Abstract
Rationale
Cystic fibrosis (CF) is characterized by bronchoalveolar neutrophilia and submucosal lymphocytosis. We hypothesized that Th17 lymphocytes are part of this submucosal infiltrate.
Objectives
Quantification and phenotyping of the lymphocytic infiltrate in the bronchial submucosa of patients with CF (n=53, of which 20 were newly diagnosed), non-CF bronchiectasis (n = 17), and healthy control subjects (n = 13).
Methods
We measured IL-17 levels in bronchoalveolar lavage and CD4+, CD8+, and IL-17+ cell counts in endobronchial biopsies. Correlations were made with infection status and other inflammatory markers. Potential cellular sources of IL-17 were determined by double staining.
Measurements and Main Results
IL-17+ cell counts (median [interquartile range] cells/mm2) were significantly higher in patients with established CF (205 [115–551]) and non-CF bronchiectasis (245 [183–436]) than in control subjects (53 [12–82]) (P<0.01 for both). Patients with newly diagnosed CF had intermediate counts (171 [91–252]). IL-17–positive CD4+ T cells, γδT cells, natural killer T cells, and neutrophils were identified. Bronchoalveolar lavage IL-17 levels (pg/ml) were highest in established CF (14.6 [2.2–38.4]), low in newly diagnosed CF and control subjects (1.7 [1.7–1.74]; 1.7 [1.7–3]), and intermediate in non-CF bronchiectasis (9.1 [1.7–34] pg/ml) (Kruskal-Wallis P = 0.001). There was a significant correlation between IL-17 and neutrophil counts (P < 0.001, R = 0.6) as well as IL-4 (P < 0.001, R = 0.84).
Conclusions
Th17 lymphocytes are present in the airway submucosa in CF, even in a young, newly diagnosed group. Other IL-17+ cells include neutrophils, γδ T cells, and natural killer T cells.
Keywords: Th17 cells, cystic fibrosis, inflammation
Cystic fibrosis (CF) is a life-limiting autosomal recessive disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. CFTR dysfunction results in recurrent pulmonary infections and inflammation, which in the majority of patients ultimately leads to respiratory failure.
Much of the research into the host inflammatory response in CF has focused on the neutrophils found in the airway lumen, the current hypothesis being that their excessive numbers overwhelm the normal physiological clearance and apoptotic mechanisms (1). They therefore undergo necrosis, with resultant release of their mediators contributing to the tissue damage characteristic of CF (2).
However, little is known about the lymphocytes that predominate in the submucosal tissue in the airway wall (3, 4). If these lymphocytes play a role in driving the overexuberant neutrophilic inflammatory response, they could represent a novel therapeutic target.
T-helper cells are delineated by their unique transcription and differentiation factors and are characterized by their cytokine signature. Examples include Th1, Th2, Tregs, and Th17 cells. Th17 cells express the transcription factor RORγt and secrete the proinflammatory cytokine IL-17 (5, 6). IL-17 acts on a wide range of cell types, including those of the hemopoietic, endothelial, epithelial, and mesenchymal lineages. It induces the expression of multiple proinflammatory cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-6, granulocyte macrophage colony stimulating factor (GMCSF), granulocyte colony stimulating factor (GCSF), and IL-8 (7–9). It is involved in granulopoiesis; is a key cytokine for the recruitment, activation, and migration of neutrophils; and has been shown to regulate neutrophil migration in the lung (10, 11). The primary function of Th17 cells appears to be the clearance of pathogens that are not adequately handled by Th1 and Th2 cells, in particular extracellular bacteria and fungi (12, 13). Although clearance of some pathogens depends on this response, certain pathogens, such as Pseudomonas aeruginosa and Aspergillus fumigatus induce the production of IL-17 and a strong neutrophilic response (14–16) but still fail to be cleared. In such situations, IL-17–driven inflammation is no longer protective, but may be detrimental.
There is preliminary evidence that Th17 lymphocytes may be involved in the pathogenesis of CF. Sputum levels of both IL-17 and IL-23, a regulator of Th17 development, are raised in adult patients with CF during respiratory exacerbations and decline after antibiotic therapy (17). IL-17 can induce matrix metalloproteinase expression; these are present in CF bronchoalveolar lavage fluid (BALF) and are believed to be involved in airway tissue destruction (18–20).
We hypothesized that Th17 cells are part of the lymphocytic infiltrate in the airway wall submucosa and that differences in the activation of the Th17 axis may be partly responsible for the differences in prognosis between CF and other causes of bronchiectasis.
Some of the results of these studies have been previously reported in the form of abstracts (21, 22).
METHODS
BALF and endobronchial biopsies were taken from four groups of children at the time of clinically indicated fiberoptic bronchoscopy (Table 1): established CF (n = 33), newly diagnosed CF (within 6 months of diagnosis) (n = 20), non-CF bronchiectasis (n = 17), and healthy control subjects (n = 13). Of the patients with established CF, 29 were having respiratory exacerbations at time of bronchoscopy. Of the patients newly diagnosed with CF, 16 were diagnosed because of symptoms and 4 on newborn screening. Of these 20, 5 had respiratory exacerbations, whereas 15 were clinically stable and undergoing routine surveillance bronchoscopy (23). Of the patients with non-CF bronchiectasis, all procedures were performed during respiratory exacerbations for microbiological surveillance. The healthy control subjects were either children with no respiratory disease whose carers had consented to a research bronchoscopy during an elective surgical procedure, such as pacemaker insertion (n = 3), or children undergoing a clinically indicated bronchoscopy for upper airway problems, such as stridor (n = 10). In the control group, a subject was excluded if the BALF showed bacterial or viral infection or if the differential cell count was not within normal range (24). Lung explant tissue from three adult patients with CF with end-stage CF lung disease was used for development of the staining protocols and the double-staining experiments. Details of the protocol for flexible bronchoscopy, BALF, and endobronchial biopsies have been reported elsewhere (25) and are described in detail in the online supplement. The Royal Brompton, Harefield, and National Heart and Lung Institute Research Ethics Committee approved the study; informed consent was obtained from parents and age-appropriate assent from the children.
TABLE 1.
CHARACTERISTICS OF PATIENTS IN STUDY
| CF Established | CF Newly Diagnosed | Non-CF Bronchiectasis | Control Subjects | |
|---|---|---|---|---|
| Patients | 33 | 20 | 17 | 13 |
| Age, median (IQR), yr | 9.3 (5.4–12.4) | 1.7 (0.4–4) | 8.9 (7.7–11.9) | 7.5 (1.2–10.3) |
| Male sex, n (%) | 11 (33) | 10 (50) | 4 (24) | 4 (31) |
| FEV1 median (IQR), % predicted | 53 (46–70) | N/A | 81 (72–88) | 92 (70–106) |
| No. of patients who could do lung function | 27 | 0 | 17 | 6 |
| No. of patients with respiratory exacerbation at time of bronchoscopy |
29 | 5 | 17 | 0 |
Definition of abbreviations: CF = cystic fibrosis; IQR = interquartile range.
Patients newly diagnosed with CF are significantly younger than the patients with established CF and non-CF bronchiectasis (P < 0.001).
Cytokine measurements were performed on BALF supernatant using a luminex bioplex assay (Human 17plex cytokine assay; Bio-Rad Inc., Hemel Hempstead, UK) according to the manufacturer’s protocol for the following: IL-17, GCSF, GMCSF, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IFN-γ, TNF-α, Monocyte Chemotactic Protein 1 (MCP1), and Macrophage Inflammatory Protein 1β (MIP1β). IL-23 levels were measured with ELISA (Quantikine Human IL-23 immunoassay, D2300B; R&D Systems, Abingdon, UK).
Biopsies were fixed in 10% formal saline and embedded in paraffin. Immunohistochemistry was performed on 3-μm sections with antibodies directed against CD4 (NCL-CD4–1F6; Novocastra, Newcastle, UK) and CD8 (M7103; Dako, Ely, UK) using the DAKO REAL Detection system (K5001; Dako).
In the absence of a commercially available kit to stain for IL-17, a protocol was developed using the primary goat anti-human IL-17 antibody (AF-317-NA; R&D Systems). Double-staining immunofluorescence experiments were performed for IL-17 with CD4, CD8, γδ T-cell receptor (TCR) (TCR1153; Thermo Scientific, Basingstoke, UK), the macrophage marker CD68 (M0876; Dako), neutrophil elastase (M0752; Dako), and the natural killer T (NKT) cell marker invariant Vα24TCR (IM1588; Beckman Coulter, High Wycombe, UK) for 10 patients in each group as well as the lung explant tissue from the three adult patients with CF. The online supplement contains further details.
Analysis
Area profile counts were performed using a Zeiss Axioskop 2 plus light microscope at ×400 magnification and Axiovision 4.6 software (Imaging Associates Ltd. Bicester, UK) for CD4-, CD8-, and IL-17–positive cells. For analysis of immunofluorescent double staining, a Leica DM2500 microscope was used with Leica Application Suite version 2.8.1 software (Leica Microsystems, Milton Keynes, UK).
Statistical Analysis
Nonparametric statistical tests were used (Kruskal-Wallis [KW] and Mann Whitney) and correction for multiple comparisons was made where appropriate by adjusting the P values with the Bonferroni method. Statistical tests were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL).
RESULTS
Endobronchial Biopsies
Biopsies were of sufficient quality to be analyzed in 58 children (see Table E1 in the online supplement). There was a significant difference in CD4 counts between groups (KW P = 0.03), with the highest counts in patients with established CF and non-CF bronchiectasis. Patients newly diagnosed with CF had similar CD4 counts to control subjects. CD8 counts were also similar across all four groups (Table 2).
TABLE 2.
BIOPSY CELL COUNT RESULTS
| CF Established | CF Newly Diagnosed | Non-CF Bronchiectasis | Control Subjects | |
|---|---|---|---|---|
| Patients with good-quality biopsy specimens | 24 | 13 | 14 | 7 |
| CD4+ cell count, median (IQR)cells/mm2 | 223 (135–389) | 162 (109–319) | 232 (194–505) | 154 (76–179) |
| CD8+ cell count, median (IQR) cells/mm2 | 218 (137–304) | 97 (59–385) | 251 (185–344) | 137 (56–351) |
| IL-17+ cell count, median (IQR) cells/mm2 | 205 (115–551) | 171 (91–252) | 245 (183–436) | 53 (12–82) |
Definition of abbreviations: CF = cystic fibrosis; IQR = interquartile range; KW = Kruskal-Wallace.
There is a significant difference in CD4 counts (KW P = 0.03) and IL-17+ counts (KW P = 0.01) between groups, but not in CD8 counts.
IL-17+ cells were visualized in the airways of all groups (Figure 1). IL-17+ cell counts were significantly different across disease groups (KW P = 0.01) with patients with established CF and non-CF bronchiectasis having significantly higher counts (P < 0.01 for both) than control subjects. IL-17+ cell counts in patients newly diagnosed with CF were also significantly higher (P < 0.05) than control subjects (Figure 2). This was still the case when the analysis was restricted to the 10 patients newly diagnosed with CF who were clinically stable and not undergoing respiratory exacerbation at the time of bronchoscopy.
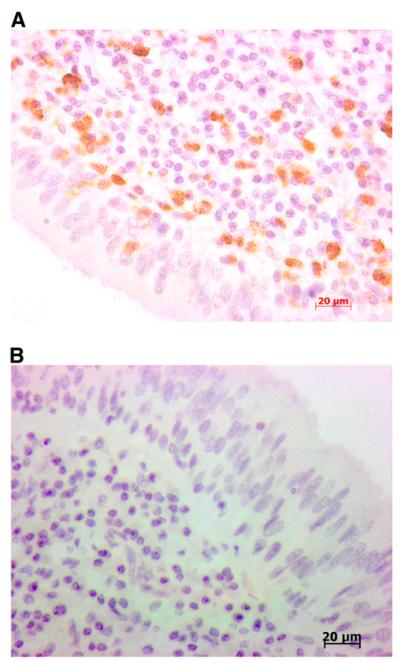
Figure 1.
(A) Example of IL-17+ staining in pediatric cystic fibrosis endobronchial biopsy. (B) Negative control.
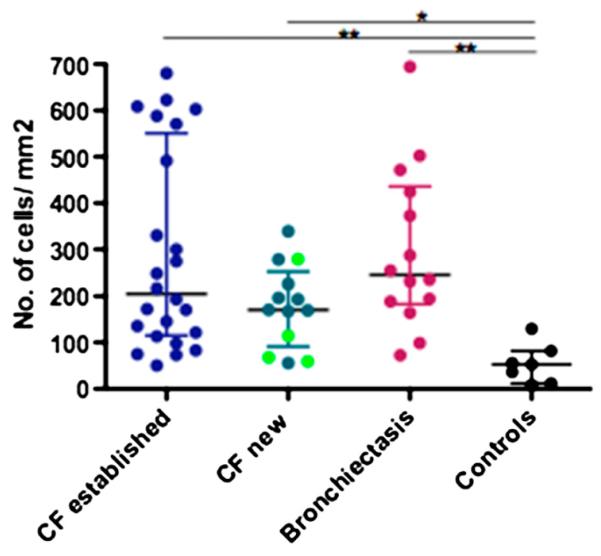
Figure 2.
IL-17+ cell counts in pediatric endobronchial biopsies. IL-17+ cell counts are significantly different across disease groups (Kruskal-Wallis P = 0.01) with patients with established cystic fibrosis (CF) and non-CF bronchiectasis having numbers significantly higher (P < 0.01 for both) than control subjects. IL-17+ cell counts in the patients newly diagnosed with CF are also significantly higher (P < 0.05) than control subjects. The four light green dots in the newly diagnosed CF group represent the four patients who were diagnosed on newborn screening.
There was no difference in IL-17+ cell counts between patients with established CF and non-CF bronchiectasis. This still held true when patients with CF (both established and newly diagnosed) undergoing respiratory exacerbations (n = 24) (median [interquartile range (IQR)] cells/mm2) (205 [102–478]) were compared with the patients with non-CF bronchiectasis (n = 14) (245 [183–436]) who were all undergoing respiratory exacerbation. There was no difference between the IL-17+ cell counts of patients with CF undergoing respiratory exacerbations and patients with CF who were stable (n = 12) (170 [122–279]).
By double staining for IL-17 and CD4, we showed that cells positive for both (i.e., Th17 cells) were present in the pediatric airway (Figure 3). The observation that IL-17 cell counts were higher than CD4 counts in sequential sections of biopsies in 45% of samples led us to hypothesize that cells other than those conventionally described as Th17 (i.e., CD4+), could be producing IL-17 in the pediatric airway. We therefore sought alternative potential sources of IL-17 by double staining for IL-17 and CD8, γδTCR, the macrophage marker CD68, neutrophil elastase, and the NKT cell marker invariant Vα24 TCR. In addition to Th17 cells, IL-17+ neutrophils and γδ T cells were identified (Figures E1 and E2) in the pediatric endobronchial biopsies. In adult end-stage CF transplant tissue, but not in the endobronchial biopsies, IL-17+ NKT cells were also identified (Figure E3). The relative proportion of Th17 (IL17+CD4+) cells compared with the total number of IL-17+ cells was similar across disease groups (Figure 4). The percentage of Th17 cells out of the total number of IL-17+ cells in the endobronchial biopsies was 88% (79–94%) median (IQR) in patients with established CF (n = 10), 75% (27–92%) in patients with newly diagnosed CF (n = 10), 58% (48–80%) in patients with non-CF bronchiectasis (n = 10), and 50% (20–88%) in control subjects (n = 9).
Figure 3.
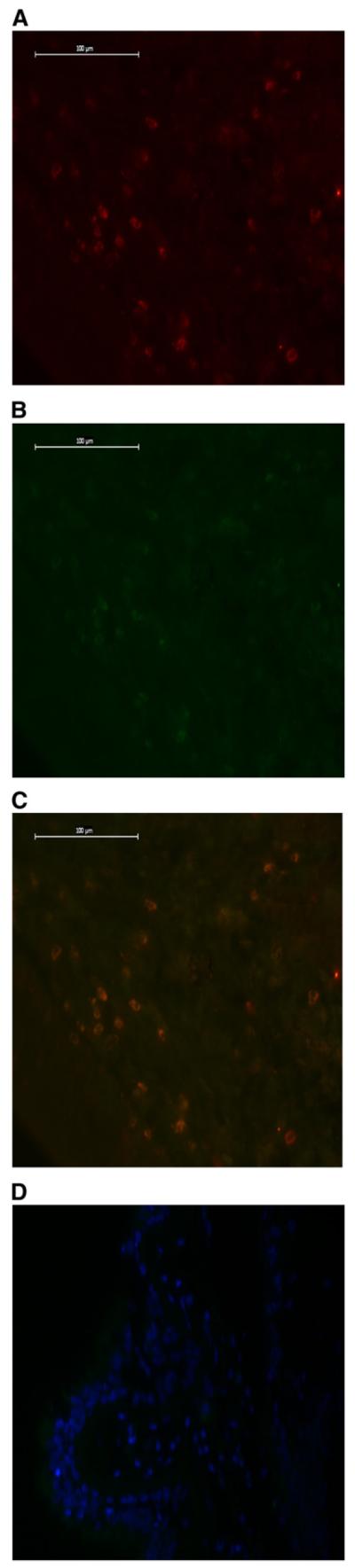
(A) IL-17+ cells staining red. (B) CD4+ cells staining green. (C) Double staining for IL-17 and CD4 in cystic fibrosis (CF) pediatric endobronchial biopsy. (D) Negative control (4′,6-diamidino-2-phenylindole).
Figure 4.
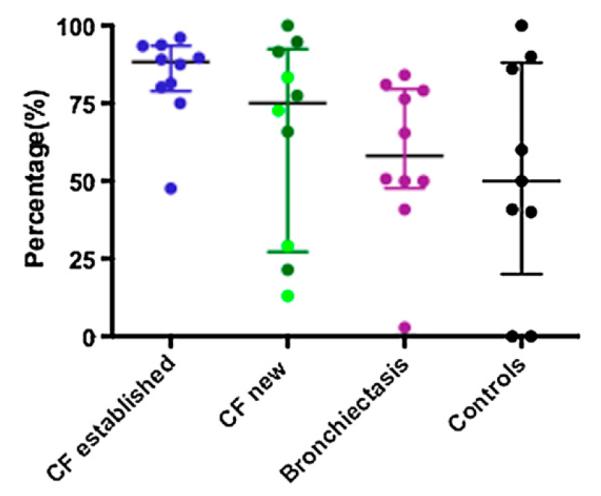
Percentage of IL-17+CD4+ cells compared with the total number of IL-17+ cells in pediatric endobronchial biopsies is the same across disease groups. Once again, the four light green dots in the newly diagnosed cystic fibrosis (CF) group represent the four patients who were diagnosed on newborn screening.
Double staining was performed on BALF cytospins from patients with established CF. IL-17+ neutrophils were identified in the cytospins of these patients (Figure E4). We were unable to identify IL-17+CD4+ cells, IL-17+ γδT cells, or IL-17+ NKT cells in BALF, which may be because the number of lymphocytes, γδ T cells, and NKT cells is very low in the predominantly neutrophilic CF BALF. Another possibility could be these cells require restimulation with antigen or TCR activation. When Aujla and colleagues isolated cells from explanted hilar lymph nodes of CF transplant patients, these cells only showed modest IL-17 production. However, on stimulation with concanavalin A, IL-17 production increased fourfold (26).
BALF
BALF was available for analysis in 67 patients (Table E2). IL-17 levels were significantly different (KW P = 0.001) across disease groups (Figure 5A).
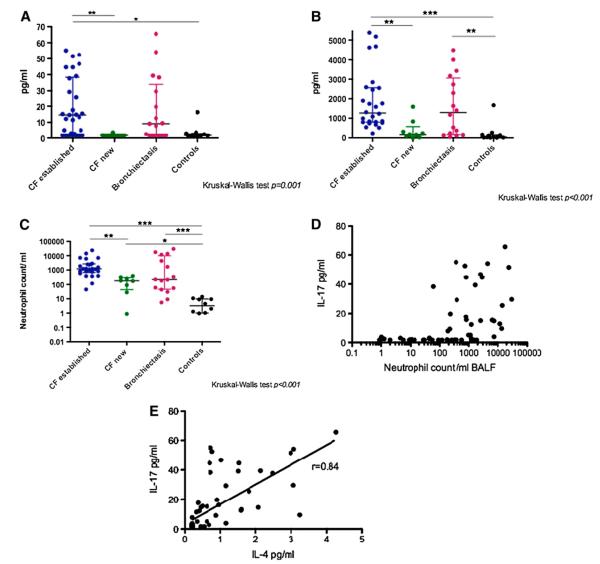
Figure 5.
(A) IL-17 levels in bronchoalveolar lavage fluid (BALF). IL-17 levels were significantly different (Kruskal-Wallis [KW] P = 0.001) across disease groups. Patients newly diagnosed with cystic fibrosis (CF) had levels (median [interquartile range] 1.7 [1.7–1.74] pg/ml) similar to control subjects (1.7 [1.7–3] pg/ml; P = 0.3). Patients with established CF had significantly higher levels (14.6 [2.2–38.4] pg/ml) than both of these groups (P < 0.01 vs. new CF, P < 0.05 vs. control subjects). Levels in patients with non-CF bronchiectasis (9.1 [1.7–34] pg/ml) appeared raised above control and newly diagnosed CF values, but after correction for multiple comparisons, this did not reach statistical significance. There was no difference in IL-17 levels between patients with established CF and patients with non-CF bronchiectasis. (B) IL-8 levels in BALF. IL-8 levels are significantly different across disease groups (KW P < 0.001). Levels in patients with established CF are significantly higher than control subjects and patients newly diagnosed with CF (P < 0.001, P < 0.01, respectively), levels in patients with non-CF bronchiectasis are also significantly higher than control subjects (P < 0.01). (C) Neutrophil counts in BALF. Neutrophil counts are significantly different across disease groups (KW P < 0.001). Neutrophil counts in patients newly diagnosed with CF are already significantly higher than control subjects (P < 0.05). (D) There is a significant correlation between BALF IL-17 levels and BALF neutrophil counts (P < 0.001, R = 0.6) (x-axis is a logarithmic scale). (E) Unexpected significant correlation of IL-17 with IL-4 in BALF (P < 0.001, R = 0.84).
Patients newly diagnosed with CF had levels (median [IQR] 1.7 [1.7–1.74] pg/ml) similar to control subjects (1.7 [1.7–3] pg/ml). In contrast, patients with established CF had significantly higher levels (14.6 [2.2–38.4] pg/ml) than both of these groups (P < 0.01 vs. newly diagnosed CF, P < 0.05 vs. control subjects). Levels in patients with non-CF bronchiectasis (9.1 [1.7–34] pg/ml) appeared higher than values from control subjects and patients newly diagnosed with CF, but after correction for multiple comparisons, this did not reach statistical significance. There was no significant difference in IL-17 levels between the patients with established CF and patients with non-CF bronchiectasis. A similar pattern was observed for the cytokines GMCSF, IFN-γ, IL-1β, IL-8 (Figure 5B), MCP1, IL-4, and TNF-α (Table E6). IL-23 could only be detected in two samples (both from patients with established CF) at concentrations of 31 and 63 pg/ml. BALF neutrophil count was the only marker that was significantly increased in patients newly diagnosed with CF compared with healthy control subjects (Figure 5C), despite none of the mediators measured, including IL-8, being significantly increased in patients newly diagnosed with CF compared with control subjects. The one outlier in the control group with high IL-17 levels was a 2-month old child who presented with stridor (her clinical details are provided in the online supplement).
Across all patients, there was a significant correlation between BALF IL-17 levels and BALF neutrophil counts (P < 0.001, R = 0.6) (Figure 5D). IL-17 was also significantly correlated with several other cytokines, but generally these correlations were weak, with the exception of IL-4 (P < 0.001, R = 0.84) (Figure 5E). There was no correlation with age (R = 0.39), IL-17 biopsy cell counts (R = 0.12), or, in the subgroup of patients (n = 47) who were old enough to perform spirometry, FEV1 (R = 0.30). However, there was a relationship with bacterial infection, with BALF IL-17 levels being significantly higher in the group of patients with positive BALF bacterial cultures (n = 21) (14.6 [7.1–39.3] pg/ml) than those with negative cultures (n = 46) (1.8 [1.7–12.3] pg/ml, P = 0.003).
Patients with CF who were having respiratory exacerbations (n = 28) also had higher IL-17 levels in their BALF (12.7 [1.7–35.6] pg/ml) compared with patients with CF who were clinically stable (n = 10) (1.7 [1.7–5.6] pg/ml, P = 0.03).
DISCUSSION
In this manuscript, we report the development of a double-staining immunofluorescence protocol, with which we demonstrate the presence of submucosal Th17 (CD4+IL-17+) lymphocytes in endobronchial biopsies of children with CF. This is the first direct evidence to support the hypothesis that the Th17 pathway is involved in the pathogenesis of CF lung disease. We also report increased numbers of IL-17+ cells in children newly diagnosed with CF with BALF neutrophilia, in the absence of elevation of IL-8, suggesting that IL-17+ cells are important in the very early stages of CF. Th17 cells are not the only source of IL-17; we have also demonstrated the presence of IL-17+ neutrophils, γδT cells, and NKT cells in the airway. Both established CF and non-CF bronchiectasis samples had higher numbers of IL-17+ cells, suggesting this is not a CFTR-specific phenomenon.
Our focus encompassing both the airway wall and lumen rather than just the latter, which is more commonly studied using BALF, stems from the belief that these two compartments may not be comparable and may yield different insights. In explanted lung tissue, although most of the neutrophils are seen in the surface epithelium (suggesting they may be migrating into the airway lumen), there is a lymphocyte-dominated infiltrate in the CF airway submucosa (3), and we have reported similar findings in children (4). In this study, the submucosal IL-17+ cell count of the endobronchial biopsies does not correlate with the levels of IL-17 or other inflammatory markers in BALF. This would seem to support our hypothesis of compartmentalization of pathogenic processes; however, we must also consider alternative explanations, including that these small proximal biopsies may not be representative of the whole of the lower airway, or that dilution of BALF is so variable that the noise in the signal masks relationships. Furthermore, not just Th17 lymphocytes can secrete IL-17 in the pediatric airway.
Of these other IL-17+ cells, IL-17+ neutrophils have been shown to be present in both patients with end-stage CF undergoing lung transplantation (27) and patients with chronic obstructive pulmonary disease undergoing surgery for lung cancer (28). We have now also demonstrated their presence in patients with non-CF bronchiectasis as well as control subjects.
The isolation of IL-17+ γδ T cells is interesting because, in murine models, they are one of the main producers of IL-17 in response to infection with Mycobacterium tuberculosis (29) and P. aeruginosa (30). Furthermore, mice with chronic granulomatous disease that have pulmonary aspergillosis have also been shown to have unrestrained IL-17+ γδ T cell activity and acute inflammatory lung injury (31).
The final group of IL-17+ cells we demonstrated were NKT cells in transplant tissue from adult lungs. NKT cells are a unique subset of T cells that express both NK cell markers and a T-cell receptor. Recent studies have reported that activated invariant NKT (iNKT) cells in the lung are able to produce IL-17, whereas airway neutrophilia induced by intranasal αGalCer or lipopolysaccharide instillation was significantly reduced in iNKT cell–deficient mice. These mice also produced significantly less IL-17 in their BALF compared with wild-type control mice (32).
Within the airway lumen, our BALF results are consistent with previously published data showing significantly increased levels of IL-17 in the BALF of children with CF and sputum of adults with CF who are having a respiratory exacerbation with P. aeruginosa infection (17, 26, 33), as well as adult patients with CF who are clinically stable (33).
The exact pathogenic mechanisms of IL-17 in CF are still to be elucidated. One possible mechanism may be through priming of airway epithelial cells lacking functional CFTR, by up-regulating pattern recognition receptors, including the bacterial sensors nucleotide binding oligomerization domain (NOD) 1, NOD2, and TLR4, and its own receptors IL-17RA and IL-17RC. IL-17 priming led to much greater NOD1 agonist and Pseudomonas diffusible material induced IL-8 secretion in ΔF508 airway epithelial cells than wild-type airway epithelial cells (34). P. aeruginosa is a gram-negative organism that often causes chronic infection in patients with CF. The host mounts a vigorous neutrophilic inflammatory response but is unable to clear the pathogen. Dubin and Kolls have used an agarose bead model of P. aeruginosa infection in mice, which mimics the chronic airway infection seen in CF, and demonstrated the importance of the IL-17/IL-23 axis in this model. IL-23p19−/− mice had significantly lower induction of IL-17, keratinocyte-derived chemokine, and IL-6, decreased BALF neutrophils, matrix metalloproteinase-9, and on histology had reduced inflammation compared with wild-type mice, but there was no difference in bacterial dissemination between the two groups (16).
The importance of the Th17 pathway is supported by the patients newly diagnosed with CF who already demonstrate significantly increased numbers of IL-17+ cells in the submucosa of their endobronchial biopsies and increased neutrophil counts in their BALF compared with control subjects. Of the four patients newly diagnosed with CF in whom the diagnosis was made on newborn screening, three already showed a neutrophilia in their BALF. The one patient who had a normal cell differential had an IL-17+ cell count of 59 cells/mm2, which is comparable to that of control subjects.
The main weakness of this study, which is unavoidable, is that it is cross-sectional, and there are no longitudinal data, largely because of the ethics of bronchoscopy in children. The findings are hypothesis generating, and proof of the importance of the Th17 pathway will require intervention studies. The differences in our findings between patients with newly diagnosed and established CF, and between culture-positive and -negative patients, suggests an association between the Th17 axis and disease stage, supporting the concept that this axis is important. Our strengths are our large numbers and inclusion of the non-CF bronchiectasis group, which enable us to differentiate CFTR-specific findings from those related to inflammation in general.
Our data generate the hypothesis that early inflammation may be Th17 driven and that subsequently a vicious cycle could occur, in which the initial production of IL-17 attracts neutrophils, which are in turn stimulated to produce more IL-17. This cycle would obviously be augmented by other cytokines (e.g., IL-8), but the intriguing possibility is raised that the Th17 axis is the initiating factor.
Unanswered Questions and Future Research
One unexpected finding was the correlation between levels of IL-17 and IL-4 in BALF. There has been evidence from a murine model suggesting that Th17 cells may up-regulate Th2 cell–mediated eosinophilic airway inflammation (35). Eosinophil cationic protein levels have been shown to be raised in the serum and sputum of patients with CF (36). However, there was no correlation between BALF IL-17 levels and serum IgE, IL-5, IL-13, blood or BALF eosinophil counts in our study. Recently, a novel sub-population of Th17 cells that produces both IL-17 and IL-4 has been described (37). These Th17/Th2 cells are found in very small numbers in blood from healthy subjects and are significantly increased in the circulation of patients with asthma. More work is needed to determine whether these cells have a role in CF. More work is also needed to dissect the individual roles and the relative importance of the various cells that secrete IL-17 and the complex interplay between the adaptive immune system and the innate immune system.
Conclusions
In summary, Th17 lymphocytes are present in the submucosa of children with CF from early in the course of the disease and could be the earliest drivers of the inflammatory response. We have also shown that IL-17+ neutrophils, γδ T cells, and NKT cells are also present in the CF airway. Both established CF and non-CF bronchiectasis samples had higher numbers of IL-17+ cells, suggesting this is not a CFTR-specific phenomenon. The Th17 pathway may be a novel therapeutic target in chronic inflammatory lung disease.
Supplementary Material
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
IL-17 is a proinflammatory cytokine that regulates granulopoiesis and neutrophil recruitment. IL-17 levels are elevated in the sputum of adult patients with cystic fibrosis (CF) undergoing respiratory exacerbations and decrease with antibiotic therapy. However, the source of this cytokine in CF has yet to be elucidated.
What This Study Adds to the Field
We have used immunohistochemistry to determine that Th17 lymphocytes are present in the submucosa of endobronchial biopsies from children with CF, even early in the course of the disease. Th17 lymphocytes are not the only source of IL-17 in the CF airway; IL-17+ neutrophils, γδT cells, and natural killer T cells have also been identified.
Acknowledgment
The authors thank the following people for their invaluable help: Dr. I. Balfour-Lynn, Dr. M. Rosenthal, Dr. C. Hogg, and Dr. S. Saglani for performing the bronchoscopies; Prof E. Alton and his research team at the NHLI, especially N. Newman for helping cut the biopsies and J. Donovan for helping with the Luminex experiments; members of Prof C. Lloyd’s research group, especially Dr. S. Trivedi and S. Mathie for advice regarding immunohistochemistry; Dr. M. Burke for kindly providing explanted lung tissue from three adult CF transplant patients for immunohistochemistry; and the statistician at RBH, W. Banya, for his help with some of the statistical analysis.
Supported by the Royal Brompton Hospital Pediatric Respiratory Department’s discretionary fund, European Respiratory Society Fellowship No.64 (N.R.), and Swiss National Science Foundation grant 1172/05b (N.R.).
Footnotes
Author Disclosure: H.-L.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.R. received grant support from the European Respiratory Society and the Swiss National Science Foundation. S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M.L. was a consultant for MedImmune Inc. J.C.D. is on the Advisory Board for Vertex and received lecture fees from Forest.
Authorship credit: H.L.T. conducted the majority of the experiments, collected patient samples, analyzed the data, and took the lead on writing the manuscript. N.R. taught H.L.T. some of the experimental techniques, collected patient samples, and compiled the clinical database. S.B. performed some of the experimental work and collected patient samples. A.B., C.M.L., and J.C.D. conceptualized, delineated the hypotheses and designed the experiments, contributed to the interpretation of the analyses, supervised the project, and helped with revising the manuscript. A.B. and J.C.D. also performed bronchoscopies and obtained samples.
References
- 1.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 2.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 3.Hubeau C, Lorenzato M, Couetil JP, Hubert D, Dusser D, Puchelle E, Gaillard D. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol. 2001;124:69–76. doi: 10.1046/j.1365-2249.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regamey N, Tsartsali L, Cornish N, Zhu J, Qiu Y, Jeffery PK, Alton EW, Bush A, Davies JC. Airway mucosa inflammation in children with cystic fibrosis. Pediatr Pulmonol. 2007;42:A175. [Google Scholar]
- 5.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 6.Steinman L. A brief history of T(H)17, the first major revision in the T (H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettelli E, Oukka M, Kuchroo VKT. (H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 11.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 14.Zelante T, Bozza S, De Luca A, D’Angelo C, Bonifazi P, Moretti S, Giovannini G, Bistoni F, Romani L. Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol. 2009;47:S162–S169. doi: 10.1080/13693780802140766. [DOI] [PubMed] [Google Scholar]
- 15.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 16.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L519–L528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prause O, Bozinovski S, Anderson GP, Linden A. Increased matrix metalloproteinase-9 concentration and activity after stimulation with interleukin-17 in mouse airways. Thorax. 2004;59:313–317. doi: 10.1136/thx.2003.008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L, Lam WK, Tipoe GL, Shum IH, Yan C, Leung R, Sun J, Ooi GC, Tsang KW. Overexpression of matrix metalloproteinase-8 and -9 in bronchiectatic airways in vivo. Eur Respir J. 2002;20:170–176. doi: 10.1183/09031936.02.00282402. [DOI] [PubMed] [Google Scholar]
- 20.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, Joosten LA, van den Berg WB. Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheum. 2005;52:975–983. doi: 10.1002/art.20885. [DOI] [PubMed] [Google Scholar]
- 21.Tan H, Regamey N, Hilliard T, Donovan J, Alton E, Lloyd C, Bush A, Davies C. The role of the novel Th17 axis in CF lung disease: Relationship of IL17 with markers of infection and inflammation in pediatric bronchoalveolar lavage fluid [abstract] Pediatr Pulmonol. 2008;43:A180. [Google Scholar]
- 22.Tan H, Regamey N, Hilliard T, Alton E, Bush A, Lloyd C, Davies J. Exploring the phenotype of the lymphocytic infiltrate in the CF airway: Do IL17+ cells play a role in disease pathogenesis [abstract] Pediatr Pulmonol. 2009;44:A126. [Google Scholar]
- 23.Hilliard TN, Sukhani S, Francis J, Madden N, Rosenthal M, Balfour-Lynn I, Bush A, Davies J. Bronchoscopy following diagnosis with cystic fibrosis. Arch Dis Child. 2007;92:898–899. doi: 10.1136/adc.2006.105825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Blic J, Midulla F, Barbato A, Clement A, Dab I, Eber E, Green C, Grigg J, Kotecha S, Kurland G, et al. Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. Eur Respir J. 2000;15:217–231. doi: 10.1183/09031936.00.15121700. [DOI] [PubMed] [Google Scholar]
- 25.Hilliard TN, Regamey N, Shute JK, Nicholson AG, Alton EW, Bush A, Davies JC. Airway remodelling in children with cystic fibrosis. Thorax. 2007;62:1074–1080. doi: 10.1136/thx.2006.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodlie M, McKean MC, Johnson GE, Anderson AE, Hilkens CM, Fisher AJ, Corris PA, Lordan JL, Ward C. Raised interleukin-17 is immuno-localised to neutrophils in cystic fibrosis lung disease. Eur Respir J. 2011;37:1378–1385. doi: 10.1183/09031936.00067110. [DOI] [PubMed] [Google Scholar]
- 28.Eustace A, Smyth LJ, Mitchell L, Williamson K, Plumb J, Singh D. Identification of cells expressing interleukin-17A and F in the lungs of COPD patients. Chest. 2011;139:1089–1100. doi: 10.1378/chest.10-0779. [DOI] [PubMed] [Google Scholar]
- 29.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 30.Dubin PJ, Eisenstatt J, Kolls J. IL-23, IL-17 and the TH17 response are critical to mediating inflammation but not protection in P. aeruginosa pulmonary infection. Pediatr Pulmonol. 2009;44:A125. [Google Scholar]
- 31.Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 32.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decraene A, Willems-Widyastuti A, Kasran A, De Boeck K, Bullens DM, Dupont LJ. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable cystic fibrosis patients. Respir Res. 2010;11:177. doi: 10.1186/1465-9921-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roussel L, Rousseau S. IL-17 primes airway epithelial cells lacking functional cystic fibrosis transmembrane conductance regulator (CFTR) to increase NOD1 responses. Biochem Biophys Res Commun. 2010;391:505–509. doi: 10.1016/j.bbrc.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 35.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 36.Suri R, Marshall LJ, Wallis C, Metcalfe C, Bush A, Shute JK. Effects of recombinant human DNase and hypertonic saline on airway inflammation in children with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:352–355. doi: 10.1164/rccm.2110015. [DOI] [PubMed] [Google Scholar]
- 37.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–230. e221–224. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.