Abstract
Intestinal barrier dysfunction is a main feature of the inflammatory bowel diseases (IBD), Crohn's disease and ulcerative colitis. Leak flux diarrhoea and a facilitated uptake of noxious antigens are the two consequences resulting from an impaired epithelial barrier. Barrier perturbations in IBD comprise alterations in epithelial tight junctions (TJ), i.e. a reduced number of horizontal TJ strands and an altered TJ protein expression and subcellular distribution. Moreover, increased incidence of apoptotic events as well as erosions and ulcerations can add to that leakiness. These barrier defects are attributed to enhanced activity of pro-inflammatory cytokines like TNFα, INFγ, IL-1β and IL-13, which are highly expressed in the chronically inflamed intestine. Although the aetiology of IBD is far from being clear, chronic inflammation is believed to result from an inadequate immune response as a consequence of genetic predisposition as well as changes in, and altered responses to, the intestinal microbiota. On the other hand, an insufficient mucosal response to bacterial stimuli results in an insufficient immune response towards intestinal pathogens. However, detailed characterization of barrier defects offers the opportunity to consider and test therapeutic interventions. Beside cytokine antagonists, different plant compounds and probiotics have been shown to stabilize the barrier function by affecting TJ protein expression and distribution.
Professor Jörg D. Schulzke (MD) and Nina A. Hering (PhD) work together at the Section of Nutrition of the GI Department and the Institute of Clinical Physiology at the Charité, Berlin. Their research focuses on the pathophysiology of the GI barrier function in inflammatory bowel diseases, coeliac disease and infectious diseases. One of the central topics is regulation of tight junction proteins and the therapeutic action of anti-inflammatory drugs, phytopharmaceuticals, probiotics and nutrition.
 |
Introduction
Patients with inflammatory bowel disease (IBD) including Crohn's disease (CD), ulcerative colitis (UC), and microscopic colitis suffer from inflammation-induced leak flux diarrhoea. This type of diarrhoea is caused by a passive loss of ions and water from the circulation into the intestinal lumen as a result of an impaired intestinal barrier. On the other hand, enhanced uptake of noxious antigens from the gut lumen enhances mucosal and systemic inflammatory processes as a permissive factor for IBD. Because of this circulus vitiosus, susceptibility of the barrier already increases with small alteration.
Numerous studies have been conducted to identify and characterize the mechanisms of barrier disruption in IBD and have highlighted the major role of the epithelial tight junction (TJ) in this respect. Having the multifactorial nature of IBD pathogenesis in mind, this review focuses on the role of cytokines, the relevance of genetic dispositions and the influence of the intestinal microbiota on intestinal barrier function with special attention on TJs.
Tight junctions – determinants of intestinal barrier function
The intestinal barrier is established by a polarized monolayer of epithelial columnar cells, which are connected by intercellular junctions. From these the TJ is the structural feature which helps to maintain a strict and regulated separation of the body against the luminal content of the gut. This separation is also necessary to prevent back leakage of absorbed ions and nutrients or to avoid the entry of luminal antigens and microorganisms. The TJ can be recognized in freeze fracture electron microscopy as a complex network of continuous strands built up by intramembranous particles (Staehelin, 1973). Structurally, the TJ is composed of four different types of integral membrane proteins: occludin (Furuse et al. 1993), the claudins (Tsukita et al. 2001), tricellulin (Ikenouchi et al. 2005) and junctional adhesion molecule (Martin-Padura et al. 1998). Claudins in particular play a critical role in barrier function. In over-expression, silencing or knock-out approaches several of the 27 claudins so far described in mammals were shown to have sealing or pore-forming properties within the gastrointestinal tract (Van Itallie & Anderson, 2006). While claudin-1, −3, −4, −5 and −8 have sealing functions (Van Itallie et al. 2001; Furuse et al. 2002; Amasheh et al. 2005; Amasheh et al. 2009b; Milatz et al. 2010), others such as claudin-2, −10b or −15 act as paracellular channels and promote a charge-selective passage of small ions (Amasheh et al. 2002; Gunzel et al. 2009; Tamura et al. 2011). In addition, claudin-2 was shown to act as a paracellular water channel (Rosenthal et al. 2010). Composition, structure and permeability of the TJ are tissue-specific, and strictly regulated by physiological as well as pathophysiological stimuli including inflammatory regulators.
Barrier defects in IBD
The importance of an intact epithelial TJ becomes evident in IBD. Clinical symptoms of IBD result from intestinal inflammation and subsequent epithelial dysfunction including impaired absorptive functions and barrier defects (leak flux diarrhoea). Investigations on sigmoid colon biopsies from patients with mild CD revealed impaired TJ complexity, characterized by a decreased number of TJ strands, reduced depth of the main TJ meshwork and more strand breaks (Fig. 1). In addition, expression of sealing claudin-3, −5 and −8 and occludin was diminished, while pore-forming claudin-2 was up-regulated. Furthermore, claudin-5 and −8 were distributed off the TJ (Zeissig et al. 2007). Similar changes were observed in UC, including down-regulation of occludin, claudin-1 and −4 and up-regulation of the pore-forming claudin-2 (Heller et al. 2005). Structurally, strand count and meshwork depth were reduced (Schmitz et al. 1999a; Heller et al. 2005).
Figure 1. Freeze fracture electron microscopy of TJ strands from control (A) and mild to moderately inflamed Crohn's disease (B and C).
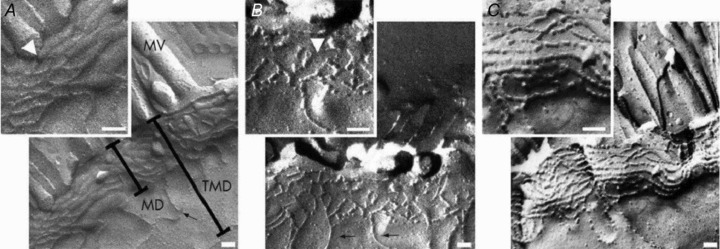
Controls show continuous TJ strands, while in Crohn's disease a reduced number of TJ strands, frequent strand breaks (arrowhead in B) and a discontinuous TJ network are obvious (C). Scale bars indicate 100 nm; MV, micro villi; MD, main TJ meshwork depth without aberrant strands; TMD, total TJ meshwork depth from the most apical to the most basal strand including aberrant strands. Reproduced from Zeissig et al. 2007.
In addition, leaks resulting from epithelial apoptosis (Gitter et al. 2000; Bojarski et al. 2001) were found to be increased in CD and UC (Heller et al. 2005; Zeissig et al. 2007). Consequently, microerosions due to arrested restitution caused by the Th2 cytokine interleukin-13 (IL-13) are an early event in UC (Heller et al. 2005). Recently, epithelial-mesenchymal-transition (EMT) has been identified as an important barrier feature in coeliac disease and could be equally important in IBD. EMT can change cell polarization within the epithelium, thereby intensifying endocytotic antigen uptake from the lumen with induction of a parallel subcellular redistribution of TJ proteins (Schumann et al. 2012).
Thus, gross lesions (erosions), apoptotic leaks, changes in TJ structure or composition as well as altered endo-/transcytosis are important barrier pathomechanisms, which enable noxious antigens derived for example from food or microorganisms to penetrate the mucosal barrier to a significant extent. Enhanced endocytosis in IBD could also be a mechanism by which bacterial translocation is enhanced in IBD. Moreover, our group has gained evidence that changes in tricellulin contribute to enhanced macromolecular passage in UC (Krug et al. 2010), as this TJ protein normally restricts macromolecular permeability in tricellular TJs by tightening the central tube (Krug et al. 2009). The main aspects of barrier dysfunction in IBD are summarized in Table 1.
Table 1.
Aspects of barrier dysfunction in IBD
| Inflammatory bowel diseases | Cytokine profile | Epithelial resistance (Ω cm2) | Tight junction proteins | Other pathomechanisms | References |
|---|---|---|---|---|---|
| Crohn's disease (sigmoid colon) | TNFα, IFNγ (Th1) | 23 ± 3 (59% of control) (active, mild to moderate inflamed) | Claudin-2 ↑ | Epithelial apoptosis ↑ | Zeissig et al. 2007 |
| Occludin ↓ | |||||
| Claudin-3 ↓ | |||||
| Claudin-5 ↓ and redistributed | |||||
| Claudin-8 ↓ and redistributed | |||||
| Ulcerative colitis (sigmoid colon) | IL-13, TNFα (Th2) | 20 ± 3 (21% of control) (active, mild to moderate inflamed) | Claudin-2 ↑ | Epithelial apoptosis ↑ | Heller et al. 2005; Schmitz et al. 1999a; Krug et al. 2010 |
| Occludin ↓ | |||||
| Claudin-1 ↓ | Ulcerations | ||||
| Claudin-4 ↓ | Microerosions | ||||
| Tricellulin ↓ | |||||
| Collagenous colitis (sigmoid colon) (microscopic colitis) | TNFα, IFNγ (Th1) | 29 ± 2 (23% of control) (macroscopically normal) | Claudin-2 ↑ | Malabsorption | Burgel et al. 2002; Tagkalidis et al. 2007 |
| Claudin-4 ↓ | |||||
| Occludin ↓ |
Regulation: ↓ down, ↑ up.
Role of inflammatory cytokines in barrier disturbance
Pro-inflammatory cytokines play a key role in the induction of barrier defects in IBD. Tumour necrosis factor-α (TNFα) and interferon-γ (IFNγ) are increased in CD (Th1 profile), while in UC the inflammatory response is characterized by an increase in TNFα and IL-13. Interestingly, in cell culture and animal models these cytokines were found to induce comparable barrier defects, as observed in CD or UC, including TJ changes, apoptosis induction and enhanced bacterial translocation (John et al. 2011). Cytokines can affect TJs in two ways, first by expression regulation and second, and perhaps more importantly, by affecting the redistribution processes. For example, TNFα as well as IL-13 can increase claudin 2 protein expression in HT-29/B6 cells. In the case of TNFα, this was induced via the phosphatidylinositol-3-kinase pathway (Fig. 2) (Mankertz et al. 2009). Exposure of native rat colon to TNFα and IFNγ revealed up-regulation of pore-forming claudin 2 and down-regulation of barrier-forming claudin-1, −5 and −7 (Amasheh et al. 2009a). Beside pro-inflammatory cytokines, we recently found the transforming growth factor-β (TGFβ), which is assumed to be rather protective in IBD (Monteleone et al. 2008), to increase claudin-4 expression by stimulating claudin-4 promoter activity directly (Hering et al. 2011a). Myosin light chain kinase (MLCK) is one important regulatory element of TJ protein regulation which was found to be affected in the intestine of IBD patients (Blair et al. 2006). Phosphorylation of MLCK leads to reorganization of perijunctional F-actin, and consequently to redistribution of TJ proteins from the tight junction domain of the enterocyte towards intracellular compartments (Shen et al. 2006). Recently, MLCK-dependent zonula occludens-1 (ZO 1) exchange was suggested to be critical for this process (Yu et al. 2010). In addition, MLCK activation stimulates IL-13 synthesis, which in turn increases claudin 2 expression (Weber et al. 2010). TNFα and interleukin-1β enhance TJ permeability by stimulating MLCK gene expression via NFκB in Caco 2 cells (Ye & Ma, 2008; Al-Sadi et al. 2011). Structural and functional TJ regulation is also affected by MLCK-induced caveolin-1-dependent endocytosis of occludin (Marchiando et al. 2010). In contrast, IFNγ was found to induce TJ redistribution via a Rho/ROCK signalling-dependent macropinocytosis-like mechanism (Bruewer et al. 2005). As already mentioned, EMT has to be considered as a further mechanism, by which TJ proteins are re-distributed due to a loss of cell polarity. In coeliac disease TJ assembly and expression was affected by dysregulated cell polarity proteins Par 3 and PP 1 (Schumann et al. 2012). How far this also occurs in IBD patients and to what extent cytokines could regulate EMT might be elucidated in forthcoming studies.
Figure 2. Involvement of phosphatidylinositol-3-kinase signalling (PI3K) in TNFα-induced claudin 2 up-regulation in HT-29/B6 cells.
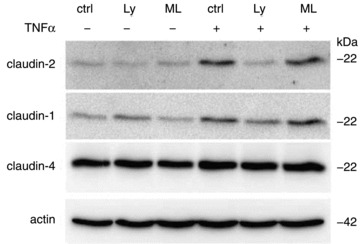
Western blot analyses represent expression level without treatment (ctrl), after TNFα treatment and after pre-incubation with PI3K inhibitor LY294002 (Ly) or MLCK inhibitor ML-7 (ML). Inhibition of PI3K prevents TNFα-induced claudin 2 up-regulation, while ML 7 has no influence. Reproduced from Mankertz et al. 2009 with kind permission from Springer Science+Business Media; copyright ©2009 Springer Verlag.
Finally, TNFα and IL-13 can also enhance apoptotic events within the intestinal epithelium (Schmitz et al. 1999b; Heller et al. 2005), at which the contribution of apoptosis to leakiness might depend on the restitution capabilities of the harmed epithelium (Marchiando et al. 2011). Furthermore, IFNγ, TNFα as well as IL-13 were observed to increase bacterial translocation in cell culture approaches (Clark et al. 2003, 2005; Troeger et al. 2007).
Aetiology of barrier dysfunction in IBD
However, despite extensive investigations and characterization of barrier determinants the aetiology of IBD is far from being clear. That is in part due to the fact that it seems to involve a multifactorial interplay of different factors. In particular, genetic disposition and changes to the intestinal microbiota are crucially linked to each other and may subsequently favour an inadequate immune response.
Genetic predisposition has been increasingly discussed as being of central importance over the last few years. For example, single-nucleotide polymorphisms (SNPs) in the genes encoding for PAR 3 and MAGI2, which both play a role in TJ assembly, were found to be associated with coeliac diseases and UC (Wapenaar et al. 2008). Furthermore, HNF4α, encoding the transcription factor hepatocyte nuclear factor 4α, was identified as susceptibility loci in a genome-wide association study on UC (Barrett et al. 2009). With regard to barrier function, this seems to be important as HNF4α is involved in transcriptional regulation of TJs, adherens junctions, and desmosome expression (Battle et al. 2006). Claudin-15 was identified as a direct target of HNF4α (Darsigny et al. 2009). In mice, conditional intestinal HNF4α deletion was associated with increased permeability, a more severe course of dextran sulfate sodium (DSS)-induced colitis (Ahn et al. 2008) and claudin-15 down-regulation (Darsigny et al. 2009), which was recently described to be linked to sodium deficiency and glucose malabsorption (Tamura et al. 2011). Moreover, claudin 2 gene expression is known to be controlled in a cooperative manner by caudal-related homeobox (Cdx) proteins, GATA 4 and HNF1α (Sakaguchi et al. 2002; Escaffit et al. 2005), which is positively regulated by HNF4α (Eeckhoute et al. 2004).
Mutations in the caspase-activated recruitment domain (CARD15) gene were assumed to be one risk factor for the development of CD. Beside other tissues, the CARD15 gene product NOD2 is expressed in ileal crypts, where it senses bacterial components (Lala et al. 2003). CARD15 mutations were found to be associated with an elevated mucosal permeability (Buhner et al. 2006; D'Incàet al. 2006). Although the mechanism has not been identified so far, CARD15 gene expression was shown to be up-regulated by TNFα and IFNγ (Rosenstiel et al. 2003). Moreover, NOD2-signalling defects result in reduced expression of antimicrobial defensins (Wehkamp et al. 2004; Voss et al. 2006). This is assumed to be critical for the initiation of barrier defects (Rosenstiel et al. 2003; Wehkamp et al. 2004), as bacterial pathogens can gain direct access to the mucosal barrier and trigger inflammation or direct barrier disturbances, e.g. by induction of epithelial apoptosis (Nielsen et al. 2011), focal leaks (Troeger et al. 2007), and TJ disruption (Bucker et al. 2009; Hering et al. 2011b).
Barrier disturbances are also caused by alterations in the composition of gut microbiota, resulting in an imbalance of protective commensals and potential pathogens in IBD (Sepehri et al. 2007). This becomes evident from different mouse models. For example, IL 2- or IL-10-deficient mice, which suffer from spontaneous chronic intestinal inflammation (Elson et al. 2005), do not develop symptoms under germ-free conditions (Sellon et al. 1998; Dieleman et al. 2004). Similar effects were observed in DSS-treated mice, which had milder symptoms when kept germ-free (Hudcovic et al. 2001). As a further consequence of altered microbial complexity, production or availability of different bacteria-derived metabolites, e.g. the short chain fatty acid (SCFA) butyrate is reduced in IBD (Chapman et al. 1994). Butyrate not only serves as an energy source for the enterocytes, but also exerts anti-inflammatory properties (Segain et al. 2000; Tedelind et al. 2007). In addition, reduced production of butyrate may also lead to less activation of SCFA-coupled electroneutral NaCl absorption in the colon and thus limit the activity of a transport mechanism that reduces luminal fluid load in the colon (Krishnan et al. 1999). Furthermore, butyrate was recently shown to reduce bacterial translocation in a cell culture model (Lewis et al. 2010) and our group has obtained experimental evidence for a barrier-regulating function of claudin 2 (Plöger et al. 2010).
Therapeutic approaches
Although IBD cannot be cured so far, the increasing understanding of inherent pathomechanisms offers the possibility of specific therapeutic interventions, which can reduce symptoms by strengthening epithelial barrier function.
Counteracting the inflammatory action of cytokines by application of cytokine antagonist is one important option. TNFα antibody therapy revealed reduction of epithelial apoptosis to normal levels in CD patients (Zeissig et al. 2004). Mucosal healing was reported in UC patients as well (Afif et al. 2009). Although first investigations revealed no change in occludin, and claudin 1 and 4 after TNFα antibody treatment (Zeissig et al. 2004), effects on claudin 2 seem likely, perhaps after a longer period of therapy. Interestingly, the expression of TNFα and IL-1β was found to be inhibited by zinc. The zinc finger protein A20 is directly involved in the negative feedback regulation of NFκB signalling (Prasad et al. 2004). Zinc therapy decreased mucosal permeability in CD patients (Sturniolo et al. 2001). The impact of zinc on TJ composition and structure has not been studied so far in humans, but up-regulation of occludin and ZO 1 was reported in animal studies (Zhang & Guo, 2009).
Direct influence on TJ protein expression was observed from several plant components, such as the flavonoid quercetin or the isoquinoline alkaloid berberine. Quercetin found in different fruits or onions enhances barrier function in Caco 2 cells by up-regulating claudin 4 expression (Amasheh et al. 2008). Berberine as used in traditional medicine has anti-inflammatory effects in experimental colitis in rats (Zhou & Mineshita, 2000) and prevented barrier dysfunction induced by TNFα and INFγ in a cell culture model, e.g. claudin 1 distribution (Fig. 3) (Amasheh et al. 2010). Furthermore, several probiotic microorganisms are known to influence the expression of pro-inflammatory cytokines (Roselli et al. 2006) or to influence TJ expression and composition directly. For example, redistribution and reduction of ZO 1, occludin and claudin-1, −3, −4 and −5 could be prevented by the probiotic mixture VSL#3 in a murine model of colitis (Mennigen et al. 2009). E. coli Nissle 1917, the active compound of the preparation Mutaflor, is proven to be effective in maintaining remission in UC (Rembacken et al. 1999), enhances mucosal integrity (Ukena et al. 2007), and its effect on barrier-relevant TJ proteins is presently under investigation by our group with experimental evidence for a TJ effect (Hering et al. 2011c).
Figure 3. Berberine protects claudin 1 from TNFα-induced disassembly from the TJ.
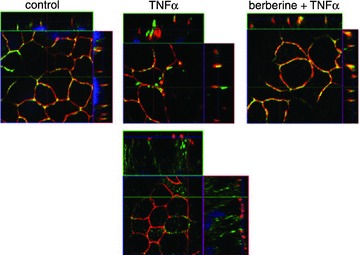
Immunofluorescent staining and subsequent confocal laser-scanning microscopy of HT-29/B6 cells shows claudin 1 in green and TJ marker ZO 1 in red. While claudin 1 is redistributed off the TJ to intracellular compartments after TNFα treatment, pre-treatment with berberine reveals colocalization of claudin 1 and ZO 1 (merge), indicated by yellow staining. Reproduced from Amasheh et al. 2010.
Conclusion
Disturbed barrier function is a key feature in IBD. The role of the microbiota and genetic disposition has been discussed to be pivotal for the aetiology of IBD but the formal pathogenesis remains to be elucidated. Misleading bacterial signalling results in a chronic inflammatory state, which can lead to barrier dysfunction represented, for example, by alterations in epithelial TJs. Genetic predisposition may influence the reactivity of the mucosal immune system and of the epithelium in IBD. In particular, the identification of single TJ components such as the claudins or tricellulin and our growing understanding of their functional nature and regulation have provided more insight into these mechanisms and will allow us to identify therapeutic interventions. The schematic diagram in Fig. 4 summarizes the main aspects of inflammatory barrier dysfunction, including our present understanding of the underlying pathomechanisms and influence of therapeutic components.
Figure 4. Main features of inflammatory barrier dysfunction comprise TJ alterations, e.g. subcellular redistribution, altered protein expression and composition as well as epithelial apoptosis.
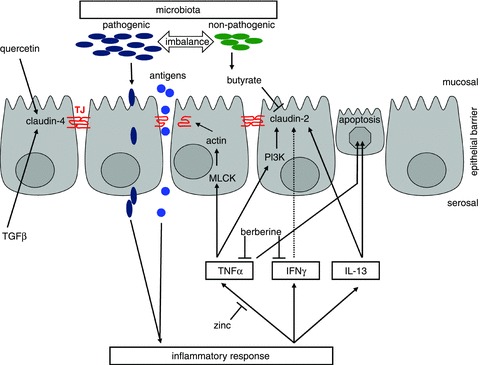
As key inductors, pro-inflammatory cytokines (TNFα, INFγ, IL-13) can trigger these changes by stimulating signalling cascades (e.g. MLCK, PI3K). Inflammation in turn is suggested to be induced or enhanced by the passage of luminal antigens or pathogens. Beside other factors, this can result from an imbalance or altered composition of the intestinal microbiota. With respect to therapeutic intervention, bacterial products (butyrate), food components (quercetin) or cytokines (TGFβ) are known to influence TJ protein expression but the inflammatory cascade can also be blocked, attenuating intestinal inflammation (berberine, zinc).
Glossary
Abbreviations
- CD
Crohn's disease
- DSS
dextran sulfate sodium
- EMT
epithelial-mesenchymal-transition
- HNF4α
hepatocyte nuclear factor 4 α
- IBD
inflammatory bowel disease
- IFNγ
interferon-γ
- IL
interleukin
- MLCK
myosin light chain kinase
- SCFA
short chain fatty acid
- TGFβ
transforming growth factor-β
- TJ
tight junction
- TNFα
tumour necrosis factor-α
- UC
ulcerative colitis
- ZO
zonula occludens
References
- Afif W, Leighton JA, Hanauer SB, Loftus EV, Jr, Faubion WA, Pardi DS, Tremaine WJ, Kane SV, Bruining DH, Cohen RD, Rubin DT, Hanson KA, Sandborn WJ. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis. 2009;15:1302–1307. doi: 10.1002/ibd.20924. [DOI] [PubMed] [Google Scholar]
- Ahn SH, Shah YM, Inoue J, Morimura K, Kim I, Yim S, Lambert G, Kurotani R, Nagashima K, Gonzalez FJ, Inoue Y. Hepatocyte nuclear factor 4α in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:908–920. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R, Ye D, Said HM, Ma TY. Cellular and molecular mechanism of interleukin-1β modulation of Caco2 intestinal epithelial tight junction barrier. J Cell Mol Med. 2011;15:970–982. doi: 10.1111/j.1582-4934.2010.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFα-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFκB signaling. J Cell Sci. 2010;123:4145–4155. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- Amasheh M, Grotjohann I, Amasheh S, Fromm A, Soderholm JD, Zeitz M, Fromm M, Schulzke JD. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009a;44:1226–1235. doi: 10.1080/00365520903131973. [DOI] [PubMed] [Google Scholar]
- Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Quercetin enhances epithelial barrier function and increases claudin 4 expression in Caco 2 cells. J Nutr. 2008;138:1067–1073. doi: 10.1093/jn/138.6.1067. [DOI] [PubMed] [Google Scholar]
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin 2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M. Na+ absorption defends from paracellular back-leakage by claudin 8 upregulation. Biochem Biophys Res Commun. 2009b;378:45–50. doi: 10.1016/j.bbrc.2008.10.164. [DOI] [PubMed] [Google Scholar]
- Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, Gitter AH, Schulzke JD, Fromm M. Contribution of claudin 5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle MA, Konopka G, Parviz F, Gaggl AL, Yang C, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4α orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci U S A. 2006;103:8419–8424. doi: 10.1073/pnas.0600246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, Wagner S, Fromm M, Schulzke JD. Permeability of human HT-29/B6 colonic epithelium as a function of apoptosis. J Physiol. 2001;535:541–552. doi: 10.1111/j.1469-7793.2001.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- Bucker R, Troeger H, Kleer J, Fromm M, Schulzke JD. Arcobacter butzleri induces barrier dysfunction in intestinal HT-29/B6 Cells. J Infect Dis. 2009;200:756–764. doi: 10.1086/600868. [DOI] [PubMed] [Google Scholar]
- Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433–443. doi: 10.1053/gast.2002.34784. [DOI] [PubMed] [Google Scholar]
- Chapman MA, Grahn MF, Boyle MA, Hutton M, Rogers J, Williams NS. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut. 1994;35:73–76. doi: 10.1136/gut.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon γ induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258–1267. doi: 10.1053/j.gastro.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Clark EC, Patel SD, Chadwick PR, Warhurst G, Curry A, Carlson GL. Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut. 2003;52:224–230. doi: 10.1136/gut.52.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsigny M, Babeu JP, Dupuis AA, Furth EE, Seidman EG, Lévy E, Verdu EF, Gendron FP, Boudreau F. Loss of hepatocyte-nuclear-factor-4α affects colonic ion transport and causes chronic inflammation resembling inflammatory bowel disease in mice. PLoS One. 2009;4:e7609. doi: 10.1371/journal.pone.0007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman LA, Hoentjen F, Qian BF, Sprengers D, Tjwa E, Torres MF, Torrice CD, Sartor RB, Tonkonogy SL. Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats. Clin Exp Immunol. 2004;136:30–39. doi: 10.1111/j.1365-2249.2004.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incà R, Annese V, Leo DiV, Latiano A, Quaino V, Abazia C, Vettorato MG, Sturniolo GC. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn's disease. Aliment Pharmacol Ther. 2006;23:1455–1461. doi: 10.1111/j.1365-2036.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- Eeckhoute J, Formstecher P, Laine B. Hepatocyte nuclear factor 4α enhances the hepatocyte nuclear factor 1α-mediated activation of transcription. Nucleic Acids Res. 2004;32:2586–2593. doi: 10.1093/nar/gkh581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin 2 along the human intestine: Implication of GATA 4 in the maintenance of claudin 2 in differentiating cells. J Cell Physiol. 2005;203:15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-αinduced single-cell apoptosis. FASEB J. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- Gunzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Muller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci. 2009;122:1507–1517. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Hering NA, Andres S, Fromm A, Tol VanEA, Amasheh M, Mankertz J, Fromm M, Schulzke JD. Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin 4 in HT-29/B6 cells. J Nutr. 2011a;141:783–789. doi: 10.3945/jn.110.137588. [DOI] [PubMed] [Google Scholar]
- Hering NA, Richter JF, Krug SM, Gunzel D, Fromm A, Bohn E, Rosenthal R, Bucker R, Fromm M, Troeger H, Schulzke JD. Yersinia enterocolitica induces epithelial barrier dysfunction through regional tight junction changes in colonic HT-29/B6 cell monolayers. Lab Invest. 2011b;91:310–324. doi: 10.1038/labinvest.2010.180. [DOI] [PubMed] [Google Scholar]
- Hering NA, Richter JF, Krug SM, Günzel D, Fromm A, Bohn E, Rosenthal R, Bücker R, Fromm M, Troeger H, Schulzke JD. Modulation of epithelial barrier function by pathogenic Y. enterocolitica or probiotic E. coli Nissle. FASEB J. 2011c;25:1036.6. [Google Scholar]
- Hudcovic T, Stepankova R, Cebra J, Tlaskalova-Hogenova H. The role of microflora in the development of intestinal inflammation: acute and chronic colitis induced by dextran sulfate in germ-free and conventionally reared immunocompetent and immunodeficient mice. Folia Microbiol (Praha) 2001;46:565–572. doi: 10.1007/BF02818004. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LJ, Fromm M, Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal. 2011;15:1255–1270. doi: 10.1089/ars.2011.3892. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Ramakrishna BS, Binder HJ. Stimulation of sodium chloride absorption from secreting rat colon by short-chain fatty acids. Dig Dis Sci. 1999;44:1924–1930. doi: 10.1023/a:1018871412748. [DOI] [PubMed] [Google Scholar]
- Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug SM, Bojarski C, Fromm A, Schulzke JD, Fromm M. Tricellulin in Crohn's disease and ulcerative colitis. FASEB J. 2010;24:998.1. [Google Scholar]
- Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, Keshav S. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010;16:1138–1148. doi: 10.1002/ibd.21177. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD. TNFα up-regulates claudin 2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336:67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJ. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–1218. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, Amasheh S, Fromm M. Claudin 3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Boirivant M, Pallone F, Macdonald TT. TGF-β1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008;1(Suppl. 1):S50–S53. doi: 10.1038/mi.2008.55. [DOI] [PubMed] [Google Scholar]
- Nielsen HL, Nielsen H, Ejlertsen T, Engberg J, Gunzel D, Zeitz M, Hering NA, Fromm M, Schulzke JD, Bucker R. Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/B6 intestinal epithelial cells. PLoS One. 2011;6:e23858. doi: 10.1371/journal.pone.0023858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plöger S, Bergann T, Fromm A, Bücker R, Fromm M, Schulzke JD. Effect of sodium butyrate on barrier function of HT-29/B6-Tet-On-GR-MR cells in conjunction with down-regulation of claudin 2. Genes Nutr. 2010;5(Suppl. 1):S28. [Google Scholar]
- Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- Roselli M, Finamore A, Britti MS, Mengheri E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco 2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br J Nutr. 2006;95:1177–1184. doi: 10.1079/bjn20051681. [DOI] [PubMed] [Google Scholar]
- Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, Schreiber S. TNF-α and IFN-γ regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin 2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC. Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1α. J Biol Chem. 2002;277:21361–21370. doi: 10.1074/jbc.M110261200. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999a;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFα) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999b;112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- Schumann M, Gunzel D, Buergel N, Richter JF, Troeger H, May C, Fromm A, Sorgenfrei D, Daum S, Bojarski C, Heyman M, Zeitz M, Fromm M, Schulzke JD. Cell polarity-determining proteins Par 3 and PP 1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–228. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- Segain JP, Blétière RaingeardDeLaD, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:675–683. doi: 10.1002/ibd.20101. [DOI] [PubMed] [Google Scholar]
- Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- Sturniolo GC, Leo DiV, Ferronato A, D'Odorico A, D'Inca R. Zinc supplementation tightens “leaky gut” in Crohn's disease. Inflamm Bowel Dis. 2001;7:94–98. doi: 10.1097/00054725-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Tagkalidis PP, Gibson PR, Bhathal PS. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol. 2007;60:382–387. doi: 10.1136/jcp.2005.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin 2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger H, Richter JF, Beutin L, Gunzel D, Dobrindt U, Epple HJ, Gitter AH, Zeitz M, Fromm M, Schulzke JD. Escherichia coliα-haemolysin induces focal leaks in colonic epithelium: a novel mechanism of bacterial translocation. Cell Microbiol. 2007;9:2530–2540. doi: 10.1111/j.1462-5822.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, Suerbaum S, Buer J, Gunzer F, Westendorf AM. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;12:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itallie VanC, Rahner C, Anderson JM. Regulated expression of claudin 4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itallie VanCM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- Wapenaar MC, Monsuur AJ, Bodegraven vanAA, Weersma RK, Bevova MR, Linskens RK, Howdle P, Holmes G, Mulder CJ, Dijkstra G, Heel vanDA, Wijmenga C. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463–467. doi: 10.1136/gut.2007.133132. [DOI] [PubMed] [Google Scholar]
- Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, Schroder JM, Bevins CL, Fellermann K, Stange EF. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal α defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-α-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12:1331–1346. doi: 10.1111/j.1582-4934.2008.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO 1 regulate tight junction barrier function. Proc Natl Acad Sci U S A. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor α antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein 1 (ZO 1) expression in weaning piglets. Br J Nutr. 2009;102:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharmacol Exp Ther. 2000;294:822–829. [PubMed] [Google Scholar]


