Abstract
Exercise training is a clinically proven, cost-effective, primary intervention that delays and in many cases prevents the health burdens associated with many chronic diseases. However, the precise type and dose of exercise needed to accrue health benefits is a contentious issue with no clear consensus recommendations for the prevention of inactivity-related disorders and chronic diseases. A growing body of evidence demonstrates that high-intensity interval training (HIT) can serve as an effective alternate to traditional endurance-based training, inducing similar or even superior physiological adaptations in healthy individuals and diseased populations, at least when compared on a matched-work basis. While less well studied, low-volume HIT can also stimulate physiological remodelling comparable to moderate-intensity continuous training despite a substantially lower time commitment and reduced total exercise volume. Such findings are important given that ‘lack of time’ remains the most commonly cited barrier to regular exercise participation. Here we review some of the mechanisms responsible for improved skeletal muscle metabolic control and changes in cardiovascular function in response to low-volume HIT. We also consider the limited evidence regarding the potential application of HIT to people with, or at risk for, cardiometabolic disorders including type 2 diabetes. Finally, we provide insight on the utility of low-volume HIT for improving performance in athletes and highlight suggestions for future research.
Martin Gibala (pictured) is Professor and Chair of the Department of Kinesiology at McMaster University. He studies the regulation of skeletal muscle energy metabolism including the impact of nutrition and training on exercise performance. Maureen MacDonald is also a Professor of Kinesiology at McMaster, where she studies the effect of exercise on cardiovascular regulation. Jonathan Little completed doctoral studies at McMaster and is currently a postdoctoral fellow at the University of British Columbia. John Hawley is Professor and Head of the Exercise Metabolism Group at RMIT University, whose focus is skeletal muscle energy metabolism related to exercise and diabetes.
 |
Introduction
High-intensity interval training (HIT) describes physical exercise that is characterized by brief, intermittent bursts of vigorous activity, interspersed by periods of rest or low-intensity exercise. HIT is infinitely variable with the specific physiological adaptations induced by this form of training determined by a myriad of factors including the precise nature of the exercise stimulus (i.e. the intensity, duration and number of intervals performed, as well as the duration and activity patterns during recovery). When compared on a matched-work basis or when estimated energy expenditure is equivalent, HIT can serve as an effective alternate to traditional endurance training, inducing similar or even superior changes in a range of physiological, performance and health-related markers in both healthy individuals and diseased populations (Wisloff et al. 2007; Tjonna et al. 2009; Hwang et al. 2011). Less is known regarding the effects of low-volume HIT, but growing evidence suggests this type of training stimulates physiological remodelling comparable with moderate-intensity continuous training despite a substantially lower time commitment and reduced total exercise volume (Gibala & McGee 2008). These findings are important from a public health perspective, given that ‘lack of time’ remains one of the most commonly cited barriers to regular exercise participation (Stutts 2002; Trost et al. 2002; Kimm et al. 2006). Moreover, recent evidence suggests that HIT is perceived to be more enjoyable than moderate-intensity continuous exercise (Bartlett et al. 2011). Here we review some of the mechanisms responsible for improved skeletal muscle metabolic control and changes in cardiovascular function in response to low-volume HIT, as well as the potential health-related implications for patients with chronic diseases including type 2 diabetes and cardiovascular disease. We also speculate on the practical application of low-volume HIT for elite performance. Although it is recognized that the underlying mechanisms are probably different compared with less-trained subjects (Iaia & Bangsbo 2010), responses in elite athletes may help our understanding of why low-volume HIT is such a potent exercise stimulus.
Physiological remodelling after low-volume HIT
The most common model employed in low-volume HIT studies has been the Wingate test, which consists of a 30 s ‘all out’ cycling effort against a supra-maximal workload. Subjects typically perform four to six work bouts separated by ∼4 min of recovery, for a total of 2–3 min of intense exercise during a training session that lasts ∼20 min. As little as six sessions of this type of training, totalling ∼15 min of all out cycle exercise over 2 weeks, increased skeletal muscle oxidative capacity as reflected by the maximal activity and/or protein content of mitochondrial enzymes (Burgomaster et al. 2005; Gibala et al. 2006). We have also directly compared 6 weeks of Wingate-based HIT with traditional endurance training that was designed according to current public health guidelines (Table 1) (Burgomaster et al. 2008; Rakobowchuk et al. 2008). We found similar training-induced improvements in various markers of skeletal muscle and cardiovascular adaptation despite large differences in weekly training volume (∼90% lower in the HIT group) and time commitment (∼67% lower in the HIT group). In addition to an increased skeletal muscle oxidative capacity (Fig. 1), other endurance-like adaptations have been documented after several weeks of low-volume HIT including an increased resting glycogen content, a reduced rate of glycogen utilization and lactate production during matched-work exercise, an increased capacity for whole-body and skeletal muscle lipid oxidation, enhanced peripheral vascular structure and function, improved exercise performance as measured by time-to-exhaustion tests or time trials and increased maximal oxygen uptake (Burgomaster et al. 2005, 2008; Gibala et al. 2006; Rakobowchuk et al. 2008).
Table 1.
Summary of protocols in studies from our laboratory that directly compared 6 weeks of either high-intensity interval training (HIT) or traditional endurance training
| Variable | HIT group | Endurance group |
|---|---|---|
| Protocol | 30 s × 4–6 repeats, 4.5 min rest (3 sessions per week) | 40–60 min cycling (5 sessions per week) |
| Training intensity (workload) | ‘All out’ maximal effort (∼500 W) | 65% of 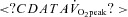 (∼150 W) (∼150 W) |
| Weekly training time commitment | ∼10 min (∼1.5 h including rest) | ∼4.5 h |
| Weekly training volume | ∼225 kJ | ∼2250 kJ |
From Burgomaster et al. (2008). 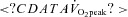 , peak oxygen uptake.
, peak oxygen uptake.
Figure 1. Peak oxygen uptake (top panel) and the maximal activity of the mitochondrial enzyme citrate synthase measured in biopsy samples (bottom panel) obtained before (PRE) and after (POST) 6 weeks of Wingate-based high-intensity interval training (HIT) or traditional moderate-intensity endurance training (ET).
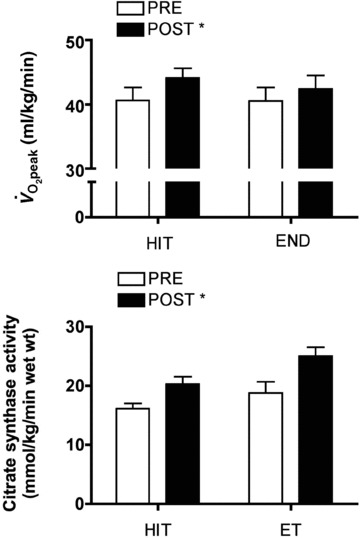
Total exercise volume was 90% lower in the HIT group. Redrawn from Burgomaster et al. (2008) with permission.* P < 0.05 vs Pre; main effect for time.
Wingate-based HIT is, however, extremely demanding and may not be safe, tolerable or appealing for some individuals. We therefore sought to design a more practical model of low-volume HIT that is time efficient while also having wider application to different populations including people at risk for chronic metabolic diseases. To accomplish this goal we decreased the absolute intensity of the work bouts, but increased their duration and shortened the rest intervals. Our new practical HIT model consists of 10 × 60 s work bouts at a constant-load intensity that elicits ∼90% of maximal heart rate, interspersed with 60 s of recovery. The protocol is still time efficient in that only 10 min of exercise is performed over a 20 min training session. Importantly, this practical, time-efficient HIT model is still effective at inducing rapid skeletal muscle remodelling towards a more oxidative phenotype, similar to our previous Wingate-based HIT studies and high-volume endurance training (Little et al. 2010b). Both types of low-volume HIT protocols are also effective for improving functional performance, as shown by cycling time trials that resemble normal athletic competition (Gibala et al. 2006; Little et al. 2010b).
The molecular mechanisms underlying skeletal muscle metabolic adaptations to low-volume HIT have recently been investigated. Given the potency of HIT to increase mitochondrial capacity, it is perhaps not surprising that investigations have examined the influence of low-volume HIT on the activation of peroxisome-proliferator activated receptor γ coactivator (PGC)-1α, which is regarded as the ‘master regulator’ of mitochondrial biogenesis in muscle (Wu et al. 1999). Evidence suggests that exercise intensity is the key factor influencing PGC-1α activation in human skeletal muscle (Egan et al. 2010). In this respect, acute low-volume Wingate-based HIT increases PGC-1α mRNA by several-fold when measured 3 h post-exercise (Gibala et al. 2009; Little et al. 2011b). This is comparable with the acute increase in PGC-1α mRNA expression observed after a bout of continuous endurance-type exercise (Norrbom et al. 2004; Egan et al. 2010). Similar to endurance exercise (Wright et al. 2007; Little et al. 2010a), acute Wingate-based HIT may activate PGC-1 by increasing its nuclear translocation (Little et al. 2011b). The increase in nuclear PGC-1 following low-volume HIT coincides with increased mRNA expression of several mitochondrial genes (Little et al. 2011b), suggesting that a program of mitochondrial adaptation is engaged with these short bursts of intensity exercise (Fig. 2).
Figure 2. Potential intracellular signalling mechanisms involved in HIT-induced mitochondrial biogenesis.
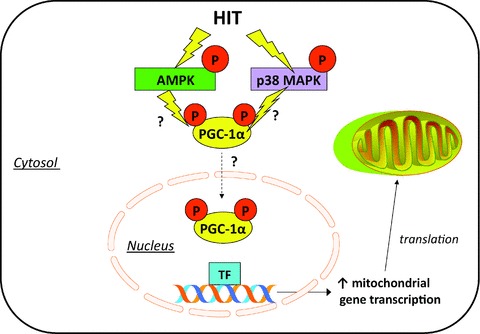
Low-volume HIT has been shown to activate 5′-AMP-activated protein kinase (AMPK) and p38 mitogen-activated protein kinase (MAPK). Both of these exercise-responsive signalling kinases are implicated in direct phosphorylation and activation of PGC-1α. Increased nuclear abundance of PGC-1α following HIT is hypothesized to co-activate transcription factors (TF) to increase mitochondrial gene transcription, ultimately resulting in accumulation of more mitochondrial proteins to drive mitochondrial biogenesis.
The upstream signals that activate PGC-1α and mitochondrial biogenesis in response to low-volume HIT have not been clearly elucidated but probably relate to robust changes in intramuscular ATP:ADP/AMP ratio following exercise (Chen et al. 2000) and the concomitant activation of 5′-adenosine monophosphate-activated protein kinase (AMPK) (Gibala et al. 2009; Little et al. 2011b). Activation of p38 mitogen-activated protein kinase (MAPK), possibly via increased generation of reactive oxygen species (ROS) (Kang et al. 2009), may also be involved (Gibala et al. 2009; Little et al. 2011b). Elevated levels of PGC-1 protein also accompany increased markers of mitochondrial content following a period of low-volume HIT. Six weeks of Wingate-based HIT increased the protein content of PGC-1 by ∼100% in young, healthy individuals (Burgomaster et al. 2008) and 2 weeks of 10 × 1 min HIT resulted in a ∼25% increase in nuclear PGC-1 protein (Little et al. 2010b). Collectively, these results indicate that PGC-1α is probably involved in regulating some of the metabolic adaptations to low-volume HIT. Given the positive effects that a modest increase in muscle PGC-1α appears to have on oxidative capacity, anti-oxidant defence, glucose uptake, resistance to age-related sarcopenia and anti-inflammatory pathways (Sandri et al. 2006; Benton et al. 2008; Wenz et al. 2009), the increase in PGC-1α following low-volume HIT may highlight potential widespread health benefits for this type of exercise.
The impact of interval types of training programs on cardiovascular structure and function has also been investigated (Wisloff et al. 2009), but few studies have utilized low-volume HIT models. However, as little as 2 weeks of Wingate-based HIT has been reported to increase cardiorespiratory capacity as reflected by changes in peak oxygen uptake (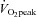 ) (Whyte et al. 2010) although this is not a universal finding (Burgomaster et al. 2005). Another study showed that 6 weeks of Wingate-based HIT increased
) (Whyte et al. 2010) although this is not a universal finding (Burgomaster et al. 2005). Another study showed that 6 weeks of Wingate-based HIT increased 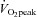 to the same extent as traditional endurance training despite a markedly reduced time commitment and total training volume (Burgomaster et al. 2008). We have also shown in young healthy men and women that low-volume HIT increases compliance in peripheral but not central arteries (Rakobowchuk et al. 2008). The protocol also increased endothelial function in the trained legs to an extent that is comparable to changes observed after a much higher volume of continuous moderate-intensity training (Rakobowchuk et al. 2008). The mechanisms regulating cardiovascular adaptations to various forms of low-volume HIT have yet to be comprehensively examined.
to the same extent as traditional endurance training despite a markedly reduced time commitment and total training volume (Burgomaster et al. 2008). We have also shown in young healthy men and women that low-volume HIT increases compliance in peripheral but not central arteries (Rakobowchuk et al. 2008). The protocol also increased endothelial function in the trained legs to an extent that is comparable to changes observed after a much higher volume of continuous moderate-intensity training (Rakobowchuk et al. 2008). The mechanisms regulating cardiovascular adaptations to various forms of low-volume HIT have yet to be comprehensively examined.
Potential application of HIT in people with or at risk for cardiometabolic disorders
While much of the work conducted to date has involved relatively high-volume protocols that are comparable in volume to traditional endurance training, HIT has been shown to improve cardiorespiratory fitness in a range of populations including those with coronary artery disease, congestive heart failure, middle age adults with metabolic syndrome and obese individuals (Warburton et al. 2005; Wisloff et al. 2007; Moholdt et al. 2009; Munk et al. 2009). In many cases, the increase in cardiorespiratory fitness after HIT was superior to after continuous moderate-intensity training (Wisloff et al. 2007; Tjonna et al. 2008, 2009; Moholdt et al. 2009). Endothelial function, assessed using flow-mediated dilatation of the brachial artery, is improved to a greater extent following HIT compared with continuous moderate-intensity training (Wisloff et al. 2007; Tjonna et al. 2008, 2009; Moholdt et al. 2009). Other studies have documented beneficial changes in various components of resting blood pressure (Rognmo et al. 2004; Schjerve et al. 2008; Whyte et al. 2010) and left ventricular morphology (Wisloff et al. 2007). It appears that this type of cardiac remodelling requires a longer duration of training and greater exercise volume than the load required to alter cardiorespiratory fitness or peripheral vascular structure and function. It could be that the short intense bursts of activity with low-volume HIT induce large-magnitude increases in cellular and peripheral vascular stress, while effectively ‘insulating’ the heart from those stresses due to the brief duration of the exercise bouts. This relative central insulation permits individuals to train at much higher intensities than they would otherwise, but may also result in different timelines and effective stimulus loads between the central and peripheral components of the cardiovascular system.
Low-volume HIT studies in persons who might be at risk for cardiometabolic disorders or patients with chronic disease are very limited. However, recent work has shown that as few as six sessions of either Wingate-based HIT and the more practical constant-load model over 2 weeks improve estimated insulin sensitivity in previously sedentary, overweight individuals (Whyte et al. 2010; Hood et al. 2011). Insulin sensitivity in these studies was calculated based on either single fasting glucose and insulin measurements (Hood et al. 2011) or the response to an oral glucose tolerance test (Whyte et al. 2010) and therefore primarily reflects hepatic as opposed to peripheral (skeletal muscle) insulin sensitivity. Peripheral insulin sensitivity following exercise training may be improved by increased skeletal muscle glucose transport capacity, mediated in part by the protein GLUT4. Skeletal muscle GLUT4 content after short-term HIT is increased by a comparable magnitude (∼2-fold) to that observed after high-volume endurance training (Hood et al. 2011). We also recently demonstrated that low-volume HIT was well tolerated and rapidly improved skeletal muscle GLUT4 content in eight patients with type 2 diabetes (Little et al. 2011a). This small pilot study also showed that six sessions of HIT over 2 weeks reduced average 24 h blood glucose concentration and postprandial glucose excursions, measured via continuous glucose monitoring under standardized diet but otherwise free-living conditions (Little et al. 2011a). These beneficial adaptations were realized even though the weekly training time commitment was much lower than common public health guidelines that generally call for at least 150 min of moderate to vigorous exercise per week to promote health. While the preliminary evidence from these small, proof-of-principle studies are intriguing, large-scale studies are clearly needed to resolve whether low-volume HIT is a realistic, time-efficient exercise alternative to reduce the risk of cardiometabolic disease or improve health and wellbeing in patients with chronic disease.
HIT and athletic performance
HIT has been an integral part of training programs for the enhancement of athletic performance since the beginning of the 19th century. Yet despite being a core component of competition preparation, the unique effect of specific training interventions on the performances of well-trained individuals is sparse. This, perhaps, is understandable for several practical reasons. First, exercise physiologists have found it difficult to convince elite athletes that it could be worthwhile to experiment with their normal training programs. Second, even if athletes (and their coaches) were willing to modify their training practices, conventional approaches to investigate the response to different doses of a treatment (i.e. interval training) using repeated-measures design in which each athlete receives all the different doses is totally impractical for studies of physical training; the long-lasting effects of any given dose of training prevent athletes from receiving more than one dose of the treatment.
Over a decade ago we embarked on a series of investigations into the effects of interval training in competitive endurance athletes using a standardized training protocol, namely, replacing a portion (∼15–20%) of an athletes’ aerobic base training with six to eight sessions of continuous (5 min) high-intensity (90% of 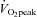 ) work bouts undertaken twice a week throughout a 3 week intervention period (see Hawley et al. 1997 for review). We systematically examined the effect of this interval training protocol on a variety of outcome measures including performance (Lindsay et al. 1996; Stepto et al. 1999), skeletal muscle metabolism (Westgarth-Taylor et al. 1997; Stepto et al. 2001), cell signalling (Yu et al. 2003; Clark et al. 2004) and the interaction of HIT with various diet manipulations (Stepto et al. 2002; Yeo et al. 2008). Stepto et al. (1999) employed a novel approach to determine the effects of divergent interval training protocols on performance lasting ∼1 h by fitting polynomial or other curves to the responses for each interval training dose for individual athletes. As we originally hypothesized, training sessions that employed work bouts that were closely matched to race-pace (8 × 4 min at 85% of peak aerobic power output (PPO)) significantly enhanced performance (2.8%, 95% CI = 4.3–1.3%). Yet, somewhat surprisingly, short-duration, supra-maximal work bouts (12 × 30 s at 175% of PPO) were just as effective in improving performance (2.4%, 95% CI = 4.0–0.7%). Consistent with this observation, Psilander et al. (2010) recently reported that a single bout of low-volume HIT (7 × 30 s ‘all out’ efforts) stimulated increases in mitochondrial gene expression that were comparable to or greater than the changes after more prolonged (3 × 20 min bouts at ∼87% of
) work bouts undertaken twice a week throughout a 3 week intervention period (see Hawley et al. 1997 for review). We systematically examined the effect of this interval training protocol on a variety of outcome measures including performance (Lindsay et al. 1996; Stepto et al. 1999), skeletal muscle metabolism (Westgarth-Taylor et al. 1997; Stepto et al. 2001), cell signalling (Yu et al. 2003; Clark et al. 2004) and the interaction of HIT with various diet manipulations (Stepto et al. 2002; Yeo et al. 2008). Stepto et al. (1999) employed a novel approach to determine the effects of divergent interval training protocols on performance lasting ∼1 h by fitting polynomial or other curves to the responses for each interval training dose for individual athletes. As we originally hypothesized, training sessions that employed work bouts that were closely matched to race-pace (8 × 4 min at 85% of peak aerobic power output (PPO)) significantly enhanced performance (2.8%, 95% CI = 4.3–1.3%). Yet, somewhat surprisingly, short-duration, supra-maximal work bouts (12 × 30 s at 175% of PPO) were just as effective in improving performance (2.4%, 95% CI = 4.0–0.7%). Consistent with this observation, Psilander et al. (2010) recently reported that a single bout of low-volume HIT (7 × 30 s ‘all out’ efforts) stimulated increases in mitochondrial gene expression that were comparable to or greater than the changes after more prolonged (3 × 20 min bouts at ∼87% of 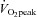 ) endurance exercise in well-trained cyclists. Given the lower volume of work and the fact that mitochondrial transcription factor A, the downstream target of PGC-1α, was only increased after the 30 s protocol, the authors concluded that brief intense interval training might be a time-efficient strategy for highly trained individuals.
) endurance exercise in well-trained cyclists. Given the lower volume of work and the fact that mitochondrial transcription factor A, the downstream target of PGC-1α, was only increased after the 30 s protocol, the authors concluded that brief intense interval training might be a time-efficient strategy for highly trained individuals.
Guellich and colleagues (2009) have recently extended our early findings (Stepto et al. 1999, Fig. 3) that ‘polarized training’ enhanced endurance performance. These workers reported that elite endurance athletes from a range of sports including rowing, running, cycling and cross-country skiing perform only a small portion of their training at competition/race-pace intensities, with the bulk of their workload comprising low-intensity, high-volume workouts, and exposure to extreme HIT sessions. In a recent review Laursen (2010) proposed that a polarized approach to training, in which ∼75% of total training volume be performed at low intensities, with 10–15% performed at supra-maximal intensities may be the optimal training intensity distribution for elite athletes who compete in intense endurance events. We suggest that the unique genetic and/or molecular signature resulting from polarized training is a fertile area for future research. Indeed, directly linking exercise-induced signalling cascades in skeletal muscle to defined metabolic responses and specific changes in gene and protein expression that occur after diverse interval training regimens may provide clues as to why HIT is such a potent intervention for promoting both health outcomes and enhancing athletic performance and exercise capacity.
Figure 3. The effect of varying the intensity of interval training on changes in 40 km time-trial performance.
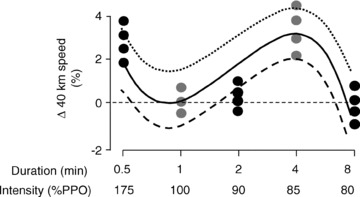
Well-trained male cyclists were randomly assigned to one of five different doses of high-intensity interval training (HIT): 12 × 30 s at 175% of peak sustained power output (PPO), 12 × 1 min s at 100% PPO, 12 × 2 min at 90% PPO, 8 × 4 min at 85% PPO, or 4 × 8 min at 80% PPO. Cyclists completed six HIT sessions over a 3 week period in addition to their habitual aerobic base training. Redrawn from Stepto et al. (1999) with permission.
Conclusion and directions for future research
Considerable evidence currently exists to support a role for low-volume HIT as a potent and time-efficient training method for inducing both central (cardiovascular) and peripheral (skeletal muscle) adaptations that are linked to improved health outcomes. Limited work has examined the application of low-volume HIT in people with, or at risk for, cardiometabolic disorders, and at present the potential benefits of this type of training are unclear. Regardless of the group studied, the majority of low-volume studies have utilized relatively short intervention periods (i.e. lasting up to several weeks). Future work involving long-term (i.e. months to years) interventions in a variety of clinical cohorts (i.e. individuals with insulin resistance, obesity, type 2 diabetes and cardiovascular disease) are urgently needed to better understand how manipulating the exercise stimulus impacts on cardiovascular and musculoskeletal remodelling in these populations. One aspect that is unclear from the present literature is the precise intensity and minimal volume of training that is needed to potentiate the effect of the stimulus-adaptation on outcomes such as mitochondrial biogenesis and relevant health markers. To answer such questions, a complex series of studies needs to be undertaken that systematically ‘titrate’ levels of the ‘training impulse’ and determine subsequent cellular, performance and clinical responses after divergent training interventions. In this regard, the perspectives gained from the use of supra-maximal interval-based training in well-trained athletes may aid in understanding why and how low-volume HIT improves health and functional performance in the general population and in many chronic disease states. Information derived from future studies will need to provide practical, evidence-based recommendations for novel exercise prescription that can be incorporated into daily living and form an integral component in the development of future combinatorial therapies for the prevention and treatment of chronic inactivity-related diseases. If achieved, these goals will simultaneously reduce the economic burden associated with an inactive lifestyle.
Acknowledgments
Work cited from the authors’ laboratories has been supported by the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research, the Canadian Diabetes Association and The Australian Research Council.
Glossary
Abbreviations
- HIT
high-intensity interval training
- PGC-1α
peroxisome-proliferator activated receptor γ coactivator
- PPO
peak aerobic power output
References
- Bartlett JD, Close GL, MacLaren DP, Gregson W, Drust B, Morton JP. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci. 2011;29:547–553. doi: 10.1080/02640414.2010.545427. [DOI] [PubMed] [Google Scholar]
- Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A. Modest PGC-1α overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J Biol Chem. 2008;283:4228–4240. doi: 10.1074/jbc.M704332200. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005;98:1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- Chen Z-P, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–E1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Clark SA, Chen ZP, Murphy KT, Aughey RJ, McKenna MJ, Kemp BE, Hawley JA. Intensified exercise training does not alter AMPK signaling in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E737–E743. doi: 10.1152/ajpendo.00462.2003. [DOI] [PubMed] [Google Scholar]
- Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O'Gorman DJ. Exercise intensity-dependent regulation of PGC-1α mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol. 2010;588:1779–1790. doi: 10.1113/jphysiol.2010.188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, Essen vanM, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev. 2008;36:58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol. 2009;106:929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- Guellich A, Seiler S, Emrich E. Training methods and intensity distribution of young world-class rowers. Int J Sports Physiol Perform. 2009;4:448–460. doi: 10.1123/ijspp.4.4.448. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Myburgh KH, Noakes TD, Dennis SC. Training techniques to improve fatigue resistance and enhance endurance performance. J Sports Sci. 1997;15:325–333. doi: 10.1080/026404197367335. [DOI] [PubMed] [Google Scholar]
- Hood MS, Little JP, Tarnopolsky MA, Myslik F, Gibala MJ. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med Sci Sports Exerc. 2011;43:1849–1856. doi: 10.1249/MSS.0b013e3182199834. [DOI] [PubMed] [Google Scholar]
- Hwang CL, Wu YT, Chou CH. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: a meta-analysis. J Cardiopulm Rehabil Prev. 2011;31:378–385. doi: 10.1097/HCR.0b013e31822f16cb. [DOI] [PubMed] [Google Scholar]
- Iaia FM, Bangsbo J. Speed endurance training is a powerful stimulus for physiological adaptations and performance improvements of athletes. Scand J Med Sci Sports. 2010;20:11–23. doi: 10.1111/j.1600-0838.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- Kang C, O'Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-γ coactivator-1α signaling is redox sensitive. Free Radic Biol Med. 2009;47:1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Kimm SY, Glynn NW, McMahon RP, Voorhees CC, Striegel-Moore RH, Daniels SR. Self-perceived barriers to activity participation among sedentary adolescent girls. Med Sci Sports Exerc. 2006;38:534–540. doi: 10.1249/01.mss.0000189316.71784.dc. [DOI] [PubMed] [Google Scholar]
- Laursen PB. Training for intense exercise performance: high-intensity or high-volume training? Scand J Med Sci Sports. 2010;20:1–10. doi: 10.1111/j.1600-0838.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- Lindsay FH, Hawley JA, Myburgh KH, Schomer HH, Noakes TD, Dennis SC. Improved athletic performance in highly trained cyclists after interval training. Med Sci Sports Exerc. 1996;28:1427–1434. doi: 10.1097/00005768-199611000-00013. [DOI] [PubMed] [Google Scholar]
- Little JP, Gillen JB, Percival M, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011a;111:1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011b;300:R1303–R1310. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010a;298:R912–R917. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010b;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moholdt TT, Amundsen BH, Rustad LA, Wahba A, Løvø KT, Gullikstad LR, Bye A, Skogvoll E, Wisløff U, Slørdahl SA. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158:1031–1037. doi: 10.1016/j.ahj.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Munk PS, Staal EM, Butt N, Isaksen K, Larsen AI. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation A randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J. 2009;159:734–741. doi: 10.1016/j.ahj.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1α mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol. 2004;96:189–194. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- Psilander N, Wang L, Westergren J, Tonkonogi M, Sahlin K. Mitochondrial gene expression in elite cyclists: effects of high-intensity interval exercise. Eur J Appl Physiol. 2002;110:597–606. doi: 10.1007/s00421-010-1544-1. [DOI] [PubMed] [Google Scholar]
- Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R236–R242. doi: 10.1152/ajpregu.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11:216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjerve IE, Tyldum GA, Tjønna AE, Stølen T, Loennechen JP, Hansen HE, Haram PM, Heinrich G, Bye A, Najjar SM, Smith GL, Slørdahl SA, Kemi OJ, Wisløff U. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci (Lond) 2008;115:283–293. doi: 10.1042/CS20070332. [DOI] [PubMed] [Google Scholar]
- Stepto NK, Carey AL, Staudacher HM, Cummings NK, Burke LM, Hawley JA. Effect of short-term fat adaptation on high-intensity training. Med Sci Sports Exerc. 2002;34:449–455. doi: 10.1097/00005768-200203000-00011. [DOI] [PubMed] [Google Scholar]
- Stepto NK, Hawley JA, Dennis SC, Hopkins WG. Effects of different interval-training programs on cycling time-trial performance. Med Sci Sports Exerc. 1999;31:736–741. doi: 10.1097/00005768-199905000-00018. [DOI] [PubMed] [Google Scholar]
- Stepto NK, Martin DT, Fallon KE, Hawley JA. Metabolic demands of intense aerobic interval training in competitive cyclists. Med Sci Sports Exerc. 2001;33:303–310. doi: 10.1097/00005768-200102000-00021. [DOI] [PubMed] [Google Scholar]
- Stutts WC. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. 2002;50:499–507. [PubMed] [Google Scholar]
- Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–534. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjønna AE, Stølen TO, Bye A, Volden M, Slørdahl SA, Odegård R, Skogvoll E, Wisløff U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond) 2009;116:317–326. doi: 10.1042/CS20080249. [DOI] [PubMed] [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Warburton DE, McKenzie DC, Haykowsky MJ, Taylor A, Shoemaker P, Ignaszewski AP, Chan SY. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol. 2005;95:1080–1084. doi: 10.1016/j.amjcard.2004.12.063. [DOI] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Westgarth-Taylor C, Hawley JA, Rickard S, Myburgh KH, Noakes TD, Dennis SC. Metabolic and performance adaptations to interval training in endurance-trained cyclists. Eur J Appl Physiol Occup Physiol. 1997;75:298–304. doi: 10.1007/s004210050164. [DOI] [PubMed] [Google Scholar]
- Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59:1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- Wisløff U, Ellingsen Ø, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37:139–146. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yeo WK, Paton CD, Garnham AP, Burke LM, Carey AL, Hawley JA. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J Appl Physiol. 2008;105:1462–1470. doi: 10.1152/japplphysiol.90882.2008. [DOI] [PubMed] [Google Scholar]
- Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. J Physiol. 2003;546:327–335. doi: 10.1113/jphysiol.2002.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]


