Abstract
Non-technical summary
Limb ischaemia commonly occurs in peripheral artery disease and blood pressure response to stimulation of muscle afferent nerves during exercise activity is augmented in this disease; however, the mechanisms responsible for this physiological process are not well-known. In this report, expression and function of acid-sensing ion channels subtype 3 (ASIC3) in muscle afferent nerves were examined following the hindlimb ischaemia induced by femoral artery occlusion. The data provided here suggest that femoral artery occlusion increases ASIC3 expression and responsiveness in muscle sensory neurons. The results help us better understand why the blood pressure response to exercise is exaggerated in patients with peripheral artery disease.
Abstract
Sympathetic nerve activity and arterial blood pressure responses to static hindlimb muscle contractions are greater in rats with femoral arteries that were previously ligated (24–72 h earlier) than in control rats. Studies further demonstrate that acid-sensing ion channel subtype 3 (ASIC3) in thin-fibre muscle afferents contributes to the amplified reflex muscle responses observed in occluded rats, probably due to enhanced ASIC3 expression in muscle sensory neurons. The purpose of this study was to characterize acid-induced current with activation of ASIC3 in dorsal root ganglion (DRG) neurons of control rats and rats with 24 h of femoral occlusion using whole-cell patch clamp methods. Also, immunohistochemistry was employed to examine existence of ASIC3 expression in DRG neurons of thin-fibre afferents. DRG neurons from 4- to 6-week-old rats were labelled by injecting the fluorescence tracer DiI into the hindlimb muscles 4–5 days prior to the recording experiments. The results of this study show that ∼90% of current responses evoked by pH 6.7 in DRG neurons innervating the hindlimb muscles are ASIC3-like. The peak current amplitude to pH 6.7 is significantly attenuated with application of rAPETx2, a specific ASIC3 antagonist. In addition, ASIC3-like current responses to pH 6.7 are observed in small, medium and large DRG neurons, and size distribution of DRG neurons is similar in control and occluded animals. However, the peak current amplitude of DRG neuron response induced by ASIC3 stimulation is larger in occluded rats than that in control rats. Moreover, the percentage of DRG neurons with ASIC3-like currents is greater after arterial occlusion compared with control. Furthermore, results from double immunofluorescence experiments show that femoral artery occlusion mainly augments ASIC3 expression within DRG neurons projecting C-fibre afferents. Taken together, these data suggest that (1) the majority of current responses to pH 6.7 are ASIC3-like in DRG neurons with nerve endings in the hindlimb muscles, (2) a greater acid-induced current responding to pH 6.7 develops when hindlimb arterial blood supply is deficient under ischaemic conditions, and (3) increased ASIC3 expression is largely observed in thin C-fibres of DRG neurons after hindlimb ischaemia.
Introduction
The thin-fibre/group III and IV afferent nerves arising from contracting skeletal muscle contribute to sympathetic nerve and cardiovascular responses during exercise via a reflex neural mechanism, termed the ‘exercise pressor reflex’ (Kaufman et al. 1983, 1984a,b; Mitchell et al. 1983). Exercise induces the production of muscle metabolites, such as lactic acid, in the activated muscle (Kaufman et al. 1996, 2002; Sinoway et al. 2005). These metabolites, along with decreased pH levels in the interstitial space of muscles, stimulate group III and IV muscle afferents, the free endings of which reside in the interstitium (Kaufman et al. 1996, 2002; Sinoway et al. 2005). Metabolite-sensitive receptors, including acid-sensing ion channels (ASICs), located on the muscle afferent nerves are excited by those muscle by-products (Hayes et al. 2007, 2008; McCord et al. 2008, 2009). Through these actions, cardiovascular nuclei in the brainstem are activated, the sympathetic nervous system is activated, blood pressure (BP) and heart rate (HR) are further increased, and the exercise pressor reflex is evoked (Hayes et al. 2007, 2008; McCord et al. 2008, 2009). These reflex mechanisms that process muscle afferent signals via sensory nerve receptors are altered in cardiovascular diseases (Sinoway et al. 2005; Smith et al. 2006; Gao et al. 2007; Xing et al. 2008a; Tsuchimochi et al. 2010).
Peripheral arterial disease (PAD) caused by a restriction of lower limb blood flow is common in older adults (Ouriel, 2001; Critchley et al. 2003; Muir, 2009). The most common symptom of this disease is intermittent claudication, which frequently occurs during physical activity but is relieved promptly by rest (Rejeski et al. 2008). When the exercise pressor reflex is activated in patients with PAD, increases in sympathetic nerve activity (SNA), BP and HR are exaggerated (Baccelli et al. 1999; Bakke et al. 2007). A rat model of femoral artery ligation has been employed to study PAD in humans (Waters et al. 2004). Studies have used this model to demonstrate that the SNA and pressor responses to static muscle contraction and lactic acid injection into the arterial blood supply of hindlimb muscles are amplified in occluded rats compared with control rats (Liu et al. 2010; Tsuchimochi et al. 2010).
A recent study has shown that arterial injection of a specific ASIC3 blocker markedly attenuates the reflex pressor response to muscle contraction in the rats with a ligated femoral artery, but has only modest effects in the rats with freely perfused hindlimbs (Tsuchimochi et al. 2011). Notably, ASIC3 expression is upregulated in dorsal root ganglion (DRG) neurons innervating the hindlimb muscles with the occluded femoral artery (Liu et al. 2010). Additionally, injecting lactic acid into the arterial blood supply of hindlimb muscles to stimulate ASIC3 of muscle afferent nerves increases SNA and BP to a greater degree in occluded rats (Liu et al. 2010). Nevertheless, the underlying mechanisms by which femoral occlusion augments sympathetic nerve and blood pressure responsiveness to muscle contraction and lactic acid injected into the hindlimb muscles remain to be determined.
Given that DRG cells are the primary sensory projections to group III and IV fibre afferent nerves, expression and characteristics of sensory receptors (i.e. ASIC3) in DRG neurons are generally examined to study receptor physiology (Deval et al. 2008, 2011; Mamet et al. 2002, 2003). The receptors in question are found in both the peripheral terminals and cell bodies of sensory DRG neurons. Receptor activity and characteristics of the DRG cell body have been used to reflect activity and characteristics of the receptors located at the nerve endings (Mamet et al. 2002, 2003; Deval et al. 2008, 2011).
In this report, whole-cell patch clamp methods were employed to examine acid-induced current responses in DRG neurons of control rats and rats whose femoral artery was ligated for 24 h. In addition, among ASICs, ASIC3 is found predominantly on sensory neurons, and maintains functional channels that open in response to proton concentration fluctuation (Waldmann et al. 1997a,b, 1999; Light et al. 2008). The pH range required to activate ASIC3 is approximately 6.5–7.0 (Deval et al. 2011, 2008), which is close to what is observed in exercising muscle and/or moderately ischaemic tissues (Rotto et al. 1989; MacLean et al. 1998, 2000; Yagi et al. 2006). Furthermore, acid-induced currents with ASIC3 activation in DRG neurons of control rats and rats with 24 h of femoral artery occlusion were characterized in this study.
Previous studies suggest that the glycolytic muscle plays a major role in reflex muscle responses evoked by static contraction (Wilson et al. 1995). Furthermore, a recent study using electrophysiological methods has demonstrated that DRG neurons with nerve endings in the white portion of the gastrocnemius muscle develop greater inward current responses to metabolic stimulation such as acid (Xing et al. 2008b). Therefore, in the current report, acid-induced currents were recorded on rat DRG neurons innervating the white portion of the gastrocnemius muscles identified by retrograde labelling with the fluorescent dye DiI.
Furthermore, dual immunofluorescence techniques were employed to examine localization of ASIC3 within DRG neurons with A- and thin C-fibres (thin-fibre group IV) in control rats and occluded rats.
Methods
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State College of Medicine and complied with the National Institutes of Health (NIH) guidelines.
Labelling DRG neurons innervating hindlimb muscle
Male Sprague–Dawley rats (4–6 weeks old) were anaesthetized by inhalation of an isoflurane–oxygen mixture (2–5% isoflurane in 100% oxygen). The skin was incised and pulled away from underlying muscle tissue, and the fluorescent retrograde tracer DiI (60 mg ml−1) was injected into the white portion of the gastrocnemius muscle (Xing et al. 2008a,b). The injection volume of 1 μl was administered, and the injection was repeated three times at different locations. The injection needle was left in the muscle for 5–10 min to prevent leakage of tracer. The skin overlying the muscle was then sutured. The animals were returned to their cages for 4–5 days to permit the retrograde tracer to be transported to DRG neurons.
Ligation of the femoral artery
At 24 h prior to the recording experiments, the rats that previously received DiI injections were anaesthetized with an isoflurane–oxygen mixture (as above). Then, the femoral artery on one limb was surgically exposed, dissected and ligated ∼3 mm distal to the inguinal ligament as previously described (Xing et al. 2008a; Liu et al. 2010). The same procedures were performed on the other limb except that a suture was placed below the femoral artery but was not tied; this served as the control.
Examination of DRG neuron responsiveness
The rats were anaesthetized with an isoflurane–oxygen mixture (as above) followed by cervical dislocation and decapitation. The L4–6 DRGs were quickly removed and transferred immediately into Dulbecco's modified Eagle's medium (DMEM). The DRGs were minced, and the ganglion fragments were processed to obtain dissociated DRG neurons as described previously (Xing et al. 2008a,b). The cell suspension was centrifuged to remove the supernatant, and the cell pellet was resuspended in DMEM. The cells were then plated onto a 35 mm culture dish containing pre-coated coverslips.
Next, patch recordings were performed within 6 h of dissociation (Xing et al. 2008a,b). Neurons were first visualized using a combination of epifluorescent illumination and differential interference contrast (DIC, 20–40×) optics on an inverted microscope (Nikon TE2000). Under DIC, images of DiI-positive neurons were displayed on a video monitor. Neurons were patched in the whole-cell configuration and recorded at a holding potential of −70 mV using a MultiClamp 700B amplifier (Axon Inc.). Seals (1–10 GΩ) between the glass electrode (2–5 MΩ resistance) and the cell were established in a modified Tyrode solution (Xing et al. 2008a,b). After the whole-cell configuration was established, the cell membrane capacitance and series resistance were electronically compensated. In order to record action potentials of DRG neurons, a mode of the whole-cell current holding was performed. All experiments were then conducted. Signals were acquired using the pCLAMP 9.0 software and experimental data were analysed using the Clampfit software program. Neurons were considered proton-sensitive if acid solution elicited an inward current of >50 pA in peak amplitude.
Drugs stored in stock solutions were diluted in extracellular solution immediately before being used and were held in a series of independent syringes connected to corresponding fused silica columns (inner diameter 200 μm) (Xing et al. 2008a,b). The ends of the parallel columns were connected to a common silica column. The distance from the column mouth to the examined cell was 100 μm. Cells in the recording chamber were continuously bathed in Tyrode solution. The gravity-fed solutions containing each drug were delivered to the cells by controlling the corresponding valve switch (WP Instruments).
A total of 241 neurons were tested. All DRG neurons used in this report were DiI-positive. At the end of each experiment, the gastrocnemius muscle was dissected to confirm that DiI was located in the white portion of the gastrocnemius muscle.
Immunohistochemistry
The rats were anaesthetized with an isoflurane–oxygen mixture (as above) and then transcardially perfused with 200 ml of ice-cold saline containing 1000 units heparin followed by 500 ml of 4% fresh prepared, ice-cold paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). L4–6 DRGs of control and occluded limbs were immediately dissected out and immersed in the same fixative at 4°C for 2 h The tissues were then stored in PBS containing 30% sucrose overnight, and a cryostat was used to obtain DRG sections (10 μm).
DRG sections on slides were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. After being washed with PBS, the tissue was permeabilized, blocked in 0.3% Triton X-100 in PBS supplemented with 5% goat serum for 1 h, and then incubated with the guinea pig polyclonal anti-ASIC3 (1:250, Neruomics) antibody overnight at 4°C. After being washed in PBS, the sections were incubated with the goat anti-guinea pig fluorescein isothiocyanate (FITC)-labelled secondary antibody (1:200, Neruomics) for 2 h at room temperature.
To examine ASIC3 localization within C- and A-fibre DRG neurons, sections were incubated with the second primary antibody (mouse anti-peripherin at 1:200, Sigma; or anti-NF200 at 1:200, Abcam) overnight. Peripherin and NF200 are used to label neurons with C- and A-fibres, respectively. Then, the sections were washed and incubated for 1 h at room temperature with a secondary antibody (Alexa Fluor-594-conjugated goat anti-mouse IgG, dilution: 1:200) for 2 h at room temperature. After that, the sections were washed in PBS and coverslipped.
FITC- and Alexa Fluor-594-labelled DRG neurons were examined using a Nikon Eclipse 80i microscope with appropriate filters, and the images were stored digitally on a computer. As described previously (Liu et al. 2010), at least five sections containing L4–6 DRGs per rat were randomly chosen for analysis of FITC and Alexa Fluor-594 staining intensity. The sections were coded, and immunolabelling was examined in a blinded fashion. A threshold value of staining intensity was set according to the mean staining intensity of background using the Nis-Elements software (Nikon, Co.). Cells with labelling more than 1.75 times the background intensity were considered positive. The number of total ASIC3 and peripherin/NF200-positive neurons was counted in each section. Percentages of double (FITC and peripherin/NF200)-labelled neurons were calculated: total number of double-labelled cells × 100/total number of peripherin/NF200-positive cells. The majority of DRG neurons showed a clear nucleus and perimeter and they were counted. To minimize the possibility of counting a single DRG neuron more than once, DRG sections were collected on five glass slides in series, and only one of these slides was assessed for immunocytochemical analysis.
Statistical analysis
Experimental data were analysed using one-way repeated measures analysis of variance (ANOVA). As appropriate, Tukey's post hoc tests were used. All values were presented as the mean ± SEM. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed using SPSS for Windows version 15.0.
Results
Determination of ASIC3-like currents in DRG neurons
As reported previously, two different types of ASIC currents were observed as pH 6.7 solution was applied to DRG neurons (Fig. 1). ASIC currents were distinguished by their inactivation kinetics. Eight of 87 DiI-DRG neurons (9%) developed acid-induced currents with a slow inactivating rate (τ = 1606 ± 35 ms) in response to pH 6.7. The currents with this characteristic are considered to be elicited by recombinant ASIC1a (Baron et al. 2008). In contrast, acid-induced currents in 79 of 87 DiI-DRG neurons (91%) showed rapid inactivation kinetics (τ = 368 ± 13 ms), which is typically observed as recombinant ASIC3 channels are activated (Waldmann et al. 1997a).
Figure 1. Whole-cell patch clamp methods were employed to study acid-induced currents to pH 6.7 in DRG neurons of control rats and rats with 24 h of femoral artery ligation.
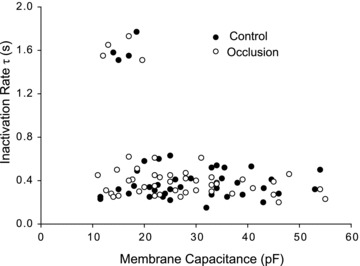
Two different ASIC currents were distinguished on the basis of their inactivation kinetics. One type of current exhibited a slow inactivating rate (larger τ value) and was only expressed in small DRG neurons with a cell capacitance of <20 pF. This group of currents represents typically an ASIC1a response to pH 6.7. Another type of current had rapid inactivation kinetics (smaller τ value), and the currents were observed in small, medium and large DRG neurons with cell capacitance ranging from 10 to 60 pF. Those currents are typically observed as recombinant ASIC3 channels are activated. Note that there were no significant differences in inactivating rate and cell capacitance for DRG neurons of control rats and occluded rats.
Also, whole-cell membrane capacitance of DiI neurons with both ASIC-type currents described above was analysed (Fig. 1). ASIC1a currents were observed in small DRG neurons with cell capacitance of <20 pF, whereas ASIC3-like currents were observed in small, medium and large DRG neurons with cell capacitance ranging from 10 to 60 pF.
In addition, no significant differences were observed for inactivating rate or cell capacitance in DiI-DRG neurons of control rats and occluded rats. The cell capacitance was 28 ± 2 pF in DRG neurons of control and 27 ± 2 pF (P > 0.05 vs. control) in DRG neurons with femoral occlusion. A slow inactivating rate was 1603 ± 58 ms in control and 1610 ± 50 ms in femoral occlusion (P > 0.05, vs. control). A rapid inactivating rate was 364 ± 20 ms in control and 372 ± 16 ms in femoral artery occlusion (P > 0.05 vs. control).
Previous studies have suggested that the toxins PcTx1 and rAPETx2 are specific antagonists to ASIC1a and ASIC3 homomeric channels, respectively (Escoubas et al. 2000; Diochot et al. 2004). Thus, in this experiment, the current responses to pH 6.7 in DiI-labelled DRG neurons were also examined after application of these specific blockers. Toxin PcTx1 (20 nm) significantly inhibited current responses induced by pH 6.7 in DRG neurons exhibiting ASIC1a currents (Fig. 2A and B). The current amplitude was 1375 ± 339 pA with pH 6.7 and 27 ± 8 pA with PcTx1 pretreatment (P < 0.05 vs. pH 6.7 alone). However, rAPETx2 had minimal effects on this type of current in DRG neurons (Fig. 2A and B). The current amplitude was 1375 ± 339 pA with pH 6.7 alone and 1355 ± 320 pA with rAPETx2 pretreatment (P > 0.05). On the other hand, pretreatment of rAPETx2 (1 μm) significantly attenuated peak amplitude of currents evoked by pH 6.7 in DRG neurons that displayed ASIC3-like current. The current amplitude was 684 ± 129 pA with pH 6.7 and 223 ± 21 pA with rAPETx2 prior to pH 6.7 (P < 0.05 vs. pH 6.7 alone). Likewise, PcTx1 had no significant effects on ASIC3-like currents (Fig. 2C and D). The current amplitude was 658 ± 141 pA with pH 6.7 alone and 653 ± 140 pA with PcTx1 pretreatment (P > 0.05 vs. pH 6.7 alone). In addition, Fig. 2 shows that the inhibitory effects of PcTx1 on ASIC1a currents, and rAPETx2 on ASIC3-like currents were both reversible.
Figure 2. Effects of blocking ASIC1a and ASIC3 homomeric channels on acid-evoked currents in DRG neurons innervating the hindlimb muscles.
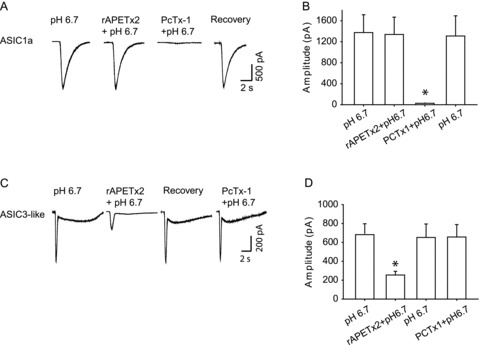
Toxins PcTx1 and rAPETx2 were used as specific antagonists to ASIC1a and to ASIC3, respectively. A, original traces of ASIC1a currents response to pH 6.7 with prior application of rAPETx2 and PcTx1. B, averaged data showing peak amplitudes of ASIC1a currents. Toxin PcTx1 (20 nm) significantly inhibited current responses induced by pH 6.7 in DRG neurons exhibiting ASIC1a currents. *P < 0.05 vs. pH 6.7 alone. However, rAPETx2 had no significant effects on this type of current in DiI- labelled neurons. C, typical ASIC3-like current response to pH 6.7 with prior exposure to rAPETx2 and PcTx1. D, averaged data showing that a prior application of rAPETx2 (1 μm) significantly attenuated peak amplitudes of currents evoked by pH 6.7 in DRG neurons that displayed ASIC3-like currents. PcTx1 had negligible effects on this type of currents. *P < 0.05 vs. pH 6.7 alone. Note that the inhibitory effects of PcTx1 on ASIC1a currents and rAPETx2 on ASIC3-like currents were both reversible.
Effects of femoral occlusion on ASIC3-like currents in DRG neurons
The size distribution of DRG neurons responding to pH 6.7 with ASIC3-like currents recorded in control and 24 h of arterial occlusion is presented in Fig. 3. DRG neurons with ASIC3-like currents were distributed in small, medium and large size neurons. However, size distribution was similar in control and occluded animals.
Figure 3. Size distribution of DRG neurons responding to pH 6.7.
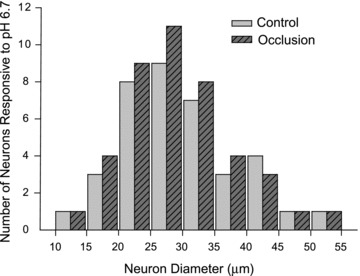
Acid-evoked current responses with activation of ASIC3 were recorded in DiI-labelled DRG neurons of control rats and rats with 24 h of femoral artery occlusion. No differences between both experimental groups were observed for the average size of DRG neurons that responded to pH 6.7.
Figure 4A shows typical recordings from two neurons that only express ASIC3-like current, which were obtained from a control rat and an occluded rat, respectively. A pH of 6.7 induced currents, with a transient and a plateau phases, which is compatible with the kinetics of the ASIC3-like current. When compared with that in the control, DRG neurons with the arterial occlusion exhibited a greater peak inward current. In order to better compare the current responsiveness in both experimental groups, the current density (amplitude/membrane capacitance) was analysed. This approach can minimize the confounding effects of cell size. The current density was 20.6 ± 4.1 pA pF−1 in 37 DiI-neurons from control rats and 35.7 ± 4.9 pA pF−1 in 42 neurons from rats with arterial occlusion (P < 0.05 vs. control) (Fig. 4B). Moreover, femoral artery ligation surgery increased the percentage of neurons expressing ASIC3-like current evoked by pH 6.7 from 45.7 ± 5.5% in control to 63.2 ± 5.6% in arterial occlusion (P < 0.05 vs. control) (Fig. 4C).
Figure 4. Effects of femoral arterial occlusion on ASIC3-like currents response to pH 6.7.
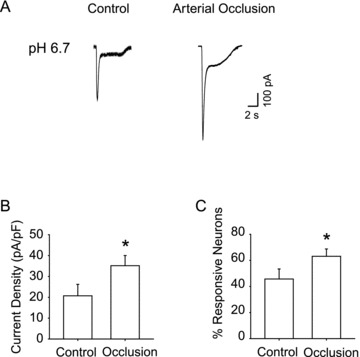
A, original traces of ASIC3-like current responsive to pH 6.7 recorded in DRG neurons innervating hindlimb muscles in control and after 24 h arterial occlusion. B, averaged data show mean current density of ASIC3-like currents activated by pH 6.7. Twenty-four hours of arterial occlusion induced a larger current density compared with control. *P < 0.05 vs. control. C, percentage of DRG neurons innervating muscle with ASIC3-like currents responsive to pH 6.7. A larger percentage of DRG neurons with ASIC3-like currents were observed in 24 h of arterial occlusion than in control. *P < 0.05 compared with control.
In addition, a mode of whole-cell current holding was performed to examine action potential (AP) of DiI-labelled DRG neurons (Fig. 5). There was no significant difference in resting membrane potential between control (–55.4 ± 1.5 mV) and occluded group (–54.5 ± 1.1 mV, P > 0.05 vs. control). Figure 5A further shows the original tracings of spontaneous AP and pH 6.7-triggered AP in DRG neurons. In addition, the prior application of rAPETx2 inhibited pH 6.7-evoked cell depolarization and AP. It is noteworthy that the number of neurons in which APs could be triggered by decreased pH was significantly increased by femoral artery ligation (Fig. 5B). The percentage of neurons with pH 6.7-triggered APs was 35.2 ± 5.2% in control and 51.2 ± 4.4% in arterial occlusion (P < 0.05 vs. control), whereas the percentage of neurons with spontaneous AP in the control group (31.5 ± 4.8%) was similar to that in femoral artery ligation group (34.5 ± 3.5%, P > 0.05 vs. control).
Figure 5. Current clamp recordings were performed on DiI-labelled neurons exhibiting ASIC3-like currents.
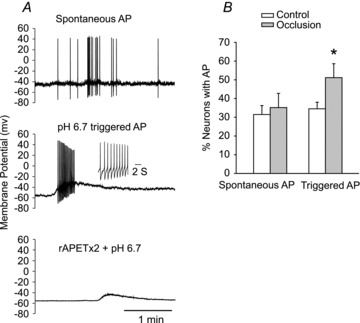
A, original traces. Top: spontaneous action potentials (AP). Middle: slight acidification using a pH 6.7 solution sufficiently depolarized the membrane to trigger firing. Action potentials are magnified in the inset. Bottom: the presence of rAPETx2 inhibited pH 6.7-evoked depolarization and depressed the AP threshold. B, percentage of recorded DRG neurons with spontaneous APs and APs triggered by pH drops to 6.7 in control and femoral artery occlusion. Averaged data showing that the percentage of neurons with spontaneous APs was similar in the control group compared with the femoral artery ligation group. However, femoral artery ligation increased the number of neurons in which APs could be triggered by a pH drop. *P < 0.05 vs. control.
Examination of ASIC3 within DRG neurons with C- and A-fibres
In this experiment, we further determined if ASIC3 exists within DRG neurons that project C- and A-fibre afferents. Dual immunofluorescence techniques were used to examine co-localization of fluorescent ASIC3 and peripherin/NF200 immunoreactivity in DRG neurons from control rats and occluded rats (Figs 6 and 7). The appearance of ASIC3 and peripherin/NF200 within DRG neurons is characterized by fluorescent green and red colour, respectively (Figs 6 and 7).
Figure 6. Immunofluorescence was employed to examine double-labelling for ASIC3 and peripherin.
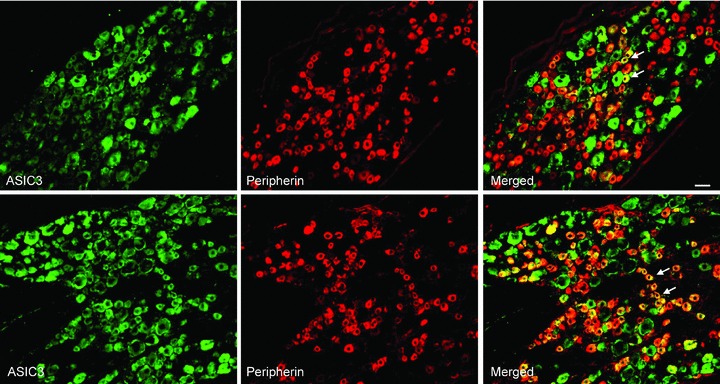
Peripherin was used to label DRG neurons that project thin C-fibres. Representative photomicrographs show ASIC3 and peripherin staining in DRG neurons of a control rat (top panel) and an occluded rat (bottom panel). Arrows indicate representative cells positive for both ASIC3 and peripherin after they were merged. The number of double-labelled DRG neurons is greater in occluded rats than in control rats. Scale bar, 50 μm.
Figure 7. Immunofluorescence was employed to examine double-labelling for ASIC3 and NF200.
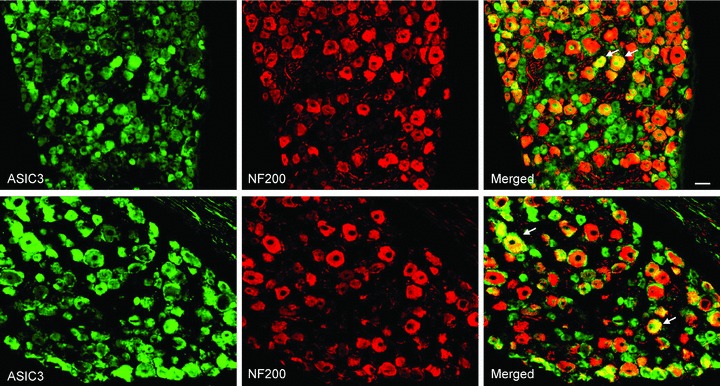
Note that NF200 was used to identify A-fibres DRG neurons. Photomicrographs are representative to illustrate staining of ASIC3 and NF200 in DRG neurons of a control rat (top panel) and an occluded rat (bottom panel). Arrows indicate examples for merged ASIC3- and NF200-positive cells. No differences in the number of double-stained ASIC3 and NF200 were observed in DRG neurons of the control and occluded groups. Scale bar, 50 μm.
The photomicrographs show that ASIC3 staining appears in C- and A-fibres of DRG neurons in both control and occlusion groups. A large proportion of DRG neurons with C-fibre were ASIC3-positive compared with A-fibre neurons (Figs 6 and 7). Figure 6 further shows that a greater number of C-fibre neurons containing ASIC3 were found in an occluded rat (bottom panel) compared with a control rat (top panel). The percentage of double-labelled neurons with ASIC3 and peripherin was significantly greater in occluded limbs than in controls. They were 15 ± 1% in the controls (n = 4) and 32 ± 1% (P < 0.05 vs. control) in the ligation group (n = 5). Figure 7 demonstrates that DRG neurons with A-fibres include ASIC3 staining in both control and ligation groups. The percentage of double-labelled neurons with ASIC3 and NF200 was similar in both experimental groups. They were 8 ± 1% in controls (n = 4) and 9 ± 1% (P > 0.05 vs. control) in the ligation group (n = 5). There was no significant difference in number of peripherin- and NF200-positve DRG neurons between both experimental groups.
Discussion
Taken together, the data of this study indicate that in DRG neurons with nerve endings in the hindlimb muscles, ASIC3-containing channels represent the majority of acid-induced currents elicited by moderate external acidosis in a range that is relevant to exercising muscle and/or hindlimb ischaemia. Additionally, a greater current response with activation of ASIC3 is observed as the arterial blood supply to the hindlimb is deficient under ischaemic conditions. Note that the size of DRG neurons that have ASIC3-like currents is typically small to large, and the size distribution is similar in control and occluded animals. Also, the percentage of DRG neurons with pH 6.7-triggered APs is greater in occluded rats than in control rats, suggesting that femoral occlusion increases the probability of sensory neurons to evoke neuronal activities. The results of immunohistochemical experiments suggest that ASIC3 appears in both C- and A-fibres of DRG neurons, and that femoral artery occlusion largely increases expression of ASIC3 in DRG neurons that project C-fibre afferents.
Previous studies have suggested that lactic acid plays an important role in mediating the exercise pressor reflex (Rotto et al. 1988, 1989). First, static exercise increases muscle SNA and BP via a reflex neural pathway and these responses are closely related to decreases in muscle pH in healthy subjects (Victor et al. 1988). Second, lactic acid injected into the arterial blood supply of hindlimb muscles reflexively increases BP via its stimulation of chemosensitive muscle afferents in anaesthetized cats (Rotto et al. 1988). Third, in healthy humans, muscle SNA and pressor responses to exercise are attenuated by muscle glycogen depletion or dichloroacetate, which can blunt contraction-induced lactic acidosis (Ettinger et al. 1991; Sinoway et al. 1992).
ASICs are members of a family of amiloride-sensitive sodium channels and are considered as molecular sensors in afferent neurons (Molliver et al. 2005; Naves et al. 2005; Sugiura et al. 2005; Yagi et al. 2006; Lingueglia, 2007; Light et al. 2008; Holzer, 2009). They are almost ubiquitous in the mammalian nervous system and are activated as pH drops below 7.0. There are six different proteins of ASICs (ASIC1a, 1b, 2a, 2b, 3 and 4), encoded by four genes (ASIC1, 2, 3 and 4). The ASIC3 protein, however, is mostly found in DRG where it forms functional channels (Waldmann et al. 1997a,b, 1999; Light et al. 2008) that are opened by proton concentrations that are observed within exercising and/or ischaemic muscles. Additionally, a prior investigation supports the idea that ASIC3 is unlikely to contribute to mechanically activated currents in mammalian sensory neurons (Drew et al. 2004). Based on the experimental results of ASIC3 receptor distribution and ionic properties, it was postulated that ASIC3 is likely to play a role in engaging in the exercise pressor reflex. First, ASIC3 receptors are activated by pH ranges that are seen in exercising muscles (MacLean et al. 2000; Street et al. 2001). Second, lactate that is accumulated in active muscle tissues can enhance ASIC3 sensitivity to protons (Immke et al. 2001, 2003). Thus, ASIC3 is a suitable sensor for lactic acidosis as muscles undergo anaerobic metabolism. Third, ASIC3 is a dominant ASIC subunit, preferentially localized in DRG neurons of thin-fibre afferent nerves (Waldmann et al. 1997a,b, 1999).
Moreover, blocking ASIC receptors using amiloride and the more selective antagonist A-317567 has been reported to attenuate the pressor response evoked by static exercise and by arterial injection of lactic acid into the hindlimb muscles (Hayes et al. 2007, 2008). Accordingly, acid sensing has been considered an important characteristic of thin-fibre afferents sensory neurons and contributes to reflex cardiovascular responses to muscle contraction. A recent study recorded discharges of thin-fibre muscle afferent nerves in cats, and reported that ASICs participate in the metabolic but not the mechanoreceptor component of the exercise pressor reflex (McCord et al. 2008, 2009).
Previous studies have shown that ASIC3 generally distributes in small- to large-size DRG neurons (Molliver et al. 2005; Deval et al. 2011). In this report, DiI was injected into the hindlimb muscles in order to label the DRG neurons that innervate muscles, and the results of the patch clamp experiments demonstrated that DiI-labelled DRG neurons with ASIC3-like currents are small, medium and large in size. In addition, immunocytochemistry has shown that ASIC3 immunolabelling appears in DRG neurons of all sizes. However, femoral occlusion largely affects ASIC3 function and expression in small to medium size neurons (i.e. ∼20–35 μm in diameter), specifically in DRG neurons that project C-fibres, which are considered to be engaged in the muscle metaboreflex (Kaufman et al. 1983, 1984b).
A recent study has further shown that arterial injection of a specific ASIC3 blocker, rAPETx2, markedly attenuates the reflex pressor response to muscle contraction in the rats with a ligated femoral artery, but has only modest effects in the rats with freely perfused hindlimbs (Tsuchimochi et al. 2011). These findings raised questions about the underlying mechanism that causes a difference in the exercise pressor reflex between rats with freely perfused hindlimbs and rats with hindlimb ischaemia. Note that ASIC3 expression is upregulated in the DRG neurons innervating the hindlimb muscles of occluded rats (Liu et al. 2010). Consistent with this result, lactic acid injected into the arterial blood supply of hindlimb muscles to stimulate ASIC3 of muscle afferent nerves increases SNA and BP to a greater degree in occluded rats than in control rats (Liu et al. 2010). Also, an acidic milieu is likely to sensitize mechanically sensitive muscle afferent nerves and augments the pressor response to static muscle contraction as the femoral artery is ligated in rats. The results of our whole-cell patch clamp analysis demonstrated that the peak current amplitude of DRG neuron response induced by ASIC3 stimulation is greater in occluded rats than in control rats. This result suggests that femoral artery occlusion is likely to augment ASIC3 activity in thin-fibre muscle afferent nerves, thereby leading to the exaggerated exercise pressor reflex.
However, it seems that it is unlikely that proton concentration/low pH directly enhances the exercise pressor reflex in rats with 24–72 h of femoral occlusion. A previous study demonstrated that there are no significant differences in resting levels of intramuscular pH in sham-control limbs and ligated limbs of rats 12 h, 4, 7 and 14 days after the surgery (Challiss et al. 1986). This result is in agreement with findings in PAD patients suggesting that muscle pH is not altered in symptomatic legs (Kemp et al. 2001; Greiner et al. 2006). Thus, it is postulated that at rest muscle pH in the occluded limb and systemic pH are unlikely to be altered 24–72 h after ligation surgery. However, 24 and 72 h of ligation increases ASIC3 expression in DRG neurons, suggesting that there may not be a direct correlation between resting muscle pH and femoral occlusion-enhanced ASIC3. It is postulated that increased ASIC3 protein expression in DRG neurons innervating the hindlimb muscles is likely to be driven by nerve growth factor (NGF), given that its levels are elevated in rat DRG tissue 24–48 h after femoral artery ligation. Other studies have suggested that NGF is responsible for basal ASIC3 expression of DRG neurons via a TrkA-activated phospholipase C/protein kinase C pathway involved in processing inflammation-induced sensitization to pain (Mamet et al. 2002, 2003).
In addition, published work suggests that activation of capsaicin-sensitive receptor TRPV1 and acid-sensitive ASIC preferentially stimulates metabolically sensitive afferents (Hayes et al. 2007, 2008). Specifically, TRPV1 expression and responsiveness are increased in DRG neurons of rats with ligated femoral arteries (Xing et al. 2008a). Furthermore, other investigators have shown that NGF sensitizes TRPV1 (Zhu et al. 2004, 2007). This sensitization may make TRPV1 functional in the same range as ASIC3. However, the results of a prior study on healthy cats suggest that TRPV1 receptors are unlikely to be involved in the exercise pressor reflex (Kindig et al. 2005). Additionally, endogenous stimulants to TRPV1 under physiological conditions are not clearly understood. Acid/low pH is considered a TRPV1 stimulant, but activation of sensory TRPV1 requires pH < 6.0, which is below a range observed in physiological responses in exercising muscles. Published studies suggest that pH 6.5–7.0 can activate ASIC3 receptors in sensory neurons (Deval et al. 2008, 2011). In the current report, acid-induced current responses were characterized in DRG neurons innervating the hindlimb muscles as a value of pH is 6.7, and a specific ASIC3 antagonist was given to effectively prevent the acid-induced currents. The data suggest a role for ASIC3 in processing muscle afferent signals under the conditions of hindlimb ischaemia. Nevertheless, the possible involvement of TRPV1 in augmented sympathetic and pressor responses in hindlimb ischaemia remains to be studied.
In conclusion, results of the present study demonstrate that DRG response to ASIC3 receptor stimulations is augmented following femoral artery ligation, and that amplified ASIC3 response is especially affected in small/medium diameter DRG neurons innervating the hindlimb muscles. Additional data suggest that femoral occlusion increases ASIC3 expression within DRG neurons projecting C-fibre afferents. Taken together, the results of the present study further suggest that among ASICs, ASIC3 plays a major role in static contraction-augmented sympathetic responsiveness via thin-fibre afferent nerves when hindlimb blood supply is insufficient, as observed in PAD.
Acknowledgments
This study was supported by NIH R01 HL090720, American Heart Association Established Investigator Award 0840130N and NIH P01 HL096570.
Glossary
Abbreviations
- ASIC
acid-sensing ion channel
- BP
blood pressure
- DRG
dorsal root ganglion
- HR
heart rate
- PAD
peripheral arterial disease
- NGF
nerve growth factor
- SNA
sympathetic nerve activity
Author contributions
J.X. and J.L. participated in the design of the experiments. J.X. contributed to the collection, analysis and interpretation of electrophysiological data, and drafting the manuscript. J.L. contributed to the collection, analysis and interpretation of immunocytochemical data, and drafting the manuscript. J.L. contributed to the conception and design of the experiments, the analysis and interpretation of the data, and revising the article critically for important intellectual content. All authors approved the final version.
References
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology. 1999;50:361–374. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg. 2007;33:20–25. doi: 10.1016/j.ejvs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28:1498–1508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss RA, Hayes DJ, Petty RFH, Radda GK. An investigation of arterial insufficiency in rat hindlimb: A combined 31P-n.m.r. and bloodflow study. Biochem J. 1986;236:461–467. doi: 10.1042/bj2360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- Deval E, Noël J, Gasull X, Delaunay A, Alloui A, Friend V, et al. Acid-sensing ion channels in postoperative pain. J Neurosci. 2011;31:6059–6066. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Noël J, Lay N, Alloui A, Diochot S, Friend V, et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;19:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23:1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, et al. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P, Weille DeJR, Lecoq A, Diochot S, Waldmann R, Champigny G, et al. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. Am J Physiol Heart Circ Physiol. 1991;261:H1653–H1658. doi: 10.1152/ajpheart.1991.261.5.H1653. [DOI] [PubMed] [Google Scholar]
- Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H939–H945. doi: 10.1152/ajpheart.00911.2006. [DOI] [PubMed] [Google Scholar]
- Greiner A, Esterhammer R, Messner H, Biebl M, Mühlthaler H, Fraedrich G, et al. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J Vasc Surg. 2006;43:978–986. doi: 10.1016/j.jvs.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol. 2007;581:1271–1282. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2008;295:H1720–H1725. doi: 10.1152/ajpheart.00623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Handbook of Experimenal Pharmacology. Vol. 194. Springer; 2009. Acid-sensitive ion channels and receptors; pp. 283–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. Chapter 10. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 381–447. [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12(6):429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol. 1984a;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984b;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Roberts N, Bimson WE, Bakran A, Harris PL, Gilling-Smith GL, et al. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg. 2001;34:1103–1110. doi: 10.1067/mva.2001.117152. [DOI] [PubMed] [Google Scholar]
- Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol. 2005;288:H1867–H1873. doi: 10.1152/ajpheart.00735.2004. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol. 2010;299:H1357–H1364. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Hayes SG, Kaufman MP. Acid-sensing ion and epithelial sodium channels do not contribute to the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2008;295:H1017–H1024. doi: 10.1152/ajpheart.00450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, Kaufman MP. Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2009;297:H443–H449. doi: 10.1152/ajpheart.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2000;278:R563–R571. doi: 10.1152/ajpregu.2000.278.3.R563. [DOI] [PubMed] [Google Scholar]
- MacLean DA, LaNoue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitial metabolite responses in the cat. J Appl Physiol. 1998;85:1583–1592. doi: 10.1152/jappl.1998.85.4.1583. [DOI] [PubMed] [Google Scholar]
- Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278:48907–48913. doi: 10.1074/jbc.M309468200. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir RL. Peripheral arterial disease: pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs. 2009;27:26–30. doi: 10.1016/j.jvn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38:1561–1569. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Tian L, Liao Y, McDermott MM. Social cognitive constructs and the promotion of physical activity in patients with peripheral artery disease. J Cardiopulm Rehabil Prev. 2008;28:65–72. doi: 10.1097/01.HCR.0000311512.61967.6e. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol. 1989;67:256–263. doi: 10.1152/jappl.1989.67.1.256. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, et al. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol. 1992;263:H1499–H1505. doi: 10.1152/ajpheart.1992.263.5.H1499. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol. 2001;537:993–998. doi: 10.1111/j.1469-7793.2001.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol. 2010;299:H106–H113. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion (Abstract) FASEB J. 2011 doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Bertocci L, Pryor S, Nunnally R. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest. 1988;82:1301–1305. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, Weille deJ, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Lingueglia E, Weille DeJR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann N Y Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol. 2004;97:773–780. doi: 10.1152/japplphysiol.00107.2004. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Dyke CK, Parsons D, Wall PT, Pawelczyk JA, Williams RS, et al. Effect of skeletal muscle fiber type on the pressor response evoked by static contraction in rabbits. J Appl Physiol. 1995;79:1744–1752. doi: 10.1152/jappl.1995.79.5.1744. [DOI] [PubMed] [Google Scholar]
- Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol. 2008a;295:H1262–H1269. doi: 10.1152/ajpheart.00271.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Sinoway L, Li J. Differential responses of sensory neurones innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol. 2008b;686:3245–3252. doi: 10.1113/jphysiol.2008.154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol. 2004;92:3148–3152. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediates acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34:689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


