Abstract
Inflammation is an underlying feature of a variety of human diseases. An important manifestation of this pathophysiological response is microvascular dysfunction, which includes the activation of vascular endothelial cells, and circulating leucocytes and platelets. While endothelial cells and leucocytes are widely accepted as critical players in the microvascular alterations induced by inflammation, recent attention has focused on the modulatory role of platelets, which act both as effector and target cells in inflamed microvessels. Evidence is presented to demonstrate the capacity for ‘cross-talk’ between platelets and other cells (endothelial cells, leucocytes) that contribute to an inflammatory response, and to illustrate the pathophysiological consequences of these interactions of platelets with other cells within the microvasculature.
Neil Granger is currently Boyd Professor and Head of the Department of Molecular and Cellular Physiology at LSUHSC-Shreveport. He is a leading expert on microvascular responses to inflammatory stimuli, in particular cardiovascular risk factors. He has served as President of the Microcirculatory Society, and the American Physiological Society. Karen Stokes received her PhD from Trinity College, Dublin, and did her post-doctoral training under Neil Granger. She is currently an Assistant Professor. Her major focus is the impact of cytomegalovirus on the microvasculature. Both authors employ intravital videomicroscopy to study arteriolar vasodilatation, leukocyte and platelet recruitment in postcapillary venules, and thrombosis.
 |
Introduction
It is now well recognized that inflammation is an underlying feature of a variety of diseases that are associated with significant morbidity and mortality. Cancer, sickle cell disease, atherosclerosis, Alzheimer's disease and other conditions have not been traditionally classified as inflammatory diseases; however there is mounting evidence that suggests a role for inflammation in their initiation and/or progression. Inflammatory conditions exhibit several characteristic responses of the microvasculature that allow the affected tissues to mount an inflammatory response, but may also lead to tissue injury and organ dysfunction. For example, impaired blood flow regulation, the recruitment of inflammatory cells, oxidative stress, and enhanced protein and water extravasation are often detected in inflamed tissue. While these responses have been linked to endothelial cell dysfunction caused by the adhesion and activation of leucocytes in the microvasculature, there is a large and growing body of evidence that implicates platelets and their activation products in the altered microvascular function that accompanies an inflammatory response (Rhodin et al. 2003; Gavins et al. 2007; Langer & Gawaz, 2008; Sabrkhany et al. 2010). Platelet adhesion and activation not only account for the increased incidence of thrombosis that is associated with acute and chronic inflammatory conditions, but also intensifies, via contact-dependent and -independent mechanisms, the activation of vascular endothelial cells and leucocytes in inflamed microvessels. This review examines the interactions between platelets, vascular endothelial cell (ECs), and leucocytes during inflammation, and addresses how platelets serve as both target and effector cells in the inflammatory response. Evidence for platelet ‘cross-talk’ in some experimental models of human disease is also summarized.
Crosstalk between platelets, leucocytes and endothelial cells
Platelets as a target in inflammation
As platelets course through the vasculature of inflamed tissue, they are exposed to soluble mediators such as lipid mediators, cytokines and chemokines released by activated leucocytes, ECs and perivascular cells (Fig. 1). These mediators engage with receptors on platelets to elicit an activation response that is characterized by the degranulation of dense granules and/or α-granules, release of platelet products, and mobilization and activation of platelet adhesion molecules. Lipid mediators (e.g. platelet activating factor), cytokines (e.g. interferon-γ (IFN-γ), interleukin-2 (IL-2)) and chemokines (e.g. CXCL12, CCL22) are examples of inflammatory mediators that can activate platelets. The oxidative stress that accompanies inflammation results in phospholipase A2 activation and the generation of PAF and other arachidonic acid metabolites that can also activate platelets. The chemokine receptors CXCR4 and CCR4 are expressed on platelets and when engaged with their chemokine ligands (CXCL12 and CCL22, respectively), platelets express P-selectin and release their own compliment of chemokines and other granular components into extracellular fluid (Gleissner et al. 2008). While some cytokines (e.g. IFN-γ) act on platelets to promote the degranulation of dense granules, others (e.g. IL-2) appear to target platelet α-granules (Li, 2008). The accumulation of immune cells in inflamed tissue can also lead to the generation of adenosine diphosphate (ADP), which activates platelets and promotes the degranulation of both dense granules and α-granules. The ecto-ATPase expressed on the surface of lymphocytes can rapidly convert ATP release from platelets and other blood cells to ADP (Stafford et al. 2003). In addition to soluble inflammatory mediators, microparticles liberated from the plasma membrane of ECs following cytokine activation can also alter platelet function. It is unclear whether this requires physical contact between the cells or simply the generation of platelet agonists, like thrombin (Dignat-George & Boulanger, 2011), and this may depend on the inflammatory stimulus.
Figure 1. A schematic diagram of the ‘cross-talk’ between platelets, vascular endothelium and leucocytes in response to stimuli released during inflammatory conditions.
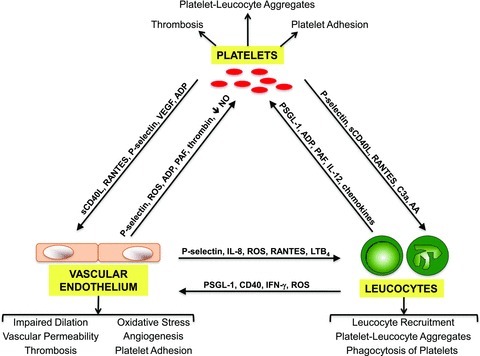
The arrows indicate the direction of the communication, and illustrate both the physical interactions (via cell adhesion molecules, such as P-selectin) and soluble factors (e.g. chemokines, cytokines, etc.) that mediate the cell–cell communication via autocrine and paracrine pathways. Some of the pathological changes resulting from this platelet ‘cross-talk’ are listed below the endothelial cells and leucocytes, and above the platelets. The information portrayed here is discussed throughout the text. AA: arachidonic acid; LTB4: leukotriene B4.
While platelets normally do not physically interact with vascular ECs, activated platelets will bind to the wall of inflamed microvessels by attaching either directly to ECs or to leucocytes that are already adherent on the vessel wall. The absence of platelet adhesion to healthy ECs has been attributed, at least in part, to inhibitory mechanisms involving nitric oxide (NO), prostacyclin, and adenosine that are normally generated by vascular endothelium. Adenosine diphosphate may accumulate in inflamed tissue either as a result of diminished capillary perfusion or due to inhibition of ectonucleotidase CD39, which is expressed on the surface of ECs and circulating immune cells, where it efficiently hydrolyses extracellular ATP and ADP (both of which stimulate platelet adhesion and aggregation) to AMP and ultimately adenosine (which inhibits platelet adhesion and aggregation) (Robson et al. 2006). Oxidative stress and proinflammatory cytokines (e.g. tumour necrosis factor-α (TNF-α)) downregulate CD39 thereby favoring the accumulation of ADP and resulting in lower adenosine levels (Robson et al. 2006). The excess generation of superoxide in inflamed tissue also results in the inactivation of NO, thereby reducing the levels of this endogenous anti-adhesion molecule. The combined effects of depleting adenosine and NO, with a corresponding accumulation of inflammatory mediators that elicit platelet activation, is likely to lead to an increased expression of adhesion molecules by platelets that enable them to interact with ECs and other circulating blood cells.
According to the classical paradigm of platelet–vessel wall interactions during thrombus formation, vessel injury and exposure of matrix material that underlies the EC layer is required for platelet adhesion. However, it is now recognized that platelets can adhere to the vascular wall in the absence of vessel injury. The EC activation that accompanies inflammation appears to be sufficient, in the presence of platelet activation, to elicit platelet–EC adhesion (May et al. 2008). This phenomenon has been demonstrated in both in vitro (Li et al. 1996; Bombeli et al. 1998) and in vivo (Massberg et al. 1999; Tailor et al. 2005) models of inflammation. Different adhesion glycoprotein-ligand pairs have been implicated in the platelet–EC adhesion associated with acute or chronic models of inflammation, including P-selectin (platelet or EC)–P-selectin glycoprotein ligand-1 (PSGL-1) (EC or platelet), GPIb (platelet)–von Willebrand factor (EC), as well as GPIIb/IIIa (platelet)–fibrinogen-intercellular adhesion molecule-1 (ICAM-1) (EC) interactions (Morrell et al. 2007; May et al. 2008). These molecular interactions allow platelets to roll and firmly adhere to the endothelial cell surface, in a manner similar to leucocyte–EC adhesion.
Platelets as effector cells that amplify the inflammatory response
The binding of platelets to vascular endothelium enables the former to modulate the activation state of the latter (and vice versa). Upon activation, platelets release >300 proteins and small molecules, most of which are biologically active molecules that can influence the function of the vascular wall and circulating immune cells (Coppinger et al. 2004). Many of these molecules, including growth factors, cytokines, chemokines, angiogenic factors, ADP/ATP, and coagulation factors, are preformed and stored in dense bodies or α-granules, for release upon platelet activation. Other bioactive molecules are either synthesized by platelets (e.g. thromboxane, reactive oxygen species (ROS), IL-1β) or shed from the cell surface (CD40 ligand (CD40L), P-selectin) upon activation. When platelets adhere to ECs, the close proximity of the two cells enables the platelets to deposit large quantities of chemokines, cytokines and growth factors (e.g. vascular endothelial growth factor; VEGF) that trigger signal transduction pathways that regulate the metabolic, adhesive and proliferative properties of ECs (Siegel-Axel & Gawaz, 2007). Co-incubation of CD40L+ platelets with EC monolayers results in EC activation, increased expression of EC adhesion molecules, including ICAM-1, enhanced production of IL-8 (a neutrophil chemoattractant), and increased leucocyte–EC adhesion (Danese et al. 2003a). These endothelial responses appear to be dependent on (1) the engagement of platelet CD40L with EC CD40, and (2) secretion of the chemokine, regulated upon activation, normal T cell expressed (RANTES), by platelets (Danese et al. 2003a), exemplifying this platelet–EC ‘cross-talk’(Fig. 1).
CD40L is a membrane glycoprotein in the TNF family that is expressed on platelets and other circulating cells. Platelet CD40L expression is increased during inflammation, as is the plasma concentration of soluble CD40L (sCD40L), 95% of which is shed from the surface of activated platelets (Danese et al. 2003b). Endothelial expression of CD40 is also increased during inflammatory bowel disease (IBD) (Vowinkel et al. 2007a). In addition to inducing an inflammatory phenotype in EC, CD40L+ platelets appear to elicit the expression of tissue factor by engaging with CD40 on ECs (Bavendiek et al. 2002). Soluble CD40L may also bind platelet glycoprotein GPIIb/IIIa, which helps to stabilize the thrombus and activate more platelets (Prasad et al. 2003). Mice that are genetically deficient in either CD40 or CD40L exhibit marked reductions in leucocyte and platelet adhesion in venules inflamed by hypercholesterolaemia (Stokes et al. 2009) or colitis (Vowinkel et al. 2007a). CD40/CD40L also appears to play a role in inflammation-enhanced microvascular thrombosis since CD40L-deficient mice exhibit an attenuated thrombogenic response in arterioles that can be restored to normal after administration of sCD40L (Gavins et al. 2011). Finally, there is evidence that supports a role for platelets and CD40L in the impaired endothelium-dependent vasodilatation that accompanies hypercholesterolaemia-induced microvascular inflammation (Stokes et al. 2006, 2009).
RANTES, a member of the CC-chemokine family, is produced by a variety of cells, but platelets are considered to be the major source in vivo (Aukrust et al. 1998). RANTES induces leucocyte chemotaxis by engaging its receptor (CCR5); however the chemokine promotes leucocyte activation/adhesion through an oligomerization-dependent interaction with EC surface glycosaminoglycans (GAGs) (Appay & Rowland-Jones, 2001). The avidity of RANTES for GAGs ensures that blood cell-derived RANTES is concentrated on the EC surface in inflamed or damaged tissue. RANTES has been implicated in the leucocyte adhesion in cerebral venules of mice with experimental autoimmune encephalitis (dos Santos et al. 2005) and stroke (Terao et al. 2008), as well as in monocyte arrest on cultured ECs (Baltus et al. 2003). CXCL7 and CXCL4 are two other platelet-derived chemokines that have been shown to promote leucocyte recruitment (Gleissner et al. 2008).
There is mounting evidence that platelets may play an important role in activation of the alternative and classical pathways of complement. Complement activation is associated with the generation of C3a and C5a, which are cytokine-like mediators that promote leucocyte recruitment and amplify the inflammatory response via leucocyte receptors (Del Conde et al. 2005; Peerschke et al. 2010) and activation of vascular endothelium (Albrecht et al. 2004). The production and deposition of complement on/by platelets is dependent on (and proportional to) platelet activation (Peerschke et al. 2006). For example, weak activation of platelets by agonists such as ADP supports less complement activation than is induced by thrombin. Furthermore different agonists may induce different pathways of complement. Platelet activation is accompanied by the expression of P-selectin, which is associated with activation of the alternative complement pathway (Del Conde et al. 2005). Classical pathway activation is linked to platelet expression of gC1qR (the complement receptor for C1q) (Peerschke et al. 2006) and secretion of the primary glycosaminoglycan on platelets, chondroitin sulfate (Hamad et al. 2008). Microparticles liberated from activated platelets may also generate/deposit complement components (Peerschke et al. 2010). Activated platelets and platelet microparticles exhibit measurable levels of complement (including C1q, C3b, C5b-9) deposition on the platelet cell membrane, which can attract and activate leucocytes by interacting with complement receptors expressed by leucocytes (Del Conde et al. 2005; Peerschke et al. 2006). Moreover, platelets can be activated by C5b-9 proteins (Sims et al. 1988), suggesting a self-propagating loop between complement and platelet activation. It was recently shown that chondroitin sulfate binds C1q (Hamad et al. 2008). This appears to amplify binding of immune complexes to the activated platelet, and may help explain how arterial thrombotic events are elevated in the immune-altered state of lupus, which is associated with deposition of C1q and C4d on platelets. Whether complement activation by platelets is in part responsible for the role of platelets in other inflammatory and thrombotic diseases remains unclear, although it is plausible because complement activation has been implicated in many of these, for example atherosclerosis (Manthey et al. 2011).
In vitro studies have revealed that, similar to interactions with ECs, leucocytes can roll on and firmly adhere to a layer of immobilized (adherent) platelets (Hammer & Apte, 1992). This results from PSGL-1 on leucocytes binding to P-selectin on activated platelets (rolling), and αMβ2 (CD11b/CD18) on leucocytes interacting with GPIb and/or fibrinogen on platelets (adhesion). CD40 (leucocyte) interactions with CD40L (platelet) may also contribute to the adhesion response (Smyth et al. 2009). In vivo studies of the responses of mesenteric (Salter et al. 2001), cerebral (Ishikawa et al. 2004), and myocardial (Kupatt et al. 2002) microvessels to ischaemia–reperfusion support a role for platelets, acting via P-selectin (or GPIIb/IIIa in heart and mesentery), in the modulation of leucocyte recruitment. The opposite can also occur, i.e. adherent neutrophils can also promote the recruitment of platelets via P-selectin: PSGL-1 and/or GPIbα: αMβ2 binding (Tailor et al. 2005; Vowinkel et al. 2007b). While P-selectin expression is greatly increased in the postischaemic vasculature, this is not observed in thrombocytopenic animals, suggesting that platelets immobilized on the vessel wall may be responsible for the upregulated P-selectin noted following ischaemia–reperfusion. Bone marrow chimeric mice, produced by the transplantation of bone marrow from P-selectin deficient mice into wild-type recipients (or vice versa), have revealed that the relative roles of endothelial vs. platelet P-selectin in the recruitment of leucocytes in venules is model dependent (Tailor et al. 2005).
Inflammation is also associated with the binding of platelets to leucocytes that are circulating in the blood (platelet–leucocyte aggregates, PLAs) (Tailor et al. 2005). The P-selectin-dependent platelet–leucocyte complexes that are observed in inflamed venules may be a precursor of the free-flowing PLAs that are detected in blood of patients with chronic inflammatory diseases. The pathophysiological significance of platelet–leucocyte adhesion remains unclear. However, there is evidence that pretreatment of platelets with pro-inflammatory cytokines (e.g. IL-1β and TNF-α) leads to enhanced leucocyte–platelet interactions (Todoroki et al. 1991), suggesting that leucocytes with attached platelets are primed for adhesion. Furthermore these leucocytes can achieve a more activated state than their platelet-free counterparts. For example, neutrophils and monocytes with attached activated platelets produce more than twice the amount of superoxide than their platelet-free counterparts (Nagata et al. 1993), and P-selectin mediated signalling is critical for this response. A recent report indicates that P-selectin dependent formation of PLAs in the circulation initiates signalling pathways in neutrophils that result in the phagocytosis of the attached platelets (Maugeri et al. 2009), which may represent a clearance programme to minimize the pathological impact of uncontrolled platelet activation. Another example of the consequences of platelet–leucocyte interactions on cell activation comes from a study showing that PAF generation by the combination of platelets and neutrophils is two times higher than that detected in either cell activated separately. However, this amplification of PAF production does not appear to be dependent on cell–cell adhesion and instead relies on transcellular phospholipid metabolism between the two cells (Coeffier et al. 1990).
Platelets also release agents that have the potential to increase or decrease vascular permeability. Exposure of EC monolayers to platelets results in a ‘dose-dependent’ reduction in albumin permeability, with the greatest inhibition of permeability occurring with the highest platelet concentration (Shepard et al. 1989). This barrier protection afforded by platelets is not due to mechanical obstruction of inter-endothelial junctions, but was initially linked to a soluble platelet product, adenosine (Paty et al. 1992). Subsequent work implicated an unidentified protein/protein-associated mediator (Patil et al. 1997), and sphingosine-1-phosphate (S1P) was later shown to be a prime candidate lipid (Schaphorst et al. 2003; Peng et al. 2004) (Fig. 2). Studies of pulmonary vascular permeability in thrombocytopenic sheep are consistent with a role for platelets in the maintenance of vascular integrity (Lo et al. 1988). However, there are reports to the contrary in inflammation. For example, depletion of circulating platelets has been shown to blunt the increased vascular permeability and leucocyte recruitment noted after aseptic cutaneous wounding (Kim et al. 2009). These opposing roles for platelets in endothelial barrier function likely reflect the diverse mediators released by these cells and the intensity of the platelet activation response elicited in different models of inflammation.
Figure 2. A proposed schematic of the opposing roles of platelets in endothelial barrier function.
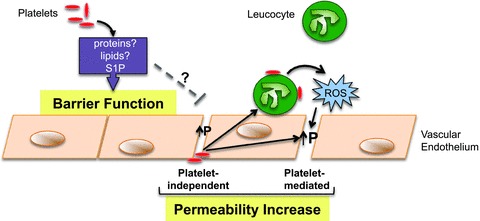
Platelets maintain normal barrier function through the release of soluble factors, most likely proteins, or lipid mediators such as sphingosine-1-phosphate (S1P). However under inflammatory conditions, this protective function of platelets may be overcome. Vascular permeability (P) may be initially increased in a platelet-independent manner, allowing platelet accumulation on the subendothelial matrix. These platelets may become activated and release factors that act directly, or indirectly via recruitment of leucocytes, to further disrupt the barrier function. This latter pathway is mediated via a burst of ROS generation from the leucocytes
It is now recognized that the leucocyte-mediated endothelial barrier dysfunction that accompanies an inflammatory response is dependent on the generation of ROS (by leucocytes), and that recruited and circulating leucocytes that are submaximally activated will not promote vascular leak due to inadequate ROS generation (Zhu et al. 2005; Zhu & He, 2006). Therefore, the role for platelets as mediators of endothelial barrier dysfunction may also depend on whether these cells can activate adherent or circulating leucocytes sufficiently to yield the required level of ROS generation. In some models, an early platelet-independent increase in vascular permeability may result in platelet attachment to the subendothelial matrix and subsequent activation. These platelets may release mediators that directly increase permeability, or the platelets could promote the recruitment of leucocytes that are sufficiently stimulated by platelets to elicit an oxidative burst and increase vascular permeability (Fig. 2). Such a scenario is consistent with the findings reported by He et al. (2006). However, there are also reports suggesting that platelets may exert opposing actions on inflammation and vascular permeability in the tumour microvaculature. Depletion of platelets reduces tumour metastasis presumably by decreasing inflammation, while enhancing the efficacy of chemotherapy by enhancing vascular permeability (Demers et al. 2011). While it may be therapeutically advantageous to transiently increase the vascular leakiness in tumours, such an approach may lead to serious consequences in tissue such as the lung. Therapeutically, perhaps a balance can be struck between enhancing the protective properties of platelets (as was shown for aprotinin treatment against bradykinin-induced oedema; O'Brien et al. 1997), and inhibiting the platelet-promoting pathways of vascular leak (including their cellular targets) in order to achieve maximal protection against platelet-mediated vascular dysfunction.
Angiogenesis is another vascular response to inflammation that can be influenced by platelets. Activated platelets release a variety of angiogenic growth factors, including VEGF, platelet derived growth factor and fibroblast growth factor. In vitro studies have revealed that both platelets and platelet microparticles stimulate EC proliferation and promote the formation of capillary-like structures (Pipili-Synetos et al. 1998). Thrombocytopenia is associated with reduced angiogenic responses to inflammation and/or tissue injury (Kisucka et al. 2006). Platelets appear to preferentially adhere in angiogenic blood vessels and interference with this adhesion process blunts blood vessel proliferation and induces haemorrhage from the angiogenic vessels (Kisucka et al. 2006). The enhanced angiogenic response elicited by erythropoietin treatment in ischaemic tissue also appears to be dependent on platelet adhesion via P-selectin (Kato et al. 2010). The identity of the platelet product that mediates the adhesion-dependent pro-angiogenic effect in these experimental models remains unknown.
Evidence for platelet ‘crosstalk’ in different diseases
From the sections above it is clear that platelets have the potential, through a variety of different mechanisms, to contribute to the pathogenesis of many diseases that have an inflammatory component, some of which were discussed above. Here we provide further discussion of a few of these diseases.
Cardiovascular diseases
Cardiovascular disease is the leading cause of death worldwide. Platelets contribute to the inflammatory process underlying large vessel disease through direct interactions with leucocytes and/or the vessel wall (dependent on P-selectin (Huo et al. 2003), GP1a, GPIIbIIIa (Massberg et al. 2002), and CD40L (Lievens et al. 2010)), and by depositing chemoattractants such as RANTES on the vessel wall (Huo et al. 2003). Activated platelets injected into atherogenic mice interact with the diseased area of the vessel in either ‘leucocyte-free’ form, or bound to leucocytes (primarily monocytes) (Lievens et al. 2010). Thus it is unclear whether the phagocytosis of injected activated platelets by neutrophils that is observed in normal mice occurs during the development of atherosclerosis. Nonetheless, there is evidence in humans that such phagocytosis occurs following a cardiac event (Maugeri et al. 2009). The entire microvasculature, including the vasa vasorum, is exposed to the same cardiovascular risk factors as large vessels, and many of the same mechanisms involved in lesion development underlie the preceding microvascular endothelial dysfunction which is characterized by impaired vasodilatation in arterioles, leucocyte and platelet recruitment in postcapillary venules, increased oxidative stress, and barrier dysfunction. Early during hypercholesterolaemia, neutrophils mediate the platelet recruitment while platelets, acting via P-selectin, reciprocate to recruit more leucocytes (Stokes et al. 2006). These cells also mediate the arteriolar dysfunction, most likely through soluble factors released into the blood and/or from leucocytes and platelets recruited to nearby venules (Kim et al. 2007). CD40L expressed by T lymphocytes (Stokes et al. 2009), and IFN-γ derived from these cells (Stokes et al. 2007), participate in this platelet-dependent inflammatory response, indicating a role for the immune system in the platelet-dependent responses in the early phases of hypercholesterolaemia. These factors are also involved in the NADPH oxidase-dependent oxidative stress induced in arterioles and venules by hypercholesterolaemia. Such a complex network is also in evidence in stroke, where T cells and IFN-γ (primarily from a non-T cell source) mediate not only the leucocyte and platelet recruitment in cerebral postcapillary venules, but also the tissue injury (Yilmaz et al. 2006). RANTES, likely to be derived from platelets, also participates in this brain injury response (Terao et al. 2008), supporting the possibility of platelet ‘cross-talk’ with immune cells and the vascular wall in ischaemic stroke. The microvascular responses to cardiovascular risk factors may predispose organs to worse injury following an ischaemic insult precipitated by thrombus formation or the lodging of emboli in major vessels of the heart or brain. There is also some evidence that the microvasculature is more vulnerable to thrombus development in the presence of risk factors such as hypertension (Senchenkova et al. 2010) and hypercholesterolaemia (Broeders et al. 2002). It remains unclear whether this enhanced vulnerability to microvascular thrombosis involves an altered communication between platelets and other cells involved in thrombogenesis.
Sickle cell disease
Sickle cell disease is a genetic disorder characterized by abnormal haemoglobin, which distorts the shape of red blood cells. These cells become lodged in the microvasculature leading to a painful sickle cell crisis. This vaso-occlusive state involves interactions between the leading edge of neutrophils (that are interacting with the vessel wall) and sickle red blood cells via the adhesion molecule αMβ2 (Hidalgo et al. 2009), supporting an inflammatory component in sickle cell disease. Others have shown that the elevated leucocyte recruitment in sickle cell transgenic mice is accompanied by higher platelet adhesion in postcapillary venules, and both of these are exacerbated by a hypoxic insult, when compared to control mice (Wood et al. 2004b). Endothelial P-selectin largely accounted for the recruitment of both leucocytes and platelets; however, the higher constitutitve expression of P-selectin in sickle cell transgenic mice could be attributed to platelet-associated P-selectin (Wood et al. 2004a), which could serve to promulgate the inflammatory response by interacting with PSGL-1 on leucocytes. In human disease, there is evidence of platelet activation and a corresponding increase in plasma sCD40L, which is likely to result from platelet shedding. The elevated platelet activation state in sickle cell patients may result from excess thrombin generation, which is supported by increased plasma thrombin levels. The clinical relevance of these findings is exemplified by the results of a phase 1 trial wherein the GPIIbIIIa antagonist eptifibatide was shown to be effective in reducing circulating levels of inflammatory mediators and sCD40L, and in attenuating platelet aggregation in sickle cell patients (Lee et al. 2007).
Inflammatory bowel disease
The two major forms of IBD are Crohn's disease and ulcerative colitis. Platelets from these patients exhibit enhanced homotypic and heterotypic aggregation responses (Andoh et al. 2006; Pamuk et al. 2006), and the active phase of IBD is associated with thrombocytosis. The activated platelets from patients with ulcerative colitis enhance the capacity of neutrophils to produce ROS, and this requires P-selectin-dependent adhesion (Suzuki et al. 2001). Both platelets and neutrophils are recruited to postcapillary venules of inflamed colons, with each recruitment process influencing the other (Vowinkel et al. 2007b). A majority of the platelets that accumulate in venules do so via interactions with adherent leucocytes, presumably via platelet P-selectin–leucocyte PSGL-1–endothelial P-selectin (based on studies employing immunoblockade or genetically deficient mice) (Mori et al. 2005; Vowinkel et al. 2007b). P-selectin also appears to mediate the enhanced vascular permeability observed during colitis, suggesting the cell–cell interactions supported by P-selectin are critical to the subsequent endothelial barrier dysfunction. Both CD40 and CD40L have been implicated in the recruitment of platelets and leucocytes in experimental colitis (Vowinkel et al. 2007a). Given that platelets from colitic patients expressed elevated levels of CD40L, and their circulating levels of sCD40L, derived primarily from platelets, is raised (Danese et al. 2003b), it is plausible that both platelet-associated CD40L and platelet-derived soluble CD40L interact with vascular endothelium to induce an inflammatory phenotype. Interestingly, it appears that the interaction between platelets and neutrophils does not end at the vessel wall, because platelets have been observed to infiltrate the colon interstitium and move into the gut lumen along with neutrophils in IBD patients. Whether the extravasation of platelets potentiates the inflammatory response remains unclear, although there is evidence suggesting that this process may exacerbate the fluid secretion and diarrhoea associated with IBD (Weissmuller et al. 2008).
Patients with IBD are at increased risk for thromboembolism, which is one of the causes of death in this population. Thrombi can form both within the bowel and in extra-intestinal tissues. While it is difficult to know whether the inflammation detected in intestinal venules is linked to the extra-intestinal thrombosis, some mediators, such as CD40L, have been linked to both processes, suggesting that platelet-derived sCD40L generated in the gut enters the bloodstream to mediate thrombosis in distant vascular beds (Gavins et al. 2011). The cells/stimuli that are responsible for promoting the platelet release of sCD40L is unclear, but it is likely to be the result of an interaction (and communication) between platelets and other cell types. Although inflammatory cytokines levels are elevated in many disease states, little is known about their role in thrombosis. However, recent work has implicated both IL-1β and TNF-α in the accelerated thrombosis that occurs in extra-intestinal tissues during experimental IBD (Yoshida et al. 2011), supporting a further link between inflammation and platelets in this group of diseases.
Sepsis
During sepsis, there is an initial activation of platelets and heightened coagulation state, which can progress towards reduced platelets counts and exhaustion of the coagulation system with severe sepsis (Mavrommatis et al. 2000). While a role for platelets in sepsis has been proposed, their overall contribution is difficult to define because platelets can have opposing roles in this condition and different strains of bacteria may activate the platelets, while others inhibit it. As one of the first responders to invading bacteria, platelets bind the bacteria primarily through toll-like receptors (TLRs) or a plasma protein bridge to GPIIb-IIIa or GPIbα, and are therefore poised to direct the ensuing immune response (Cox et al. 2011). TLR activation on platelets results in an outpouring of TNF-α (Leslie, 2010) and it promotes the binding of platelets to neutrophils. The neutrophils respond by releasing DNA fibres that are composed of histones and proteases. The sticky DNA fibres, called neutrophil extracellular traps (NETs), ensnare and kill bacteria that gain access to blood (Clark et al. 2007). In addition, sepsis is associated with enhanced thrombosis (Patel et al. 2010) and NETs may also provide a stimulus and scaffold for the formation of a red blood cell rich thrombus (Fuchs et al.), further supporting the link between inflammatory and platelet responses.
During bacterial infection, neutrophils are activated in such a manner as to guide them to tissues that have not been exposed to the bacteria, and this inappropriate neutrophil accumulation in tissues begets the multi-organ injury that leads to the high mortality rate in septic patients. Neutrophils have been shown to promote platelet recruitment in postcapillary venules of the small bowel and liver (Cerwinka et al. 2003; Singer et al. 2006) of mice with endotoxaemia, via a ROS-mediated pathway. Sepsis is also associated with capillary bed plugging that has been linked to platelet adhesion to capillary endothelium via a mechanism that is dependent on P-selectin expression, activation of coagulation, and the production of ROS by NADPH oxidase (Tyml, 2011). This intensive sequestration of platelets in capillaries may contribute to the low platelet count that occurs during severe sepsis. There is also evidence that lymphocytes contribute to the leucocyte and platelet adhesion in postcapillary venules during the early stages of sepsis, but may be protective against the tissue injury (Singer et al. 2008), underlining the different roles each cell type may play in this systemic condition. In contrast, while neutrophil depletion conferred protection against the tissue inflammation and oedema in a murine model of Streptococcus pyrogenes-induced sepsis, induction of thrombocytopenia had no effect despite evidence of microthrombosis (Zhang et al. 2011). Therefore caution must be taken when extrapolating a role for platelets in sepsis induced by one bacterial pathogen to other models of infection.
Cancer
The tumour environment is one of inflammation, in which the tumour cells release chemokines and cytokines that attract inflammatory cells, which in turn release factors that the tumour cell uses to survive, proliferate and metastasize (Coussens & Werb, 2002). Platelets too have been implicated, with thrombocytosis common in a number of cancers, and platelet count inversely correlated with survival (Bambace & Holmes, 2011). Tumour cells secrete factors such as ADP and thrombin that can activate platelets. Both platelets and leucocytes can form aggregates with tumour cells that can facilitate tumour cell attachment to vessels and invasion of the tissue. The selectins, including P-selectin on both ECs and platelets (Kim et al. 1998), and the α4β1 integrin on myeloid cells (Schmid et al. 2011) have been implicated in metastasis. An angiogenic response is necessary to supply the growing tumour mass with oxygen and nutrients and to offer vascular access for further metastasis. Platelets and inflammatory leucocytes can release factors that destabilize existing vessels, promote capillary sprout formation and enhance EC proliferation. In support of these concepts, platelet depletion has been shown to inhibit pulmonary metastasis in a mouse model, and this could be reversed by administration of platelets, but not platelets that had been preincubated with a GPIIbIIIa antibody (Nierodzik et al. 1995). Therapeutic thrombocytopenia may also be beneficial if applied before chemotherapy since platelets normally act to maintain the endothelial barrier function in tumour microvessels, and depleting platelets would enhance vascular leak, thereby enhancing the delivery of chemotherapeutic drugs into the tumour (Demers et al. 2011). Several clinical studies also suggest that anti-platelet therapies may be of benefit as an adjuvant to current anti-cancer treatments (Bambace & Holmes, 2011). Inasmuch as platelets potentiate inflammatory responses and minimize tumour vascular permeability, such an approach may have pluripotent effects. However, a role for platelets cannot be globally applied to all cancers, and a recent study was unable to extrapolate promising in vitro findings to an in vivo murine model of glioblastoma (Brockmann et al. 2011). While these conflicting findings may relate to the specific type and/or stage of cancer, work is ongoing to determine the efficacy of targeting platelets to treat the inflammatory and metastatic components of this disease.
Conclusion
While platelets are best known as primary mediators of haemostasis, there is growing recognition that these anuclear cellular fragments may play an equally important role in inflammation. The widely held view that haemostasis and inflammation are intimately linked pathophysiological processes can, in large part, be explained by the capacity of activated platelets to avidly bind to, and communicate with, other platelets as well as ECs and leucocytes. A consequence of this ‘cross-talk’ between platelets and other cells that participate in the inflammatory response is a more robust reaction of the microvasculature and other tissue components to inflammatory stimuli. The capacity of platelets to both respond and contribute to inflammatory signals places this cell population at the centre of different pathophysiological processes that underlie the tissue injury and organ dysfunction that are associated with a variety of diseases characterized by high morbidity and mortality. The rapidly expanding knowledge about how platelets can function to mediate haemostasis and modulate inflammation may lead to novel and effective therapeutic strategies for the long and growing list of pathological conditions that involve both thrombosis and inflammation. However, consideration must be given to the beneficial actions of platelets that are related to the release of factors such as adenosine and prostacyclin, and the potential negative consequences of depressing platelet function. Similarly, blocking steps in the thrombosis pathway may be deleterious in some conditions. Therapeutic advances are likely to result from a continued effort to reveal the mechanisms that underlie the platelet's capacity to communicate with and excite other circulating blood cells and cellular components of the vascular wall, and how intervening in these pathways alters the protective properties of platelets.
Acknowledgments
The authors are supported by grants from the National Heart Lung and Blood Institute (R01 HL26441, D.N.G.) and the National Centre for Research Resources (P20 RR018724, K.Y.S.).
Glossary
Abbreviations
- AA
arachidonic acid
- CD40L
CD40 ligand
- EC
endothelial cell
- GAG
glycosaminoglycan
- IBD
inflammatory bowel disease
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon-γ
- IL
interleukin
- LTB4
leukotriene B4
- NET
neutrophil extracellular trap
- NO
nitric oxide
- PAF
platelet-activating factor
- PLA
platelet-leucocyte aggregate
- PSGL-1
P-selectin glycoprotein ligand-1
- RANTES
regulated upon activation, normal T cell expressed
- ROS
reactive oxygen species
- S1P
sphingosine-1-phosphate
- TLR
toll-like receptor
- TNF-α
tumour necrosis factor-α
- VEGF
vascular endothelial growth factor.
References
- Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164:849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A, Yoshida T, Yagi Y, Bamba S, Hata K, Tsujikawa T, Kitoh K, Sasaki M, Fujiyama Y. Increased aggregation response of platelets in patients with inflammatory bowel disease. J Gastroenterol. 2006;41:47–54. doi: 10.1007/s00535-005-1721-x. [DOI] [PubMed] [Google Scholar]
- Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, Gullestad L. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation. 1998;97:1136–1143. doi: 10.1161/01.cir.97.12.1136. [DOI] [PubMed] [Google Scholar]
- Baltus T, Weber KS, Johnson Z, Proudfoot AE, Weber C. Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood. 2003;102:1985–1988. doi: 10.1182/blood-2003-04-1175. [DOI] [PubMed] [Google Scholar]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- Bavendiek U, Libby P, Kilbride M, Reynolds R, Mackman N, Schonbeck U. Induction of tissue factor expression in human endothelial cells by CD40 ligand is mediated via activator protein 1, nuclear factor κB, and Egr-1. J Biol Chem. 2002;277:25032–25039. doi: 10.1074/jbc.M204003200. [DOI] [PubMed] [Google Scholar]
- Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), αvβ3 integrin, and GPIbα. J Exp Med. 1998;187:329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann MA, Bender B, Plaxina E, Nolte I, Erber R, Lamszus K, Groden C, Schilling L. Differential effects of tumor-platelet interaction in vitro and in vivo in glioblastoma. J Neurooncol. 2011;105:45–56. doi: 10.1007/s11060-011-0560-2. [DOI] [PubMed] [Google Scholar]
- Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, Egbrink MG. Hypercholesterolemia enhances thromboembolism in arterioles but not venules: complete reversal by L-arginine. Arterioscler Thromb Vasc Biol. 2002;22:680–685. doi: 10.1161/01.atv.0000013287.08141.74. [DOI] [PubMed] [Google Scholar]
- Cerwinka WH, Cooper D, Krieglstein CF, Ross CR, McCord JM, Granger DN. Superoxide mediates endotoxin-induced platelet-endothelial cell adhesion in intestinal venules. Am J Physiol Heart Circ Physiol. 2003;284:H535–541. doi: 10.1152/ajpheart.00311.2002. [DOI] [PubMed] [Google Scholar]
- Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- Coeffier E, Delautier D, Couedic LeJP, Chignard M, Denizot Y, Benveniste J. Cooperation between platelets and neutrophils for paf-acether (platelet-activating factor) formation. J Leukoc Biol. 1990;47:234–243. doi: 10.1002/jlb.47.3.234. [DOI] [PubMed] [Google Scholar]
- Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost. 2011;9:1097–1107. doi: 10.1111/j.1538-7836.2011.04264.x. [DOI] [PubMed] [Google Scholar]
- Danese S, Motte delaC, Sturm A, Vogel JD, West GA, Strong SA, Katz JA, Fiocchi C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003a;124:1249–1264. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003b;52:1435–1441. doi: 10.1136/gut.52.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde IDel, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers M, Ho-Tin-Noe B, Schatzberg D, Yang JJ, Wagner DD. Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 2011;71:1540–1549. doi: 10.1158/0008-5472.CAN-10-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- Santos dosAC, Barsante MM, Arantes RM, Bernard CC, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis – an intravital microscopy study. J Neuroimmunol. 2005;162:122–129. doi: 10.1016/j.jneuroim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavins F, Yilmaz G, Granger DN. The evolving paradigm for blood cell-endothelial cell interactions in the cerebral microcirculation. Microcirculation. 2007;14:667–681. doi: 10.1080/10739680701404903. [DOI] [PubMed] [Google Scholar]
- Gavins FN, Li G, Russell J, Perretti M, Granger DN. Microvascular thrombosis and CD40/CD40L signaling. J Thromb Haemost. 2011;9:574–581. doi: 10.1111/j.1538-7836.2010.04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleissner CA, Hundelshausen vonP, Ley K. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol. 2008;28:1920–1927. doi: 10.1161/ATVBAHA.108.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad OA, Ekdahl KN, Nilsson PH, Andersson J, Magotti P, Lambris JD, Nilsson B. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J Thromb Haemost. 2008;6:1413–1421. doi: 10.1111/j.1538-7836.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer DA, Apte SM. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J. 1992;63:35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Zhang H, Zhu L, Jiang Y, Zhou X. Leukocyte-platelet aggregate adhesion and vascular permeability in intact microvessels: role of activated endothelial cells. Am J Physiol Heart Circ Physiol. 2006;291:H591–599. doi: 10.1152/ajpheart.01228.2005. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004;24:907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- Kato S, Amano H, Ito Y, Eshima K, Aoyama N, Tamaki H, Sakagami H, Satoh Y, Izumi T, Majima M. Effect of erythropoietin on angiogenesis with the increased adhesion of platelets to the microvessels in the hind-limb ischemia model in mice. J Pharmacol Sci. 2010;112:167–175. doi: 10.1254/jphs.09262fp. [DOI] [PubMed] [Google Scholar]
- Kim MH, Carter PR, Harris NR. P-selectin-mediated adhesion impairs endothelium-dependent arteriolar dilation in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2007;292:H632–638. doi: 10.1152/ajpheart.00780.2006. [DOI] [PubMed] [Google Scholar]
- Kim MH, Curry FR, Simon SI. Dynamics of neutrophil extravasation and vascular permeability are uncoupled during aseptic cutaneous wounding. Am J Physiol Cell Physiol. 2009;296:C848–856. doi: 10.1152/ajpcell.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisucka J, Butterfield CE, Duda DG, Eichenberger SC, Saffaripour S, Ware J, Ruggeri ZM, Jain RK, Folkman J, Wagner DD. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci U S A. 2006;103:855–860. doi: 10.1073/pnas.0510412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupatt C, Wichels R, Horstkotte J, Krombach F, Habazettl H, Boekstegers P. Molecular mechanisms of platelet-mediated leukocyte recruitment during myocardial reperfusion. J Leukoc Biol. 2002;72:455–461. [PubMed] [Google Scholar]
- Langer HF, Gawaz M. Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost. 2008;99:480–486. doi: 10.1160/TH07-11-0685. [DOI] [PubMed] [Google Scholar]
- Lee SP, Ataga KI, Zayed M, Manganello JM, Orringer EP, Phillips DR, Parise LV. Phase I study of eptifibatide in patients with sickle cell anaemia. Br J Haematol. 2007;139:612–620. doi: 10.1111/j.1365-2141.2007.06787.x. [DOI] [PubMed] [Google Scholar]
- Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328:562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- Li JM, Podolsky RS, Rohrer MJ, Cutler BS, Massie MT, Barnard MR, Michelson AD. Adhesion of activated platelets to venous endothelial cells is mediated via GPIIb/IIIa. J Surg Res. 1996;61:543–548. doi: 10.1006/jsre.1996.0161. [DOI] [PubMed] [Google Scholar]
- Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, Wijnands E, Goossens P, Kruchten vanR, Thevissen L, Boon L, Flavell RA, Noelle RJ, Gerdes N, Biessen EA, Daemen MJ, Heemskerk JW, Weber C, Lutgens E. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SK, Burhop KE, Kaplan JE, Malik AB. Role of platelets in maintenance of pulmonary vascular permeability to protein. Am J Physiol Heart Circ Physiol. 1988;254:H763–771. doi: 10.1152/ajpheart.1988.254.4.H763. [DOI] [PubMed] [Google Scholar]
- Manthey HD, Thomas AC, Shiels IA, Zernecke A, Woodruff TM, Rolfe B, Taylor SM. Complement C5a inhibition reduces atherosclerosis in ApoE–/– mice. FASEB J. 2011;25:2447–2455. doi: 10.1096/fj.10-174284. [DOI] [PubMed] [Google Scholar]
- Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Enders G, Matos FC, Tomic LI, Leiderer R, Eisenmenger S, Messmer K, Krombach F. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;94:3829–3838. [PubMed] [Google Scholar]
- Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MT, Piccoli A, Totani L, Cianflone D, Maseri A, Manfredi AA. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and β2 integrin-dependent cell clearance program. Blood. 2009;113:5254–5265. doi: 10.1182/blood-2008-09-180794. [DOI] [PubMed] [Google Scholar]
- Mavrommatis AC, Theodoridis T, Orfanidou A, Roussos C, Christopoulou-Kokkinou V, Zakynthinos S. Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit Care Med. 2000;28:451–457. doi: 10.1097/00003246-200002000-00027. [DOI] [PubMed] [Google Scholar]
- May AE, Seizer P, Gawaz M. Platelets: inflammatory firebugs of vascular walls. Arterioscler Thromb Vasc Biol. 2008;28:s5–10. doi: 10.1161/ATVBAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- Mori M, Salter JW, Vowinkel T, Krieglstein CF, Stokes KY, Granger DN. Molecular determinants of the prothrombogenic phenotype assumed by inflamed colonic venules. Am J Physiol Gastrointest Liver Physiol. 2005;288:G920–926. doi: 10.1152/ajpgi.00371.2004. [DOI] [PubMed] [Google Scholar]
- Morrell CN, Sun H, Swaim AM, Baldwin WM., 3rd Platelets an inflammatory force in transplantation. Am J Transplant. 2007;7:2447–2454. doi: 10.1111/j.1600-6143.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- Nagata K, Tsuji T, Todoroki N, Katagiri Y, Tanoue K, Yamazaki H, Hanai N, Irimura T. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62) J Immunol. 1993;151:3267–3273. [PubMed] [Google Scholar]
- Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost. 1995;74:282–290. [PubMed] [Google Scholar]
- O'Brien JG, Battistini B, Farmer P, Johnson RJ, Zaharia F, Plante GE, Sirois P. Aprotinin, an antifibrinolytic drug, attenuates bradykinin-induced permeability in conscious rats via platelets and neutrophils. Can J Physiol Pharmacol. 1997;75:741–749. [PubMed] [Google Scholar]
- Pamuk GE, Vural O, Turgut B, Demir M, Umit H, Tezel A. Increased circulating platelet-neutrophil, platelet-monocyte complexes, and platelet activation in patients with ulcerative colitis: a comparative study. Am J Hematol. 2006;81:753–759. doi: 10.1002/ajh.20655. [DOI] [PubMed] [Google Scholar]
- Patel KN, Soubra SH, Lam FW, Rodriguez MA, Rumbaut RE. Polymicrobial sepsis and endotoxemia promote microvascular thrombosis via distinct mechanisms. J Thromb Haemost. 2010;8:1403–1409. doi: 10.1111/j.1538-7836.2010.03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S, Kaplan JE, Minnear FL. Protein, not adenosine or adenine nucleotides, mediates platelet decrease in endothelial permeability. Am J Physiol Heart Circ Physiol. 1997;273:H2304–2311. doi: 10.1152/ajpheart.1997.273.5.H2304. [DOI] [PubMed] [Google Scholar]
- Paty PS, Sherman PF, Shepard JM, Malik AB, Kaplan JE. Role of adenosine in platelet-mediated reduction in pulmonary vascular permeability. Am J Physiol Heart Circ Physiol. 1992;262:H771–777. doi: 10.1152/ajpheart.1992.262.3.H771. [DOI] [PubMed] [Google Scholar]
- Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke EI, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- Pipili-Synetos E, Papadimitriou E, Maragoudakis ME. Evidence that platelets promote tube formation by endothelial cells on matrigel. Br J Pharmacol. 1998;125:1252–1257. doi: 10.1038/sj.bjp.0702191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KS, Andre P, Yan Y, Phillips DR. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Curr Opin Hematol. 2003;10:356–361. doi: 10.1097/00062752-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Rhodin JA, Thomas TN, Clark L, Garces A, Bryant M. In vivo cerebrovascular actions of amyloid beta-peptides and the protective effect of conjugated estrogens. J Alzheimers Dis. 2003;5:275–286. doi: 10.3233/jad-2003-5403. [DOI] [PubMed] [Google Scholar]
- Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabrkhany S, Griffioen AW, Egbrink MG. The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta. 2010;1815:189–196. doi: 10.1016/j.bbcan.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Salter JW, Krieglstein CF, Issekutz AC, Granger DN. Platelets modulate ischemia/reperfusion-induced leukocyte recruitment in the mesenteric circulation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1432–1439. doi: 10.1152/ajpgi.2001.281.6.G1432. [DOI] [PubMed] [Google Scholar]
- Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F, Garcia JG. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, Acevedo LM, Manglicmot JR, Song X, Wrasidlo W, Blair SL, Ginsberg MH, Cheresh DA, Hirsch E, Field SJ, Varner JA. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchenkova EY, Russell J, Almeida-Paula LD, Harding JW, Granger DN. Angiotensin II-mediated microvascular thrombosis. Hypertension. 2010;56:1089–1095. doi: 10.1161/HYPERTENSIONAHA.110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JM, Moon DG, Sherman PF, Weston LK, Vecchio PJDel, Minnear FL, Malik AB, Kaplan JE. Platelets decrease albumin permeability of pulmonary artery endothelial cell monolayers. Microvasc Res. 1989;37:256–266. doi: 10.1016/0026-2862(89)90044-7. [DOI] [PubMed] [Google Scholar]
- Siegel-Axel DI, Gawaz M. Platelets and endothelial cells. Semin Thromb Hemost. 2007;33:128–135. doi: 10.1055/s-2007-969025. [DOI] [PubMed] [Google Scholar]
- Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263:18205–18212. [PubMed] [Google Scholar]
- Singer G, Stokes KY, Granger DN. Hepatic microcirculation in murine sepsis: role of lymphocytes. Pediatr Surg Int. 2008;24:13–20. doi: 10.1007/s00383-007-2037-0. [DOI] [PubMed] [Google Scholar]
- Singer G, Urakami H, Specian RD, Stokes KY, Granger DN. Platelet recruitment in the murine hepatic microvasculature during experimental sepsis: role of neutrophils. Microcirculation. 2006;13:89–97. doi: 10.1080/10739680500466343. [DOI] [PubMed] [Google Scholar]
- Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- Stafford NP, Pink AE, White AE, Glenn JR, Heptinstall S. Mechanisms involved in adenosine triphosphate-induced platelet aggregation in whole blood. Arterioscler Thromb Vasc Biol. 2003;23:1928–1933. doi: 10.1161/01.ATV.0000089330.88461.D6. [DOI] [PubMed] [Google Scholar]
- Stokes KY, Calahan L, Hamric CM, Russell JM, Granger DN. CD40/CD40L contributes to hypercholesterolemia-induced microvascular inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H689–697. doi: 10.1152/ajpheart.00962.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KY, Calahan L, Russell JM, Gurwara S, Granger DN. Role of platelets in hypercholesterolemia-induced leukocyte recruitment and arteriolar dysfunction. Microcirculation. 2006;13:377–388. doi: 10.1080/10739680600745877. [DOI] [PubMed] [Google Scholar]
- Stokes KY, Gurwara S, Granger DN. T-cell derived interferon-gamma contributes to arteriolar dysfunction during acute hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2007;27:1998–2004. doi: 10.1161/ATVBAHA.107.146449. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugimura K, Hasegawa K, Yoshida K, Suzuki A, Ishizuka K, Ohtsuka K, Honma T, Narisawa R, Asakura H. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301–1306. doi: 10.1080/003655201317097164. [DOI] [PubMed] [Google Scholar]
- Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirculation. 2005;12:275–285. doi: 10.1080/10739680590925691. [DOI] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todoroki N, Watanabe Y, Akaike T, Katagiri Y, Tanoue K, Yamazaki H, Tsuji T, Toyoshima S, Osawa T. Enhancement by IL-1β and IFN-γ of platelet activation: adhesion to leukocytes via GMP-140/PADGEM protein (CD62) Biochem Biophys Res Commun. 1991;179:756–761. doi: 10.1016/0006-291x(91)91881-c. [DOI] [PubMed] [Google Scholar]
- Tyml K. Critical role for oxidative stress, platelets, and coagulation in capillary blood flow impairment in sepsis. Microcirculation. 2011;18:152–162. doi: 10.1111/j.1549-8719.2010.00080.x. [DOI] [PubMed] [Google Scholar]
- Vowinkel T, Anthoni C, Wood KC, Stokes KY, Russell J, Gray L, Bharwani S, Senninger N, Alexander JS, Krieglstein CF, Grisham MB, Granger DN. CD40-CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 2007a;132:955–965. doi: 10.1053/j.gastro.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Vowinkel T, Wood KC, Stokes KY, Russell J, Tailor A, Anthoni C, Senninger N, Krieglstein CF, Granger DN. Mechanisms of platelet and leukocyte recruitment in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007b;293:G1054–1060. doi: 10.1152/ajpgi.00350.2007. [DOI] [PubMed] [Google Scholar]
- Weissmuller T, Campbell EL, Rosenberger P, Scully M, Beck PL, Furuta GT, Colgan SP. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J Clin Invest. 2008;118:3682–3692. doi: 10.1172/JCI35874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K, Russell J, Hebbel RP, Granger DN. Differential expression of E- and P-selectin in the microvasculature of sickle cell transgenic mice. Microcirculation. 2004a;11:377–385. doi: 10.1080/10739680490437559. [DOI] [PubMed] [Google Scholar]
- Wood KC, Hebbel RP, Granger DN. Endothelial cell P-selectin mediates a proinflammatory and prothrombogenic phenotype in cerebral venules of sickle cell transgenic mice. Am J Physiol Heart Circ Physiol. 2004b;286:H1608–1614. doi: 10.1152/ajpheart.01056.2003. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Yilmaz CE, Granger DN. Role of tumor necrosis factor-α in the extraintestinal thrombosis associated with colonic inflammation. Inflamm Bowel Dis. 2011;17:2217–2223. doi: 10.1002/ibd.21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Rahman M, Herwald H, Thorlacius H. Streptococcal M1 protein-induced lung injury is independent of platelets in mice. Shock. 2011;35:86–91. doi: 10.1097/SHK.0b013e3181ea4476. [DOI] [PubMed] [Google Scholar]
- Zhu L, Castranova V, He P. fMLP-stimulated neutrophils increase endothelial [Ca2+]i and microvessel permeability in the absence of adhesion: role of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2005;288:H1331–1338. doi: 10.1152/ajpheart.00802.2004. [DOI] [PubMed] [Google Scholar]
- Zhu L, He P. fMLP-stimulated release of reactive oxygen species from adherent leukocytes increases microvessel permeability. Am J Physiol Heart Circ Physiol. 2006;290:H365–372. doi: 10.1152/ajpheart.00812.2005. [DOI] [PubMed] [Google Scholar]


