Abstract
Size at birth is related to adult health outcomes. Twins are born smaller than singletons; this has been assumed to be secondary to limited nutrient supply in late gestation. We hypothesised that growth trajectory in twins, and the adult consequences of being conceived a twin, are determined in early gestation. Twin pregnancies in sheep were randomised to reduction of one twin on day 42 of a 148 day pregnancy by intra-thoracic KCl (Reductions, n = 46) or a sham procedure (Twins, n = 22). Singleton-bearing ewes also underwent a sham procedure (n = 27). Ewes lambed spontaneously. Linear measures of size at birth were similar in Twins and Reductions, and significantly less than in Singletons. Birthweight was lower in Twins and Reductions than in Singletons, and less in Twins than in Reductions (means (SEM): Singletons, liveborn n = 23: 6.59 (0.17) kg; Twins, liveborn n = 36: 5.23 (0.16) kg; Reductions, liveborn n = 27: 5.76 (0.15) kg; all comparisons P < 0.05). Reductions grew most rapidly between birth and weaning (Singletons, 20.0 (0.4) g kg−1 day−1; Twins, 20.0 (0.3) g kg−1 day−1; Reductions, 21.0 (0.3) g kg−1 day−1, P < 0.05) and were of similar weight as Singletons by weaning; Twins remained smaller by weaning but grew most rapidly thereafter (Singletons, 1.6 (0.1) g kg−1 day−1; Twins, 2.1 (0.1) g kg−1 day−1; Reductions, 1.6 (0.1) g kg−1 day−1, P < 0.01), so that all groups had similar weight at 2 years. However, Twins and Reductions had greater percentage fat mass than Singletons at 2 years (Singletons, 11.1 (1.1)%; Twins, 14.8 (1.2)%; Reductions, 15.5 (1.1)%, P < 0.05). Thus, in twins, fetal growth trajectory, linear size at birth and adult fat mass are largely determined in early gestation. If this is also true in humans, there are important implications for interventions aimed at optimising fetal growth and pregnancy outcome.
Key points
Reduced size at birth and shorter gestation length are both associated with increased risks of non-communicable diseases (NCD) in later adult life.
Twins are born both smaller and earlier than singletons and adult twins also are reported to be at increased risk of common NCDs such as diabetes.
The smaller size and shorter gestation length of twins has been presumed to be due to a lack of intrauterine space and/or limitations of placental nutrient supply in late gestation, but there are few data to support this.
We show that size at birth and adult fat mass in twin sheep are determined largely in early gestation.
Knowledge of the mechanisms underlying early pregnancy determination of fetal growth and gestation length in twins are likely to increase understanding of how early pregnancy factors influence lifelong health for offspring from all pregnancies.
Introduction
The risks for adult obesity and type 2 diabetes, major contemporary health issues, are inversely related to size at birth in singletons (Newsome et al. 2003; Simmons, 2008). Twins are born smaller than singletons, even when adjusted for gestational age (Papageorghiou et al. 2008), and also are at increased risk of abdominal adiposity and type 2 diabetes in adulthood (Poulsen et al. 2009). Lower leg fat mass in elderly twins, thought to be protective against insulin resistance, has been reported to be positively associated with differences in birthweight within twin pairs (Monrad et al. 2009), such that the twin within a pair with the lower birthweight had lesser lower leg fat mass and also lower insulin sensitivity, suggesting that the origin of increased risks of adiposity and insulin resistance may lie before birth.
We have previously demonstrated in sheep that maternal undernutrition only around the time of conception results in altered fetal growth, preterm birth, epigenetic changes in appetite regulatory genes in the fetal ventral hypothalamus, altered postnatal growth and impaired glucose tolerance in adulthood (Bloomfield et al. 2003; Rumball et al. 2008a; Stevens et al. 2010). Elegant studies in rodents have demonstrated that a maternal low protein diet only during the pre-implantation period results in reduced size at birth, altered postnatal growth and increased postnatal blood pressure (Kwong et al. 2000). Embryo transfer experiments confirmed that this was an effect on the blastocyst and was independent of later maternal environment (Watkins et al. 2008), and the finding of reduced mRNA expression of the imprinted genes igf2 and H19 in livers of male offspring suggests that epigenetic changes in the pre-implantation embryo may mediate some of these postnatal effects (Kwong et al. 2006).
Twin conception is also a periconceptional event and, in sheep, results in epigenetic changes in the fetal ventral hypothalamus (Begum et al. 2011), perturbed fetal insulin secretion with increased insulin secretion to glucose early in the third trimester (Rumball et al. 2008a) switching to impaired insulin secretion in late gestation (Green et al. 2011), altered postnatal growth and altered adult hypothalamic–pituitary–adrenal (HPA) axis function (Bloomfield et al. 2007). Consistent with the human data, altered adult HPA axis function in sheep was related to within-twin pair coefficients for birthweight (Bloomfield et al. 2007).
It is generally considered that reduced size at birth in twins is due to late-gestation growth restriction (Muhlhausler et al. 2011). However, there are few good data supporting this concept. Indeed, human data demonstrating relationships between first trimester fetal size and both size at birth and gestation length in singletons (Smith et al. 1998; Bukowski et al. 2007b,a; Salomon et al. 2011), and between fetal growth trajectories and gestation length in twins (Hediger et al. 2005), suggest that both size at birth and gestation length may be determined, at least in part, in early gestation.
We therefore hypothesised that prenatal and postnatal growth, and adult body composition, all may be determined in early pregnancy in twins. We tested this experimentally by determining whether twin conception per se, regardless of the number of fetuses present in utero in late gestation, would result in reduced size at birth and gestation length, altered postnatal growth, and increased adult fat mass.
Methods
Ethical approval
This study was approved by the animal ethics committee of the University of Auckland. All experiments were conduced in accordance with National Animal Ethics Advisory Committee guidelines and institutional Standard Operating Procedures.
Animals
Multiparous Romney ewes were mated after synchronisation of oestrus with an intravaginal progesterone-containing controlled internal drug-release device (Wheaton et al. 1993). Ewes were mated outdoors as a single flock on a good nutritional plane (3–4% dry matter (kg of body weight)−1 day−1) increasing up to 5% dry matter (kg of body weight)−1 day−1 through gestation to maintain recommended weight gains according to fetal number. At day 40 of gestation, ewes were identified as being single or twin bearing by ultrasonography. On day 41 of gestation, fetal crown–rump length (CRL) was measured and twin-bearing ewes were randomly allocated to one of two treatment groups (Twins or Reductions).
On day 42–43 of gestation, all ewes (total 100; 25 singleton, 35 reduction, 25 twin) underwent general anaesthesia (induction with propofol (2,6-diisopropylphenol), 5 mg kg−1, Astra Zeneca, New Zealand; maintenance with 2% isoflurane and oxygen) with monitoring of maternal oxygen saturations throughout. Following surgical skin preparation, fetal position was identified by ultrasonography (Phillips HDI1000, Phillips Healthcare, Best, the Netherlands). In the Reduction group, one fetus was randomly assigned to killing via ultrasound-guided intrathoracic injection of 1.5 ml 2 m KCl using a spinal needle (22 gauge, 31/2 inch (8.9 cm)). Fetuses were observed ultrasonographically for 5 min following injection to confirm demise. The ewe was then allowed to recover from anaesthesia. Singletons and Twins underwent a sham procedure consisting of insertion of the needle into the gestational sac for 3 min. After recovery from surgery, animals were scanned each day for 7 days to monitor the heart beat of the remaining fetus and then returned to the paddock. Ewes randomised to Reduction but which lost both fetuses or had both fetuses survive were removed from the experiment (n = 4).
Parturition and postnatal management
Ewes were moved into individual indoor pens with open mesh sides allowing visualisation of other animals on day 130 of gestation to enable frequent blood samples and to monitor well-being and delivery. The housing facility had a 12 h light–dark cycle. Within 6 h of birth, lambs were weighed, blood sampled, measured and tagged. Lambs were then weighed and measured on days 3 and 7, fortnightly until weaning at 3 months, then monthly until 12 months (young adulthood) and again at 18 months of age. From weaning onwards females were run as one flock and males were run in a separate flock; males were not castrated.
Growth velocity was calculated using an exponential method (Patel et al. 2005). Milk intake between day 7 and day 14 in a randomly selected subgroup (Singletons, n = 9; Twins, n = 9 pairs; Reductions, n = 9) was assessed by modifications to the deuterium oxide dilution technique described previously (Van Kreel et al. 1996; Auchtung et al. 2002). Three hundred milligrams of D2O were injected intravenously with the precise dose determined gravimetrically and the time recorded. Blood samples were taken before injection, 2 and 6 h after injection and then daily for 7 days. Lambs were weighed after the last sample and remained in individual pens with their mothers throughout, with no access to other ewes. Plasma samples were analysed for D2O concentrations by isotope ratio mass spectrometry, with modifications to published protocols (Previs et al. 1996; Van Kreel et al. 1996). Instead of configuring the ion mass spectrometer to detect the mass-to-charge ratios (m/z) 26 and 27, which are associated with the acetylene 1H and 2H isotopomers derived from the H2O and CaC2 reaction, a pyrolysis furnace was used to pyrolyse the acetylene to H2 and an isotope ratio mass spectrometer (IRMS) was used to measure m/z 2 (1H1H) and 3 (2H1H) directly. The acetylene gas was injected into the gas chromatographer (GC) (Pora PLOT Q, 30 m × 0.32 mm ID, Supelco Inc., Bellafonte, PA, USA) with helium as carrier gas (1.5 ml min−1) for the separation of acetylene from air components. A headspace volume of 20 μl was injected in the split mode (1:20 split ratio) at 110°C and at 150 kPa by an autosampler (CTC A200S, CTC analytics, Zwingen, Switzerland) fitted with a 100 μl headspace syringe (SGE analytical science, Ringwood, Victoria, Australia). The GC oven temperature was maintained isothermally at 110°C for the duration of the run (6 min). Between the GC and IRMS there was an interface which consisted of a high-temperature pyrolysis furnace operated at 1450°C. In the furnace, acetylene from the GC column was pyrolysed to H2. After drying the gas stream by a Nafion membrane, gases were introduced into the ion source. The determination of 2H enrichment was carried out with a Thermo Finnigan delta v plus continuous flow isotope ratio mass spectrometer (Finnigan MAT, Bremen, Germany). Prior to the analysis of a batch of samples, a H3+ calibration was performed to correct for H3+ formation associated with source ionisation. Data processing was performed by the vendor-provided software ISODAT (Finnigan MAT). For each set of samples analysed, a D2O calibration curve was produced. Estimated total water intake was computed from the disappearance curve of D2O in plasma in each lamb.
All blood sampling was by jugular venepuncture with collection into heparin-coated vacutainers. Samples were centrifuged for 10 min at 1106 g and 4°C. Plasma was removed and frozen until later analysis of hormones and metabolites.
At 14 months of age a randomly selected subgroup of animals (Singletons, n = 9; Twin pairs, n = 7 pairs; Reductions, n = 9) were killed by captive bolt and exsanguination. Organs were rapidly dissected and weighed. The right and left ventricular free walls and the inter-ventricular septum thickness were measured at predefined anatomical landmarks using calipers. The remaining animals in each group were retained for growth measurements to 2 years and for body composition analysis by dual X-ray absorptiometry (DXA, Norland XR-800, Cooper Surgical Ltd, Fort Atkinson, WI, USA). Prior to DXA, sheep were fasted overnight with free access to water. Scans were performed under sedation using an equi-volume mixture of diazepam (5 mg ml−1) and ketamine (100 mg ml−1) intravenously. Fat and lean mass were calculated (using Norland software) in an area defined by the thoracic inlet proximally and the base of the tail distally, and from the animal's back superiorly to the base of the humerus and femur inferiorly, and expressed as a percentage of body weight.
Hormone and metabolite analysis
Metabolite concentrations were measured on a Hitachi 902 autoanalyser (Hitachi High Technologies Corporation, Tokyo, Japan): glucose by enzymatic colorimetric assay (Roche, Mannheim, Germany); urea by kinetic ultra-violet assay (Roche); lactate and free fatty acids (FFA) by enzymatic colorimetric assays (Randox Laboratories Ltd, Ardmore, Crumlin, UK) and β-hydroxybutyrate (βHBA) by kinetic ultra-violet assay (Randox).
Plasma hormone concentrations were measured by specific radioimmunoassay (RIA) established and validated for maternal and fetal sheep plasma. Plasma insulin was measured according to previously published methods (Oliver et al. 1993) except that ovine insulin was used as the standard (Sigma Chemical, St Louis, MO, USA, batch no. I9254). The minimal detectable concentration was 0.03 ng ml−1 plasma and the inter- and intra-assay coefficients of variation (CVs) were 14.0% and 9.5%, respectively. Plasma insulin-like growth factor 1 (IGF-1) was measured using an IGF binding protein-blocked RIA (Blum & Breier, 1994; Vickers et al. 1999). The detection limit was 0.7 ng ml−1 and the inter- and intra-assay CVs were 11.4% and 11.5%, respectively.
Steroids were measured using mass spectrometry as previously described (Rumball et al. 2008b). The inter-assay CVs for cortisol, oestradiol and progesterone were 4.8, 6.8 and 11.3%, respectively; the intra-assay CVs for cortisol, oestradiol and progesterone were 1.9, 13.9 and 9.8%, respectively.
Statistics
Data were analysed by factorial or repeated-measures ANOVA with the Tukey post hoc test with group and sex, and the interaction term between them, included as co-variates in all analyses. Ewe identification was included as a random effect to account for the non-independence of twin pairs. Gestation length was analysed by Kaplan–Meier survival analysis. Associations between adult body composition and early life factors including size at birth and growth velocity were explored using uni- and multivariate linear regression. In twin lambs, within- and between-twin pair coefficients for birthweight were entered into the regression models to separate maternal factors affecting fetal growth from intrinsic fetal factors (Carlin et al. 2005; Bloomfield et al. 2007). Data are presented as least-square means (SEM) unless otherwise stated, and significance was assumed when P < 0.05.
Results
Gestation and size at birth
A total of 95 ewes entered the experiment with 86 delivering liveborn lambs (23 Singleton, 36 Twin lambs and 27 Reduction lambs). The outcome for all fetuses and lambs is shown in Fig. 1. Fetal crown–rump length on day 41 of gestation was not significantly different amongst groups (Singletons, 38.1 (0.5) mm, Twin 37.0 (0.5) mm, Reductions 37.1 (0.4) mm, P = 0.2). Gestational length was significantly less in Twins compared to Singletons (146.9 (0.2) vs. 148.2 (0.4) days, P < 0.05); gestation length in Reductions (147.2 (0.3) days) was not significantly different from Singletons or Twins. Birthweight of Twin lambs was significantly less than that of Singletons (Table 1); birthweight of Reductions was intermediate between Singletons and Twins and was significantly different from both (Table 1). Measures of linear size at birth were similar in Twins and Reductions and, except for biparietal diameter and abdominal girth, were significantly less than those in Singletons (Table 1). Female lambs were smaller than male lambs in all groups throughout the study (data not shown), but there was no significant interaction between group and sex for any measures of growth at any time; thus, data are presented for both sexes combined.
Figure 1. Animal numbers and fate throughout the experiment.
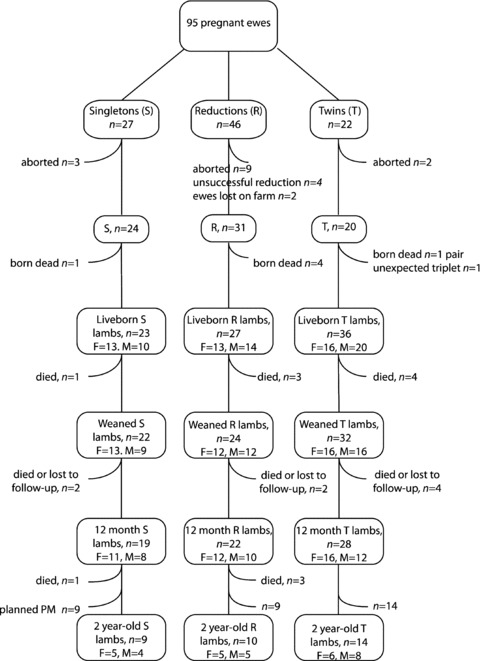
S, singleton; R, reduction; T, twin; F, female; M, male. Weaning, day 84 of life.
Table 1.
Growth measurements and growth velocity from birth to adulthood
| Singleton (n = 23) | Twin (n = 36) | Reductions (n = 27) | P value | |
|---|---|---|---|---|
| Birth | ||||
| Weight (kg) | 6.59 (0.17)a | 5.23 (0.16)b | 5.76 (0.15)c | P < 0.0001 |
| Crown–rump length (cm) | 53.3 (0.7)a | 48.7 (0.6)b | 49.8 (0.6)b | P < 0.0001 |
| Biparietal diameter (mm) | 64.2 (0.5)a | 61.9 (0.4)b | 63.0 (0.4)ab | P = 0.002 |
| Hock to toe (cm) | 21.9 (0.3)a | 20.7 (0.2)b | 20.8 (0.2)b | P = 0.0002 |
| Hind limb length (cm) | 40.4 (0.4)a | 38.1 (0.4)b | 38.8 (0.3)b | P = 0.004 |
| Chest girth (cm) | 40.7 (0.5)a | 38.0 (0.4)b | 38.9 (0.4)b | P = 0.0001 |
| Abdominal girth (cm) | 43.0 (0.7)a | 40.5 (0.5)b | 41.5 (0.6)ab | P = 0.01 |
| Weaning | ||||
| Weight (kg) | 35.0 (0.8)a | 27.9 (0.7)b | 34.0 (0.8)a | P < 0.0001 |
| Crown–rump length (cm) | 94.0 (1.2)a | 89.8 (0.9)b | 92.5 (1.0)a,b | P < 0.02 |
| Biparietal diameter (mm) | 85.0 (0.8)a | 82.3 (0.6)b | 83.5 (0.7)a,b | P = 0.03 |
| Hock to toe (cm) | 28.2 (0.3)a | 27.3 (0.2)b | 27.9 (0.2)a,b | P < 0.02 |
| Hind limb length (cm) | 55.4 (0.5) | 54.1 (0.4) | 55.2 (0.4) | P = NS |
| Chest girth (cm) | 73.3 (0.9)a | 69.4 (0.7)b | 72.6 (0.8)a | P < 0.001 |
| Abdominal girth (cm) | 87.2 (1.3) | 83.8 (1.1) | 87.2 (1.2) | P = NS |
| GV birth–weaning (g kg−1 day−1) | 20.0 (0.4) | 20.0 (0.3) | 21.0 (0.3) | P = 0.03 |
| GV weaning–12 months (g kg−1 day−1) | 1.6 (0.1)a | 2.1 (0.1)b | 1.6 (0.1)a | P = 0.005 |
| 12 months body weight (kg) | 52.8 (1.7) | 47.6 (1.5) | 51.5 (1.5) | P = 0.06 |
| 18 months body weight (kg)* | 89.0 (0.3) | 83.0 (3.0) | 86.7 (2.7) | P = NS |
| 24 months body weight (kg)* | 86.6 (2.9) | 83.0 (2.9) | 88.3 (2.8) | P = NS |
| Fat mass (% body weight)* | 11.1 (1.1)a | 14.8 (1.2)b | 15.5 (1.1)b | P < 0.0001 |
| Lean mass (% body weight)* | 72.8 (1.2)a | 69 (0.8)b | 68.2 (1.1)b | P < 0.05 |
Data are least-square means (SEM). Groups with different superscripts are significantly different by Tukey post hoc test. *n at 2 years: Singleton, 9; Twin, 14; Reductions, 10. For n at each time point, refer to Fig. 1. GV, growth velocity.
Maternal pregnancy hormones and metabolites
Plasma metabolite, insulin and IGF-1 concentrations were not different in twin, reduction or singleton bearing ewes in early and mid gestation (data not shown). In late gestation, Twin ewes had decreased plasma glucose and insulin concentrations, and elevated βHBA and free fatty acid concentrations, compared with Singleton and Reduction ewes, which had similar values (Fig. 2A–D). Plasma IGF-1 concentrations on days 133 and 140 of gestation were significantly lower in Twin compared with Singleton and Reduction ewes (Fig. 2E). Preparturient rises in cortisol concentrations were not different amongst groups (Fig. 3B). Progesterone concentrations and the progesterone/oestradiol ratio were higher, and fell more rapidly before birth, in Twin ewes than in Reduction and Singleton ewes (P < 0.001, Fig. 3A and D). The preparturient rise in oestradiol concentrations was more rapid and greater in Twins than in Singletons and Reductions (Fig. 3C, P < 0.001). However, there were no significant differences in the timing and rate of rise or fall in concentrations of these hormones relative to the time of birth (Fig. 3).
Figure 2. Ewe metabolite and hormone plasma concentations in late gestation.
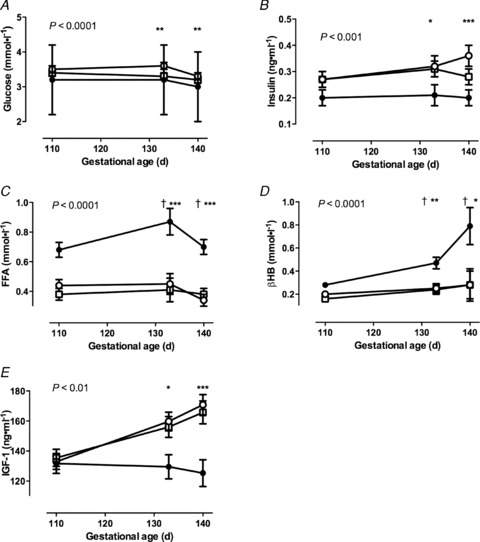
A, glucose; B, insulin; C, free fatty acids (FFA); D, beta hydroxybutyrate (βHB); E, IGF-1. Data are mean ± SEM. Open squares, Singletons; filled circles, Twins; open circles, Reductions. The P value is for the repeated-measures ANOVA; Tukey post hoc: *P < 0.05, **P < 0.01, ***P < 0.001 for Reductions vs. Twins; †P < 0.001 for Singletons vs. Twins.
Figure 3. Maternal plasma concentrations of progesterone (A), cortisol (B), oestradiol (C) and the progesterone: oestradiol ratio (D) plotted against days before delivery.
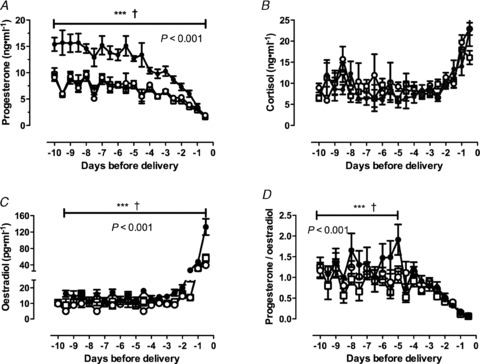
Data are mean ± SEM. Open squares, Singletons; filled circles, Twins; open circles, Reductions. The P value is for the repeated measures ANOVA; Tukey post hoc: ***P < 0.001 for Reductions vs. Twins; †P < 0.001 for Singletons vs. Twins.
Lamb metabolites and hormones
Consistent with the maternal data, lamb plasma glucose and insulin concentrations in the blood samples taken within 6 h of birth were significantly lower in Twin lambs compared with Reduction and Singleton lambs (Fig. 4). Insulin concentrations remained elevated in Reductions through to weaning at 3 months of age and glucose concentrations were also higher in this group until day 42 (Fig. 4). IGF-1 concentrations were not different amongst groups in the first week of life, but were significantly greater in Reductions and Singletons than in Twins at day 42 and were still elevated in Reductions compared with Twins at 3 months of age (day 84) (Fig. 4). There were no differences in lactate or urea concentrations amongst groups (data not shown). There were no significant differences amongst groups in any of the hormones or metabolites measured between weaning and 12 months of age apart from significantly greater plasma IGF-1 concentrations in Reductions compared with Singletons at 12 months of age (Reductions 216.7 (12.4) ng ml−1; Twins 200.0 (10.0) ng ml−1; Singletons 176.0 (10.6) ng ml−1; P < 0.05).
Figure 4. Lamb metabolite and hormone plasma concentrations from birth to weaning.
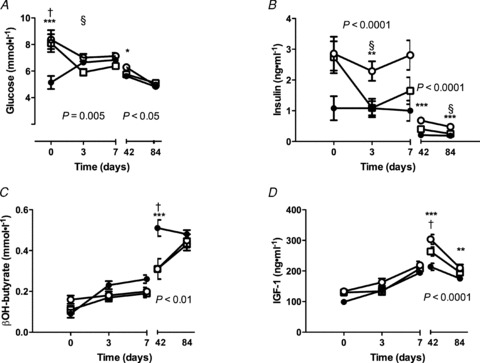
A, glucose; B, insulin; C, β hydroxybutyrate (βOH-butyrate); D, IGF-1. Data are mean ± SEM. Open squares, Singletons; filled circles, Twins; open circles, Reductions. The P value is for the repeated-measures ANOVA from days 0–7 or days 42 and 84. Tukey post hoc: *P < 0.05, **P < 0.01, ***P < 0.001 for Reductions vs. Twins; †P < 0.001 for Singletons vs. Twins; §P < 0.05 for Reductions vs. Singletons.
Postnatal growth and body composition
There was no difference in milk intake amongst the groups in the second week of life (Singletons, n = 9: 147 (5) ml kg−1 day−1; Twins, n = 18: 143 (4) ml kg−1 day−1; and Reductions, n = 9: 151 (5) ml kg−1 day−1). Female lambs had a greater milk intake than males (157 (4) vs. 138 (4) ml kg−1 day−1, P = 0.001), although there was no interaction with group. Despite similar milk intakes, Reduction lambs grew more quickly between birth and weaning than Singletons or Twins (P = 0.03, Table 1 and Fig. 5). Following weaning, Twins grew more quickly than Singletons or Reductions (P = 0.005, Table 1 and Fig. 5). By 12 months of age (young adulthood) there was no significant difference in body weight amongst the groups, although Twins were still 4–5 kg lighter than Singletons and Reductions (Table 1). At post-mortem at 14 months of age, total thymus weight (neck + chest thymus) was ∼22% less in Twins and Reductions than in Singletons and remained so when expressed per kilogram bodyweight (Table 2). In females, ovary weight was significantly greater in Twins than in Singletons, with Reductions intermediate (Table 2). In males, testes weight was not significantly different amongst groups. Kidney and lung weights were significantly less, and peri-renal fat significantly greater, in females than in males (data not shown). There were no other significant differences in organ weights amongst groups, or between the sexes, either in absolute weight or when expressed relative to body weight (Table 2). However, right ventricular free wall thickness was 15% less in Twins and Reductions than in Singletons, although this was only statistically significant in Twins (Table 2). Left ventricular free wall and interventricular septal thicknesses were not significantly different amongst groups.
Figure 5. Postnatal growth from birth to 12 months of age.
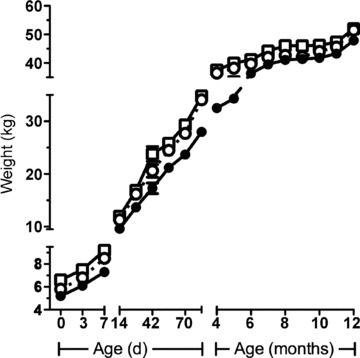
Data are mean ± SEM. Open squares, Singletons; filled circles, Twins; open circles, Reductions. Growth velocities from birth to weaning and from weaning to adulthood were calculated using an exponential method (Patel et al. 2005) and data analysed by ANOVA. Growth velocity was greatest in Reductions from birth to weaning, and in Twins from weaning to 12 months (see Table 1 for details and Fig. 1 for animal numbers at birth, weaning and 12 months of age).
Table 2.
Organ weights at post-mortem at 14 months of age
| Singleton (n = 9) | Twins (n = 14) | Reductions (n = 9) | P value | |
|---|---|---|---|---|
| Body weight (kg) | 66.8 (2.2) | 66.5 (1.9) | 67.7 (2.1) | NS |
| Carcass weight (kg) | 46.3 (1.4) | 45.8 (1.4) | 46.9 (1.3) | NS |
| Pancreas (g) | 77.4 (3.7) | 69.7 (3.0) | 77.8 (3.8) | NS |
| Kidneys (g)† | 206.7 (2.8) | 189.3 (6.3) | 202.1 (6.5) | NS |
| Adrenal glands (g) | 4.35 (0.27) | 5.00 (0.26) | 4.49 (0.26) | NS |
| Perirenal fat (g)† | 408.4 (69.7) | 397.0 (53.0) | 443.7 (66.1) | NS |
| Spleen (g) | 109.0 (6.5) | 103.1 (5.6) | 100.3 (6.1) | NS |
| Total thymus (g)† | 65.9 (5.1)a | 51.5 (4.6)b | 52.0 (4.8)b | P < 0.05 |
| Thymus (g (kg body weight)−1)† | 10.20 (0.91) | 9.28 (0.81) | 8.70 (0.86) | NS |
| Liver (g) | 1244 (57) | 1187 (53) | 1278 (54) | NS |
| Heart (g) | 344.5 (12.9) | 320.0 (7.6) | 320.8 (12.3) | NS |
| RV free wall (mm) | 9.00 (0.37)a | 7.61 (0.23)b | 7.80 (0.38)a,b | P = 0.01 |
| LV free wall (mm) | 14.50 (0.54) | 14.41 (0.85) | 13.70 (0.97) | NS |
| IVS (mm) | 20.83 (1.04) | 18.56 (0.98) | 20.27 (1.08) | NS |
| Lung (g)‡ | 696.3 (38.5) | 712.6 (31.2) | 704.2 (36.6) | NS |
| Ovaries (g) | 1.33 (0.12)a | 1.79 (0.12)b | 1.74 (0.15)a,b | P < 0.05 |
| Ovaries (g (kg body weight)−1) | 0.020 (0.001)a | 0.027 (0.001)b | 0.026 (0.001)a,b | P < 0.05 |
| Testes (g) | 561.5 (79.3) | 692.4 (9.2) | 654.6 (61.4) | NS |
Data are least square means (SEM). Different superscripts represent significantly different by Tukey post hoc test. †P < 0.05, ‡P < 0.01 for effect of sex (kidneys and lungs were significantly lighter in females than males; peri-renal fat mass was significantly greater in females than males. Singletons: 3 males, 6 females; Twins: 7 males, 7 females; Reductions: 5 males, 4 females). RV, right ventricle; LV, left ventricle; IVS, inter-ventricular septum.
In the animals that continued to 2 years of age (Singletons, n = 9; Twins, n = 14; Reductions, n = 10), there was no significant difference in weight amongst the groups at this age. However, percentage fat mass was significantly greater, and percentage lean mass significantly less, in both Twins and Reductions compared with Singletons at 2 years of age, with no difference between Twins and Reductions (Table 1). Adult percentage fat mass was significantly greater in females (18.0 ± 0.9%) than in males (9.7 ± 0.9%), but there was no group by sex interaction (data not shown). Adult fat mass was significantly and positively associated with growth velocity between weaning and 12 months of age only in Reductions (1% increase in fat mass for each 0.19 (0.01) g kg−1 day−1 increase in GV in females and 0.12 (0.12) g kg−1 day−1 in males, P = 0.04; effect of sex P = 0.002). There were no other associations between adult fat mass and birthweight or measures of growth velocity in any of the groups. In the Twin group alone, there were no statistically significant correlations between the between-twin pair and within-twin pair coefficients for birth weight and fat mass.
Discussion
These data demonstrate that reduced size at birth, reduced gestation length and adult fat mass following twin conception in sheep are largely determined in early gestation. Human twins are also born earlier and smaller than singletons, even when only spontaneous births are considered (Chauhan et al. 2010). Observational data suggest that reduced gestation length and size at birth also may not be completely abrogated by fetal reduction of higher order multiples to twins or to singletons in humans, although this finding is not universal (Sebire et al. 1997; Wimalasundera, 2010). However, fetal reduction in humans is not random, with selection of smaller fetuses where there is size discrepancy and this is technically possible, meaning that these observational data in humans are likely to overestimate size at birth.
The death of one twin could have had effects on the surviving co-twin due to either release of material from the demised fetus or from haemodynamic disturbances. In human twin pregnancies with demise of one twin, data are only available for the pregnancy outcomes discussed above and for neurodevelopmental outcomes. The latter are significantly worse in surviving co-twins from monochorionic pregnancies than in surviving co-twins from dichorionic pregnancies with fetal death of one twin (Hillman et al. 2011). This is thought to be due to haemodynamic factors. In late gestation sheep, complete surgical removal of one twin has no effect on gestation length of the remaining co-twin, whereas in pregnancies in which one twin has a cord ligation resulting in death and is left in utero, gestation length is decreased (Rueda et al. 1995). However, it is not clear whether this is because the deceased twin has an effect on reducing gestation length or whether the presence of a surviving twin has a restraining effect on the normal prompt expulsion of a dead fetus that occurs in sheep. In our study, the finding that the surviving co-twin in the Reduction group had a phenotype very similar to that of twins in the sham Twin group, even through to adulthood, suggests that the death of a co-twin did not have a significant effect on growth, gestation length or development.
It is possible that Reductions are more similar to Twins because of limitations of placental supply secondary to a smaller placenta for each individual twin within a pair. The very similar maternal progesterone concentrations between Reductions and Singletons in late gestation, significantly less than those in Twin-bearing ewes, indicate that placentae were similar in Singletons and Reductions, at least in terms of progesterone-synthesising capacity (Gur et al. 2011). We were unable to record placental weights, as to determine gestational length ewes were allowed to deliver spontaneously, usually at night, meaning that accurate placental data were not obtained.
Our finding that Twins had a significantly reduced birth weight compared with Reductions indicates that there is additional constraint of growth in Twins that occurred after fetal reduction. Measures of linear growth at birth, however, were similar between Twins and Reductions, indicating that the reduced birthweight in Twins probably reflects an impaired ability of fetal twin lambs to lay down body stores in preparation for birth. This is probably due to the increased demand two fetuses place on the mother in late gestation, an interpretation supported by decreased plasma glucose and increased plasma free fatty acid and β-hydroxybutyrate concentrations in twin-bearing ewes in late gestation, and decreased plasma concentrations of glucose, insulin and IGF-1 on the first day of life in Twin lambs compared with Reductions and Singletons. These maternal metabolic outcomes in late gestation are consistent with the findings of Rumball et al. (2008a).
The different postnatal growth trajectories between Reductions and Twins are intriguing. Reductions demonstrated accelerated growth immediately after birth, with a growth velocity between birth and weaning that was greater than Singletons or Twins, consistent with a release of antenatal constraint of growth. This rapid ‘catch-up’ growth in Reductions was not due to differences in milk intake. Previous studies comparing milk intake between singleton and twin lambs have reported both lesser and similar intakes in twins compared with singletons (Burris & Baugus, 1955; Wohlt et al. 1984). Milk production is greater in twin-bearing ewes (Torres-Hernandez & Hohenboken, 1980; Wohlt et al. 1984), and singleton-bearing ewes have greater milk residuals following feeding (Wohlt et al. 1984), suggesting that there is tighter regulation of demand and supply in twins. Further, it has been reported that the association between the percentage of milk protein and lamb growth rates in twins is much weaker than in singletons (Torres-Hernandez & Hohenboken, 1980) and that early pregnancy events, including maternal nutrition and twin status, affect the regulation of postnatal growth and the associations between lamb growth rate, size at birth and milk intake (Jaquiery et al. 2011). Altered relationships between milk intake and lamb growth could be related to altered milk composition, which can be affected by factors during pregnancy (Meyer et al. 2011) but which we were unable to measure in this study, or to altered regulation of energy balance at the level of the hypothalamus. We have previously reported that twin fetal lambs have epigenetic modifications of appetite-regulating genes in the ventral hypothalamus (Begum et al. 2011) and, if fetal growth trajectory in twins is determined in early gestation as proposed in this paper, it is likely that these modifications are also present in Reductions, potentially leading to altered regulation of postnatal metabolism and growth. The elevated insulin and IGF-1 concentrations in Reductions at 6 weeks of age and weaning also could be secondary to either increased nutrient intake, secondary to either increased intake or altered milk composition, or to altered set-points in the regulation of these hormones. Although we did not measure milk intake beyond the second week of life, previous data suggest that milk intakes are not different in twins and singletons at a month of age (Hatfield et al. 1995).
After weaning, Reductions and Singletons had similar growth velocities which were less than that of Twins, with Twins now demonstrating accelerated growth such that by 1 year of age there was no longer a significant difference in body weight amongst groups. This post-weaning growth acceleration would be most consistent with removal of constraints of nutrients from the mother allowing twins the ability to grow more rapidly when grazing.
We found that both Twins and Reductions had increased fat mass at 2 years of age, despite similar body weight and similar organ composition at 1 year of age. Although the association between twin conception and later risk of adult disease in humans has been controversial (Phillips et al. 2001), more recent data utilising within-twin pair statistical techniques suggest that, indeed, twins are at increased risk of diabetes and altered fat deposition in later life (Vaag & Poulsen, 2007; Poulsen et al. 2009). Although we did not find significant associations with size at birth or, in Twins, with the within-twin pair coefficient for birthweight (Carlin et al. 2005), this may be due to a lack of power as half the animals were killed at 1 year of age for organ weights and tissue collection. Fat mass in Reductions was associated with greater growth velocity between weaning and 12 months, indicating that Reductions which continued to grow quickly after weaning had greater fat mass. This is consistent with the literature linking later adiposity with childhood growth trajectory (Yliharsila et al. 2008; Eriksson, 2011), but it is not clear why this association was only present in Reductions.
As Twins and Reductions had similar, increased, fat mass at 2 years of age, this strongly suggests that propensity for fat mass is set early in pregnancy. Early development is a time when epigenetic marks are erased and re-established (Morgan et al. 2005), and this, therefore, may be a mechanism that can explain our findings. Indeed, we have recently demonstrated that late gestation twin fetal sheep have altered acetylation and methylation of the glucocorticoid receptor and proopiomelanocortin promotor regions in the ventral hypothalamus (Begum et al. 2011), the site of the appetite regulatory pathways, although it is not yet known whether this translates into altered appetite regulation and energy balance in postnatal life. Human monozygotic twins within a twin pair, which are genetically identical, have been shown to have different levels of gene expression in blood mononuclear cells and umbilical cord endothelial cells at birth (Gordon et al. 2011) suggesting that antenatal environmental factors may alter gene expression in utero. Indeed, 19% of monozygotic twins differ with respect to the X chromosome inactivated (Wong et al. 2010; Wong et al. 2011), suggesting that epigenetic differences in twins are likely to occur as early as the wave of epigenetic reprogramming in early embryonic life; however, differences in X chromosome inactivation at this time are likely to be stochastic. In contrast, reports that epigenetic differences in twins increase with increasing age (Fraga et al. 2005; Wong et al. 2010) provide evidence that phenotypic differences within twin pairs are epigenetically determined; however, it is not yet clear whether these epigenetic differences are causal for phenotypic differences and, if so, whether they have their origin before or after birth (Bell & Spector, 2011).
These data provide further evidence that significant aspects of fetal developmental trajectory, including size at birth, are determined around the time of conception. It is now clear that factors acting in the periconceptional period which influence fetal growth and the timing of birth, including nutritional influences and twin conception, also result in altered postnatal physiology which could predispose to adult disease (Bloomfield et al. 2007; Poulsen et al. 2009; Todd et al. 2009).
Increased understanding of the mechanisms by which factors operating around the time of conception affect pregnancy outcomes, fetal growth and long-term physiology would have implications for twins, for babies born after use of assisted reproductive techniques and for potential preventative public health measures leading to optimal pregnancy outcomes in all pregnancies.
Acknowledgments
We would like to thank: staff of Ngapouri Research Station, University of Auckland, Reporoa, New Zealand for technical assistance; Eric Thorstensen at the Liggins Institute, University of Auckland for hormone and metabolite analyses; Mike Tavendale, AgResearch Grasslands, Palmerston North, New Zealand for D2O analysis, and the Health Research Council of New Zealand and the National Research Centre for Growth and Development, New Zealand, for funding support.
Glossary
Abbreviations
- βHBA
beta hydroxybutyrate
- CRL
crown–rump length
- CV
coefficient of variation
- DXA
dual energy X-ray absorptiometry
- FFA
free fatty acids
- HPA
hypothalamic–pituitary–adrenal
- IGF-1
insulin-like growth factor-1
- RIA
radioimmunoassay
Author's present address
S. N. Hancock: Department of Agriculture and Food, 3 Baron-Hay Court, South Perth, WA 6151, Western Australia.
Author contributions
The animal experiments were performed at Ngapouri Research Station, University of Auckland, Reporoa, New Zealand, and the laboratory analyses were performed at the Liggins Institute, University of Auckland, Auckland, New Zealand. S.N.H. conducted the animal experiments up to 1 year of age, collected samples, analysed the data and drafted the manuscript. M.H.O. assisted in the design and execution of the animal experiments and contributed to interpretation of data and critical revision of the manuscript. C.M. conducted the experiments at 2 years of age and analysed and interpreted the DXA data. A.L.J. supervised the experiments at 2 years of age, analysis and interpretation of the DXA data and contributed to critical revision of the manuscript. F.H.B. conceived and designed the experiments, assisted with experimental procedures, oversaw analysis and interpretation of data and had substantial input into writing of the manuscript. All authors have approved the final version of the manuscript.
References
- Auchtung TL, Baer DJ, Erdman RA, Barao SM, Dahl GE. Relation of growth hormone response to growth hormone-releasing hormone to estimation of milk production via deuterium oxide dilution in beef cattle. J Anim Sci. 2002;80:1270–1274. doi: 10.2527/2002.8051270x. [DOI] [PubMed] [Google Scholar]
- Begum G, Stevens A, Smith BoltonE, Connor KL, Challis JRG, Bloomfield FH, White A. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J. 2012. In press. Doi: 10.1096/fj.11-198762. [DOI] [PMC free article] [PubMed]
- Bell JT, Spector TD. A twin approach to unraveling epigenetics. Trends Genet. 2011;27:116–125. doi: 10.1016/j.tig.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Harding JE. Effects of twinning, birth size and postnatal growth on glucose tolerance and hypothalamo-pituitary-adrenal function in post-pubertal sheep. Am J Physiol Endocrinol Metab. 2007;292:E231–E237. doi: 10.1152/ajpendo.00210.2006. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Hawkins P, Campbell M, Phillips DJ, Gluckman PD, Challis JRG, Harding JE. A periconceptional nutritional origin for noninfectious preterm birth. Science. 2003;300:606. doi: 10.1126/science.1080803. [DOI] [PubMed] [Google Scholar]
- Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs. Growth Regul. 1994;4:11–19. [PubMed] [Google Scholar]
- Bukowski R, Smith GC, Malone FD, Ball RH, Nyberg DA, Comstock CH, et al. Fetal growth in early pregnancy and risk of delivering low birth weight infant: prospective cohort study. BMJ. 2007a;334:836. doi: 10.1136/bmj.39129.637917.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski R, Smith GC, Malone FD, Ball RH, Nyberg DA, Comstock CH, et al. Human sexual size dimorphism in early pregnancy. Am J Epidemiol. 2007b;165:1216–1218. doi: 10.1093/aje/kwm024. [DOI] [PubMed] [Google Scholar]
- Burris MJ, Baugus CA. Milk consumption and growth of suckling lambs. J Anim Sci. 1955;14:186. [Google Scholar]
- Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Chauhan SP, Scardo JA, Hayes E, Abuhamad AZ, Berghella V. Twins: prevalence, problems, and preterm births. Am J Obstet Gynecol. 2010;203:305–315. doi: 10.1016/j.ajog.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Eriksson JG. Early growth and coronary heart disease and type 2 diabetes: findings from the Helsinki Birth Cohort Study (HBCS) Am J Clin Nutr. 2011. DOI: 10.3945/ajcn.110.000638. [DOI] [PubMed]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L, Joo JH, Andronikos R, Ollikainen M, Wallace EM, Umstad MP, et al. Clues to the effect of intrauterine milieu on gene expression from a study of two tissues from neonatal monozygotic twins. Epigenetics. 2011;6:579–592. doi: 10.4161/epi.6.5.15072. [DOI] [PubMed] [Google Scholar]
- Green AS, Macko AR, Rozance PJ, Yates DT, Chen X, Hay WW, Jr, Limesand SW. Characterization of glucoseinsulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab. 2011;300:E817–E823. doi: 10.1152/ajpendo.00572.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur S, Turk G, Demirci E, Yuce A, Sonmez M, Ozer S, Aksu E. Effect of pregnancy and foetal number on diameter of corpus luteum, maternal progesterone concentration and oxidant/antioxidant balance in ewes. Reprod Domest Anim. 2011;46:289–295. doi: 10.1111/j.1439-0531.2010.01660.x. [DOI] [PubMed] [Google Scholar]
- Hatfield PG, Snowder GD, Head WA, Jr, Glimp HA, Stobart RH, Besser T. Production by ewes rearing single or twin lambs: effects of dietary crude protein percentage and supplemental zinc methionine. J Anim Sci. 1995;73:1227–1238. doi: 10.2527/1995.7351227x. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Luke B, Gonzalez-Quintero VH, Martin D, Nugent C, Witter FR, Mauldin JG, Newman RB. Fetal growth rates and the very preterm delivery of twins. Am J Obstet Gynecol. 2005;193:1498–1507. doi: 10.1016/j.ajog.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: a systematic review and metaanalysis. Obstet Gynecol. 2011;118:928–940. doi: 10.1097/AOG.0b013e31822f129d. [DOI] [PubMed] [Google Scholar]
- Jaquiery AL, Oliver MH, Bloomfield FH, Harding JE. Periconceptional events perturb postnatal growth regulation in sheep. Pediatr Res. 2011;70:261–266. doi: 10.1203/PDR.0b013e3182242deb. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond C, et al. Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction. 2006;132:265–277. doi: 10.1530/rep.1.01038. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Reed JJ, Neville TL, Thorson JF, Maddock-Carlin KR, Taylor JB, et al. Nutritional plane and selenium supply during gestation affect yield and nutrient composition of colostrum and milk in primiparous ewes. J Anim Sci. 2011;89:1627–1639. doi: 10.2527/jas.2010-3394. [DOI] [PubMed] [Google Scholar]
- Monrad RN, Grunnet LG, Rasmussen EL, Malis C, Vaag A, Poulsen P. Age-dependent nongenetic influences of birth weight and adult body fat on insulin sensitivity in twins. J Clin Endocrinol Metab. 2009;94:2394–2399. doi: 10.1210/jc.2008-1858. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14:R47–R58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- Mühlhäusler BS, Hancock SN, Bloomfield FH, Harding R. Are twins growth restricted? Pediatr Res. 2011;70:117–122. doi: 10.1203/PDR.0b013e31821f6cfd. [DOI] [PubMed] [Google Scholar]
- Newsome CA, Shiell AW, Fall CHD, Phillips DIW, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?– A systematic review. Diabet Med. 2003;20:339–348. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Harding JE, Breier BH, Evans PC, Gluckman PD. Glucose but not a mixed amino acid infusion regulates plasma insulin-like growth factor-I concentrations in fetal sheep. Pediatr Res. 1993;34:62–65. doi: 10.1203/00006450-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Papageorghiou AT, Bakoulas V, Sebire NJ, Nicolaides KH. Intrauterine growth in multiple pregnancies in relation to fetal number, chorionicity and gestational age. Ultrasound Obstet Gynecol. 2008;32:890–893. doi: 10.1002/uog.6140. [DOI] [PubMed] [Google Scholar]
- Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics. 2005;116:1466–1473. doi: 10.1542/peds.2004-1699. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Davies MJ, Robinson JS. Fetal growth and the fetal origins hypothesis in twins – problems and perspectives. Twin Res. 2001;4:327–331. doi: 10.1375/1369052012669. [DOI] [PubMed] [Google Scholar]
- Poulsen P, Grunnet LG, Pilgaard K, Storgaard H, Alibegovic A, Sonne MP, et al. Increased risk of type 2 diabetes in elderly twins. Diabetes. 2009;58:1350–1355. doi: 10.2337/db08-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previs SF, Hazey JW, Diraison F, Beylot M, David F, Brunengraber H. Assay of the deuterium enrichment of water via acetylene. J Mass Spectrom. 1996;31:639–642. doi: 10.1002/(SICI)1096-9888(199606)31:6<639::AID-JMS336>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rueda BR, Dunn TG, Anthony RV, Moss GE. Influence of fetal death and fetectomy on gestation and the initiation of parturition in the ewe. Reprod Fertil Dev. 1995;7:1221–1225. doi: 10.1071/rd9951221. [DOI] [PubMed] [Google Scholar]
- Rumball CW, Harding JE, Oliver MH, Bloomfield FH. Effects of twin pregnancy and periconceptional undernutrition on maternal metabolism, fetal growth and glucose-insulin axis function in ovine pregnancy. J Physiol. 2008a;586:1399–1411. doi: 10.1113/jphysiol.2007.144071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumball CW, Oliver MH, Thorstensen EB, Jaquiery AL, Husted SM, Harding JE, Bloomfield FH. Effects of twinning and periconceptional undernutrition on late-gestation hypothalamic-pituitary-adrenal axis function in ovine pregnancy. Endocrinology. 2008b;149:1163–1172. doi: 10.1210/en.2007-1306. [DOI] [PubMed] [Google Scholar]
- Salomon LJ, Hourrier S, Fanchin R, Ville Y, Rozenberg P. Is first-trimester crown-rump length associated with birthweight? BJOG. 2011;118:1223–1228. doi: 10.1111/j.1471-0528.2011.03009.x. [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Sherod C, Abbas A, Snijders RJ, Nicolaides KH. Preterm delivery and growth restriction in multifetal pregnancies reduced to twins. Hum Reprod. 1997;12:173–175. doi: 10.1093/humrep/12.1.173. [DOI] [PubMed] [Google Scholar]
- Simmons R. Perinatal programming of obesity. Semin Perinatol. 2008;32:371–374. doi: 10.1053/j.semperi.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Smith MF, McNay MB, Fleming JE. First-trimester growth and the risk of low birth weight. N Engl J Med. 1998;339:1817–1822. doi: 10.1056/NEJM199812173392504. [DOI] [PubMed] [Google Scholar]
- Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, et al. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151:3652–3664. doi: 10.1210/en.2010-0094. [DOI] [PubMed] [Google Scholar]
- Todd SE, Oliver MH, Jaquiery AL, Bloomfield FH, Harding JE. Periconceptional undernutrition of ewes impairs glucose tolerance in their adult offspring. Pediatr Res. 2009;65:409–413. doi: 10.1203/PDR.0b013e3181975efa. [DOI] [PubMed] [Google Scholar]
- Torres-Hernandez G, Hohenboken W. Relationships between ewe milk production and composition and preweaning lamb weight gain. J Anim Sci. 1980;50:597–603. [Google Scholar]
- Vaag A, Poulsen P. Twins in metabolic and diabetes research: what do they tell us? Curr Opin Clin Nutr Metab Care. 2007;10:591–596. doi: 10.1097/MCO.0b013e3282ab9ea6. [DOI] [PubMed] [Google Scholar]
- Kreel VanBK, Vegt VanderF, Meers M, Wagenmakers T, Westerterp K, Coward A. Determination of total body water by a simple and rapid mass spectrometric method. J Mass Spectrom. 1996;31:108–111. doi: 10.1002/(SICI)1096-9888(199601)31:1<108::AID-JMS279>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Casey PJ, Champion ZJ, Gravance CG, Breier BH. IGF-I treatment increases motility and improves morphology of immature spermatozoa in the GH-deficient dwarf (dw/dw) rat. Growth Horm IGF Res. 1999;9:236–240. doi: 10.1054/ghir.1999.0114. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod. 2008;78:299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- Wheaton JE, Carlson KM, Windels HF, Johnston LJ. CIDR: A new progesterone-releasing intravaginal device for induction of estrus and cycle control in sheep and goats. Anim Reprod Sci. 1993;33:127–141. [Google Scholar]
- Wimalasundera RC. Selective reduction and termination of multiple pregnancies. Semin Fetal Neonatal Med. 2010;15:327–335. doi: 10.1016/j.siny.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Wohlt JE, Foy WL, Jr, Kniffen DM, Trout JR. Milk yield by Dorset ewes as affected by sibling status, sex and age of lamb, and measurement. J Dairy Sci. 1984;67:802–807. doi: 10.3168/jds.S0022-0302(84)81370-3. [DOI] [PubMed] [Google Scholar]
- Wong CC, Caspi A, Williams B, Craig IW, Houts R, Ambler A, et al. A longitudinal study of epigenetic variation in twins. Epigenetics. 2010;5:516–526. doi: 10.4161/epi.5.6.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Caspi A, Williams B, Houts R, Craig IW, Mill J. A longitudinal twin study of skewed X chromosome-inactivation. PLoS One. 2011;6:e17873. doi: 10.1371/journal.pone.0017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56–70 y. Am J Clin Nutr. 2008;87:1769–1775. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]


