-9199-1. [DOI] [PubMed] [Google Scholar]
- 31.Saunders MA, Liang H, Li WH. Human polymorphism at microRNAs and microRNA target sites. Proc Natl Acad Sci U S A. 2007;104:3300–3305. doi: 10.1073/pnas.0611347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP. Vertebrate microRNA genes. Science. 2003;299:1540. doi: 10.1126/science.1080372. [DOI] [PubMed] [Google Scholar]
- 33.Matkovich SJ, Van Booven DJ, Youker KA, et al. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009;119:1263–1271. doi: 10.1161/CIRCULATIONAHA.108.813576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsuguchi M, Seok HY, Callis TE, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn M, Voss M, Mitko D, et al. Left ventricular assist device support reverses altered cardiac expression and function of natriuretic peptides and receptors in end-stage heart failure. Cardiovasc Res. 2004;64:308–314. doi: 10.1016/j.cardiores.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Tsuneyoshi H, Oriyanhan W, Kanemitsu H, et al. Does the beta2-agonist clenbuterol help to maintain myocardial potential to recover during mechanical unloading? Circulation. 2005;112:I51–I56. doi: 10.1161/CIRCULATIONAHA.104.525097. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Tsukashita M, Nishina T, et al. Chronic partial unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in failing rat hearts. J Thorac Cardiovasc Surg. 2009;137:465–470. doi: 10.1016/j.jtcvs.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Pieroni M, Corti A, Tota B, et al. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J. 2007;28:1117–1127. doi: 10.1093/eurheartj/ehm022. [DOI] [PubMed] [Google Scholar]
- 40.Wohlschlaeger J, von Winterfeld M, Milting H, et al. Decreased myocardial chromogranin a expression and colocalization with brain natriuretic peptide during reverse cardiac remodeling after ventricular unloading. J Heart Lung Transplant. 2008;27:442–449. doi: 10.1016/j.healun.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Margulies KB, Matiwala S, Cornejo C, Olsen H, Craven WA, Bednarik D. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ Res. 2005;96:592–599. doi: 10.1161/01.RES.0000159390.03503.c3. [DOI] [PubMed] [Google Scholar]
- 42.Drakos SG, Kfoury AG, Hammond EH, et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol. 2010;56:382–391. doi: 10.1016/j.jacc.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann J, Ciechanover A, Lerman LO, Lerman A. The ubiquitin-proteasome system in cardiovascular diseases-a hypothesis extended. Cardiovasc Res. 2004;61:11–21. doi: 10.1016/j.cardiores.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Klotz S, Foronjy RF, Dickstein ML, et al. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation. 2005;112:364–374. doi: 10.1161/CIRCULATIONAHA.104.515106. [DOI] [PubMed] [Google Scholar]
- 46.Greenberg B, Quinones MA, Koilpillai C, et al. Effects of Long-term Enalapril Therapy on Cardiac Structure and Function in Patients With Left Ventricular Dysfunction : Results of the SOLVD Echocardiography Substudy. Circulation. 1995;91:2573–2581. doi: 10.1161/01.cir.91.10.2573. [DOI] [PubMed] [Google Scholar]
- 47.van Krimpen C, Smits JF, Cleutjens JP, et al. DNA synthesis in the non-infarcted cardiac interstitium after left coronary artery ligation in the rat: effects of captopril. J Mol Cell Cardiol. 1991;23:1245–1253. doi: 10.1016/0022-2828(91)90082-w. [DOI] [PubMed] [Google Scholar]
- 48.Lisy O, Redfield MM, Jovanovic S, et al. Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function In vivo. Circulation. 2000;102:338–343. doi: 10.1161/01.cir.102.3.338. [DOI] [PubMed] [Google Scholar]
- 49.Lisy O, Redfield MM, Schirger JA, Burnett JC., Jr Atrial BNP endocrine function during chronic unloading of the normal canine heart. Am J Physiol Regul Integr Comp Physiol. 2005;288:R158–R162. doi: 10.1152/ajpregu.00444.2004. [DOI] [PubMed] [Google Scholar]
- 50.Tevaearai HT, Walton GB, Eckhart AD, Keys JR, Koch WJ. Heterotopic transplantation as a model to study functional recovery of unloaded failing hearts. J Thorac Cardiovasc Surg. 2002;124:1149–1156. doi: 10.1067/mtc.2002.127315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recovered cohort. Gene expression changes in the beta-integrin signaling pathway in the recovered group. Gene expression changes were significantly different at the receptor level (beta-integrins). The differential expression of beta-integrin receptors is associated with the differential expression of genes distal to the receptor, including vinculin (downregulated in the recovered group, where green indicates downregulation after VAD), a gene previously shown to play a role in heart failure, and the Rho family member cdc42. (Birks et al, 2005).
Heart failure and hypertrophy are associated with increased expression of ANP, BNP and Chromogranin A due to tensile stretch during cardiac dilatation in the heart as well as in the blood. After mechanical support ANP and BNP levels decrease in both in the heart and in the blood. In contrast, chromogranin A only decreases in the heart. (modified from Wohlschlaeger et al. 2008)