Abstract
Muscle protein synthesis (MPS) is the driving force behind adaptive responses to exercise and represents a widely adopted proxy for gauging chronic efficacy of acute interventions, (i.e. exercise/nutrition). Recent findings in this arena have been progressive. Nutrient-driven increases in MPS are of finite duration (∼1.5 h), switching off thereafter despite sustained amino acid availability and intramuscular anabolic signalling. Intriguingly, this ‘muscle-full set-point’ is delayed by resistance exercise (RE) (i.e. the feeding × exercise combination is ‘more anabolic’ than nutrition alone) even ≥24 h beyond a single exercise bout, casting doubt on the importance of nutrient timing vs. sufficiency per se. Studies manipulating exercise intensity/workload have shown that increases in MPS are negligible with RE at 20–40% but maximal at 70–90% of one-repetition maximum when workload is matched (according to load × repetition number). However, low-intensity exercise performed to failure equalises this response. Analysing distinct subcellular fractions (e.g. myofibrillar, sarcoplasmic, mitochondrial) may provide a readout of chronic exercise efficacy in addition to effect size in MPS per se, i.e. while ‘mixed’ MPS increases similarly with endurance and RE, increases in myofibrillar MPS are specific to RE, prophetic of adaptation (i.e. hypertrophy). Finally, the molecular regulation of MPS by exercise and its regulation via ‘anabolic’ hormones (e.g. IGF-1) has been questioned, leading to discovery of alternative mechanosensing–signalling to MPS.
Phil Atherton (left) completed his PhD charting signal transduction pathways regulating skeletal muscle metabolism and plasticity. After this, he completed a 3 year post-doc, providing molecular biology input to a large scale exercise-training programme into the effects of ageing on physiological and metabolic adaptations to exercise. In 2008 he took up a Research Councils UK Fellowship (HEFCE funded beyond 2012) with the view to developing an independent career and is currently pursuing work to define the molecular regulation of protein turnover by nutrition and exercise, in health and disease. A particular interest of Phil's, is back-translating ‘hits and leads’ from humans into more tractable in vitro models, with the objective of achieving both observational and mechanistic understanding. Ken Smith (right) completed his PhD at the University of Dundee under the tutelage of Prof. Mike Rennie where he developed his career long interest in the development and application of stable isotopic methodologies to understand the regulation of human fuel metabolism, in particular amino acid and protein turnover in skeletal muscle, in health and disease; with particular focus on the role of both nutrition and exercise in maintaining muscle mass and function. Currently he is a principal research fellow in the Division of Metabolic Physiology at the University of Nottingham where he oversees the Mass Spectrometry Core facility, a core component of the recently awarded MRC/ARUK ‘centre for musculoskeletal ageing’.
 |
Background
Skeletal muscles are highly plastic tissues that adapt to cope with the increased locomotory and metabolic demands of exercise. However, successful adaptation to exercise in terms of altered muscle physiology and improved performance varies exquisitely according to the activities imposed (e.g. force, duration, etc.) and by an individual's genetic makeup, which designates his or her ‘responder status’ (Timmons, 2011). It follows that selectivity over the quantity (i.e. individual proteins or ‘bulk’ subfractions such as myofibrillar, mitochondrial and sarcoplasmic) of muscle proteins synthesised underlies the exquisite adaptive specificity to distinct exercise training regimens, and perhaps even the marked heterogeneity of responsiveness to training (Timmons, 2011).
In healthy, recreationally active individuals, skeletal muscle proteins display turnover rates of ∼1.2% day-1 and exist in dynamic equilibrium: muscle protein breakdown (MPB) exceeds muscle protein synthesis (MPS) in the fasted state, and MPS exceeds MPB in the fed state. In response to exercise, MPS is transiently increased whereas MPB also increases, or remains the same (the latter of which is on the proviso of sufficient exogenous nutrient supply; Kumar et al. 2009a). It follows that on a cumulative basis, increases in MPS after each exercise bout ‘drives’ adaptation to exercise training.
Stable isotopes: capturing protein turnover in vivo
Dynamic measures of muscle protein turnover can be determined in muscle tissue using stable isotope methodologies (Rennie et al. 1982; Wolfe, 1982). Stable isotopes are non-radioactive naturally occurring ‘heavy atoms’ (NB safe for use in man), which are essentially identical to their endogenous counterparts but can be distinguished by their mass difference (using mass spectrometric techniques). This allows us to measure incorporation of these isotopic ‘motifs’ into biological samples, i.e. isotopically labelled amino acids to measure MPS in protein obtained from biopsy tissue (Rennie et al. 1982; Trappe et al. 2002; Katsanos et al. 2005; Koopman et al. 2008). However, since these methods require constant tracer infusions, they are only suitable for measuring ‘acute’ (∼hours) MPS in a controlled laboratory setting. Therefore it is of great interest that new tracer methods have been recently developed where measures of MPS are possible in free-living subjects over weeks to months. This method involves ingestion of deuterated water (D2O) to assess cumulative incorporation of deuterium into muscle proteins via deuterium exchange through alanine (Robinson et al. 2011).
The choice of labelled amino acid will determine the method of measurement. Using deuterium (in place of hydrogen) labelling allows measurement of synthesis using gas chromatography–mass spectrometry (GC-MS), whereas the use of 13C or 15N is traditionally measured using isotope ratio mass spectrometry (IRMS) of fixed gases, i.e. CO2 or N2, which requires combustion or release of CO2, e.g. by reaction with ninhydrin or in the case of 15N, combustion to NO2 followed by reduction to produce N2. The free amino acids (AAs) from hydrolysis of proteins are separated by chromatography (gas or liquid) and combusted prior to mass spectrometric analysis, e.g. gas chromatography–combustion (GC-C)–IRMS or liquid chromatography–combustion (LC-C)–IRMS (for an introductory review of tracer approaches see Rennie, 1999).
Recent advances in the stability and sensitivity of mass spectrometers, coupled with the availability of multiply ‘heavy atom’ labelled amino acids, e.g. [1,2-13C2]leucine (Atherton et al. 2010), [D5]- or [13C6]phenylalanine (Koopman et al. 2008; Burd et al. 2011), has permitted greater resolution of the acute responses of MPS even over 30–45 min periods (Atherton et al. 2010) and thus measurement of the temporal nature of the MPS response. Technical development and application of methods to measure muscle protein breakdown (MPB) has, however, lagged behind that of MPS and as a result much less is known about the responses of MPB to exercise and nutrition. However, stable isotopes do allow for estimates of MPB by dilution of the tracer across a limb (using an arterio-venous balance model) when assessed in conjunction with limb blood flow, i.e. a greater difference in labelling of an essential amino acid (EAA) between arterial-venous samples indicates a higher rate of release of AAs via MPB (Wilkes et al. 2009).
Regulation of MPS by nutrition
The two principal determinants of adult skeletal muscle proteostasis are physical activity (discussed subsequently) and nutrient availability. The anabolic effects of nutrition are principally driven by the transfer and incorporation of amino acids captured from dietary protein sources, into skeletal muscle proteins. The purpose of this is to compensate for muscle protein that is lost in fasted (postabsorptive) periods due to, for example, amino acid oxidation and/or carbon donation for liver gluconeogenesis (Wackerhage & Rennie, 2006). Critically (assuming good health and mobility), it is this dynamic ‘fasted-loss/fed-gain’ cycle in proteostasis that ensures muscle mass remains constant. But what are the ‘anabolic components’ of nutrition? After early work defining that the anabolic effects of mixed-meal feeding were entirely attributable to essential amino acids (EAA) (Smith et al. 1992), we and others have gone on to show dose-dependent and saturable effects at 10 g EAAs (Cuthbertson et al. 2005) equivalent to ∼20 g protein (Moore et al. 2009). Perhaps unsurprisingly, this anabolic response is transient in nature, which makes sense, as forsaking adaptive increases in MPB one could achieve hypertrophy simply by eating excess protein! The time course of the feeding response with a saturable amount of protein is as follows. After a lag of around 30 min there is a large increase (∼3-fold) with MPS peaking around 1.5 h before returning to baseline by 2 h (Atherton et al. 2010) despite continued increased availability of circulating amino acids and sustained ‘anabolic signalling’ (Bohe et al. 2001; Atherton et al. 2010). It is at this point the muscle becomes refractory to stimulation despite sustained elevations of AAs (see Fig. 1). We have termed this phenomenon ‘muscle-full’ (Bohe et al. 2001; Atherton et al. 2010) based on the developmental concept introduced by Joe Millward wherein muscle protein accretion is physically limited by the inelastic collagen connective tissue of the endomysium surrounding each fibre (the ‘bag-full’ hypothesis)(Millward et al. 1994).
Figure 1.
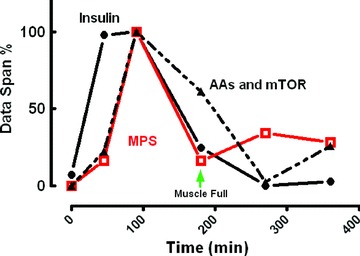
The ‘muscle-full’ effect. Relationship between MPS, AA and intramuscular signalling
What of a role for insulin in regulating anabolic responses to nutrition (via nutrient-induced secretion)? While it is noteworthy that provision of protein alone (i.e. without carbohydrate) causes a rise in insulin similar to that seen following a mixed meal (Atherton et al. 2010), insulin apparently does not contribute to the anabolic effects of EAAs on MPS. To exemplify this, EAA infusates robustly stimulate MPS even when insulin is ‘clamped’ at postabsorptive concentrations (5 μIU ml−1 with the β-cell inhibitor octreotide; Greenhaff et al. 2008). However, this does not mean there is no postprandial anabolic role for insulin. Indeed, in addition to the 3-fold rise in MPS, there is also a significant anti-proteolytic (∼40–50%) effect of feeding on skeletal muscle which is apparently entirely attributable to insulin. To illustrate this, a rise in insulin to just 15 μ IU ml−1 (3× postabsorptive concentrations) is sufficient to mimic the 50% inhibition of MPB (NB the maximal effect size) caused by a mixed meal (Wilkes et al. 2009). Moreover, this anti-catabolic effect cannot be recapitulated via large-dose AA infusions (18 g h−1 over 3 h) when insulin is clamped at postabsorptive concentrations (5 μU ml−1) (Greenhaff et al. 2008). Thus, to summarise, EAA regulates anabolic responses via large increases in MPS, while insulin release regulates anti-catabolic (depressions in MPB) responses. It follows that as the change in MPS is far greater than that in MPB, MPS is the major driving force behind nutrient induced anabolism.
Regulation of MPS by acute exercise
The magnitude of acute response of muscle to resistance exercise in terms of MPS is dependent upon both workload and intensity. For example, at intensities ≤40% of one-repetition maximum (1-RM), there are no detectable increases in MPS, whereas at intensities greater than 60% 1-RM, exercise increases MPS 2- to 3-fold (Kumar et al. 2009b). However, this does not mean that lower intensity exercise cannot yield anabolic effects. Indeed, increases in MPS at 30% 1-RM of comparable magnitude to a group performing 90% 1-RM are possible but only when exercise is performed to failure and not when work is matched between 30 and 90% 1-RM (Burd et al. 2010). In essence this means that increasing the volume of work at a lower intensity can overcome and even surpass the blunted MPS response with work-matched low-intensity exercise, probably as a consequence of increased type II fibre recruitment due to the fatiguing nature of the contractions (Burd et al. 2010). As such, low-load, fatiguing contractions may represent a feasible approach to stimulate muscle hypertrophy and a means of escape from lifting heavy weights.
In terms of contraction mode, although eccentric-type exercise training (i.e. lengthening contractions, not backward running) has been shown to result in greater muscle hypertrophy (Roig et al. 2009), measurement of MPS after both concentric and eccentric contractions has demonstrated only relatively small temporal differences (Cuthbertson et al. 2006). Moreover, when total work is matched between eccentric and concentric contractions there is no difference in training-induced muscle hypertrophy (Moore et al. 2011). As such, increased external loading encountered during eccentric contractions may explain the greater efficacy of eccentric training, rather than contraction mode per se.
It perhaps comes as no surprise that, as with the ‘muscle-full’ response to feeding, the anabolic response to exercise must also be of limited duration. In terms of the time course of MPS response, immediately after exercise there is a latent period (prior to rises in MPS) of a duration which seems to relate to the magnitude of energy/mechanical stress associated with the exercise. This premise was exemplified in a rodent study showing that MPS is suppressed during intense contraction in a duty cycle (i.e. work)-dependent manner (Atherton & Rennie, 2006; Rose et al. 2009). Furthermore, although there exist no equivalent studies in humans (i.e. MPS during exercise), there have been measures made in the acute period of recovery of exercise bouts which may allude to similar mechanisms. For example, while MPS remained unchanged up to 3 h after extremely fatiguing and damaging eccentric contractions (step-up/step-down carrying weight) (Cuthbertson et al. 2006), the latency for lower intensity exercise (6 × 8 repetitions at 75% 1-RM) is <1 h (Kumar et al. 2009b).
After this latent period, MPS rises sharply between 45 and 150 min and may be sustained for up to 4 h (Kumar et al. 2009b) in the fasted state (limited by substrate availability), and in the presence of increased AA availability, up to and beyond 24 h (Cuthbertson et al. 2006). Interestingly, the time course of changes in MPS to the exercise bout is mimicked by that of the epimysial collagen and tendon collagen, thus demonstrating a high degree of coordination between tissues of the musculoskeletal system in response to exercise (Miller et al. 2005).
Exercise × nutrient interactions regulating MPS
A key aspect surrounding acute responses to exercise and subsequent adaptation is nutrient × exercise interactions. This is exemplified by the fact that acute increases in MPS after exercise in the absence of EAA nutrition provide a more prolonged rise in MPB such that the net effect is negative muscle protein balance (Biolo et al. 1995). If such EAA deficiency persisted throughout training, this would lead to maladaptation; you can't build or remodel muscle without amino acids! It follows that increasing dietary EAA availability after exercise enhances both the magnitude and duration of the increase in MPS (Pennings et al. 2011). Therefore, in essence, exercise is able to pre-condition muscle to delay the muscle full ‘set-point’ (illustrated in Fig. 1). Interestingly, addition of carbohydrate to protein affords no greater anabolic effects on protein turnover (neither increases in MPS nor depressions in MPB) after exercise, highlighting the central role of EAAs as the principal (and perhaps only!) macronutrients required to optimise anabolic responses in protein turnover to exercise (Staples et al. 2011).
There has been considerable work undertaken to determine the optimal timing of nutritional intake in order to maximise post-exercise MPS and ensuing adaptations to training (Cribb & Hayes, 2006; Hoffman et al. 2009). In general, we believe that it is largely irrelevant whether the feed is given pre-, during or post-exercise. This is because the delaying of the muscle-full response appears to last at least 24 h (Burd et al. 2011) after a single bout of exercise, which may help explain chronic adaptations such as hypertrophy/remodelling of muscle over time, independent of proximity-dependent feeding patterns (see Fig. 2). Therefore, we contend that nutrient sufficiency per se, rather than timing of intake, is the more important aspect to successful hypertrophic adaptation (that is not to say some acute performance/recovery benefits may be afforded by consumption of nutrition in close proximity to exercise) (Ferguson-Stegall et al. 2011). Moreover, there are still limits to how hard the system can be pushed and increasing protein loading to an identical bout of exercise still demonstrates a saturable response at around 20 g (equivalent to the 10 g EAA maximum dose observed with EAAs in the absence of exercise), above which amino acid oxidation is increased and excess protein is thus catabolised (Moore et al. 2009). Therefore increasing the EAA load will not fully overcome the muscle-full effect afforded by exercise; rather, it prolongs the anabolic window. As such moderate feeding strategies may be better (∼20 g PRO aliquots) but, perhaps, more often (the frequency of which remains to be determined, i.e. how long the muscle remains refractory to the anabolic effects of AAs).
Figure 2.
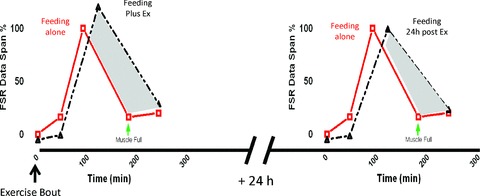
Delaying of the ‘muscle-full’ signal in response to nutrition persists even 24 h beyond a single exercise bout
Regulation of MPS by exercise training
The effect of exercise training on MPS is less well studied. Although a number of studies cite increases in ‘basal or postabsorptive’ MPS as a result of training per se, they may simply be confirming the prolonged acute effects, especially where measurements were made less than 24 h following the last bout of exercise (Hasten et al. 2000). Nonetheless, there are data which suggest that exercise training shortens the duration of the anabolic response, which could be due to greater acute adaptive efficiency (Hartman et al. 2006; Tang et al. 2008), or perhaps the laws of diminishing returns in terms of adaptive responses.
Responses in MPS to different exercise modes
As a field we are often guilty of focusing on resistance exercise and nutrition and ways to make muscles bigger. Nonetheless, most studies support the notion that MPS responses are similar irrespective of the mode of exercise, i.e. resistance vs. non-resistance (though the duration of sensitisation may differ). For instance, endurance-type exercise such as running or cycling is also associated with increased synthesis of mixed muscle proteins acutely (∼50–60%) (Harber et al. 2010). However, these acute responses are not associated with significant changes in muscle mass, i.e. hypertrophy observed with resistance exercise. So what do these changes mean? Clearly extrapolating the amplitude of increase in mixed muscle MPS cannot inform on adaptation – so what can we do? As was stated in the initial section of this review, for adaptation to display exercise-mode specificity, there must be distinct responses of different protein fractions (and indeed individual proteins) within muscle. Indeed, this proposition was elegantly displayed in a study where the same individuals performed a 10 weeks resistance (weight-lifting) programme in one leg and a 10 weeks endurance (cycling) programme in the other. After training, post-exercise myofibrillar not mitochondrial protein synthesis increased with resistance exercise (Wilkinson et al. 2008). Conversely, after training mitochondrial protein synthesis increased only in the endurance-trained leg whereas myofibrillar did not. These data seem to suggest a ‘matching’ between MPS responses and phenotypic changes, i.e. muscle hypertrophy in resistance training versus mitochondrial biogenesis in endurance training. Nonetheless, although it would be tempting to conclude that acute responses within specific muscle pools may provide insight into chronic adaptations ensuing, responses in the untrained individual may be less specific (Wilkinson et al. 2008) and be more related to the unfamiliarity of exercise per se (Coffey et al. 2006). Therefore, extrapolation of acute MPS in subfractions to potential adaptive responses after a single bout of unfamiliar exercise should be cautiously interpreted.
Sensing and signalling regulating MPS
Despite being a hot-bed of research, the ‘black box’ question relating to the mechanisms regulating MPS and adaptation to exercise still remains poorly defined. Exercise triggers complex mechanotransduction and physico-chemical (i.e. endocrine, auto/paracrine) sensory mechanisms (Glass, 2010; West et al. 2010). Subsequent activation of receptor and non-receptor mediated intramuscular signalling modulates cellular apparatus regulating both short-term post-translational (phosphorylation) control of protein turnover and gene expression (mRNA/miRNA) and long term changes in cellular metabolic capacity.
But what do we know of this black box? First, it is well established that the mammalian target of rapamycin (mTOR) is a key signalling pathway regulating exercise/nutrient-induced alterations in MPS (Drummond et al. 2009; Dickinson et al. 2011). Indeed, mTOR activation ultimately induces phosphorylation of multiple translational initiation factor substrates (4E-binding protein (4EBP1), ribosomal protein S6 kinase (p70S6K1), eukaryotic initiation factors 4 G/A/B (eIF4G/A/B) and formation of the eIF3F scaffold) to promote assembly of the 48S pre-initiation complex. In a parallel pathway, activation of the key guanine exchange factor, eukaryotic initiation factor 2B (eIF2B) eIF2 shuttles the initiator tRNA (Met-tRNAi) to the ribosome during formation of the 48S pre-initiation complex, thereby promoting ‘global’ protein synthesis and co-ordinately enhancing translational efficiency (for detailed reviews of mTOR and associated signalling see Proud, 2009; Goodman et al. 2011).
In terms of ‘what is upstream of mTOR?’, it has for a long time been known that nutrients (EAAs) signal through mTOR independent of proximal insulin signalling (for detailed reviews beyond the scope of this one see Proud, 2009, 2011). However, exercise-induced inputs upstream of mTOR have been more controversial. Much of the early animal (Stitt et al. 2004) and cell (Rommel et al. 2001) work pointed to a canonical signalling pathway whereby increases in insulin-like growth factor (IGF-1, or splice variants like mechano-growth factor (MGF)) production stimulates proximal insulin signalling pathways (IGFr–AKT–mTOR), and thereafter key substrates of mTOR regulating translational initiation. However, there are a number of lines of evidence from both in vivo and in vitro systems arguing against such a canonical IGFr–AKT–mTOR pathway in the regulation of exercise-induced MPS. In an elegantly designed study, resistance exercise was performed in human arm muscles under conditions of either high endogenous hormone (HH; concurrent bilateral leg exercise) or low endogenous hormone (LH; no concurrent leg exercise) concentrations (West et al. 2009). Yet, despite considerable differences in growth hormone, testosterone and IGF-1 concentrations between the LH and HH groups, there were no differences in mTOR signalling, MPS, or in chronic adaptations to training in terms of mass or strength gains (West et al. 2010). These data suggest that systemic induction of IGF-1 is not a pivotal part of the adaptive process. Nonetheless, it could be argued that IGF-1 regulates AKT–mTOR signalling via more ‘local’ auto/paracrine signalling mechanisms. Yet this is also difficult to reconcile as ablation of the IGFr does not compromise chronic adaptations, i.e. hypertrophic responses to loading in pre-clinical models (Spangenburg et al. 2008; Hamilton et al. 2010).
So what else may be upstream of mTOR in response to exercise? Mechanotransduction is the process of converting mechanical (i.e. exercise) stimuli into cellular responses and represents a viable means by which cells can distinguish mechanical inputs and, thus, perhaps confer adaptive specificity (for detailed review see Hornberger, 2011). Importantly, recent work has highlighted that phospholipase D (PLD) and its membrane-derived lipid second messenger phosphatidic acid (PA) are upstream of contraction-induced activation of mTOR, since pharmacological inhibition of PLD effectively ablated activation of mTOR in response to contractions (O'Neil et al. 2009). Perhaps this represents at least one of the intrinsic mechanisms by which muscle can adapt independently of systemic or even locally derived membrane receptor-based signals.
In terms of generation of an endurance phenotype, perhaps the major signalling axis implicated in mitochondrial biogenesis is the 5′-AMP-activated protein kinase (AMPK)–peroxisome proliferator-activated receptor γ co-activator (PGC-1) pathway, probably activated by heightened AMP:ATP ratios due to high energy demands (and/or stress) associated with endurance (Atherton et al. 2005) or even unfamiliar activities (Coffey et al. 2006). Overexpression of PGC-1 promotes mitochondrial biogenesis (Viscomi et al. 2011), and activation of AMPK can both put the brakes on MPS and induce MPB via proteaosomal and autophagy related mechanisms (Bolster et al. 2002).
This latter notion that the control of MPS and MPB is co-ordinately regulated via flux through the AMPK–AKT–mTOR ‘pathways’ is intriguing and it is speculated that the balance of these signals (governed by energetic and mechanical impositions) may to some degree determine adaptive specificity and perhaps, capacity.
Conclusions and future work
As workers in the field, we tend to ‘pigeonhole’ exercise training regimens into ‘endurance’ activities composing prolonged low-intensity efforts (e.g. prolonged running and cycling), or ‘resistance’ activities (Kumar et al. 2009a) comprising high-intensity efforts (e.g. lifting weights). However, this classification belies the fact that there are exercise regimens that utilise both modalities. For example, high intensity training (HIT) involves very brief bouts of high-intensity, Wingate style contractions but primarily elicits an endurance-type adaptation as its main feature (Burgomaster et al. 2008). Moreover, for Joe Public at the gym and critically for elite athletes, the goal is often to perform cross-style training (also called concurrent training) in order to prepare for events requiring mixtures of strength, endurance and power, the contribution of each required varying according to the demands of the specific event(s). However, whether there exists a conflict between different training modes on a molecular, MPS or adaptive basis still remains largely to be defined.
Despite considerable advances in our biochemical understanding of ‘implicated signalling pathways’ we are a considerable way off understanding their involvement in adaptive specificity in man. For example, how do apparently similar changes in cellular signals regulate specific muscle fractions (mitochondrial, myofibrillar, etc.) in a manner according to the nature of the exercise? Indeed even comparison of exercise regimens providing adaptations at opposite ends of the spectrum (classic endurance vs. resistance) has failed to reach consensus on distinct regulatory signalling events. This is perhaps because responses are profoundly driven by training status (Coffey et al. 2006; Wilkinson et al. 2008; Vissing et al. 2011), genetic heterogeneity (Timmons, 2011) and even technical limitations of poor temporal resolution from ‘snap-shot’ measures of phosphorylation. On the other hand we may have to face the prospect that seeking ‘master regulators’ such as AMPK, AKT and mTOR in humans is naive and that spreading our nets wider, i.e. to encompass genomic mRNA/miRNA measures, is necessary to truly understand the role of protein turnover in determining heterogeneity in adaptive specificity and capacity.
Acknowledgments
P.J.A. is a designated Research Councils UK Fellow, supported by the Royal Society and Ajinomoto Inc. We also acknowledge the outstanding and lifelong contribution of Professor Michael J. Rennie PhD, FRSE (Emeritus Professor, University of Nottingham) to this field. We graciously apologise to colleagues whose work we could not include in this review due to space restrictions.
References
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Rennie MJ. Protein synthesis a low priority for exercising muscle. J Physiol. 2006;573:288–289. doi: 10.1113/jphysiol.2006.110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141:568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5:e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- Cribb PJ, Hayes A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med Sci Sports Exerc. 2006;38:1918–1925. doi: 10.1249/01.mss.0000233790.08788.3e. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Stegall L, McCleave E, Ding Z, Iii DoernerPG, Liu Y, Wang B, Healy M, Kleinert M, Dessard B, Lassiter DG, Kammer L, Ivy JL. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. J Nutr Metab. 2011;2011:623182. doi: 10.1155/2011/623182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13:225–229. doi: 10.1097/mco.0b013e32833862df. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011;23:1896–1906. doi: 10.1016/j.cellsig.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DL, Philp A, MacKenzie MG, Baar K. A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS One. 2010;5:e11624. doi: 10.1371/journal.pone.0011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1254–R1262. doi: 10.1152/ajpregu.00348.2010. [DOI] [PubMed] [Google Scholar]
- Hartman JW, Moore DR, Phillips SM. Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males. Appl Physiol Nutr Metab. 2006;31:557–564. doi: 10.1139/h06-031. [DOI] [PubMed] [Google Scholar]
- Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Hoffman JR, Ratamess NA, Tranchina CP, Rashti SL, Kang J, Faigenbaum AD. Effect of protein-supplement timing on strength, power, and body-composition changes in resistance-trained men. Int J Sport Nutr Exerc Metab. 2009;19:172–185. doi: 10.1123/ijsnem.19.2.172. [DOI] [PubMed] [Google Scholar]
- Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. 2011;43:1267–1276. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, Loon vanLJ. Coingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–580. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009a;106:2026–2039. doi: 10.1152/japplphysiol.91481.2008. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009b;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward DJ, Bowtell JL, Pacy P, Rennie MJ. Physical activity, protein metabolism and protein requirements. Proc Nutr Soc. 1994;53:223–240. doi: 10.1079/pns19940024. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- Moore DR, Young M, Phillips SM. Similar increases in muscle size and strength in young men after training with maximal shortening or lengthening contractions when matched for total work. Eur J Appl Physiol. 2012 doi: 10.1007/s00421-011-2078-x. (2012) [DOI] [PubMed] [Google Scholar]
- O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol. 2009;587:3691–3701. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93:322–331. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- Proud CG. mTORC1 signalling and mRNA translation. Biochem Soc Trans. 2009;37:227–231. doi: 10.1042/BST0370227. [DOI] [PubMed] [Google Scholar]
- Proud CG. mTOR Signalling in Health and Disease. Biochem Soc Trans. 2011;39:431–436. doi: 10.1042/BST0390431. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. An introduction to the use of tracers in nutrition and metabolism. Proc Nutr Soc. 1999;58:935–944. doi: 10.1017/s002966519900124x. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J. 2011;25:3240–3249. doi: 10.1096/fj.11-186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M, O'Brien K, Kirk G, Murray R, McKinnon P, Shadgan B, Reid WD. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med. 2009;43:556–568. doi: 10.1136/bjsm.2008.051417. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI3K/Akt/mTOR and PI3K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Alsted TJ, Jensen TE, Kobbero JB, Maarbjerg SJ, Jensen J, Richter EA. A Ca2+-calmodulin-eEF2K-eEF2 signalling cascade, but not AMPK, contributes to the suppression of skeletal muscle protein synthesis during contractions. J Physiol. 2009;587:1547–1563. doi: 10.1113/jphysiol.2008.167528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol Endocrinol Metab. 1992;262:E372–E376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Le RD, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586:283–291. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, Rennie MJ, Macdonald MJ, Baker SK, Phillips SM. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med Sci Sports Exerc. 2011;43:1154–1161. doi: 10.1249/MSS.0b013e31820751cb. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol. 2008;294:R172–R178. doi: 10.1152/ajpregu.00636.2007. [DOI] [PubMed] [Google Scholar]
- Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol. 2011;110:846–853. doi: 10.1152/japplphysiol.00934.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab. 2002;282:E551–E556. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, Schon EA, Lamperti C, Zeviani M. In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell Metab. 2011;14:80–90. doi: 10.1016/j.cmet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing K, McGee SL, Farup J, Kjolhede T, Vendelbo MH, Jessen N. Differentiated mTOR but not AMPK signaling after strength vs endurance exercise in training-accustomed individuals. Scand J Med Sci Sports. 2012 doi: 10.1111/j.1600-0838.2011.01395.x. (2012) [DOI] [PubMed] [Google Scholar]
- Wackerhage H, Rennie MJ. How nutrition and exercise maintain the human musculoskeletal mass. J Anat. 2006;208:451–458. doi: 10.1111/j.1469-7580.2006.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK, Phillips SM. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol. 2010;108:60–67. doi: 10.1152/japplphysiol.01147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De LM, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol. 2009;587:5239–5247. doi: 10.1113/jphysiol.2009.177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr. 2009;90:1343–1350. doi: 10.3945/ajcn.2009.27543. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–3717. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR. Stable isotope approaches for study of energy substrate metabolism. Fed Proc. 1982;41:2692–2697. [PubMed] [Google Scholar]


