Abstract
One of the many actions of the carbonic anhydrase inhibitor, acetazolamide (ACZ), is to accelerate acclimatisation and reduce periodic breathing during sleep. The mechanism(s) by which ACZ may improve breathing stability, especially at high altitude, remain unclear. We tested the hypothesis that acute i.v. ACZ would enhance cerebrovascular reactivity to CO2 at altitude, and thereby lower ventilatory drive and improve breathing stability during wakefulness. We measured arterial blood gases, minute ventilation (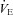 ) and middle cerebral artery blood flow velocity (MCAv) before and 30 min following ACZ administration (i.v. 10 mg kg−1) in 12 healthy participants at sea level and following partial acclimatisation to altitude (5050 m). Measures were made at rest and during changes in end-tidal
) and middle cerebral artery blood flow velocity (MCAv) before and 30 min following ACZ administration (i.v. 10 mg kg−1) in 12 healthy participants at sea level and following partial acclimatisation to altitude (5050 m). Measures were made at rest and during changes in end-tidal 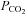 and
and 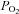 (isocapnic hypoxia). At sea level, ACZ increased resting MCAv and its reactivity to both hypocapnia and hypercapnia (P < 0.05), and lowered resting
(isocapnic hypoxia). At sea level, ACZ increased resting MCAv and its reactivity to both hypocapnia and hypercapnia (P < 0.05), and lowered resting 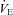 , arterial O2 saturation (
, arterial O2 saturation (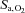 ) and arterial
) and arterial 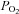 (
(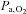 ) (P < 0.05); arterial
) (P < 0.05); arterial 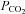 (
(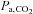 ) was unaltered (P > 0.05). At altitude, ACZ also increased resting MCAv and its reactivity to both hypocapnia and hypercapnia (resting MCAv and hypocapnia reactivity to a greater extent than at sea level). Moreover, ACZ at altitude elevated
) was unaltered (P > 0.05). At altitude, ACZ also increased resting MCAv and its reactivity to both hypocapnia and hypercapnia (resting MCAv and hypocapnia reactivity to a greater extent than at sea level). Moreover, ACZ at altitude elevated 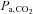 and again lowered resting
and again lowered resting 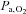 and
and 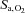 (P < 0.05). Although the
(P < 0.05). Although the 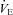 sensitivity to hypercapnia or isocapnic hypoxia was unaltered following ACZ at both sea level and altitude (P > 0.05), breathing stability at altitude was improved (e.g. lower incidence of ventilatory oscillations and variability of tidal volume; P < 0.05). Our data indicate that i.v. ACZ elevates cerebrovascular reactivity and improves breathing stability at altitude, independent of changes in peripheral or central chemoreflex sensitivities. We speculate that
sensitivity to hypercapnia or isocapnic hypoxia was unaltered following ACZ at both sea level and altitude (P > 0.05), breathing stability at altitude was improved (e.g. lower incidence of ventilatory oscillations and variability of tidal volume; P < 0.05). Our data indicate that i.v. ACZ elevates cerebrovascular reactivity and improves breathing stability at altitude, independent of changes in peripheral or central chemoreflex sensitivities. We speculate that 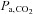 -mediated elevations in cerebral perfusion and an enhanced cerebrovascular reactivity may partly account for the improved breathing stability following ACZ at high altitude.
-mediated elevations in cerebral perfusion and an enhanced cerebrovascular reactivity may partly account for the improved breathing stability following ACZ at high altitude.
Key points
Acetazolamide improves breathing stability during sleep in newcomers to high altitude, but the mechanism remains unclear.
We examined the effects of a single i.v. dose of acetazolamide on brain vascular function and breathing at sea level and following 7 days at high altitude (5050 m).
We demonstrated that acute i.v. acetazolamide at high altitude enhances the brain blood flow response to changes in CO2 and improves breathing stability.
We speculate that the enhanced brain blood flow responses following acetazolamide ingestion may account for the well-documented acetazolamide-induced improvement in abnormal breathing at high altitude.
Introduction
The stability of breathing rhythm is thought to depend strongly on the ‘CO2 reserve’, i.e. the difference between the eupnoeic pressure of CO2 in arterial blood (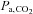 ) and the hypocapnic
) and the hypocapnic 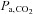 threshold for apnoea (see Dempsey (2005) for review). The CO2 reserve is reduced when breathing hypoxic air because of increased ventilation (due to the peripheral chemoreflex activation) lowering
threshold for apnoea (see Dempsey (2005) for review). The CO2 reserve is reduced when breathing hypoxic air because of increased ventilation (due to the peripheral chemoreflex activation) lowering 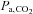 , thereby increasing the risk of periodic breathing during sleep (Xie et al. 2001, 2006). Ascent to high altitude in newcomers often leads to unstable breathing during both wakefulness (Brusil et al. 1980; Waggener et al. 1984) and sleep (Sutton et al. 1979, 1980; Lahiri et al. 1983; Normand et al. 1990; Lahiri & Data, 1992). Typically an oscillatory pattern of breathing can be distinguished, especially during sleep, with changes in tidal volume that can develop into periodic breathing with central apnoea periods. This development of breathing instability is probably due to a greater increase in the hypoxic ventilatory response associated with high altitude-induced hypoxaemia compared with the increase in background ventilatory drive, resulting in a reduced CO2 reserve (Khoo et al. 1982; Dempsey, 2005). The carbonic anhydrase inhibitor acetazolamide (ACZ) diminishes breathing pattern disturbances upon ascent to high altitude, especially during sleep (Weil et al. 1978; Sutton et al. 1979, 1980). At sea level, ACZ increases the CO2 reserve in both humans (Teppema et al. 2010) and anaesthetised cats (Teppema et al. 2001). However, since both the peripheral (Hackett et al. 1987) and central chemoreflex sensitivities (Burki et al. 1992) appear to be preserved following ACZ at high altitude, it seems unlikely that a blunted ventilatory drive would account for the increase in CO2 reserve. Accordingly, the prophylactic mechanisms of action by which ACZ improves breathing stability during wakefulness and sleep remain poorly understood.
, thereby increasing the risk of periodic breathing during sleep (Xie et al. 2001, 2006). Ascent to high altitude in newcomers often leads to unstable breathing during both wakefulness (Brusil et al. 1980; Waggener et al. 1984) and sleep (Sutton et al. 1979, 1980; Lahiri et al. 1983; Normand et al. 1990; Lahiri & Data, 1992). Typically an oscillatory pattern of breathing can be distinguished, especially during sleep, with changes in tidal volume that can develop into periodic breathing with central apnoea periods. This development of breathing instability is probably due to a greater increase in the hypoxic ventilatory response associated with high altitude-induced hypoxaemia compared with the increase in background ventilatory drive, resulting in a reduced CO2 reserve (Khoo et al. 1982; Dempsey, 2005). The carbonic anhydrase inhibitor acetazolamide (ACZ) diminishes breathing pattern disturbances upon ascent to high altitude, especially during sleep (Weil et al. 1978; Sutton et al. 1979, 1980). At sea level, ACZ increases the CO2 reserve in both humans (Teppema et al. 2010) and anaesthetised cats (Teppema et al. 2001). However, since both the peripheral (Hackett et al. 1987) and central chemoreflex sensitivities (Burki et al. 1992) appear to be preserved following ACZ at high altitude, it seems unlikely that a blunted ventilatory drive would account for the increase in CO2 reserve. Accordingly, the prophylactic mechanisms of action by which ACZ improves breathing stability during wakefulness and sleep remain poorly understood.
One potential mechanism by which ACZ may improve the CO2 reserve is via an effect on cerebral blood flow (CBF). Several studies have demonstrated a link between resting CBF and its response to CO2 (termed cerebrovascular CO2 reactivity), and the CO2 reserve (Xie et al. 2005, 2009). For example, pharmacologically induced reductions in resting CBF and cerebrovascular CO2 reactivity reduce the CO2 reserve during sleep (Xie et al. 2009) and cause breathing instability during wakefulness (Fan et al. 2010b). It has been well documented that, at sea level, acute i.v. administration of ACZ rapidly elevates resting CBF (Ehrenreich et al. 1961; Hauge et al. 1983; Lassen et al. 1987; Jensen et al. 1990) without altering cerebral metabolism (Posner & Plum, 1960; Vorstrup et al. 1984). However, no studies have examined the acute effects of a single dose of ACZ on CBF, cerebrovascular CO2 reactivity and breathing stability at both sea level and at high altitude. Therefore, although plausible, it remains unclear whether acute i.v. ACZ administration at high altitude alters cerebrovascular function and subsequently modulates breathing stability.
In the present study, we examined the effect of i.v. ACZ on cerebrovascular and ventilatory responsiveness to CO2 and O2 at sea level and following partial acclimatisation to 5050 m. We selected to use the i.v. route of administration to avoid the confounding factor of altered acid–base balance associated with oral ACZ administration (Swenson, 1998). Based on previous studies that show a worsening of breathing stability following experimental reductions in CBF and cerebrovascular CO2 reactivity (Xie et al. 2009; Fan et al. 2010b), we tested the hypothesis that acute ACZ-induced elevations in CBF and reactivity would lead to stabilisation of breathing control in partially acclimatised newcomers to 5050 m. These experiments were part of a series investigating the cardiorespiratory and cerebrovascular adaptations and mechanisms of regulation over 14 days at 5050 m. Although some of the experiments performed during this expedition have already been published (Fan et al. 2010a,b; Thomas et al. 2010; Lucas et al. 2011), apart from the experimental design and control data at high altitude from half of this group (Lucas et al. 2011), there is no overlap or duplication of data with the present study described herein.
Methods
Participants
Twelve sea-level residents (eight male and four female) with a mean age of 30 ± 10 years (mean ± SD) and body mass index of 23 ± 2 kg m−2 participated in this study. Participants were non-smokers, had no previous history of cardiovascular, cerebrovascular or respiratory diseases and were not taking any medication.
Ethical approval
The study was approved by the Lower South Regional Ethics Committee of Otago and conformed to the standards set by the Declaration of Helsinki. All participants were informed regarding the purposes and procedures of this study, and informed consent was given prior to participation.
Experimental design
After a full familiarisation with the experimental procedures outlined below (on the first visit), the participants underwent experimental trials at sea level and at 5050 m (the Ev-K2-CNR Pyramid Laboratory, Nepal; barometric pressure 413 ± 1 mmHg). At sea level, participants underwent three experimental trials in randomised order (ACZ, indomethacin and placebo). Due to time constraints, no placebo trial was carried out at 5050 m. The ascent profile from sea level (Dunedin, New Zealand) to the Pyramid Laboratory has been described previously (Fan et al. 2010a). To avoid any confounding influence of acute mountain sickness (AMS), experimental sessions were carried out between days 5–11 (7 ± 2 days) after arrival to 5050 m, after any symptoms from AMS had subsided. The present study was part of a larger experiment – to be reported elsewhere – in which the participants were administrated indomethacin (100 mg, oral) on a separate day (single-blinded, randomised order and separated by at least 3 days). Before each experimental session, participants abstained from exercise and alcohol for 24 h, caffeine for 12 h and a heavy meal for 4 h prior.
With the exception of the arterial blood gas sampling, which was conducted following a 10 min supine rest, all experiments were performed with participants in a semi-recumbent position. Following 10–15 min of quiet rest, each experimental testing session consisted of: (a) an arterial blood gas sample; (b) instrumentation; (c) 5 min resting baseline; (d) modified hyperoxic rebreathing and isocapnic hypoxia (see details of methods below); (e) ACZ administration (i.v. 10 mg kg−1) or placebo (sea level only); (f) 30 min rest; and (g) repeat testing of a–d. The order of the modified rebreathing and isocapnic hypoxic rebreathing was randomised and 5 min recovery was permitted between each trial to restore end-tidal gases to baseline resting values.
Ventilatory stability
Details of the ventilatory stability analysis have been previously described (Fan et al. 2010b). In brief, we first established whether unstable breathing patterns were present by visually analysing the ventilation (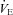 ) traces obtained during spontaneous room air breathing during the final 2 min of the 5 min baseline period, based on absence or presence of Cheyne–Stokes-like respiratory patterns, characterised by distinct waxing and waning of tidal volume (VT) and breathing frequency (f) pattern (Cherniack & Longobardo, 1973). If the trace was considered unstable, the difference between the peak and trough of any oscillation in
) traces obtained during spontaneous room air breathing during the final 2 min of the 5 min baseline period, based on absence or presence of Cheyne–Stokes-like respiratory patterns, characterised by distinct waxing and waning of tidal volume (VT) and breathing frequency (f) pattern (Cherniack & Longobardo, 1973). If the trace was considered unstable, the difference between the peak and trough of any oscillation in 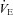 was measured. The average of this
was measured. The average of this 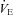 difference (magnitude of
difference (magnitude of 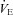 oscillation) was subsequently used as an index of ventilatory stability. The number of oscillations during this 2 min period was also noted. In addition, we calculated the co-efficient of variation of the breath-by-breath
oscillation) was subsequently used as an index of ventilatory stability. The number of oscillations during this 2 min period was also noted. In addition, we calculated the co-efficient of variation of the breath-by-breath 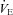 , VT and f data (variability) during the 2 min period.
, VT and f data (variability) during the 2 min period.
Modified rebreathing method
The modified rebreathing method is a well-established method for assessing both ventilatory and cerebrovascular CO2 reactivities (Ainslie & Duffin, 2009). We selected the modified rebreathing method over a steady-state method to limit the confounding influence of a 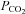 gradient on ventilatory control (Fan et al. 2010b). Since hyperoxia (
gradient on ventilatory control (Fan et al. 2010b). Since hyperoxia (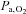 ≥ 150 mmHg) diminishes peripheral chemoreceptors’ output (Cunningham et al. 1963; Gardner, 1980), the ventilatory response to the modified rebreathing method can be interpreted as the ventilatory CO2 sensitivity primarily from the central chemoreflex. The details of the modified rebreathing method have been previously described in Fan et al. (2010a). In brief, the participants wore a nose clip and breathed through a mouthpiece connected to a Y-valve which allowed switching from room air to a 6 l rebreathing bag filled with 7% CO2 and 93% O2. Following baseline room air breathing, participants were instructed to hyperventilate for 5 min to lower and then maintain a partial pressure of CO2 (
≥ 150 mmHg) diminishes peripheral chemoreceptors’ output (Cunningham et al. 1963; Gardner, 1980), the ventilatory response to the modified rebreathing method can be interpreted as the ventilatory CO2 sensitivity primarily from the central chemoreflex. The details of the modified rebreathing method have been previously described in Fan et al. (2010a). In brief, the participants wore a nose clip and breathed through a mouthpiece connected to a Y-valve which allowed switching from room air to a 6 l rebreathing bag filled with 7% CO2 and 93% O2. Following baseline room air breathing, participants were instructed to hyperventilate for 5 min to lower and then maintain a partial pressure of CO2 (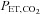 ) at 22 ± 2 mmHg (sea level) or 17 ± 3 mmHg (5050 m). Participants were then switched to the rebreathing bag following an expiration and instructed to take three deep breaths to ensure rapid equalisation of
) at 22 ± 2 mmHg (sea level) or 17 ± 3 mmHg (5050 m). Participants were then switched to the rebreathing bag following an expiration and instructed to take three deep breaths to ensure rapid equalisation of 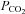 in the rebreathing circuit. The rebreathing tests were terminated when either: (i)
in the rebreathing circuit. The rebreathing tests were terminated when either: (i) 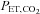 reached 60 mmHg; (ii) partial pressure of end-tidal O2 (
reached 60 mmHg; (ii) partial pressure of end-tidal O2 (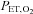 ) dropped below 160 mmHg; (iii)
) dropped below 160 mmHg; (iii) 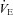 exceeded 100 l min−1; or (iv) the participant reached the end of their tolerance.
exceeded 100 l min−1; or (iv) the participant reached the end of their tolerance.
The rebreathing data were analysed on a breath-by-breath basis using a specially designed programme (Full Fit Rebreathing programme, Version 3.1, University of Toronto, Toronto, Canada) and the analysis has been described in detail previously (Mohan et al. 1999; Duffin et al. 2000).
Isocapnic hypoxia
The soda-lime rebreathing technique was used to assess the ventilatory O2 sensitivity as an index of peripheral chemoreflex sensitivity (Mathew et al. 1983). Participants wore a nose clip and breathed through a mouthpiece connected to a Y-valve allowing switching from room air to a circuit consisting of a 6 l rebreathing bag and a soda-lime reservoir. The protocol began with baseline room air breathing, before participants were switched to the rebreathing circuit at the end of an inspiration. Participants filled the rebreathing bag with room air drawn in through their nose and expired into the bag. Once the bag was filled (ensuring that this was at the end of expiration) the nose clip was attached and rebreathing began. The isocapnic hypoxia was terminated when either: (i) peripheral O2 saturation (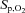 ) reached 80% at sea level and 70% at 5050 m; (ii)
) reached 80% at sea level and 70% at 5050 m; (ii) 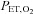 decreased to 45 mmHg at sea level and 30 mmHg at 5050 m; (iii) the
decreased to 45 mmHg at sea level and 30 mmHg at 5050 m; (iii) the 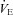 exceeded 100 l min−1; or (iv) the participant reached the end of their tolerance.
exceeded 100 l min−1; or (iv) the participant reached the end of their tolerance.
The breath-by-breath 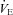 data during the isocapnic hypoxic rebreathing were plotted against
data during the isocapnic hypoxic rebreathing were plotted against 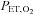 and an inverse first-order polynomial function was used to obtain the hypoxic ventilatory response curve (Day & Wilson, 2009):
and an inverse first-order polynomial function was used to obtain the hypoxic ventilatory response curve (Day & Wilson, 2009):
where y0 is the y asymptote, x is the 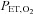 in mmHg and c is the curvature (representing the responsiveness).
in mmHg and c is the curvature (representing the responsiveness).
Steady-state hypocapnia
Voluntary hyperventilation was used to assess cerebrovascular reactivity to hypocapnia (Xie et al. 2005). Steady-state hypocapnic cerebrovascular reactivity was estimated from the slope of the reduction in mean MCAv from the final 2 min of baseline to the voluntary hyperventilation which preceded rebreathing, relative to the reduction in 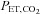 (see below). We did not control for
(see below). We did not control for 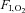 during the voluntary hyperventilation because the small increases in
during the voluntary hyperventilation because the small increases in 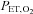 (by 29 ± 11 and 16 ± 12 mmHg at sea level and 5050 m, respectively) were considered unlikely to influence either MCAv or its reactivity.
(by 29 ± 11 and 16 ± 12 mmHg at sea level and 5050 m, respectively) were considered unlikely to influence either MCAv or its reactivity.
ACZ administration
Slow (∼60 s) i.v. administration of ACZ (10 mg kg−1; Diamox) or placebo (saline; sea level only) was achieved via an indwelling catheter located in an antecubital vein. The ACZ dose was reconstituted in approximately 5 ml of saline; a comparable volume (7–10 ml, depending on body mass) of i.v. saline was administered in the placebo trial. At sea level, the order was randomised and the participants were blinded to the condition.
Measurements
Respiratory variables
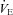 and its components of VT and f were measured using a heated pneumotachograph (Hans-Rudolph 3813) and expressed in units adjusted to BTPS.
and its components of VT and f were measured using a heated pneumotachograph (Hans-Rudolph 3813) and expressed in units adjusted to BTPS. 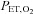 and
and 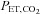 were measured using fast-responding gas analysers (model CD-3A, AEI Technologies, Pittsburgh, PA, USA; ML206 and ML240, ADInstruments, Colorado Springs, CO, USA). The pneumotachograph was calibrated using a 3 l syringe (Hans-Rudolph 5530) and the gas analysers were calibrated using gas mixtures of known concentrations of O2 and CO2 prior to each testing session.
were measured using fast-responding gas analysers (model CD-3A, AEI Technologies, Pittsburgh, PA, USA; ML206 and ML240, ADInstruments, Colorado Springs, CO, USA). The pneumotachograph was calibrated using a 3 l syringe (Hans-Rudolph 5530) and the gas analysers were calibrated using gas mixtures of known concentrations of O2 and CO2 prior to each testing session.
Cerebrovascular variables
Cerebral blood flow velocity was measured in the right middle cerebral artery using a 2 MHz pulsed Doppler ultrasound system (MCAv; DWL, Compumedics Ltd, Germany). The Doppler ultrasound probe was positioned over the right temporal window and held in place with an adjustable headband. Optimal signals were obtained using search techniques described elsewhere (Aaslid et al. 1982). Beat-to-beat mean arterial blood pressure (MAP) was monitored using finger photoplethysmography (Finometer, Finapress Medical Systems, the Netherlands). Manual blood pressure measurements by auscultation were also made periodically to check and validate the automated recordings. Cerebrovascular conductance index (CVCi) was subsequently estimated by dividing mean MCAv by MAP within each breath cycle to reveal intrinsic vascular responses to CO2.
Arterial blood gases
Arterial blood gas samples from a radial artery were obtained at rest using a 25-gauge needle into a pre-heparinised syringe. Following standardised calibration, all blood samples were analysed using an arterial blood-gas analysing system (NPT 7 series, Radiometer, Copenhagen, Denmark) for pH, partial pressure of arterial O2 (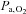 ) and CO2 (
) and CO2 (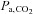 ), bicarbonate concentration ([HCO3−]) and arterial O2 saturation (
), bicarbonate concentration ([HCO3−]) and arterial O2 saturation (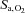 ).
).
With the exception of the arterial blood gas variables, all data were acquired at 1000 Hz using an analog-to-digital converter (PowerLab; ADInstruments) with commercially available software (Chart version 5.5.6, ADInstruments), and stored on computer for later analysis.
Statistical analysis
The effects of altitude and ACZ on resting variables, cardiorespiratory and cerebrovascular responsiveness to CO2, as well as the ventilatory variability were assessed using two-way (altitude and drug) repeated-measures ANOVA with an α-level of 0.05 (SPSS version 17.0, SPSS, Chicago, IL, USA). Pair-wise comparisons (Bonferroni corrected) were performed to isolate the effect of altitude and ACZ on the dependent measures within participants. Data are reported as mean ± SD.
Results
All 12 participants were able to complete the whole experimental protocol. Comparison of 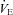 -CO2 sensitivity could be carried out in only 11 participants, both at sea level and at 5050 m, due to a poor ventilatory flow trace during hyperoxic rebreathing. Comparisons of MAP-CO2 reactivity were conducted in 9 and 10 participants at sea level and 5050 m, respectively, due to poor BP traces in remaining participants.
-CO2 sensitivity could be carried out in only 11 participants, both at sea level and at 5050 m, due to a poor ventilatory flow trace during hyperoxic rebreathing. Comparisons of MAP-CO2 reactivity were conducted in 9 and 10 participants at sea level and 5050 m, respectively, due to poor BP traces in remaining participants.
Resting variables (Table 1)
Table 1.
Resting cerebrovascular, respiratory, cardiovascular and arterial blood gas variables before and after i.v. acetazolamide (ACZ) at sea level and following partial acclimatisation to 5050 m
| Sea level | 5050 m | |||
|---|---|---|---|---|
| Control | ACZ | Control | ACZ | |
| Cerebrovascular | ||||
| MCAv (cm s−1) | 70 ± 13 | 80 ± 16† | 73 ± 12 | 94 ± 15† |
| CVCi (cm s−1 mmHg−1) | 0.88 ± 0.21 | 0.95 ± 0.20 | 0.82 ± 0.17 | 1.08 ± 0.26† |
| Respiratory | ||||
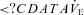 (l min−1)a (l min−1)a
|
14.3 ± 3.9a | 13.0 ± 3.7† | 17.4 ± 3.0c | 16.6 ± 2.7*†a |
| f (breaths min−1) | 15 ± 4a | 14 ± 4† | 18 ± 5* | 17 ± 3*† |
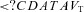 (l) (l) |
0.95 ± 0.13a | 0.95 ± 0.16 | 1.02 ± 0.31c | 0.99 ± 0.22a |
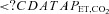 (mmHg) (mmHg) |
42 ± 5 | 40 ± 5† | 24 ± 3* | 25 ± 3*† |
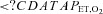 (mmHg) (mmHg) |
103 ± 6 | 103 ± 5 | 47 ± 4* | 49 ± 7* |
| Cardiovascular | ||||
| MAP (mmHg) | 80 ± 10 | 84 ± 7 | 90 ± 9 | 89 ± 15 |
| HR (beats min−1) | 68 ± 9 | 60 ± 8† | 77 ± 11* | 77 ± 11* |
| Arterial blood gases | ||||
| pH | 7.46 ± 0.04b | 7.44 ± 0.04†b | 7.46 ± 0.02 | 7.44 ± 0.02† |
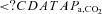 (mmHg) (mmHg) |
42 ± 4b | 42 ± 4b | 27 ± 4* | 31 ± 3*† |
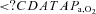 (mmHg) (mmHg) |
97 ± 6b | 93 ± 8†b | 46 ± 4* | 42 ± 3*† |
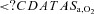 (%) (%) |
98.1 ± 0.5b | 97.5 ± 0.5†b | 82.9 ± 3.3* | 79.2 ± 2.7*† |
| [HCO3−] (mmol l−1) | 29.8 ± 3.0b | 28.7 ± 3.1b | 18.7 ± 3.0* | 20.7 ± 2.7*† |
| SBE− | 6.1 ± 2.9bg | 4.7 ± 3.2b | −3.9 ± 4.0* | −2.8 ± 2.7* |
Values are means ± SD.
Different from sea level (P < 0.05)
different from control (P < 0.05).
n = 12, except for an = 11, bn = 9 and cn = 10.
Cerebrovascular variables
No differences were observed in either MCAv or CVCi following ∼7 days of living at 5050 m (P = 0.489 and 0.452 vs. sea level, respectively). The administration of ACZ increased MCAv at sea level and at high altitude, with greater increases at altitude following partial acclimatisation to 5050 m (interaction effect: P = 0.007). Specifically, ACZ administration at sea level elevated MCAv by 15 ± 15% (P = 0.003 vs. control) without increasing CVCi (P = 0.325), whereas ACZ administration at 5050 m increased MCAv by 28 ± 11% (P < 0.001 vs. control) and CVCi by 33 ± 23% (P = 0.001 vs. control; interaction effect: P = 0.046).
Respiratory variables
Residing at high altitude elevated both 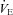 and f (P = 0.030 and 0.009, respectively), whereas administration of ACZ lowered these variables (P = 0.045 and 0.030), but not by a different extent at sea level vs. high altitude (interactions: P = 0.636 and 0.895). The VT was unaffected by altitude (P = 0.509) and ACZ administration (P = 0.674). High altitude lowered
and f (P = 0.030 and 0.009, respectively), whereas administration of ACZ lowered these variables (P = 0.045 and 0.030), but not by a different extent at sea level vs. high altitude (interactions: P = 0.636 and 0.895). The VT was unaffected by altitude (P = 0.509) and ACZ administration (P = 0.674). High altitude lowered 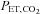 (P < 0.001), whereas ACZ administration had a differential effect on
(P < 0.001), whereas ACZ administration had a differential effect on 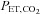 at high altitude compared with at sea level (interaction effect: P = 0.010). Specifically,
at high altitude compared with at sea level (interaction effect: P = 0.010). Specifically, 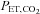 was lowered by ACZ at sea level (P = 0.027 vs. control) but elevated at 5050 m (P = 0.02). In contrast, ACZ administration did not alter
was lowered by ACZ at sea level (P = 0.027 vs. control) but elevated at 5050 m (P = 0.02). In contrast, ACZ administration did not alter 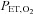 at sea level or its reduction at high altitude (altitude, P < 0.001; drug, P = 0.427; interaction, P = 0.572).
at sea level or its reduction at high altitude (altitude, P < 0.001; drug, P = 0.427; interaction, P = 0.572).
Cardiovascular variables
High altitude tended to elevate MAP (P = 0.057), whereas no effect of ACZ on MAP was evident for pooled altitudes (P = 0.654) or differentially between altitudes (interaction: P = 0.294). Resting HR was elevated at high altitude (P = 0.017). The administration of ACZ lowered resting HR at sea level (P = 0.002 vs. control) but not at 5050 m (P = 0.650; interaction effect: P = 0.021).
Arterial blood gas variables
At 5050 m, pH was similar to sea-level values (P = 0.700), indicating complete renal correction of the hypoxia-induced respiratory alkalosis and therefore well-advanced acclimatisation to this altitude at this time point. The 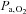 and SBE− both remained lower than at sea level (P = 0.001). Regardless of the altitude, ACZ administration lowered pH and
and SBE− both remained lower than at sea level (P = 0.001). Regardless of the altitude, ACZ administration lowered pH and 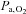 , while SBE-remained unchanged (drug effects: P = 0.015, 0.012 and 0.562, respectively; interaction effects: P = 0.831, 0.936 and 0.134). Ascent to 5050 m lowered resting
, while SBE-remained unchanged (drug effects: P = 0.015, 0.012 and 0.562, respectively; interaction effects: P = 0.831, 0.936 and 0.134). Ascent to 5050 m lowered resting 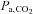 ,
, 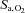 and [HCO3−] (all P < 0.001 vs. sea level). The effect of ACZ on
and [HCO3−] (all P < 0.001 vs. sea level). The effect of ACZ on 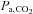 ,
, 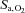 and [HCO3−] was greater at 5050 m compared with at sea level (interaction effects: P = 0.003, 0.018 and 0.024). Specifically, ACZ at sea level lowered
and [HCO3−] was greater at 5050 m compared with at sea level (interaction effects: P = 0.003, 0.018 and 0.024). Specifically, ACZ at sea level lowered 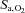 (P = 0.013) without measurably changing
(P = 0.013) without measurably changing 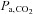 or [HCO3−] (P = 1.000 and 0.342), but at high altitude it caused a larger reduction in
or [HCO3−] (P = 1.000 and 0.342), but at high altitude it caused a larger reduction in 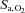 (P = 0.002) with elevations in resting
(P = 0.002) with elevations in resting 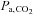 and [HCO3−] (both P < 0.001).
and [HCO3−] (both P < 0.001).
Following the placebo trial at sea level, cardiorespiratory, cerebrovascular and blood gas variables were unchanged (data not shown, for clarity).
Breathing stability (Table 2)
Table 2.
Effect of acetazolamide (ACZ) on breathing stability at sea level and following ascent to 5050 m
| Sea level | 5050 m | |||
|---|---|---|---|---|
| Control | ACZ | Control | ACZ | |
Magnitude of 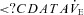 oscillation (Δ l min−1) oscillation (Δ l min−1) |
5.8 ± 5.8 | 2.5 ± 5.3† | 7.7 ± 5.2 | 5.9 ± 6.1† |
Frequency of 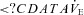 oscillation (event min−1) oscillation (event min−1) |
1.3 ± 1.2 | 0.3 ± 0.6† | 3.0 ± 0.5* | 1.8 ± 0.4*† |
| Variability | ||||
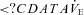 (%) (%) |
29 ± 17a | 29 ± 17†a | 36 ± 17a | 22 ± 10†a |
| f (%) | 18 ± 5a | 16 ± 8†a | 23 ± 10a | 16 ± 7†a |
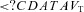 (%) (%) |
26 ± 14a | 27 ± 19a | 37 ± 17a | 21 ± 10†a |
Values are means ± SD.
Different from sea level (P < 0.05)
different from control (P < 0.05).
n = 12, except for a n = 11.
Ascent to 5050 m increased the frequency of 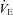 oscillation (P < 0.001; interaction effect, P = 0.551), but did not alter its magnitude, or the variability of f, VT or
oscillation (P < 0.001; interaction effect, P = 0.551), but did not alter its magnitude, or the variability of f, VT or  (P = 0.172, 0.249, 0.076 and 0.929). At sea level and 5050 m, ACZ administration reduced both the magnitude and frequency of the
(P = 0.172, 0.249, 0.076 and 0.929). At sea level and 5050 m, ACZ administration reduced both the magnitude and frequency of the  oscillations (P = 0.027 and < 0.001 vs. control, respectively; interaction effect, P = 0.489 and 0.551); the variability in f and
oscillations (P = 0.027 and < 0.001 vs. control, respectively; interaction effect, P = 0.489 and 0.551); the variability in f and  were also reduced (P = 0.033 and 0.014, respectively; interaction effect, P = 0.283 and 0.067). The ACZ administration reduced the variability of VT at 5050 m (P = 0.011) but not at sea level (P = 0.615; interaction effect, P = 0.042).
were also reduced (P = 0.033 and 0.014, respectively; interaction effect, P = 0.283 and 0.067). The ACZ administration reduced the variability of VT at 5050 m (P = 0.011) but not at sea level (P = 0.615; interaction effect, P = 0.042).
Modified rebreathing
Cerebrovascular CO2 reactivity (Fig. 1)
Figure 1. Effects of acetazolamide (ACZ) and high altitude (5050 m) on cerebrovascular responsivess to hypercapnia and hypocapnia.
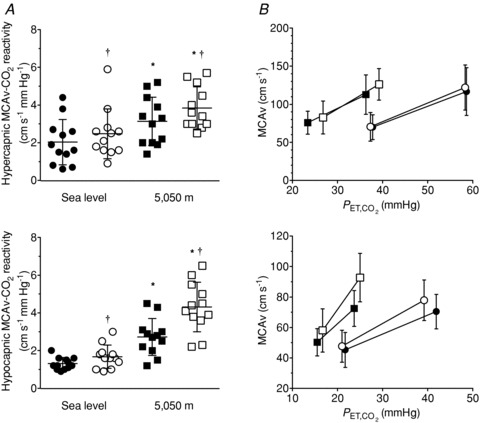
A, individual slopes; B, group data (mean ± SD). Filled circles, sea level control; open circles, sea level ACZ; filled squares, 5050 m control; open squares, 5050 m ACZ. ACZ elevated the hypercapnic and the hypocapnic cerebrovascular reactivity at both sea level and at 5050 m. *Different from sea level (P < 0.05); † different from control (P < 0.05).
Ascent to 5050 m elevated the rebreathing hypercapnic MCAv-CO2 reactivity by 114 ± 146% (P = 0.008 vs. sea level). ACZ administration elevated hypercapnic MCAv-CO2 reactivity (P = 0.030) both at sea level and 5050 m (interaction effect, P = 0.355). One participant displayed a high cerebrovascular reactivity to hypercapnic following ACZ at sea level. Re-analysis of the data with this individual excluded revealed a tendency for the hypercapnic MCAv-CO2 to be elevated with ACZ at sea level (P = 0.052). Ascent to 5050 m elevated the hypocapnic MCAv-CO2 reactivity by 114 ± 71% (P < 0.001 vs. sea level). The administration of ACZ elevated hypocapnic MCAv-CO2 reactivity at sea level (by 31 ± 43%; P = 0.026) and by a greater amount at 5050 m (by 69 ± 60%; P < 0.001; interaction effect, P = 0.003).
Ventilatory CO2 sensitivity (Fig. 2)
Figure 2. Effects of acetazolamide (ACZ) and high altitude (5050 m) on ventilatory and cardiovascular responsivess to hypercapnia.
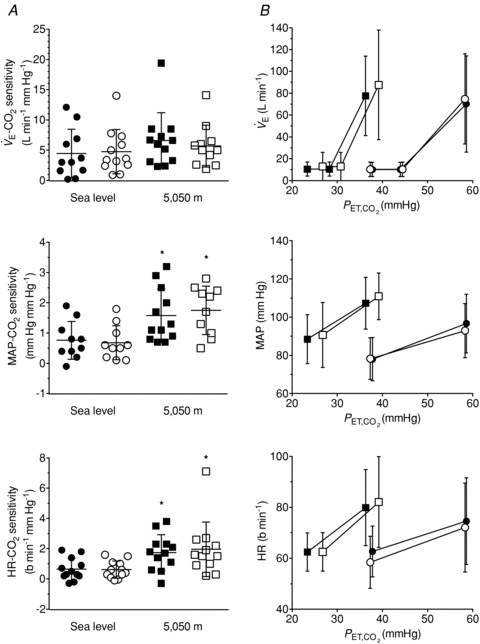
A, individual slopes; B, group data (mean ± SD). Filled circles, sea level control; open circles, sea level ACZ; filled squares, 5050 m control; open squares, 5050 m ACZ. Ascent to 5050 m enhanced the cardiovascular response to CO2, while no changes were observed following ACZ at either sea level or 5050 m. *Different from sea level (P < 0.05).
High altitude tended to elevate the 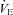 -CO2 sensitivity (P = 0.085). Meanwhile, no changes were observed in the
-CO2 sensitivity (P = 0.085). Meanwhile, no changes were observed in the 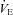 -CO2 sensitivity following ACZ administration (P = 0.883), irrespective of the altitude (interaction, P = 0.127).
-CO2 sensitivity following ACZ administration (P = 0.883), irrespective of the altitude (interaction, P = 0.127).
Cardiovascular CO2 reactivity (Fig. 2)
Ascent to 5050 m elevated both MAP-CO2 reactivity and HR-CO2 reactivity (P = 0.003 and 0.006 vs. sea level, respectively). In contrast, these reactivities were unchanged following ACZ administration (P = 0.866 and 0.611), irrespective of the altitude (interaction, P = 0.105 and 0.475, respectively).
Isocapnic hypoxia
Ascent to 5050 m elevated the 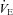 -O2 sensitivity by (2420 ± 1521 vs. 1020 ± 908 units; P = 0.002 vs. sea level). No changes were observed in the
-O2 sensitivity by (2420 ± 1521 vs. 1020 ± 908 units; P = 0.002 vs. sea level). No changes were observed in the 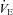 -O2 sensitivity with ACZ administration (P = 0.559 vs. control) at either sea level (981 ± 1152 vs. 1057 ± 563 units) or following ascent to 5050 m (2358 ± 1496 vs. 2482 ± 1616 units; interaction effect, P = 0.958, data not shown).
-O2 sensitivity with ACZ administration (P = 0.559 vs. control) at either sea level (981 ± 1152 vs. 1057 ± 563 units) or following ascent to 5050 m (2358 ± 1496 vs. 2482 ± 1616 units; interaction effect, P = 0.958, data not shown).
Discussion
We have examined the acute effects of a single i.v. dose of ACZ on cerebrovascular and ventilatory responsiveness to CO2 and hypoxia in healthy resting individuals at sea level and following partial acclimatisation to 5050 m. The main novel findings were that ACZ at 5050 m: (1) elevated resting MCAv (to a greater extent than at sea level) and 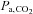 , while it lowered both
, while it lowered both 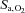 and
and 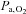 ; (2) improved breathing stability as reflected in a reduced variability of
; (2) improved breathing stability as reflected in a reduced variability of 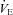 , VT and incidence of breathing oscillations, while the ventilatory responsiveness to hypercapnia and hypoxia were unchanged; and (3) elevated both hypercapnic and hypocapnic cerebrovascular CO2 reactivity, with the hypocapnia reactivity increases greater than observed at sea level. Our data thus indicate that ACZ administration at 5050 m improves breathing stability during wakefulness without concurrent improvements in arterial O2 saturation or
, VT and incidence of breathing oscillations, while the ventilatory responsiveness to hypercapnia and hypoxia were unchanged; and (3) elevated both hypercapnic and hypocapnic cerebrovascular CO2 reactivity, with the hypocapnia reactivity increases greater than observed at sea level. Our data thus indicate that ACZ administration at 5050 m improves breathing stability during wakefulness without concurrent improvements in arterial O2 saturation or 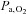 . Moreover, the improvement in breathing patterns following ACZ occurred independently of any measurable changes in the sensitivity of the central or peripheral chemoreflexes. We therefore speculate that
. Moreover, the improvement in breathing patterns following ACZ occurred independently of any measurable changes in the sensitivity of the central or peripheral chemoreflexes. We therefore speculate that 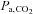 -mediated elevations in cerebral perfusion and an enhanced cerebrovascular reactivity may partly account for the improved breathing stability following ACZ at high altitude
-mediated elevations in cerebral perfusion and an enhanced cerebrovascular reactivity may partly account for the improved breathing stability following ACZ at high altitude
Limitations
An important limitation of the present study is the assumption that MCAv represents global CBF changes. Subudhi et al. (2011) pointed out that since CBF is markedly heterogenous during hypoxia, ACZ may have regional specific effects not detected with transcranial Doppler. ACZ administration (10 mg kg−1, i.v.) in rats uniformly elevates CBF to the frontal and parietal cerebral cortex, the striatum, the hippocampus and the cerebellum, reaching a maximum at 1 h and progressively returning to baseline over 6 h (LaManna & McCracken, 1990). Likewise, ACZ (1 g) elevated CBF in numerous cortical grey matter areas (occipital lobe, superior frontal gyrus, cingulate gyrus, primary sensory-motor cortex, middle and superior temporal gyrus) and putamen and white matter, which was quantified using both MRI and PET measurements in healthy humans (Grandin et al. 2005). In addition, a MR imaging study by Schreiber et al. (2000) reported no changes in MCA diameter with ACZ administration in patients with internal carotid occlusion. Finally, a recent report showed a preserved MCA diameter at 5300 m compared with sea level (Wilson et al. 2011). Taken together, we contend that: (i) ACZ elevates CBF in all major brain regions; (ii) the ACZ-induced changes in MCAv reflect these global changes in CBF; and (iii) MCA diameter is unlikely to be altered at 5050 m.
We selected to use the i.v. route rather than oral administrations to examine the influence of the acute direct effect of ACZ on CBF without the major confounding influence of changes in acid–base balance following oral administration (Hauge et al. 1983; Vorstrup et al. 1989; see Swenson, 1998 for review). Nevertheless, upon injection of ACZ, an unexpected observation was the apparent changes in pH and 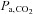 at 5050 m (Table 1). Carbonic anhydrase inhibition causes disequilibrium of the CO2 buffer system, which results from an incomplete hydration of CO2 during the passage of circulating blood through the vascular beds. There is a progressive elevation of arterial CO2 tension from the lungs to the peripheral tissues (Brzezinski et al. 1967). In the present study, there were several minutes of delay between the arterial blood sampling and analysis, thus allowing sufficient time for the equilibrium to complete. Therefore, it is likely that the true in vivo[H+] and
at 5050 m (Table 1). Carbonic anhydrase inhibition causes disequilibrium of the CO2 buffer system, which results from an incomplete hydration of CO2 during the passage of circulating blood through the vascular beds. There is a progressive elevation of arterial CO2 tension from the lungs to the peripheral tissues (Brzezinski et al. 1967). In the present study, there were several minutes of delay between the arterial blood sampling and analysis, thus allowing sufficient time for the equilibrium to complete. Therefore, it is likely that the true in vivo[H+] and 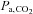 values might be overestimated by our arterial blood gas measurements following ACZ. Nevertheless, Brzezinski et al. (1967) found a consistent relationship between
values might be overestimated by our arterial blood gas measurements following ACZ. Nevertheless, Brzezinski et al. (1967) found a consistent relationship between 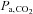 with direct cerebral tissue CO2 tension (∼6 mmHg difference) before and following ACZ in anaesthetised dogs. Accordingly, we believe that the arterial blood gas values obtained in the present study provide an insight to the differential effect of ACZ on acid–base balance at 5050 m. Our findings of a greater elevation in MCAv at 5050 m following ACZ is probably explained by the greater ACZ-induced elevations in
with direct cerebral tissue CO2 tension (∼6 mmHg difference) before and following ACZ in anaesthetised dogs. Accordingly, we believe that the arterial blood gas values obtained in the present study provide an insight to the differential effect of ACZ on acid–base balance at 5050 m. Our findings of a greater elevation in MCAv at 5050 m following ACZ is probably explained by the greater ACZ-induced elevations in 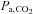 (Table 1).
(Table 1).
Acetazolamide elevates cerebral blood flow at high altitude
While the influence of ACZ on CBF has been studied extensively during normoxia (Posner & Plum, 1960; Ehrenreich et al. 1961; Hauge et al. 1983; Vorstrup et al. 1984), its effect on cerebrovascular function during hypoxic exposure is poorly understood and entirely based on oral administration of the drug. For example, studies have found either reduced (Subudhi et al. 2011) or preserved (Teppema et al. 2007) CBF velocity in response to short-duration hypoxia (4–8 h) following 1 and 3 day oral ingestion of ACZ, respectively (250 mg every 8 h). At 3475 m, Jensen et al. (1990) reported a 22% increase in CBF velocity 2 h following oral ACZ ingestion (1.5 g). Vuyk et al. (2006) found higher cerebral oxygenation during exercise at 3700 m and with ACZ (750 mg daily) compared with untreated controls. In the present study, acute ACZ administration at 5050 m increased resting MCAv by 28% and elevated the hypercapnic and hypocapnic MCAv-CO2 reactivity by 51% and 69%, respectively (Table 1 and Fig. 1). Our data thereby demonstrate, for the first time, that acute i.v. ACZ administration elevates CBF and enhances the cerebrovascular response to changes in CO2 in partially acclimatised newcomers to 5050 m. Such elevation in resting CBF with ACZ at 5050 m would also serve to improve cerebral oxygen delivery and thus potentially attenuate cerebral hypoxaemia.
Acetazolamide exacerbates hypoxaemia at 5050 m
Previous studies have found either unchanged (Teppema et al. 2006, 2007, 2010) or improved (Schoene et al. 1983; Burki et al. 1992; Mirrakhlmov et al. 1993; Subudhi et al. 2011) arterial O2 saturation with oral ACZ – presumably related to the changes in resting 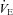 associated with carbonic anhydrase inhibition. In the present study, we observed a slight reduction in both
associated with carbonic anhydrase inhibition. In the present study, we observed a slight reduction in both 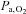 and
and 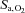 following ACZ at both sea level and following ascent to 5050 m (Table 1). We attributed this increase in arterial hypoxaemia to hypoventilation (see below) and/or the Bohr effect, whereby ACZ-induced CO2 retention causes less O2 loading of the red blood cells in the lungs.
following ACZ at both sea level and following ascent to 5050 m (Table 1). We attributed this increase in arterial hypoxaemia to hypoventilation (see below) and/or the Bohr effect, whereby ACZ-induced CO2 retention causes less O2 loading of the red blood cells in the lungs.
Carbonic anhydrase inhibition and breathing control
Despite the large body of literature (see Swenson, 1998 for review), much controversy still surrounds the effects of ACZ-induced carbonic anhydrase inhibition on ventilatory control. The effect of carbonic anhydrase inhibition on ventilatory control depends critically on the dose and the route of ACZ administration (Teppema et al. 2001). For example, oral administration of ACZ usually results in metabolic acidosis, which is frequently (but not always) associated with a rise in 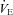 (Teppema & Dahan, 1999). Meanwhile, i.v. ACZ administration does not immediately lead to metabolic acidosis, but may be followed by inhibition of ventilatory control (Swenson, 1998).
(Teppema & Dahan, 1999). Meanwhile, i.v. ACZ administration does not immediately lead to metabolic acidosis, but may be followed by inhibition of ventilatory control (Swenson, 1998).
One important factor of carbonic anhydrase inhibition on resting 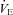 is the effect of elevated CBF and associated brain CO2 washout. Hauge et al. (1983) proposed that, assuming constant cerebral CO2 production, an increase in resting CBF and its responses to CO2 associated with cerebral capillary carbonic anhydrase inhibition would lower brain tissue
is the effect of elevated CBF and associated brain CO2 washout. Hauge et al. (1983) proposed that, assuming constant cerebral CO2 production, an increase in resting CBF and its responses to CO2 associated with cerebral capillary carbonic anhydrase inhibition would lower brain tissue 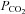 via an increase of CO2 washout, thus attenuating central chemoreceptor activation. In support of this, they observed an initial depression of
via an increase of CO2 washout, thus attenuating central chemoreceptor activation. In support of this, they observed an initial depression of 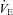 with carbonic anhydrase inhibition, which coincided with the ACZ-induced increase in resting CBF. In the present study, we also observed a reduction in resting
with carbonic anhydrase inhibition, which coincided with the ACZ-induced increase in resting CBF. In the present study, we also observed a reduction in resting 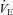 following ACZ at both sea level and 5050 m (Table 1). Our finding contradicts previous reports of elevated resting
following ACZ at both sea level and 5050 m (Table 1). Our finding contradicts previous reports of elevated resting 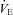 following chronic oral ACZ administration (Swenson & Hughes, 1993; Teppema & Dahan, 1999; Teppema et al. 2007, 2010). Since we observed a ∼15% increase in resting MCAv at 30 min following acute ACZ administration at sea level, while arterial pH was lowered (Table 1), we speculate that the reduction in resting
following chronic oral ACZ administration (Swenson & Hughes, 1993; Teppema & Dahan, 1999; Teppema et al. 2007, 2010). Since we observed a ∼15% increase in resting MCAv at 30 min following acute ACZ administration at sea level, while arterial pH was lowered (Table 1), we speculate that the reduction in resting 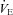 may be due to the effect of increased CBF and associated central H+ washout, overriding any stimulating effect of ACZ-induced CO2 retention (Coates et al. 1991; Teppema et al. 1995, 2010). Accordingly, it appears that the ventilatory effect observed with ACZ may be closely linked with the changes in the control of CBF.
may be due to the effect of increased CBF and associated central H+ washout, overriding any stimulating effect of ACZ-induced CO2 retention (Coates et al. 1991; Teppema et al. 1995, 2010). Accordingly, it appears that the ventilatory effect observed with ACZ may be closely linked with the changes in the control of CBF.
In the present study, both 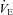 -CO2 and
-CO2 and 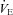 -O2 sensitivities remained unchanged with acute ACZ administration (10 mg kg−1, i.v.) at both sea level and 5050 m (Fig. 2). However, in agreement with previous findings (Teppema et al. 1992, 2006; Swenson & Hughes, 1993; Teppema & Dahan, 2004), we did observe complete abolishment of the ventilatory response to hypoxia in some participants at both sea level (n = 3) and 5050 m (n = 1) with ACZ. In addition, there was a rightward shift of the
-O2 sensitivities remained unchanged with acute ACZ administration (10 mg kg−1, i.v.) at both sea level and 5050 m (Fig. 2). However, in agreement with previous findings (Teppema et al. 1992, 2006; Swenson & Hughes, 1993; Teppema & Dahan, 2004), we did observe complete abolishment of the ventilatory response to hypoxia in some participants at both sea level (n = 3) and 5050 m (n = 1) with ACZ. In addition, there was a rightward shift of the 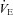 -CO2 slope during modified rebreathing with ACZ at 5050 m (Fig. 2). Our finding is in contrast to previous reports of a leftward shift of the
-CO2 slope during modified rebreathing with ACZ at 5050 m (Fig. 2). Our finding is in contrast to previous reports of a leftward shift of the 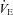 -CO2 slope with ACZ (Teppema & Dahan, 1999). However, since that study assessed the ventilatory CO2 sensitivity following 3 days of oral ACZ ingestion (250 mg 8 h−1), differences in the method of administration (oral vs. i.v.), drug dosage and assessment of ventilatory response (end-tidal forcing vs. rebreathing) probably account for these discrepant findings.
-CO2 slope with ACZ (Teppema & Dahan, 1999). However, since that study assessed the ventilatory CO2 sensitivity following 3 days of oral ACZ ingestion (250 mg 8 h−1), differences in the method of administration (oral vs. i.v.), drug dosage and assessment of ventilatory response (end-tidal forcing vs. rebreathing) probably account for these discrepant findings.
Acetazolamide and breathing instability
In the present study, we found acute ACZ administration at 5050 m improved breathing stability (Table 2), despite preserved 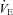 -CO2 and
-CO2 and 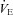 -O2 sensitivities (Fig. 2). Our data indicate that this improved breathing stability is mediated by reductions in variability of the tidal breath volumes and the frequency of breathing oscillations (i.e. Cheyne–Stokes respiration, Table 2). Our findings provide support to the notion that ACZ ameliorates breathing pattern disturbances following ascent to high altitude (Weil et al. 1978; Sutton et al. 1979, 1980; Hackett et al. 1987), independent of changes in either peripheral (Hackett et al. 1987) or central chemoreflexes (Burki et al. 1992). Furthermore, our findings corroborate with those of Gotoh et al. (1969) who found that i.v. ACZ injection (500 mg) improved breathing stability by reducing the incidence of Cheyne–Stokes respiration cycles in patients with cerebrovascular disease. We partly attribute the improvement in breathing stability with ACZ to the elevations in CBF velocity and related elevations in cerebrovascular responsiveness to changes in CO2 (Fig. 1). Indeed, it has been shown that reduction in resting CBF and cerebrovascular hypocapnic reactivity lowers the CO2 reserve (the difference between eupnoeic
-O2 sensitivities (Fig. 2). Our data indicate that this improved breathing stability is mediated by reductions in variability of the tidal breath volumes and the frequency of breathing oscillations (i.e. Cheyne–Stokes respiration, Table 2). Our findings provide support to the notion that ACZ ameliorates breathing pattern disturbances following ascent to high altitude (Weil et al. 1978; Sutton et al. 1979, 1980; Hackett et al. 1987), independent of changes in either peripheral (Hackett et al. 1987) or central chemoreflexes (Burki et al. 1992). Furthermore, our findings corroborate with those of Gotoh et al. (1969) who found that i.v. ACZ injection (500 mg) improved breathing stability by reducing the incidence of Cheyne–Stokes respiration cycles in patients with cerebrovascular disease. We partly attribute the improvement in breathing stability with ACZ to the elevations in CBF velocity and related elevations in cerebrovascular responsiveness to changes in CO2 (Fig. 1). Indeed, it has been shown that reduction in resting CBF and cerebrovascular hypocapnic reactivity lowers the CO2 reserve (the difference between eupnoeic 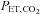 and apnoea threshold) and increases the risk of unstable breathing during sleep (Xie et al. 2009). Xie and colleagues attributed this reduction in CO2 reserve to an increased slope of ventilatory response to CO2below eupnoea (i.e. ventilatory controller gain) associated with reduced cerebrovascular hypocapnic reactivity. As such, it seems reasonable that increases in CBF and cerebrovascular hypocapnic reactivity associated with ACZ would therefore blunt the ventilatory slope to CO2 below eupnoea, thereby increasing the CO2 reserve and improve breathing stability. Moreover, since hypoventilation lowers the CO2 reserve (Dempsey, 2005), we speculate that the stabilising effect of ACZ must be greater than the destabilising effect of the reduced ventilation observed in the present study (Table 1). In support of this, ACZ has been found to increase the CO2 reserve by lowering the apnoeic threshold in humans (Teppema et al. 2010) and in the anaesthetised cat models (Teppema et al. 2001). This improvement in the CO2 reserve would account for the therapeutic effect of ACZ on breathing stability at high altitude. Together with data from the present study, these findings indicate that ACZ-induced changes in cerebrovascular function serve to modulate ventilatory controller gain below eupnoea, and thus increase the CO2 reserve and improve breathing stability at 5050 m. However, we cannot exclude the possibility that ACZ may improve breathing stability via alterations in the peripheral chemoreceptor response, which was not detected in the present study, since carbonic anhydrase inhibition is known to (i) increase carotid body sinus activity delay (Lahiri et al. 1982; Iturriaga et al. 1991), (ii) lower carotid body activity in response to steady state CO2in vivo (Hayes et al. 1976; Lahiri et al. 1976), and (iii) attenuate the speed of response to CO2in vitro (Iturriaga et al. 1991). Nevertheless, the increased cerebrovascular hypocapnic reactivity with ACZ may also account for the reduction in the incidence of central sleep apnoea in patients with congestive heart failure at sea level (Javaheri, 2006; Fontana et al. 2011). Indeed, Xie et al. (2005) previously reported a lower overall cerebrovascular CO2 reactivity, especially to hypocapnia, in congestive heart failure patients with central sleep apnoea compared with patients without. Collectively, along with the related elevations in
and apnoea threshold) and increases the risk of unstable breathing during sleep (Xie et al. 2009). Xie and colleagues attributed this reduction in CO2 reserve to an increased slope of ventilatory response to CO2below eupnoea (i.e. ventilatory controller gain) associated with reduced cerebrovascular hypocapnic reactivity. As such, it seems reasonable that increases in CBF and cerebrovascular hypocapnic reactivity associated with ACZ would therefore blunt the ventilatory slope to CO2 below eupnoea, thereby increasing the CO2 reserve and improve breathing stability. Moreover, since hypoventilation lowers the CO2 reserve (Dempsey, 2005), we speculate that the stabilising effect of ACZ must be greater than the destabilising effect of the reduced ventilation observed in the present study (Table 1). In support of this, ACZ has been found to increase the CO2 reserve by lowering the apnoeic threshold in humans (Teppema et al. 2010) and in the anaesthetised cat models (Teppema et al. 2001). This improvement in the CO2 reserve would account for the therapeutic effect of ACZ on breathing stability at high altitude. Together with data from the present study, these findings indicate that ACZ-induced changes in cerebrovascular function serve to modulate ventilatory controller gain below eupnoea, and thus increase the CO2 reserve and improve breathing stability at 5050 m. However, we cannot exclude the possibility that ACZ may improve breathing stability via alterations in the peripheral chemoreceptor response, which was not detected in the present study, since carbonic anhydrase inhibition is known to (i) increase carotid body sinus activity delay (Lahiri et al. 1982; Iturriaga et al. 1991), (ii) lower carotid body activity in response to steady state CO2in vivo (Hayes et al. 1976; Lahiri et al. 1976), and (iii) attenuate the speed of response to CO2in vitro (Iturriaga et al. 1991). Nevertheless, the increased cerebrovascular hypocapnic reactivity with ACZ may also account for the reduction in the incidence of central sleep apnoea in patients with congestive heart failure at sea level (Javaheri, 2006; Fontana et al. 2011). Indeed, Xie et al. (2005) previously reported a lower overall cerebrovascular CO2 reactivity, especially to hypocapnia, in congestive heart failure patients with central sleep apnoea compared with patients without. Collectively, along with the related elevations in 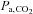 , it appears that changes in the hypocapnic cerebrovascular CO2 reactivity may alter the CO2 reserve, thus influencing breathing stability during both wakefulness and sleep. The potential implications for the role of altered cerebrovascular reactivity in the pathogenesis of breathing instability certainly warrant further investigation.
, it appears that changes in the hypocapnic cerebrovascular CO2 reactivity may alter the CO2 reserve, thus influencing breathing stability during both wakefulness and sleep. The potential implications for the role of altered cerebrovascular reactivity in the pathogenesis of breathing instability certainly warrant further investigation.
Conclusions
To our knowledge, the present study is the first to examine the effects of a single i.v. dose of ACZ on cerebrovascular function and breathing control in partially acclimatised newcomers to high altitude. We demonstrated that acute ACZ at 5050 m increased resting CBF (probably via elevations in 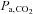 ) and enhanced the CBF responsiveness to both hypercapnia and hypocapnia, and improved breathing stability. We speculate that such elevations in CBF and cerebrovascular reactivity may account, in part, for the well-documented reductions in periodic breathing during sleep following oral ACZ ingestion at high altitude.
) and enhanced the CBF responsiveness to both hypercapnia and hypocapnia, and improved breathing stability. We speculate that such elevations in CBF and cerebrovascular reactivity may account, in part, for the well-documented reductions in periodic breathing during sleep following oral ACZ ingestion at high altitude.
Acknowledgments
The authors are thankful to Professor J. Duffin who kindly providing his technical assistance and the rebreathing analysis programme. We would like to thank Dr R. I. A. Lucas, Dr R. Basnyat and Joseph Donnelly for their assistance during the experimental testing. Special thanks to our participants for giving up their time for this study. We extend our thanks to ADInstruments and Compumedics Ltd for the use of their laboratory equipment. This study was supported by the Otago Medical Research Foundation, SPARC New Zealand, the Peninsula Health Care p/l and Air Liquide p/l. This study was carried out within the framework of the Ev-K2-CNR Project in collaboration with the Nepal Academy of Science and Technology as foreseen in the Memorandum of Understanding between Nepal and Italy, and thanks to a contribution from the Italian National Research Council.
Glossary
Abbreviations
- ACZ
acetazolamide
- AMS
acute mountain sickness
- CBF
cerebral blood flow
- CVCi
cerebrovascular conductance index
- MAP
mean arterial blood pressure
- MCAv
middle cerebral artery blood flow velocity
Author contributions
P.N.A. and K.R.B. contributed to the conception and design of the experiment, the interpretation of the data and the writing of the manuscript. J.-L.F. carried out data collection, and led the analysis, interpretation and writing of the manuscript. K.C.P., K.N.T. and S.J.E.L. contributed equally in the study design and collection of data. J.D.C. and B.K. contributed to the data interpretation and manuscript preparation. All authors approved the final version of this manuscript. The sea-level work was carried out in the Human Physiology Laboratory, Department of Physiology, University of Otago, while the experiments at high altitude were carried out at the Pyramid Laboratory, Italian Research Council.
References
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Brusil PJ, Waggener TB, Kronauer RE, Gulesian P., Jr Methods for identifying respiratory oscillations disclose altitude effects. J Appl Physiol. 1980;48:545–556. doi: 10.1152/jappl.1980.48.3.545. [DOI] [PubMed] [Google Scholar]
- Brzezinski J, Kjallquist A, Siesjo BK. Mean carbon dioxide tension in the brain after carbonic anhydrase inhibition. J Physiol. 1967;188:13–23. doi: 10.1113/jphysiol.1967.sp008120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki NK, Khan SA, Hameed MA. The effects of acetazolamide on the ventilatory response to high altitude hypoxia. Chest. 1992;101:736–741. doi: 10.1378/chest.101.3.736. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Longobardo GS. Cheyne–Stokes breathing. An instability in physiologic control. N Engl J Med. 1973;288:952–957. doi: 10.1056/NEJM197305032881810. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li AH, Nattie EE. Acetazolamide on the ventral medulla of the cat increases phrenic output and delays the ventilatory response to CO2. J Physiol. 1991;441:433–451. doi: 10.1113/jphysiol.1991.sp018760. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Cunningham DJ, Hey EN, Patrick JM, Lloyd BB. The effect of noradrenaline infusion on the relation between pulmonary ventilation and the alveolar
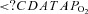 and
and 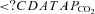 in man. Ann N Y Acad Sci. 1963;109:756–771. doi: 10.1111/j.1749-6632.1963.tb13504.x. [DOI] [PubMed] [Google Scholar]
in man. Ann N Y Acad Sci. 1963;109:756–771. doi: 10.1111/j.1749-6632.1963.tb13504.x. [DOI] [PubMed] [Google Scholar] - Day TA, Wilson RJ. A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude. J Physiol. 2009;587:883–896. doi: 10.1113/jphysiol.2008.160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- Duffin J, Mohan RM, Vasiliou P, Stephenson R, Mahamed S. A model of the chemoreflex control of breathing in humans: model parameters measurement. Respir Physiol. 2000;120:13–26. doi: 10.1016/s0034-5687(00)00095-5. [DOI] [PubMed] [Google Scholar]
- Ehrenreich DL, Burns RA, Alman RW, Fazekas JF. Influence of acetazolamide on cerebral blood flow. Arch Neurol. 1961;5:227–232. doi: 10.1001/archneur.1961.00450140109011. [DOI] [PubMed] [Google Scholar]
- Fan JL, Burgess KR, Basnyat R, Thomas KN, Peebles KC, Lucas SJ, Lucas RA, Donnelly J, Cotter JD, Ainslie PN. Influence of high altitude on cerebrovascular and ventilatory responsiveness to CO2. J Physiol. 2010a;588:539–549. doi: 10.1113/jphysiol.2009.184051. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Fan JL, Burgess KR, Thomas KN, Peebles KC, Lucas SJ, Lucas RA, Cotter JD, Ainslie PN. Influence of indomethacin on ventilatory and cerebrovascular responsiveness to CO2 and breathing stability: the influence of
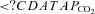 gradients. Am J Physiol Regul Integr Comp Physiol. 2010b;298:R1648–R1658. doi: 10.1152/ajpregu.00721.2009. [DOI] [PubMed] [Google Scholar]
gradients. Am J Physiol Regul Integr Comp Physiol. 2010b;298:R1648–R1658. doi: 10.1152/ajpregu.00721.2009. [DOI] [PubMed] [Google Scholar] - Fontana M, Emdin M, Giannoni A, Iudice G, Baruah R, Passino C. Effect of acetazolamide on chemosensitivity, Cheyne–Stokes respiration, and response to effort in patients with heart failure. Am J Cardiol. 2011;107:1675–1680. doi: 10.1016/j.amjcard.2011.01.060. [DOI] [PubMed] [Google Scholar]
- Gardner WN. The pattern of breathing following step changes of alveolar partial pressures of carbon dioxide and oxygen in man. J Physiol. 1980;300:55–73. doi: 10.1113/jphysiol.1980.sp013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh F, Meyer JS, Takagi Y. Cerebral venous and arterial blood gases during Cheyne-Stokes respiration. Am J Med. 1969;47:534–545. doi: 10.1016/0002-9343(69)90184-3. [DOI] [PubMed] [Google Scholar]
- Grandin CB, Bol A, Smith AM, Michel C, Cosnard G. Absolute CBF and CBV measurements by MRI bolus tracking before and after acetazolamide challenge: repeatabilily and comparison with PET in humans. Neuroimage. 2005;26:525–535. doi: 10.1016/j.neuroimage.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Hackett PH, Roach RC, Harrison GL, Schoene RB, Mills WJ., Jr Respiratory stimulants and sleep periodic breathing at high altitude. Almitrine versus acetazolamide. Am Rev Respir Dis. 1987;135:896–898. doi: 10.1164/arrd.1987.135.4.896. [DOI] [PubMed] [Google Scholar]
- Hauge A, Nicolaysen G, Thoresen M. Acute effects of acetazolamide on cerebral blood flow in man. Acta Physiol Scand. 1983;117:233–239. doi: 10.1111/j.1748-1716.1983.tb07202.x. [DOI] [PubMed] [Google Scholar]
- Hayes MW, Maini BK, Torrances RW. Reduction of the responses of carotid chemoreceptors by acetazolamide. In: Paintal AS, editor. Morphology and Mechanisms of Chemoreceptors. Delhi, India: Valabhbhai Patel Chest Institute; 1976. pp. 36–45. [Google Scholar]
- Iturriaga R, Lahiri S, Mokashi A. Carbonic anhydrase and chemoreception in the cat carotid body. Am J Physiol Cell Physiol. 1991;261:C565–C573. doi: 10.1152/ajpcell.1991.261.4.C565. [DOI] [PubMed] [Google Scholar]
- Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Wright AD, Lassen NA, Harvey TC, Winterborn MH, Raichle ME, Bradwell AR. Cerebral blood flow in acute mountain sickness. J Appl Physiol. 1990;69:430–433. doi: 10.1152/jappl.1990.69.2.430. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Data PG. Chemosensitivity and regulation of ventilation during sleep at high altitudes. Int J Sports Med. 1992;13:S31–S33. doi: 10.1055/s-2007-1024585. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Delaney GA, Fisheman AP. Peripheral and central effects of acetazolamide on control of ventilation. Physiologist. 1976;19:261. [Google Scholar]
- Lahiri S, Maret K, Sherpa MG. Dependence of high altitude sleep apnea on ventilatory sensitivity to hypoxia. Respir Physiol. 1983;52:281–301. doi: 10.1016/0034-5687(83)90086-5. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Mulligan E, Mokashi A. Adaptive response of carotid body chemoreceptors to CO2. Brain Res. 1982;234:137–147. doi: 10.1016/0006-8993(82)90478-4. [DOI] [PubMed] [Google Scholar]
- LaManna JC, McCracken KA. Carbonic anhydrase inhibition and cerebral cortical oxygenation in the rat. Adv Exp Med Biol. 1990;277:335–343. doi: 10.1007/978-1-4684-8181-5_39. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Friberg L, Kastrup J, Rizzi D, Jensen JJ. Effects of acetazolamide on cerebral blood flow and brain tissue oxygenation. Postgrad Med J. 1987;63:185–187. doi: 10.1136/pgmj.63.737.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SJ, Burgess KR, Thomas KN, Donnelly J, Peebles KC, Lucas RA, Fan JL, Cotter JD, Basnyat R, Ainslie PN. Alterations in cerebral blood flow and cerebrovascular reactivity during 14 days at 5050 m. J Physiol. 2011;589:741–753. doi: 10.1113/jphysiol.2010.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew L, Gopinath PM, Purkayastha SS, Gupta SenJ, Nayar HS. Chemoreceptor sensitivity in adaptation to high altitude. Aviat Space Environ Med. 1983;54:121–126. [PubMed] [Google Scholar]
- Mirrakhlmov M, Brimkulov N, Cieslicki J, Tobiasz M, Kudaiberdiev Z, Moldotashev I, Shmidt G, Zielinski J. Effects of acetazolamide on overnight oxygenation and acute mountain sickness in patients with asthma. Eur Respir J. 1993;6:536–540. [PubMed] [Google Scholar]
- Mohan RM, Amara CE, Cunningham DA, Duffin J. Measuring central-chemoreflex sensitivity in man: rebreathing and steady-state methods compared. Respir Physiol. 1999;115:23–33. doi: 10.1016/s0034-5687(99)00003-1. [DOI] [PubMed] [Google Scholar]
- Normand H, Barragan M, Benoit O, Bailliart O, Raynaud J. Periodic breathing and O2 saturation in relation to sleep stages at high altitude. Aviat Space Environ Med. 1990;61:229–235. [PubMed] [Google Scholar]
- Posner JB, Plum F. The toxic effects of carbon dioxide and acetazolamide in hepatic encephalopathy. J Clin Invest. 1960;39:1246–1258. doi: 10.1172/JCI104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene RB, Bates PW, Larson EB, Pierson DJ. Effect of acetazolamide on normoxic and hypoxic exercise in humans at sea level. J Appl Physiol. 1983;55:1772–1776. doi: 10.1152/jappl.1983.55.6.1772. [DOI] [PubMed] [Google Scholar]
- Schreiber SJ, Gottschalk S, Weih M, Villringer A, Valdueza JM. Assessment of blood flow velocity and diameter of the middle cerebral artery during the acetazolamide provocation test by use of transcranial Doppler sonography and MR imaging. AJNR Am J Neuroradiol. 2000;21:1207–1211. [PMC free article] [PubMed] [Google Scholar]
- Subudhi AW, Dimmen AC, Julian CG, Wilson MJ, Panerai RB, Roach RC. Effects of acetazolamide and dexamethasone on cerebral hemodynamics in hypoxia. J Appl Physiol. 2011;110:1219–1225. doi: 10.1152/japplphysiol.01393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JR, Gray GW, Houston CS, Powles AC. Effects of duration at altitude and acetazolamide on ventilation and oxygenation during sleep. Sleep. 1980;3:455–464. doi: 10.1093/sleep/3.3-4.455. [DOI] [PubMed] [Google Scholar]
- Sutton JR, Houston CS, Mansell AL, McFadden MD, Hackett PM, Rigg JR, Powles AC. Effect of acetazolamide on hypoxemia during sleep at high altitude. N Engl J Med. 1979;301:1329–1331. doi: 10.1056/NEJM197912133012406. [DOI] [PubMed] [Google Scholar]
- Swenson ER. Carbonic anhydrase inhibitors and ventilation: a complex interplay of stimulation and suppression. Eur Respir J. 1998;12:1242–1247. doi: 10.1183/09031936.98.12061242. [DOI] [PubMed] [Google Scholar]
- Swenson ER, Hughes JM. Effects of acute and chronic acetazolamide on resting ventilation and ventilatory responses in men. J Appl Physiol. 1993;74:230–237. doi: 10.1152/jappl.1993.74.1.230. [DOI] [PubMed] [Google Scholar]
- Teppema L, Berkenbosch A, DeGoede J, Olievier C. Carbonic anhydrase and control of breathing: different effects of benzolamide and methazolamide in the anaesthetized cat. J Physiol. 1995;488:767–777. doi: 10.1113/jphysiol.1995.sp021008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Balanos GM, Steinback CD, Brown AD, Foster GE, Duff HJ, Leigh R, Poulin MJ. Effects of acetazolamide on ventilatory, cerebrovascular, and pulmonary vascular responses to hypoxia. Am J Respir Crit Care Med. 2007;175:277–281. doi: 10.1164/rccm.200608-1199OC. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Bijl H, Romberg RR, Dahan A. Antioxidants reverse depression of the hypoxic ventilatory response by acetazolamide in man. J Physiol. 2006;572:849–856. doi: 10.1113/jphysiol.2005.104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. Acetazolamide and breathing. Does a clinical dose alter peripheral and central CO2 sensitivity? Am J Respir Crit Care Med. 1999;160:1592–1597. doi: 10.1164/ajrccm.160.5.9903088. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. Low-dose acetazolamide reduces the hypoxic ventilatory response in the anesthetized cat. Respir Physiol Neurobiol. 2004;140:43–51. doi: 10.1016/j.resp.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A, Olievier CN. Low-dose acetazolamide reduces CO2–O2 stimulus interaction within the peripheral chemoreceptors in the anaesthetised cat. J Physiol. 2001;537:221–229. doi: 10.1111/j.1469-7793.2001.0221k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Rochette F, Demedts M. Ventilatory effects of acetazolamide in cats during hypoxemia. J Appl Physiol. 1992;72:1717–1723. doi: 10.1152/jappl.1992.72.5.1717. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dorp vanEL, Dahan A. Arterial [H+] and the ventilatory response to hypoxia in humans: influence of acetazolamide-induced metabolic acidosis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L89–L95. doi: 10.1152/ajplung.00255.2009. [DOI] [PubMed] [Google Scholar]
- Thomas KN, Burgess KR, Basnyat R, Lucas SJ, Cotter JD, Fan JL, Peebles KC, Lucas RA, Ainslie PN. Initial orthostatic hypotension at high altitude. High Alt Med Biol. 2010;11:163–167. doi: 10.1089/ham.2009.1056. [DOI] [PubMed] [Google Scholar]
- Vorstrup S, Henriksen L, Paulson OB. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Invest. 1984;74:1634–1639. doi: 10.1172/JCI111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstrup S, Jensen KE, Thomsen C, Henriksen O, Lassen NA, Paulson OB. Neuronal pH regulation: constant normal intracellular pH is maintained in brain during low extracellular pH induced by acetazolamide–31P NMR study. J Cereb Blood Flow Metab. 1989;9:417–421. doi: 10.1038/jcbfm.1989.61. [DOI] [PubMed] [Google Scholar]
- Vuyk J, Bos VanDenJ, Terhell K, Bos DeR, Vletter A, Valk P, Beuzekom VanM, Kleef VanJ, Dahan A. Acetazolamide improves cerebral oxygenation during exercise at high altitude. High Alt Med Biol. 2006;7:290–301. doi: 10.1089/ham.2006.7.290. [DOI] [PubMed] [Google Scholar]
- Waggener TB, Brusil PJ, Kronauer RE, Gabel RA, Inbar GF. Strength and cycle time of high-altitude ventilatory patterns in unacclimatized humans. J Appl Physiol. 1984;56:576–581. doi: 10.1152/jappl.1984.56.3.576. [DOI] [PubMed] [Google Scholar]
- Weil JV, Kryger MH, Scoggin CH. Sleep and breathing at high altitude. In: Guilleminault C, Dement WC, editors. Sleep Apnea Syndromes. New York: Liss; 1978. pp. 119–136. [Google Scholar]
- Wilson MH, Edsell ME, Davagnanam I, Hirani SP, Martin DS, Levett DZ, Thornton JS, Golay X, Strycharczuk L, Newman SP, Montgomery HE, Grocott MP, Imray CH. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia - an ultrasound and MRI study. J Cereb Blood Flow Metab. 2011;31:2019–2029. doi: 10.1038/jcbfm.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Barczi SR, Reichmuth K, Morgan BJ, Mont S, Dempsey JA. Influence of cerebral blood flow on breathing stability. J Appl Physiol. 2009;106:850–856. doi: 10.1152/japplphysiol.90914.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Dempsey JA. Effect of hypoxia on the hypopnoeic and apnoeic threshold for CO2 in sleeping humans. J Physiol. 2001;535:269–278. doi: 10.1111/j.1469-7793.2001.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, Russell D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med. 2005;172:371–378. doi: 10.1164/rccm.200406-807OC. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Dempsey JA. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol. 2006;100:171–177. doi: 10.1152/japplphysiol.00440.2005. [DOI] [PubMed] [Google Scholar]


