Abstract
Non-technical summary
The distinctive umami taste elicited by l-glutamate and some other amino acids is thought to be initiated by G-protein-coupled receptors, such as heteromers of taste receptor type 1, members 1 and 3, and metabotropic glutamate receptors 1 and 4. We demonstrate the existence of multiple types of glutamate-sensitive gustatory nerve fibres and the contribution of multiple receptors and transduction pathways to umami taste. Such multiple systems for umami taste may differentially contribute to the behavioural preference for glutamate and discriminability of glutamate taste.
Abstract
The distinctive umami taste elicited by l-glutamate and some other amino acids is thought to be initiated by G-protein-coupled receptors. Proposed umami receptors include heteromers of taste receptor type 1, members 1 and 3 (T1R1+T1R3), and metabotropic glutamate receptors 1 and 4 (mGluR1 and mGluR4). Multiple lines of evidence support the involvement of T1R1+T1R3 in umami responses of mice. Although several studies suggest the involvement of receptors other than T1R1+T1R3 in umami, the identity of those receptors remains unclear. Here, we examined taste responsiveness of umami-sensitive chorda tympani nerve fibres from wild-type mice and mice genetically lacking T1R3 or its downstream transduction molecule, the ion channel TRPM5. Our results indicate that single umami-sensitive fibres in wild-type mice fall into two major groups: sucrose-best (S-type) and monopotassium glutamate (MPG)-best (M-type). Each fibre type has two subtypes; one shows synergism between MPG and inosine monophosphate (S1, M1) and the other shows no synergism (S2, M2). In both T1R3 and TRPM5 null mice, S1-type fibres were absent, whereas S2-, M1- and M2-types remained. Lingual application of mGluR antagonists selectively suppressed MPG responses of M1- and M2-type fibres. These data suggest the existence of multiple receptors and transduction pathways for umami responses in mice. Information initiated from T1R3-containing receptors may be mediated by a transduction pathway including TRPM5 and conveyed by sweet-best fibres, whereas umami information from mGluRs may be mediated by TRPM5-independent pathway(s) and conveyed by glutamate-best fibres.
Introduction
Umami taste is elicited by l-glutamate and a few other amino acids (e.g. l-aspartate), some peptides and certain ribonucleotides. Psychophysical studies in humans (Yamaguchi, 1970) and behavioural and/or electrophysiological studies in mice (Ninomiya et al. 1989a,b, 2000; Nakashima et al. 2001), rats (Stapleton et al. 2002) and rhesus monkeys (Hellekant et al. 1997) indicate that responses to umami tastants are distinct from those of sweet, salty, sour and bitter tastants. A characteristic feature of umami taste is the synergistic enhancement of potency when glutamate is mixed with the ribonucleotides inosine monophosphate (IMP) or guanine monophosphate (GMP; Yamaguchi, 1970). Recent studies demonstrated that Maillard reacted peptides and N-geranyl cyclopropylcarboxamide also enhanced chorda tympani (CT) nerve responses to glutamate in rats (Dewis et al. 2006; Katsumata et al. 2008).
Molecular studies have identified multiple potential umami receptors. The first candidate reported was a taste-specific variant of brain-type metabotropic glutamate receptor, type 4 (taste-mGluR4), missing most of the N-terminal extracellular domain (Chaudhari et al. 1996). This variant was identified in circumvallate and foliate taste buds in the posterior taste fields of rats; when expressed in Chinese hamster ovary cells, this receptor responded to glutamate and the group III mGluR agonist l-(+)-2-amino-4-phosphonobutyrate (l-AP4), although the affinity of taste-mGluR4 to glutamate (EC50 = 280 μm) and l-AP4 (EC50 = 0.1–1 mm) is more than 100 times lower than that of brain-type receptors (EC50 = 2 and 1 μm, respectively; Chaudhari et al. 1996, 2000; Yang et al. 1999). The next potential umami receptor to be discovered was a heteromer of T1R1 and T1R3 (taste receptor type 1, members 1 and 3; Nelson et al. 2001). In mice, T1R1 expression is prevalent in the fungiform taste buds of the anterior tongue, innervated by the chorda tympani nerve, but rare in the posterior circumvallate taste buds. Mouse T1R1+T1R3 heterologously expressed in human embryonic kidney (HEK) cells responds to a variety of l-amino acids, some of which elicit taste qualities other than umami (e.g. bitterness, sourness and sweetness), whereas the human-type heteromer preferentially responds to glutamate (Li et al. 2002; Nelson et al. 2002). Apparently, mouse T1R1+T1R3 acts as a broadly sensitive amino acid receptor, while human T1R1+T1R3 is a more narrowly tuned receptor. T1R1+T1R3 from either species exhibits great enhancement of responses to glutamate and/or certain other amino acids by the addition of IMP. Additional candidate umami receptors include full-length mGluR1 and mGluR4 (Toyono et al. 2002) and a variant of mGluR1 (taste-mGluR1; San Gabriel et al. 2005). Full-length mGluR1 and mGluR4 are expressed in a subset of taste cells in fungiform, foliate and circumvallate papillae in rats. Taste-mGluR1 (San Gabriel et al. 2005, 2009) is expressed in the rat foliate and circumvallate papillae and, like taste-mGluR4, lacks much of the N-terminal extracellular domain and has more than 100-fold lower affinity for glutamate than does the brain-type receptor.
To date, the physiological roles in umami taste perception of each of these receptors and their downstream signalling molecules are unclear. Studies in T1R3-knockout (KO) mice showed that behavioural preference for and CT whole-nerve responses to glutamate and/or glutamate with IMP were reduced (Damak et al. 2003) or abolished (Zhao et al. 2003). In addition to T1R3-KO mouse studies, studies of KO mice lacking downstream signalling components have shed light on the intracellular signal transduction pathway(s) underlying umami taste. Mice genetically lacking α-gustducin and/or α-transducin, inositol 1,4,5-trisphosphate receptor 3 (IP3R3) or TRPM5 lacked synergistic responses to glutamate and IMP in the CT nerve, and displayed substantial, albeit reduced, responses to glutamate in both CT and glossopharyngeal (GL) nerves (He et al. 2004; Damak et al. 2006; Hisatsune et al. 2007). These KO mice showed diminished preference for glutamate, but displayed avoidance in response to high concentrations of glutamate comparable to those of wild-type (WT) mice (He et al. 2004; Damak et al. 2006; Hisatsune et al. 2007). These data imply that the remaining responses to glutamate in these mice use other transduction pathways.
Recently, we investigated taste responses of individual mouse fungiform taste bud cells that express α-gustducin or glutamate decarboxylase 67 (GAD67), and found that the following three distinguishable types of taste cells responded to monosodium glutamate (MSG): sweet-best (S-type) and MSG-best (M-type) cells that express gustducin, and electrolyte-responsive (E-type) cells that express GAD67 (Yoshida et al. 2009). Monosodium glutamate contains sodium ions, which may activate sodium-sensing ion channels, such as amiloride-sensitive epithelial sodium channels (ENacs; Ninomiya & Funakoshi, 1988; Chandrashekar et al. 2010), which may mediate amiloride-sensitive sodium-specific responses (N-type), and transient receptor potential V1 variant (TRPV1t; Lyall et al. 2004), which may mediate amiloride-insensitive sodium non-specific responses (E-type). However, amiloride-sensitive sodium responses (N-type) were found in taste cells that do not express gustducin or GAD67 (Yoshida et al. 2009; Yoshida & Ninomiya, 2010). As with the classification of the responsiveness of taste cells, mouse single taste nerve fibres sensitive to MSG have been classified into S-type, M-type, E-type and N-type (Ninomiya et al. 1989b, 2000). It seems likely that MSG responses in E-type and N-type fibres may be due mainly to the ionic component of MSG (sodium ion), whereas S-type and M-type fibre responses to MSG may be mediated by activation of receptors by glutamate. At present, which candidate receptor(s) and transduction component(s) underlie glutamate responses of S-type and M-type cells and fibres is largely unknown.
To address these issues, we recorded single CT nerve fibre responses of WT, T1R3-KO and TRPM5-KO mice to various taste stimuli, including glutamate with and without IMP, l-amino acids and other basic taste compounds. Based on their response profiles, we assessed fibre types among these three mouse strains. We also examined effects of mGluR antagonists on the glutamate responses in each fibre type. Our results suggest that multiple receptors and transduction pathways underlie glutamate taste responses in mice. We find T1R3-dependent components, mGluR components, TRPM5-dependent and TRPM5-independent components. Taste information derived from each receptor and transduction pathway may be conveyed by different fibre types of the CT nerve.
Methods
All experimental procedures were approved by the committee for Laboratory Animal Care and Use at Kyushu University (Fukuoka Japan). The animals used were adult male and female C57BL/6JCrj (WT) mice (Charles River Japan, Tokyo, Japan), T1R3- and TRPM5-KO mice, originally developed at Mount Sinai Medical School (Damak et al. 2003, 2006) from the C57BL/6J strain, 8–20 weeks of age, ranging in weight from 20 to 35 g.
Recordings of single-fibre responses from CT nerves
Mice were anaesthetized with an injection of sodium pentobarbital (40–50 mg kg−1i.p.) and maintained at a surgical level of anaesthesia with supplemental injections of sodium pentobarbital (8–10 mg kg−1i.p. approximately every hour). The anaesthetic level was evaluated by testing the withdrawal reflex to a paw pinch. The procedures for dissection and recording of responses were as used previously (Ninomiya, 1998; Yasumatsu et al. 2003). Under pentobarbital anaesthesia, the trachea of each mouse was cannulated, and the mouse was then fixed in the supine position with a head holder to allow dissection of the CT nerve. The right CT nerve was dissected free from surrounding tissues after removal of the pterygoid muscle and cut at the point of its entry to the tympanic bulla. A single fibre or a few fibres of the nerve were teased apart with a pair of needles and lifted onto an Ag–AgCl electrode. An indifferent electrode was placed in nearby tissue. Impulse discharges resulting from chemical stimulations of the tongue were fed into an amplifier (K-1; Iyodenshikogaku, Nagoya, Japan), monitored on an oscilloscope and audiomonitor, and recorded on a computer for later analysis using a PowerLab system (PowerLab/sp4; ADInstruments, Bella Vista, NSW, Australia). At the end of the experiment, animals were killed by the administration of an overdose of the anaesthetic agent.
Chemical stimulation of the tongue
The anterior half of the tongue was enclosed in a flow chamber made of silicone rubber (Ninomiya & Funakoshi, 1981). Solutions were delivered into the chamber by gravity flow and flowed over the tongue for a controlled period. Solutions used were as follows: 0.1 m NaCl with and without 30 μm amiloride (Ami; Sigma-Aldrich, St Louis, MO, USA), 10 mm and 0.1 m KCl, 0.01 m HCl, 0.02 m quinine hydrochloride (QHCl), 0.5 m sucrose (Suc), 0.1 m l-alanine (l-Ala), 0.1 m l-cysteine (l-Cys), 0.1 m l-arginine hydrochloride (l-Arg; Wako Pure Chemicals, Osaka, Japan), 0.1 m monopotassium glutamate (MPG; Sigma), 0.1 m l-lysine hydrochloride (l-Lys) and 0.5 mm IMP (Ajinomoto, Tokyo, Japan). These chemicals were dissolved in distilled water. Agonists and antagonists for glutamate receptors used were as follows: 10 mm quisqualic acid (Quis), 10 mm l(+)-2-amino 4-phosphonobutyric acid (l-AP4), 10 mmN-methyl-d-aspartic acid (NMDA), 0.03–10 mm (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA) and 0.03–10 mm (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG; Tocris, Bristol, UK). Quisqualic acid is an agonist of group I mGluRs (mGluR1 and 5) and AMPA receptors, l-AP4 is an agonist of group III mGluRs (mGluR4, 6–8), NMDA is an agonist of NMDA receptors, AIDA is an antagonist of group I mGluRs, and CPPG is an antagonist of group III mGluRs. These agonists and antagonists were dissolved in distilled water with KOH to adjust their pH to 7.0. As previous studies have demonstrated that taste mGluRs have lower affinities than the corresponding brain-type receptors (Chaudhari et al. 2000; San Gabriel et al. 2005) and behavioural taste responses to glutamate were inhibited by high concentrations of CPPG (1 mm; Nakashima et al. 2001; Eschle et al. 2009), we used high concentrations of agonists and antagonists. To test potential effects of K+ from KOH in these solutions, KCl at the same final concentration as the added KOH was added to the array of stimulus solutions. We did not find any significant response to KCl in any tested fibres except E-type (electrolyte-best and amiloride-insensitive) fibres.
All chemicals were used at ∼24°C. During chemical stimulation of the tongue, the test solution flowed for ∼10 s at the same flow rate as the distilled water used for rinsing the tongue (∼0.1 ml s−1). The tongue was rinsed with distilled water for an interval of ∼1 min between successive stimulations. The order of chemical stimulation for the initial survey to identify a specific taste-responsive fibre was 0.5 m Suc, 0.1 m NaCl, 0.1 m MPG without and with 0.5 mm IMP, 0.01 m HCl and 0.02 m QHCl. If the fibre responded to any of the stimuli, then stimulation continued with taste compounds in the following order: 0.1 m KCl, 0.5 mm IMP, 0.1 m NaCl with 30 μm Ami, 0.1 m l-Ala, 0.1 m l-Cys, 0.1 m l-Lys, 0.1 m l-Arg, and each of these four amino acids with 0.5 mm IMP, and 10 mm l-AP4, 10 mm Quis, 10 mm NMDA, and each of these three agonists with 0.5 mm IMP, and 15 mm KCl (vehicle for agonists). In some fibres, responses were also examined to 0.1 m MPG with 0.03–10 mm AIDA or with 0.03–10 mm CPPG, and each of these solutions with 0.5 mm IMP.
Data analysis
In the analysis of single-fibre responses, single fibres were identified with the help of spike waveform analysis (PowerLab/sp4; ADInstruments). We used waveform shape parameters (width, height, peak amplitude, antipeak amplitude and interspike interval) to segregate each single unit (Ninomiya, 1998; Kawai et al. 2000; Yasumatsu et al. 2003). Frequency–time histograms of impulse discharges before, during and after chemical stimulation of the tongue were calculated by means of spike-analysis programs (SAS-1; Iyodensikogaku; and Spike histogram; ADInstruments). For data analysis, we used the net average frequency for the first 10 s after the stimulus onset, which was obtained by subtracting the spontaneous frequency for the 10 s period before stimulation. The final criteria for the occurrence of a response were as follows: the number of spikes was larger than the mean plus two standard deviations of the spontaneous discharge for three 10 s periods before stimulation, and at least three spikes were evoked by taste stimulation. Thus, for fibres without any spontaneous discharge or with very low rates of spontaneous discharge, three spikes were considered a response.
For classification of fibre types, we defined the occurrence of synergistic responses between MPG and IMP as the response magnitude of the fibre to the mixture of MPG and IMP as being 110% or larger than the sum of responses to each component.
Hierarchical cluster analysis was used to determine the extent to which the various response profiles fell into meaningful clusters (Ninomiya et al. 1982). The clustering program (Excel ad-in software for statistical analysis; SSRI, Tokyo, Japan) processed the fibre profiles based on a matrix of the Pearson correlation coefficients between all possible pairs of profiles and amalgamated the fibre sequentially into the cluster solution using the Ward method.
The χ2 test was used to test whether the frequency of each type of fibre differed statistically between two strains of mice. Repeated-measures ANOVA and Fisher's post hoc test or Student's paired t test were used to evaluate statistically the effects of the addition of IMP on CT fibre responses to amino acids and the effect of mGluR antagonists on CT fibre responses to MPG. Multivariate ANOVA (MANOVA) and Tukey's post hoc HSD test were used to assess response profiles of each type of fibre among three strains of mice. Calculations were performed using the statistical software package IBM SPSS Statistics (IBM, New York, NY, USA).
Results
Classification of single chorda tympani nerve fibres in wild-type mice
In an initial survey of 86 single fibres in WT mice that responded to MPG and/or NaCl, we determined spike activities in response to various taste stimuli. Among the MPG- and/or NaCl-responsive fibres we first segregated S-type and M-type fibres that, respectively, evoked the maximal net response (i.e. the best stimulus for the response) to sucrose or MPG among five taste stimuli (NaCl, Suc, HCl, QHCl and MPG; Fig. 1). Next we segregated N-type and E-type fibres according to their response characteristics to electrolytes and the susceptibility of their NaCl responses to Ami (Fig. 2), as reported previously (Ninomiya, 1998). That is, N-type fibres are selectively responsive to NaCl and their responses to NaCl were largely inhibited by Ami, whereas E-type fibres are broadly sensitive to various electrolytes, including acids and salts (best responsive to HCl or NaCl among the above-mentioned five stimuli) and their responses to NaCl were not inhibited by Ami (Fig. 2). We could not find any QHCl-best fibres among the MPG and/or NaCl-responsive fibres, although we found QHCl-responsive fibres that were not sensitive to either NaCl or MPG. We further classified S- and M-type fibres into subgroups according to whether or not fibres showed synergistic responses to MPG plus IMP (see Methods); two fibre subtypes showed synergistic effects between MPG and IMP (S1 and M1; Fig. 1), while the other two subtypes exhibited no synergism (S2 and M2; Fig. 1). The number of fibres of S1-, S2-, M1-, M2-, E- and N-type was 18, 13, 8, 12, 19 and 16, respectively (Table 1). In addition to the above-mentioned classification, we also classified these fibres by using the hierarchical cluster analysis according to responses to five basic tastes and MPG + IMP (Fig. 3). In this analysis, fibres were roughly classified into four groups: S-, M-, N- and E-type. The S- and M-type fibres were further divided into two subgroups: S1, S2, M1 and M2. Although some fibres were placed into another group by the hierarchical cluster analysis, in most cases the fibres were classified in a similar manner by these two approaches. In this study, we focused on umami-sensitive fibres, and synergism between glutamate and IMP is a characteristic of umami taste. Therefore, we used the former classification rather than the classification by cluster analysis, which depends upon the overall similarity of response profiles to five basic taste stimuli and MPG + IMP.
Figure 1. Sample recordings of S1-, S2-, M1- and M2-type single fibres from the chorda tympani (CT) nerves of wild-type (WT) mice.
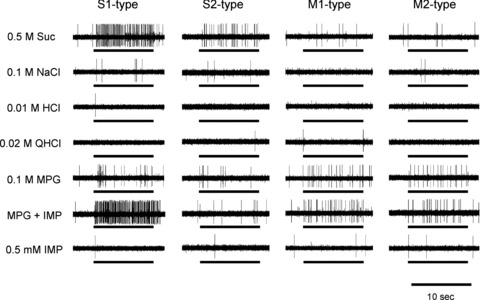
Stimuli were 0.5 m sucrose (Suc), 0.1 m NaCl, 0.01 m HCl, 0.02 m quinine hydrochloride (QHCl), 0.1 m monopotassium glutamate (MPG), 0.1 m MPG + 0.5 mm inosine monophosphate (IMP) and 0.5 mm IMP. Bars indicate application of stimuli.
Figure 2. Sample recordings of N- and E-type single fibres from the CT nerves of WT mice.
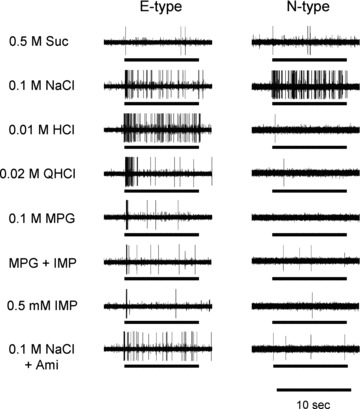
Stimuli were 0.5 m Suc, 0.1 m NaCl, 0.01 m HCl, 0.02 m QHCl, 0.1 m MPG, 0.1 m MPG + 0.5 mm IMP (MSG + IMP), 0.5 mm IMP, 0.1 m NaCl + 30 μm amiloride (Ami). Bars indicate application of stimuli.
Table 1.
Number of S1-, S2-, M1-, M2-, E- and N-type fibres in the chorda tympani of wild-type (WT), T1R3-knockout (KO) and TRPM5-KO mice
| Strain | S1 | S2 | M1 | M2 | E | N | Total |
|---|---|---|---|---|---|---|---|
| WT | 18 (20.9%) | 13 (15.1%) | 8 (9.3%) | 12 (14%) | 19 (22.1%) | 16 (18.6%) | 86 |
| T1R3-KO | 0*** (0%) | 10 (12.8%) | 6 (7.7%) | 17 (21.8%) | 26 (33.3%) | 19 (24.4%) | 78 |
| TRPM5-KO | 0*** (0%) | 11 (18%) | 8 (13.1%) | 16 (26.2%) | 17 (27.9%) | 9 (14.8%) | 61 |
Each value in parentheses indicates the percentage of total fibres in each mouse.
P < 0.001, χ2 test (vs. WT).
Figure 3. Cluster dendrogram showing the relationships among response profiles of MPG-sensitive chorda tympani (CT) fibres in WT mice.
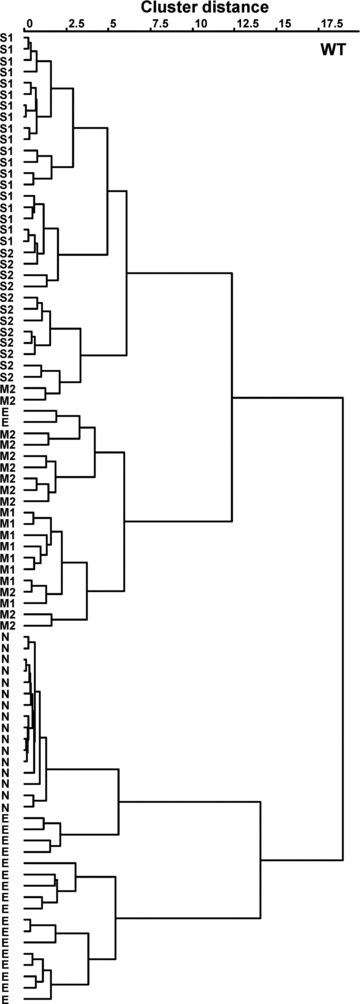
S1, S2, M1, M2, N and E indicate classification of fibre types according to the stimulus producing the maximal response, synergism by IMP and susceptibility to amiloride.
Response properties of each fibre type in wild-type mice
Figure 4 shows response profiles of six types of fibres (S1-, S2-, M1-, M2-, E- and N-type) from the CT nerves of WT mice. Each column indicates the mean number of net impulses per 10 s (mean response) of each fibre type responding to basic taste stimuli, l-amino acids or glutamate agonists with or without 0.5 mm IMP. In descending order, the mean response to MPG was: E-type (20.5 ± 2.5, mean ± SEM) > M1-type (17.4 ± 1.7) ≍ M2-type (17.0 ± 3.3) > S1-type (11.3 ± 3.0) ≍ S2-type (13.0 ± 2.5) > N-type (1.0 ± 0.5). The S1-type fibres responded robustly to 0.5 m Suc, with a mean response of 99 ± 11.3 (SEM), and to the mixture of MPG plus IMP, with a mean response of 80 ± 11.4. Mean responses to the mixture of MPG with IMP were about 4.4 times larger than the sum of responses to each component, suggesting a large synergistic effect in S1-type fibres. The addition of IMP showed synergism for S1 fibre l-Cys, l-Ala, l-Arg, l-Lys, l-AP4, Quis and NMDA, despite the very small responses to each l-amino acid and agonist alone (ANOVA; Table 2). Responses of S1 fibres to mixtures of IMP with each l-amino acid, l-AP4, Quis and NMDA were significantly larger than the sum of responses to each component (Fig. 4; Student's paired t test; P < 0.001 for MPG, l-Cys, l-Arg and l-AP4; P < 0.01 for l-Ala; P < 0.05 for l-Lys, NMDA and Quis).
Figure 4. Response profiles of S1-, S2-, M1-, M2-, E- and N-type single fibres from the CT nerves of WT mice.
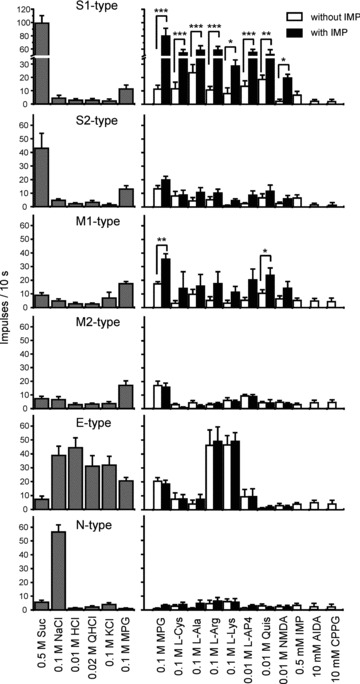
Stimuli were 0.5 m Suc, 0.1 m NaCl, 0.01 m HCl, 0.02 m QHCl, 0.1 m MPG, 0.1 m l-Cys, 0.1 m l-Ala, 0.1 m l-arginine hydrochloride (l-Arg), 0.1 m l-lysine hydrochloride (l-Lys), 10 mm l-(+)-2-amino-4-phosphonobutyrate (l-AP4), 10 mm quisqualic acid (Quis), 10 mm NMDA, 0.5 mm IMP, 10 mm (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA) and 10 mm (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG). l-Amino acids and agonists were applied with and without 0.5 mm IMP. Asterisks indicate that sum of responses to the stimulus, and 0.5 mm IMP is significantly different from the response to the mixture. Values indicated are means ± SEM. Student's paired t test; *P < 0.05; **P < 0.01; ***P < 0.001.
Table 2.
ANOVA results for the effect of addition of inosine monophosphate (IMP) on chorda tympani (CT) fibre responses to amino acids in WT, T1R3-KO and TRPM5-KO mice
| Fibre type | ||||
|---|---|---|---|---|
| Strain | S1 | S2 | M1 | M2 |
| WT | F(1,106) = 106.6*** | F(1,94) = 1.76 | F(1,72) = 4.84* | F(1,85) = 0.39 |
| T1R3-KO | Not found | F(1,68) = 3.08 | F(1,56) = 4.76 | F(1,118) = 0.09 |
| TRPM5-KO | Not found | F(1,70) = 0.08 | F(1,71) = 7.36** | F(1,124) = 0.08 |
Differences between CT fibre responses to amino acids (0.1 m MPG, 0.1 m Cys, 0.1 m l-Ala, 0.1 m l-ArgHCl, 0.1 m l-LysHCl, 10 mm l-AP4, 10 mm Quis and 10 mm NMDA) with 0.5 mm IMP and the sum of each component were assessed by two-way ANOVA. ‘Not found’, no such fibre was identified.
P < 0.05
P < 0.01
P < 0.001.
The mean response to 0.5 m Suc (42.9 ± 11.2) of S2-type sucrose-best fibres was about half that of S1-type. In S2-type fibres, the addition of IMP did not significantly potentiate responses to MPG or l-amino acids (ANOVA; Table 2). The M-type fibres were MPG-best fibres, with mean responses to 0.1 m MPG of 17.4 ± 1.7 for M1-type and 17.0 ± 3.3 for M2-type. Responses to l-amino acids were significantly enhanced by the addition of IMP in M1-type fibres but not in M2-type fibres (ANOVA; Table 2). As shown in Fig. 4, post hoc tests indicated that the addition of IMP significantly enhanced responses to MPG (Student's paired t test; P < 0.001) and Quis (P < 0.05) in M1-type fibres. The magnitude of potentiation of MPG responses by IMP in M1-type fibres was about 1.6 times (mean response, 35.5 ± 4.0), which is much smaller than that of S1-type fibres (4.4 times). The total number of impulses to MPG potentiated by IMP over the sum of responses to each component in eight M1 fibres was 121 impulses, which is about one-ninth of that (1114 impulses) in 18 S1 fibres. In M2-type fibres, l-AP4 was the most potent stimulus among various agonists for glutamate receptors tested. The E-type fibres responded to various electrolytes, such as 0.01 m HCl, 0.1 m NaCl, 0.02 m QHCl, 0.1 m KCl, 0.1 m MPG, 0.1 m l-Arg hydrochloride and 0.1 m l-Lys hydrochloride (Figs 2 and 4). Responses of E-type fibres to 0.1 m l-Arg hydrochloride and 0.1 m l-Lys hydrochloride may be due to acidic components, because the pH of these solutions was 5.3–5.4. The N-type fibres responded to NaCl specifically, and the response was greatly inhibited by Ami (Figs 2 and 4).
Response properties of each fibre type in T1R3- and TRPM5-KO mice
It is noteworthy that in T1R3- and TRPM5-KO mice, no S1-type fibres were found (Table 1). Also in the hierarchical cluster analysis, the S1-type group was not observed in T1R3- and TRPM5-KO mice (Fig. 5). Figure 6 shows response profiles of the S2-, M1-, M2-, E- and N-type fibres from the CT nerves in T1R3- and TRPM5-KO mice. Response profiles of M1-, M2-, E- and N-type fibres did not differ significantly among WT, T1R3- and TRPM5-KO mice, suggesting that glutamate responses of these fibre types are independent of T1R3 and TRPM5 (MANOVA; Table 3). In contrast, the response profiles of S2-type fibres in T1R3- and TRPM5-KO mice differed from those of WT mice (MANOVA; Table 3), where mean responses to 0.5 m sucrose were much smaller in T1R3-KO mice (13.4 ± 1.3) and TRPM5-KO mice (16.3 ± 3.0) than in WT mice (42.9 ± 11.2, Tukey's post hoc HSD test; Table 4). Thus, the overall responses to sucrose were very small in the two KO strains, as previously reported (Damak et al. 2003, 2006). As with WT mice, M1-type, but not S2- and M2-type fibres, in both T1R3- and TRPM5-KO mice showed IMP potentiation of responses to MPG or l-amino acids (ANOVA; Table 2). In M1-type fibres, responses to MPG and Quis were significantly enhanced by the addition of IMP (Fig. 6; Student's paired t test; P < 0.01 for T1R3-KO; P < 0.05 for TRPM5-KO).
Figure 5. Cluster dendrogram showing the relationships among response profiles of MPG-sensitive CT fibres in T1R3-knockout (KO) mice (A) and TRPM5-KO mice (B).
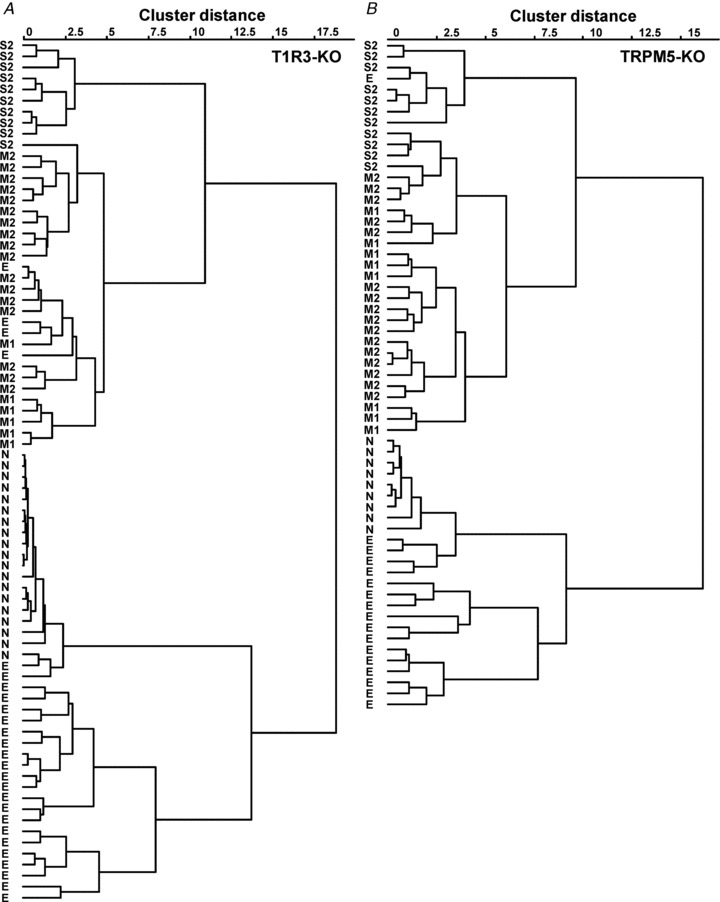
S2, M1, M2, N and E indicate classification of fibre types according to the stimulus producing the maximal response, synergism by IMP and susceptibility to amiloride.
Figure 6. Response profiles of S2-, M1-, M2-, E- and N-type single fibres from the CT nerves of T1R3- and TRPM5-KO mice.
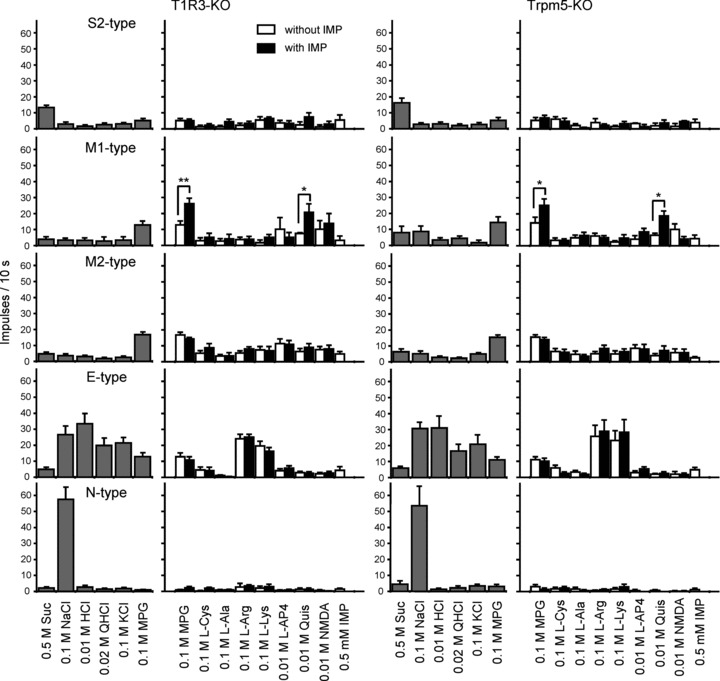
Stimuli were 0.5 m Suc, 0.1 m NaCl, 0.01 m HCl, 0.02 m QHCl, 0.1 m MPG, 0.1 m l-Cys, 0.1 m l-Ala, 0.1 m l-Arg, 0.1 m l-Lys, 10 mm l-AP4, 10 mm Quis, 10 mm NMDA and 0.5 mm IMP. l-Amino acids and agonists are applied with and without 0.5 mm IMP. Asterisks indicate that the sum of responses to the stimulus and 0.5 mm IMP is significantly different from response to the mixture. Values indicated are means ± SEM. Student's paired t test; *P < 0.05; **P < 0.01; ***P < 0.001.
Table 3.
MANOVA results for comparisons of the response profiles of S2-, M1-, M2-, E- and N-type fibres in the CT nerves of WT, T1R3-KO and TRPM5-KO mice
| Fibre type | |||||
|---|---|---|---|---|---|
| MANOVA result | S2 | M1 | M2 | E | N |
| Degree of freedom | 10, 56 | 10, 32 | 10, 78 | 10, 112 | 10, 76 |
| F value | 2.020 | 0.714 | 1.735 | 1.004 | 1.240 |
| P value | 0.048 | 0.705 | 0.088 | 0.444 | 0.280 |
The effects of the strain (WT, T1R3-KO and TRPM5-KO) on the response profiles of each fibre type (0.5 m Suc, 0.1 m NaCl, 0.01 m HCl, 0.02 m QHCl and 0.1 m MPG) were tested with a MANOVA using Pillai's trace statistic.
Table 4.
Results of Tukey's post hoc HSD test for comparisons of response profiles of S2-type fibres between WT, T1R3-KO and TRPM5-KO mice
| Strains compared | Suc | NaCl | HCl | QHCl | MPG |
|---|---|---|---|---|---|
| WT vs. T1R3-KO | 0.001** | 0.945 | 1.000 | 0.957 | 0.064 |
| WT vs. TRPM5-KO | 0.001** | 0.826 | 0.339 | 0.987 | 0.084 |
| T1R3-KO vs. TRPM5-KO | 0.997 | 0.967 | 0.371 | 0.896 | 0.982 |
The values are P values.
P < 0.01.
Inhibition of M-type fibre responses by mGluR1 and mGluR4 antagonists
We next tested potential effects of antagonists of mGluR1 (AIDA; group I mGluR antagonist) and mGluR4 (CPPG; group III mGluR antagonist) on MPG responses of five types of CT fibres in WT mice. In M1-type fibres, 0.1–10 mm AIDA significantly inhibited responses to 0.1 m MPG (repeated-measures ANOVA; F(1,40) = 11.40, P < 0.01; Fisher's post hoc test; P < 0.05 for 0.1–10 mm) and MPG with IMP (F(1,40) = 18.31, P < 0.01; post hoc P < 0.05 for 0.1–10 mm), while CPPG had no significant effect on M1-fibre responses to MPG with or without IMP (P > 0.05; Fig. 7). In contrast, in M2-type fibres, 0.1–10 mm CPPG significantly inhibited responses to MPG (F(1,50) = 23.72, P < 0.001; post hoc P < 0.05 for 0.1 and 1 mm; P < 0.01 for 0.3, 3 and 10 mm) and MPG with IMP (F(1,40) = 60.96, P < 0.001; post hoc P < 0.05 for 0.1 mm, P < 0.01 for 0.3, 3 and 10 mm), while AIDA did not influence M2 fibre responses to umami compounds (P > 0.05; Fig. 7). These two mGluR antagonists specifically affected either M1- or M2-type fibres (but not both) and did not influence responses to umami compounds of any other fibre types (P > 0.05; Fig. 8), indicating a differential and selective contribution of mGluR1 and mGluR4 to umami responses in M1 and M2 fibres, respectively. We also tested antagonists for glutamate receptors, AIDA and CPPG, at the highest concentration we used, and there were no significant responses in all types of fibres.
Figure 7. Dose-dependent effect of AIDA and CPPG on responses to 0.1 m MPG (open circles) or 0.1 m MPG + 0.5 mm IMP (filled circles) in M1-type (n = 5) and M2-type fibres (n = 6).
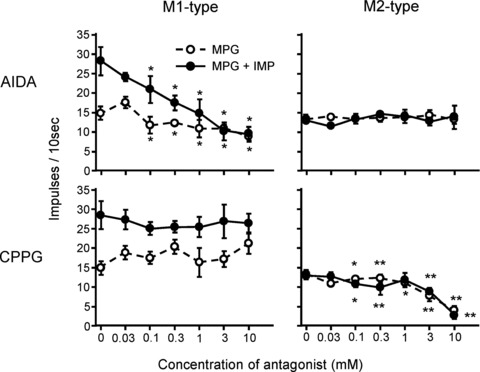
Top panels show the effect of AIDA and bottom panels show the effect of CPPG. Values indicated are means ± SEM. Fisher's post hoc test; *P < 0.05; **P < 0.01.
Figure 8. The effect of metabotropic glutamate receptor (mGluR) antagonists on taste responses of gustatory fibres.
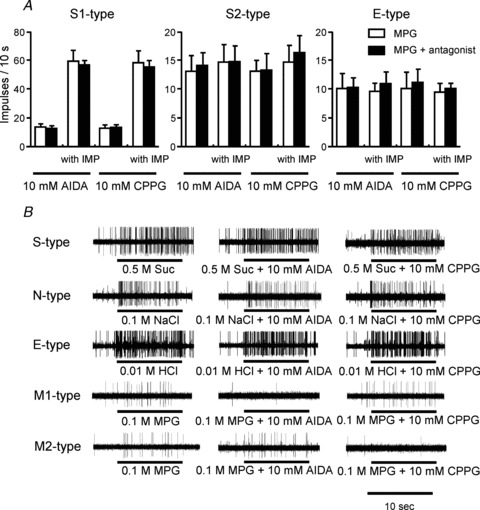
A, the effect of mGluR antagonists on responses to 0.1 m MPG or 0.1 m MPG + 0.5 mm IMP in S1- (n = 7), S2- (n = 5) and E-type fibres (n = 6). Columns indicate sum of responses to antagonist and 0.1 m MPG or 0.1 m MPG + 0.5 mm IMP with (filled) or without (open) 10 mm AIDA or 10 mm CPPG. Values indicated are means + SEM. There are no significant differences (Student's paired t test; P > 0.05). B, sample recordings showing the effect of mGluR antagonists on responses to 0.5 m Suc, 0.1 m NaCl, 0.01 m HCl and 0.1 m MPG in S-, N-, E-, M1 and M2-type fibres, respectively.
Discussion
The following five potential umami receptors have been identified in taste cells: T1R1+T1R3, taste-mGluR1, taste-mGluR4, full-length mGluR1 and full-length mGluR4. We used single-fibre nerve recording to investigate the potential roles of these candidate umami receptors and their associated transduction mechanisms in vivo. We first examined single CT nerve fibres in WT, T1R3- and TRPM5-KO mice that responded to glutamate according to their best responding stimulus among five basic taste compounds, and the occurrence of potentiation of glutamate responses by IMP. In WT mice glutamate-responding nerve fibres could be classified into the following four types: Suc-best S1 and S2, and MPG-best M1 and M2. Potentiation of glutamate responses by IMP was seen in S1- and M1-type fibres, but not in S2- and M2-type fibres. In T1R3- and TRPM5-KO mice, only S1-type fibres were absent; S2-, M1- and M2-type fibres were still present.
Among the four types of fibres, S1 is characterized by robust responses to sweet compounds and a large potentiation of glutamate responses by IMP. In addition, S1 fibres also exhibited substantial responses to l-amino acids, such as l-Cys, l-Ala, l-Arg and l-Lys, and to l-AP4 and Quis; these responses were enhanced by the addition of IMP. Such response characteristics are comparable with those found when mouse T1R1+T1R3 is expressed in HEK 293 cells (Fig. 1 in Nelson et al. 2002), suggesting that T1R1+T1R3 plays a crucial role in the amino acid responses potentiated by IMP in S1-type fibres. The loss of S1-type fibres by genetic deletion of T1R3 further supports the involvement of T1R1+T1R3 in amino acid responses. Mice genetically lacking T1R3 selectively lose their S1-type fibres, show greatly reduced responses to sweet compounds, and mostly lose the synergism of IMP with MPG. These observations are consistent with previous results of whole-CT response recordings in T1R3-KO mice (Damak et al. 2003). Expression studies in mice reported that some T1R1-positive cells coexpress T1R2 (and T1R3) in taste buds (58%, Kim et al. 2003; 10–17%, Stone et al. 2007), and studies of single taste cell responses in mice showed that some glutamate-responsive taste cells also respond to sweet compounds in the fungiform (60%, Yoshida et al. 2009) and circumvallate papillae (30%, Tomchik et al. 2007). Furthermore, our recent study showed that glutamate-sensitive Suc-best taste cells, analogous to S1- and S2-types, exist in mouse fungiform taste buds (Niki et al. 2011). Such consistent findings from taste cell to single fibre suggest strongly that glutamate-responsive S1-type fibres may receive taste information from taste cells that express all three T1Rs and thus possess both sweet (T1R2+T1R3) and umami (T1R1+T1R3) taste receptors.
The S2-type fibres remain in T1R3-KO mice, although the responses of these fibres to sucrose were largely reduced (Fig. 6). Therefore, responses of S2-type fibres to sucrose may be derived from two pathways, both T1R3-dependent and independent pathways. This remaining component of sucrose responses may be comparable to those observed in previous studies with whole-CT nerve response recordings (Damak et al. 2003; Ohkuri et al. 2009) and behavioural experiments in T1R3-KO mice (Damak et al. 2003; Zhao et al. 2003). It has been reported in T1R3-KO mice that complete loss of sweet responses is only observed for non-caloric sweetener, such as SC45647 (Damak et al. 2003; Ohkuri et al. 2009), indicating that such sweetener requires the presence of T1R3. Other sweet compounds, such as sugars, may bind to T1R2 (Nie et al. 2005) or utilize proposed T1R-independent sugar sensors, such as glucose transportes (GLUTs), sodium-glucose transporter 1 (SGLT1) and ATP-gated K+ channel (KATP) (Yee et al. 2011). The remaining weak responses of S2-type fibres to sucrose in T1R3-KO mice found in this study may originate from taste cells expressing T1R-independent sugar sensors.
Our results showed that two different mGluR antagonists, AIDA for group I mGluRs and CPPG for group III mGluRs, separately affected MPG responses in either M1- or M2-type fibres and did not influence responses to umami compounds in any other types of fibres (S1-, S2- and E-type). In addition, M1- and M2-type fibres remained in T1R3-KO mice, and their response profiles were not significantly different from those in WT mice. Several reports demonstrated the expression of brain- and taste-mGluR1 and mGluR4 receptors in taste buds and the lower affinity of taste-mGluR1 and mGluR4 to glutamate than that of the brain-type receptors (Chaudhari et al. 2000, 2009; Toyono et al. 2002, 2003; San Gabriel et al. 2005, 2009). Both mGluR1 and mGluR4 may function as umami taste receptors in taste bud cells independent of T1R1 or T1R3. Previous studies reported that CPPG inhibited responses to glutamate and l-AP4 in isolated rat taste cells (100 μm, Lin & Kinnamon, 1999), as well as behavioural responses to these compounds in rats (1 mm, Eschle et al. 2009) and mice (1 mm, Nakashima et al. 2001). We found that M2-type fibres transmit the CPPG-sensitive components of glutamate responses in mice. Furthermore, we found the existence of AIDA-sensitive components of glutamate responses that were transmitted via M1-type fibres. We do not know why M1-type fibres showed responses to the mGluR4 agonist 10 mm l-AP4, but this may be due to non-specific effects of using high concentrations of l-AP4 on glutamate receptors in M1-type taste cells. These other glutamate receptor systems present in WT and T1R3-KO mice, perhaps together with NMDA receptors (Hayashi et al. 1996; Lin and Kinnamon, 1999), may underlie the persistence of behavioural discrimination between umami and other basic tastes (Delay et al. 2006) and umami-evoked Ca2+ responses of rare T1R1-expressing circumvallate taste bud cells (Maruyama et al. 2006) even in the absence of T1R3.
Responses of MPG-best M1 fibres to l-amino acids were significantly enhanced by IMP (Table 2). As noted above, a substantial portion of glutamate responses of M1 fibres may be derived from taste cells expressing mGluR1. The T1Rs and all mGluRs are group C G-protein-coupled receptors, and it is well known that mGluR1 forms a homodimer (Okamoto et al. 1998; Robbins et al. 1999). A recent molecular study proposed that the extracellular Venus flytrap domain of T1R1 is the key site responsible for the large synergism of umami responses, where glutamate binds close to the hinge region, and IMP binds to an adjacent site close to the opening of the flytrap to increase the stability of the closed formation (Zhang et al. 2008). Several positive and selective allosteric potentiators of mGluR1 (e.g. Ro 01-6128 and Ro 67-4853) have been reported (Hemstapat et al. 2006); however, these potentiators are thought to bind to the transmembrane domain of mGluR1. To our knowledge, it is unknown whether IMP could act as a positive potentiator for mGluR1. It is most likely that T1R1 underlies the synergism of glutamate and IMP observed in M1 cells.
Our results provide new insights into umami taste transduction. The finding that both T1R3-KO and TRPM5-KO mice lacked S1-type fibres suggests that TRPM5 acts as a downstream signalling molecule in those taste cells that express T1R3. The potentiation of glutamate by IMP is greatly reduced in T1R3-KO mice (Damak et al. 2003) and TRPM5-KO mice (Zhang et al. 2003; Damak et al. 2006), as well as in mice lacking other transduction components, such as α-gustducin, α-transducin (He et al. 2004), phospholipase C β2 and IP3R3 (Hisatsune et al. 2007). Some double-labelling studies reported coexpression of T1Rs and α-gustducin in mouse fungiform papillae (Kim et al. 2003), T1Rs and TRPM5 in the mouse circumvallate papilla (Zhang et al. 2003), and IP3R3 and phospholipase C β2 in the rat circumvallate papilla (Asano-Miyoshi et al. 2001). These reports, together with the present data, indicate that these proteins function downstream of T1R1+T1R3 to mediate umami responses. In contrast, S2-, M1-, M2-, E- and N-type fibres remain in the absence of TRPM5, suggesting that taste cells connecting with those fibres can respond to taste stimuli via transduction pathways other than the above-mentioned TRPM5 pathway. Several reports have shown that mGluR1a activation increases cAMP accumulation in oocytes and transfected Chinese hamster ovary cells (Aramori & Nakanishi, 1992), baby hamster kidney cells (Thomsen 1996; Hermans et al. 2000) or HEK 293 cells (Francesconi & Duvoisin, 1998; Parmentier et al. 1998). It is generally accepted that mGluR4 is linked to inhibition of adenyl cyclase to decrease cAMP (Pin & Duvoisin, 1995). Thus, cAMP may play a role in transduction of M1- and M2-type taste cells.
In this study, we demonstrated the existence of multiple receptors and transduction pathways for umami taste. What, then, are the roles of signals mediated by the transduction pathways involving T1R3 vs. taste mGluRs?Zhao et al. (2003) demonstrated that the behavioural preference for glutamate and other amino acids in the short-term lick test was absent in their T1R3-KO mice. However, Damak et al. (2003) demonstrated that preference for glutamate in 24 h two-bottle preference tests was reduced but not abolished in another T1R3-KO mouse model. Thus, both T1R3-KO models showed significantly reduced preference for glutamate, indicating that the T1R3-dependent signal may play a major role in preference behaviour of mice. Consistent with this, T1R3-dependent and TRPM5-dependent S1-type fibres convey not only glutamate signals but also sweet signals that induce strong preference behaviour. In addition, Delay et al. (2006) demonstrated that detection thresholds of T1R3-KO mice and wild-type mice were nearly identical for sucrose and MSG, and T1R3-KO mice were still able to discriminate between the tastes of sucrose and MSG even when the cue function of the sodium component of MSG was reduced or neutralized. This indicates that T1R3-KO mice still have the ability to encode information about the qualitative features of sweet and umami taste substances. Thus, T1R3-independent mGluR pathways for glutamate taste may contribute to transmission of qualitative features of umami taste and discrimination between umami and other taste compounds. However, further studies are required to reveal the behavioural significance of each component of glutamate signals in the gustatory nerve.
Concerning the KO mice, somewhat different data were obtained from two independently generated T1R3-KO mouse models. In one T1R3-KO model, Zhao et al. (2003) showed that a behavioural (lickometer) preference for glutamate and CT nerve responses to glutamate were totally absent, suggesting that T1R1+T1R3 is essential for glutamate detection and perception in mice. In contrast, using a different T1R3-KO model, Damak et al. (2003) found that preference for glutamate in 24 h two-bottle preference tests was reduced but not abolished. Furthermore, their T1R3-KO mice exhibited a severe loss of synergistic responses of the CT nerve to glutamate and IMP, but no large reduction was observed in response to glutamate alone in both CT and GL nerves, although the sodium component of MSG was not controlled by amiloride. It should be noted that the CT nerve contains the amiloride-sensitive Na+-responsive fibres, whereas the GL nerve lacks those fibres (Ninomiya, 1998), suggesting that GL nerve responses were not inhibited by amiloride. Furthermore, Yasuo et al. (2008) demonstrated that the same line of T1R3-KO mice showed a severe loss of synergistic responses of the CT nerve to MPG and IMP and no large reduction of CT and GL nerve responses to MPG, which do not contain the sodium component. These data indicate that T1R3 is crucial for the synergistic response to glutamate and ribonucleotides, but receptors other than T1R1+T1R3 may also be involved in taste responses to glutamate in mice. Significant differences between the two different T1R3-KO models and how they were analysed may contribute to some of these differences in nerve responses to umami stimuli. The T1R3-KO model of Damak et al. (2003) lacks the entire T1R3 coding region and the gene promoter and expresses no T1R3 protein. This mouse was generated in C57BL/6J embryonic stem cells, maintained in that background and compared with littermates of the same C57BL/6J background. The T1R3-KO model of Zhao et al. (2003) deleted the amino terminal extracellular domain but not the seven transmembrane helices of T1R3. This mouse was generated in 129 embryonic stem cells, backcrossed for two generations with C57BL/6 mice and compared with 129X1/SvJ and C57BL/6 mice. In behavioural analysis, 24 h two-bottle preference tests may retain the variable of postingestive cues that may affect the animal's preference behaviour, whereas the effect of postinjestive cues may be little in lickometer preference tests. As same as T1R3-KO models, somewhat contradictory results were obtained from the TRPM5-KO models generated by Damak et al. (2006) and Zhang et al. (2003). The TRPM5-KO model of Damak et al. (2006) lacks the promoter and exons 1–4 and expresses no TRPM5 protein. This mouse was generated in C57BL/6J embryonic stem cells, maintained in that background and compared with littermates of the same C57BL/6J background. In the TRPM5-KO model of Zhang et al. (2003), five of the six transmembrane helices of TRPM5 were deleted, but upstream promoter region of the gene was retained, along with exons 1–14 encoding most of the amino-terminal portion of the gene. This mouse was generated in 129 embryonic stem cells, backcrossed for two generations with C57BL/6 mice and compared with 129X1/SvJ and C57BL/6 mice. Even if there are some differences in methods, we think it is necessary to investigate umami reception and transduction pathways by using many kinds of approaches in future study.
In conclusion, the data from the present study provide the first physiological evidence for the existence of three distinct umami taste information pathways in the mouse CT nerve, comprised of different receptors, transduction pathways and nerve fibres. Information for l-amino acids, including glutamate, initiated from the T1R1+T1R3 heterodimer may be mediated by phospholipase C β2, IP3 and TRPM5, and conveyed by S1 sweet-best fibres. In addition, glutamate-selective taste information initiated by AIDA-sensitive (mGluR1) or CPPG-sensitive (mGluR4) receptors may be transduced by a pathway(s) that does not require TRPM5, and conveyed by M1 and M2 glutamate-best fibres, respectively. The existence of multiple glutamate taste pathways may account for the persistence of behavioural discrimination between glutamate and other basic taste compounds in the absence of T1R3 or TRPM5.
Acknowledgments
This work was supported by KAKENHI 18109013, 18077004 and 23249081 (to Y.N.), 19592149 (to K.Y.) and 23689076 (to R.Y.) for Scientific Research from Japan Society for the Promotion of Science.
Glossary
Abbreviations
- AIDA
(RS)-1-aminoindan-1,5-dicarboxylic acid
- Ami
amiloride
- CPPG
(RS)-α-cyclo-propyl-4-phosphonophenylglycine
- CT
chorda tympani
- ENaC
epithelial sodium channel
- GAD67
glutamate decarboxylase 67
- GL
glossopharyngeal
- GMP
guanosine monophosphate
- HEK
human embryonic kidney
- IMP
inosine monophosphate
- IP3R3
inositol 1,4,5-trisphosphate receptor 3
- KO
knockout
- l-Ala
l-alanine
- l-AP4
l-(+)-2-amino-4-phosphonobutyrate
- l-Arg
l-arginine hydrochloride
- l-Cys
l-cysteine
- l-Lys
l-lysine hydrochloride
- mGluR1(mGluR4)
metabotropic glutamate receptor type 1 (type 4)
- MPG
monopotassium glutamate
- MSG
monosodium glutamate
- NMDA
N-methyl-d-aspartic acid
- QHCl
quinine hydrochloride
- Quis
quisqualic acid
- Suc
sucrose
- TRPM5
transient receptor potential cation channel subfamily M member 5
- WT
wild-type
Author contributions
Y.N. and K.Y. designed and performed the research and wrote the initial draft. K.Y., Y.O., S.T. and R.Y. performed recordings of taste responses and analysed data. K.I. and K.T. designed the research and analysed data. R.F.M. provided knockout mice and critically revised multiple drafts of this article. All authors read and approved the final version. All experiments were done in the Graduate School of Dental Sciences, Kyushu University.
References
- Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Asano-Miyoshi M, Abe K, Emori Y. IP3 receptor type 3 and PLCβ2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 2001;26:259–265. doi: 10.1093/chemse/26.3.259. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nature Neurosci. 2000;3:113–119. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr. 2009;90:738S–742S. doi: 10.3945/ajcn.2009.27462H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper SD. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci. 1996;16:3817–3826. doi: 10.1523/JNEUROSCI.16-12-03817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1R3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 2006;31:351–357. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- Dewis ML, DeSimone JA, Phan THT, Heck GL, Lyall V. Effect of N-geranyl cyclopropylcarboxamide (NGCC) on TRPV1 variant salt taste receptor (TRPV1t) Chem Senses. 2006;31:A105. [Google Scholar]
- Eschle BK, Eddy MC, Delay ER. Antagonism of metabotropic glutamate receptor 4 receptors by (RS)-alpha-cyclopropyl-4-phosphonophenylglycine alters the taste of amino acids in rats. Neuroscience. 2009;163:1292–1301. doi: 10.1016/j.neuroscience.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Francescini A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998;273:5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Zviman MM, Brand JG, Teeter JH, Restrepo D. Measurement of membrane potential and [Ca2+]i in cell ensembles: application to the study of glutamate taste in mice. Biophys J. 1996;71:1057–1070. doi: 10.1016/S0006-3495(96)79306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. Umami taste responses are mediated by α-transducin and α-gustducin. J Neurosci. 2004;24:7674–7680. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellekant G, Danilova V, Ninomiya Y. Primate sense of taste: behavioral and single chorda tympani and glossopharyngeal nerve fiber recordings in the rhesus monkey, Macaca mulatta. J Neurophysiol. 1997;77:978–993. doi: 10.1152/jn.1997.77.2.978. [DOI] [PubMed] [Google Scholar]
- Hemstapat K, Paulis deT, Chen Y, Brady AE, Grover VK, Alagille D, Tamagnan GD, Conn PJ. A novel class of positive allosteric modulators of metabotropic glutamate receptor subtype 1 interact with a site distinct from that of negative allosteric modulators. Mol Pharmacol. 2006;70:616–626. doi: 10.1124/mol.105.021857. [DOI] [PubMed] [Google Scholar]
- Hermans E, Saunders R, Selkirk JV, Mistry R, Nahorski SR, Challiss RAJ. Complex involvement of pertussis toxin-sensitive G proteins in the regulation of type 1α metabotropic glutamate receptor signaling in baby hamster kidney cells. Mol Pharmacol. 2000;58:352–360. doi: 10.1124/mol.58.2.352. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2007;282:37225–37231. doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- Katsumata T, Nakakuki H, Tokunaga C, Fujii N, Egi M, Phan TH, Mummalaneni S, DeSimone JA, Lyall V. Effect of Maillard reacted peptides on human salt taste and the amiloride-insensitive salt taste receptor (TRPV1t) Chem Senses. 2008;33:665–680. doi: 10.1093/chemse/bjn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun. 2003;312:500–506. doi: 10.1016/j.bbrc.2003.10.137. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kinnamon SC. Physiological evidence for ionotropic and metabotropic glutamate receptors in rat taste cells. J Neurophysiol. 1999;82:2061–2069. doi: 10.1152/jn.1999.82.5.2061. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci. 2006;26:2227–2234. doi: 10.1523/JNEUROSCI.4329-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Katsukawa H, Sasamoto K, Ninomiya Y. Behavioral taste similarities and differences among monosodium L-glutamate and glutamate receptor agonists in C57BL mice. J Nutr Sci Vitaminol (Tokyo) 2001;47:161–166. doi: 10.3177/jnsv.47.161. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Niki M, Takai S, Kusuhara Y, Ninomiya Y, Yoshida R. Responses to apical and basolateral application of glutamate in mouse fungiform taste cells with action potentials. Cell Mol Neurobiol. 2011;31:1033–1040. doi: 10.1007/s10571-011-9702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y. Reinnervation of cross-regenerated gustatory nerve fibers into amiloride-sensitive and amiloride-insensitive taste receptor cells. Proc Natl Acad Sci USA. 1998;95:5347–5350. doi: 10.1073/pnas.95.9.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Role of ions in generation of taste nerve responses to electrical tongue stimulation in rats. Jpn J Physiol. 1981;31:891–902. doi: 10.2170/jjphysiol.31.891. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res. 1988;451:319–325. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Behavioral discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol. 1989a;92:365–370. doi: 10.1016/0300-9629(89)90577-x. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol. 1989b;92:371–376. doi: 10.1016/0300-9629(89)90578-1. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr. 2000;130:950S–953S. doi: 10.1093/jn/130.4.950S. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Tonosaki K, Funakoshi M. Gustatory neural response in the mouse. Brain Res. 1982;244:370–373. doi: 10.1016/0006-8993(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu Y, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R960–R971. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Sekiyama N, Otsu M, Shimada Y, Sato A, Nakanishi S, Jingami H. Expression and purification of the extracellular ligand binding region of metabotropic glutamate receptor subtype 1. J Biol Chem. 1998;273:13089–13096. doi: 10.1074/jbc.273.21.13089. [DOI] [PubMed] [Google Scholar]
- Parmentier ML, Loly C, Restituito S, Bockaert J, Greu Y, Pin JP. The G protein-coupling profile of metabotropic glutamate receptors, as determined with exogenous G proteins, is independent of their ligand recognition domain. Mol Pharmacol. 1998;53:778–786. [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and function. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Robbins MJ, Ciruela F, Rhodes A, McIlhinney RA. Characterization of the dimerization of metabotropic glutamate receptors using an N-terminal truncation of mGluR1α. J Neurochem. 1999;72:2539–2547. doi: 10.1046/j.1471-4159.1999.0722539.x. [DOI] [PubMed] [Google Scholar]
- Gabriel SanA, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr. 2009;90:743S–746S. doi: 10.3945/ajcn.2009.27462I. [DOI] [PubMed] [Google Scholar]
- Gabriel SanA, Uneyama H, Yoshie S, Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for l-glutamate stimuli. Chem Senses. 2005;30:i25–i26. doi: 10.1093/chemse/bjh095. [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Luellig M, Roper SD, Delay ER. Discrimination between the tastes of sucrose and monosodium glutamate in rats. Chem Senses. 2002;13:95–113. doi: 10.1093/chemse/27.4.375. [DOI] [PubMed] [Google Scholar]
- Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and gustducin in palatal taste buds of mice. Chem Senses. 2007;32:255–262. doi: 10.1093/chemse/bjl053. [DOI] [PubMed] [Google Scholar]
- Thomsen C. Metabotropic glutamate receptor subtype 1A activates adenylate cyclase when expressed in baby hamster kidney cells. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:709–726. doi: 10.1016/0278-5846(96)00042-5. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyono T, Seta Y, Kataoka S, Harada H, Morotomi T, Kawano S, Shigemoto R, Toyoshima K. Expression of the metabotropic glutamate receptor, mGluR4a, in the taste hairs of taste buds in rat gustatory papillae. Arch Histol Cytol. 2002;65:91–96. doi: 10.1679/aohc.65.91. [DOI] [PubMed] [Google Scholar]
- Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res. 2003;313:29–35. doi: 10.1007/s00441-003-0740-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. The synergistic taste effect of monosodium glutamate and disodium 5′-inosinate. J Food Sci. 1970;32:473–478. [Google Scholar]
- Yang H, Wanner IB, Roper SD, Chaudhari N. An optimized method for in situ hybridization with signal amplification that allows the detection of rare mRNAs. J Histochem Cytochem. 1999;47:431–446. doi: 10.1177/002215549904700402. [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Katsukawa H, Sasamoto K, Ninomiya Y. Recovery of amiloride-sensitive neural coding during regeneration of the gustatory nerve: behavioral/neural correlation of salt discrimination. J Neurosci. 2003;23:4362–4368. doi: 10.1523/JNEUROSCI.23-10-04362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. Multiple receptor systems for glutamate detection in the taste organ. Biol Pharm Bull. 2008;31:1833–1837. doi: 10.1248/bpb.31.1833. [DOI] [PubMed] [Google Scholar]
- Yee KK, Sukumaran SK, Kotha R, Gilbertson T, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA. 2011;108:5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 2009;587:4425–4439. doi: 10.1113/jphysiol.2009.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Ninomiya Y. New insights into signal transmission from taste cells to gustatory nerve fibers. Int Rev Cell Mol Biol. 2010;279:101–134. doi: 10.1016/S1937-6448(10)79004-3. [DOI] [PubMed] [Google Scholar]
- Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA. 2008;105:20930–20934. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJP. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]


