Abstract
House dust mites (HDM; Dermatophagoides sp.) are one of the commonest aeroallergens worldwide and up to 85% of asthmatics are typically HDM allergic. Allergenicity is associated both with the mites themselves and with ligands derived from mite-associated bacterial and fungal products. Murine models of allergic airways disease for asthma research have recently switched from the use of surrogate allergen ovalbumin together with adjuvant to use of the HDM extract. This has accelerated understanding of how adaptive and innate immunity generate downstream pathology. We review the myriad ways in which HDM allergic responses are orchestrated. Understanding the molecular pathways that elicit HDM-associated pathology is likely to reveal novel targets for therapeutic intervention.
House dust mites are a major source of allergen
Asthma is a chronic inflammatory disease of the conducting airways affecting 300 million people worldwide and is the commonest chronic disease among children. The disease is characterized by reversible airway obstruction, airway hyper-responsiveness (AHR), infiltration of eosinophils and CD4+ T helper (Th) type 2 cells into the airway submucosa, mucus hypersecretion and airway remodeling. The greatest risk factors for developing asthma are a combination of genetic predisposition and environmental influences such as birth order, childhood infection history and exposure to inhaled substances that provoke allergic reactions, including pollutants and environmental allergens such as pollen, animal dander and mites [1]. Mites belong to the taxonomical subclass Acari and >50,000 species are identified. In this review, we restrict our discussion to the perennial indoor house dust mite (HDM) Dermatophagoides pteronyssinus and Dermatophagoides farinae and their associated allergens of the Der p and Der f families. In addition to asthma, other common allergic disorders caused by HDM are rhinitis, rhinoconjunctivitis and atopic dermatitis [2,3]. Although there are geographical differences, 50 – 85% of asthmatics are typically HDM allergic [4]. It has become clear that, although allergen-specific CD4+ Th2 cells orchestrate HDM allergic responses, the innate immune system plays a critical role in HDM-induced allergy pathogenesis. This finding is particularly important because, historically, research into pathogenesis of asthma and development of novel treatments was focused largely on Th2-driven pathways. However, potential therapeutics directed at components of the allergic pathways of asthmatic inflammation have not proven successful in clinical trials and treatment for asthma has not advanced beyond inhaled bronchodilators and corticosteroids. A broader understanding of the complex immune response to allergens is likely to reveal novel mediators and pathways for therapeutic intervention. This Review summarizes insights into the diverse determinants that contribute to HDM allergenicity through the activation of adaptive and innate immunity.
House dust mites and potential for allergenicity
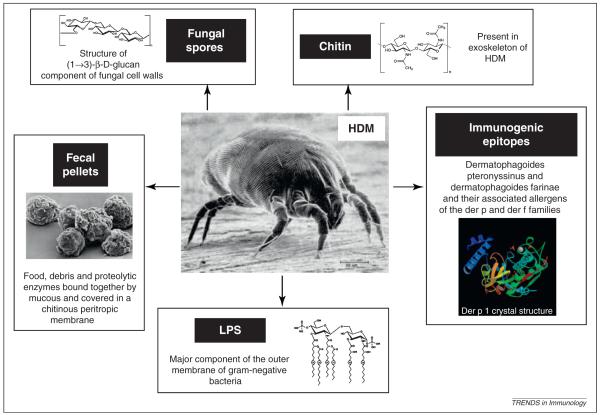
The allergenic potential of house dust mites rests with the mites themselves and with their fecal pellets (Figure 1) [5–10] (Box 1). Allergens belong to protein families with diverse biological functions [11,12] that contribute to allergenicity, as demonstrated by the protease activity of group 1 mite allergens Der p 1 and Der f 1 and the interaction with the innate immune system by the highly allergenic group 2 mite allergens Der p 2 and Der f 2. In addition to the mite-derived Der p allergens summarized in Table 1, innate pattern recognition receptor (PRR) ligands derived from microbial compounds, including lipopolysaccharide (LPS) and β-glucans, can be detected routinely in HDM extracts [13]. Moreover, chitin, a glucosamine-based polymer that forms the mite exoskeleton as well as fungal cell walls also stimulates the immune system [14] (Figure 2). Mite fecal pellets consist of three to five balls that contain food, debris and proteolytic enzymes bound together by mucus are covered in a chitinous peritropic membrane and egested. In a lifetime of 10 weeks, a house dust mite will produce ~2000 fecal particles and an even larger number of partially digested enzyme-covered dust particles [15]. The average intact mite dropping is 10 – 40 μm in diameter and can be inhaled and deposited in the conducting airways.
Figure 1.
House dust mite allergenicity. The various components of HDM, and their associated fecal pellets and dust, which activate the immune system to initiate an inflammatory response, are illustrated.
Box 1. HDM preparations.
Laboratory investigations have used preparations of HDM extract in order to elicit allergic responses in vitro and in vivo. In murine models of allergic asthma, HDM is administered topically into the airways of naïve mice via the intranasal or tracheal route resulting in pulmonary eosinophilic and Th2-type inflammation in the lung, AHR and airway remodeling. Amounts of pulmonary Th2 cytokines IL-4, -5 and -13 are increased, as well as chemokines that are involved in the recruitment, maturation and activation of other leukocytes, including neutrophils, monocytes and alveolar macrophages [5–9]. By contrast, the classical OVA/aluminum hydroxide models used to determine mechanisms of allergic airways disease involve sensitization of mice with OVA via intraperitoneal administration of OVA in conjunction with the Th2 skewing adjuvant aluminum hydroxide. Inflammation is localized to the lung via inhaled aerosolized or intranasal OVA in aqueous solution. A major advantage of the inhaled HDM model is that pathology results after mucosal sensitization within the lungs, as is presumed to occur in man, and does not require peripheral sensitization with adjuvant. Although humans are exposed to both HDM and their fecal pellets, commercially available preparations of HDM extract are typically 95% pure lyophilized mites and 5% ‘contaminating’ fecal pellets. However, because the mites consume the egested pellets, these are present in the gut and, therefore, the subsequent preparation. It should be noted that extracts of commercially available HDM vary according to their preparation, resulting in markedly different biochemical properties such as protease activity, exo- and endochitinase levels and endotoxin content [30]. Soluble HDM extract is increasingly being used but a recent study has shown that pathology is worsened when the allergens are administered in particulate form mimicking inhaled airborne particulate matter. Mast cells have been implicated in this divergent response, as the differences in airway inflammatory responses provoked by the physical nature of the allergens (particulate versus soluble) are attenuated in mast cell-deficient mice. Delayed endocytosis of particulate allergen/IgE/FcεRI complexes within lipid raft-enriched compartments prolongs IgE/FcεRI-initiated signaling, resulting in heightened cytokine responses [10]. Additionally, some investigators choose to use individual Der p or Der f proteins chemically synthesized either with or without peripheral sensitization with adjuvant. These differences are likely to affect the nature of the resulting pathology; therefore, it is important to consider the characteristics of HDM that promote particular immune pathways.
Table 1.
Characterised allergens of Dermatophagoides pteronyssinus. Information is derived from the following allergen websites. www.allergen.org – the official site for the systematic allergen nomenclature approved by the World health organization and International Union of Immunological Societies. www.allergome.org – contains information on allergenic molecules causing allergic diseases. http://pfam.sanger.ac.uk. - protein classification database www.meduniwien.ac.at/allergens/allfam – database for classifying allergens into protein families.
| Allergen | Biological Action | Mol Weight kDa |
Allergenicity Reference |
Functional Consequence |
|---|---|---|---|---|
| Der p 1 | Cysteine protease (papain-like) |
24 | 97 | Disruption of tight junctions. Cytokine, chemokine & growth factor production. Eosinophil and mast cell degranulation. Fibroblast maturation & collagen production. |
| Smooth muscle proliferation. | ||||
| Der p 2 | MD-2 related lipid recognition domain |
15 | 98 | Molecular mimicry of MD-2. |
| Presents LPS to TLR4 resulting in activation of inflammatory genes |
||||
| Der p 3 | Trypsin (serine protease). | 31 | 99 | Disruption of tight junctions. |
| Cytokine, chemokine & growth factor production. Eosinophil and mast cell degranulation. Fibroblast maturation & collagen production. |
||||
| Smooth muscle proliferation. | ||||
| Der p 4 | Alpha amylase | 60 | 100 | |
| Der p 5 | alpha-helical protein of unknown function found exclusively in mites moderately cross reactive with Der p 21 |
14 | Thought to bind hydrophobic ligands resulting in stimulation of the innate immune system |
|
| Der p 6 | Chymotrypsin (serine protease) | 25 | 101 | Disruption of tight junctions. Cytokine, chemokine & growth factor production. Eosinophil and mast cell degranulation. Fibroblast maturation & collagen production. Smooth muscle proliferation. |
| Der p 7 | Binds lipopeptide polymyxin B Strucurally homologous to lipid binding proteins |
26, 30 and 31 |
102 | Does not specifically bind LPS but can be a ligand for other bacterial lipids. Structurally similar to LPS binding protein. Interaction with innate immune system. |
| Der p 8 | Glutathione S-transferase | 27 | 103 | |
| Der p 9 | Collagenolytic serine protease | 29 | 104 | Disruption of tight junctions. Cytokine, chemokine & growth factor production. Eosinophil and mast cell degranulation. Fibroblast maturation & collagen production. Smooth muscle proliferation. |
| Der p 10 | Tropomyosin | 36 | 105 | |
| Der p 11 | Paramyosin | 103 | 106 | |
| Der p 12 | Chitinase (lacks a catalytic domain) | |||
| Der p 13 | Lipocalin Lipid transporter |
|||
| Der p 14 | Apolipophorin High molecular weight allergen found in lipid bodies and transport particles |
177 | 49 | IL-4 and IL-13 release from peripheral blood mononuclear cells of allergic donors |
| Der p 15 | Chitinase | |||
| Der p 18 | Chitinase | |||
| Der p 20 | Arginine kinase | 107 | ||
| Der p 21 | alpha-helical protein of unknown function found exclusively in mites moderately cross reactive with Der p 5 |
|||
| Der p 23 | Unknown function, homology to peritrophin-A domain (PF01607) |
14 |
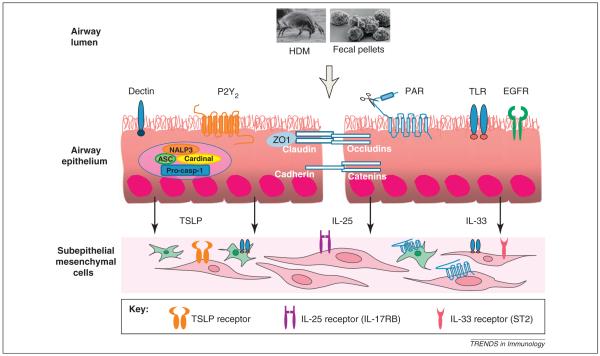
Figure 2.
Activation of airway epithelial cells. The figure shows the various pattern recognition receptors that are expressed on epithelial cells that can bind HDM-associated or induced ligands, including β-glucans, ATP, proteases (Der p 1, 3, 6 and 9) and endotoxin. Expression of these receptors on sub-epithelial fibroblasts and smooth muscle cells and the receptors for the epithelium-derived cytokines TSLP, IL-25 and IL-33 are depicted.
Induction of HDM allergenicity
Bronchial epithelial cells instruct dendritic cells (DCs), which form a dense lining of the airways, to induce Th2 immunity to inhaled allergens via the release of innate pro-Th2 cytokines that include GM-CSF, TSLP, IL-25 and IL-33 [16–18] (Box 2). Inhalation of HDM leads to the recruitment of eosinophils to the lung and IL-4-competent basophils that, together with activated inflammatory DCs, can traffic to the draining mediastinal nodes [19]. Basophils have been suggested to function as antigen-presenting cells in the gut and skin in murine models of helminth infection and after injection of the model cysteine protease papain, which is of interest because the major HDM allergen Der p 1 is a cysteine protease [20–22]. It was shown recently that FcεRI+ DCs are necessary for the initiation of Th2 immunity in a model of mucosal sensitization to inhaled HDM, and the role of basophils, which are recruited to the mediastinal lymph nodes, is limited to amplification of this response in the lung [19]. Interestingly, accumulation of basophils in the draining lymph nodes of the lung has been shown to depend upon activation of the innate adaptor protein MyD88, which is involved in sig naling via TLR4 and the IL-33 receptor ST2 [23], both of which are activated by components of the HDM extract.
Box 2. Bronchial epithelial cells coordinate the innate and adaptive responses to inhaled allergen.
It has become clear that, in addition to their function as a physical barrier, bronchial epithelial cells are central participants in generating mucosal innate and adaptive immune responses that develop in asthmatic airways. Complex allergens such as HDM contain allergenic epitopes and pathogen-associated molecular patterns for PRRs expressed on the apical surface of epithelial cells and activate these cells to initiate the development of innate and adaptive host immune responses. Innate, epithelium-derived cytokines, including TSLP, IL-33 and IL-25, shape the local accumulation and activation of Th2 responses and immunoglobulin production. Pulmonary DCs and macrophages also bridge the innate and adaptive immune response by simultaneously expressing PRRs such as TLRs and by presenting antigens to naïve T cells in lung-draining lymph nodes. Our understanding of the complex interplay between the innate and adaptive immune response initiated in the conducting airways is in its infancy and it is essential to use models of allergic disease that are reliant on local mucosal sensitization to inhaled allergens rather than peripheral sensitization, where different populations of APCs will be encountered, and the particular contribution of epithelial cells can be incorporated. Models using inhaled allergen extracts will extend our understanding of these processes and identify new potential targets for therapy. Although the scope of this Review is limited to HDM-associated airways disease, most of the concepts discussed are equally applicable to other airborne allergens such as animal danders, fungi and pollens.
Protease activity, PARs and tight junctions
Protease activity is a common feature of many human allergens, including fungi, pollen, animal dander and bee venom. HDMs and their fecal pellets contain several proteolytic enzymes. Group 1 allergens are cysteine proteases that share sequence identity with the catalytic site of the plant enzyme papain, whereas those of groups 3, 6 and 9 are serine proteases [24], which account for 79% of the proteolytic activity of house dust [25]. Der p 1 cleaves intercellular epithelial tight junctions (TJs), allowing allergen delivery from the airway lumen to submucosal antigen-presenting cells. Putative Der p 1 proteolysis sites have been identified in peptides from an extracellular domain of occludin and in the TJ adhesion protein claudin-1 [26] and serine proteases of HDM fecal pellets have been shown to activate intracellular proteolysis of both ZO-1 and occludin [27]. However, the impaired barrier function observed in poorly controlled asthmatics [28] is not replicated following a single dose of HDM to mice in vivo [29] and, therefore, might be a function of chronic as opposed to acute exposure. Alternatively, these differences could reflect the variability of protease activity exhibited by different commercial preparations of extract used by various laboratories [30].
In addition to direct effects on junctional proteins, exogenous proteases can react with cell surface protease-activated receptors (PARs) in the airways to generate leukocyte infiltration and to amplify the response to allergens [31]. Signaling through PARs, G protein-coupled receptors, typically involves the cleavage of an extracellular region of the receptor by proteases to reveal a tethered ligand sequence capable of auto-activating the receptor [32]. PAR-2 has been shown to be increased on the epithelium of patients with asthma compared to healthy controls [33]. Stimulation of epithelial PARs indirectly opens TJs and results in cytokine, chemokine and growth factor production. Activation of PARs on eosinophils and mast cells results in their degranulation. Fibroblasts mature and proliferate and produce collagen in response to activation of PARs on their cell surface [34]. Proteases can induce bronchial smooth muscle contraction and proliferation [35,36] and are capable of activating basophils in the absence of antigen-specific IgE [37]. By contrast, PAR activation induces cyclo-oxygenase activation and expression resulting in synthesis and release of prostaglandins and addition of PAR-activating peptides to isolated bronchial segments induces relaxation. The bronchodilator effect has been observed in vivo when PAR-activating peptide is administered immediately before a single aerosolized challenge in a rabbit model of peripheral sensitization to pollen of the Asthma Weed Parietaria judaica. Although there are clearly some model-specific effects, the data suggest that PAR-2 activation might play a detrimental role favoring chronic airway allergic inflammation. It should be noted that the involvement of proteases in disease pathology is not limited to asthma but has been suggested for atopic dermatitis, an inflammatory skin disease that, in common with asthma, is characterized by genetic barrier defects and allergic inflammation [38]. In addition, protease activity has been shown to cleave CXCR1 on neutrophils, inducing the release of glycosylated CXCR1 fragments that function as danger signals and stimulate IL-8 production from bronchial epithelial cells via TLR 2 [39].
Endotoxin and TLRs
The relationship between allergen exposure and sensitization is complex and the immunomodulatory effects of LPS on allergic airways disease both from epidemiological studies and murine models are often contradictory. Both inverse correlations between LPS exposure and development of atopy and positive associations between indoor LPS exposure and development of asthma have been reported. The timing and pattern of LPS exposure (transient high exposure versus chronic moderate exposure) might be crucial but other factors, such as co-exposure to allergens, β-glucans etc. might be more important. It is recognized that environmental exposure to microbial compounds that do not result in clinical disease but act through innate immune response mechanisms might influence the development of adaptive immunity and consequently allergy. In murine OVA models, antigen-specific immune responses are seen when animals are challenged via the airways in the presence of both high-dose and low-dose LPS inducing Th1/Th17 and Th2 responses, respectively [40,41].
Allergen extracts prepared from mites contain endotoxin and Gram-negative Bartonella species are thought to be the source of this LPS [42]. Endotoxin can activate both TLR4 and TLR2 directly, which are expressed by bronchial epithelial cells and DCs [7,9]. Furthermore, expression of TLR4 on airway epithelial cells is up-regulated after airway challenge with HDM extract [43]. Activation of TLR signaling leads to recruitment of cytosolic adaptor molecules such as MyD88, mal, Trif and Tram, which activate protein kinases (IRAK1, IRAK4 and IKK) that amplify the signal and activate proinflammatory transcription factors, which induce the expression of genes involved in the inflammatory response. HDM-induced epithelial TLR4 signaling activates NF-κB and induces GM-CSF, which in turn results in alveolar macrophage maturation and up-regulation of the co-stimulatory molecules CD40 and CD86. There is a tight correlation between epithelial TLR4 expression and expression of these costimulatory molecules on macrophages [43].
TLR activation on epithelial cells also results in transactivation of the epidermal growth factor receptor (EGFR) [44], which can result in cell migration and proliferation and production of proteins involved in innate immune responses including cytokines and mucins. A positive correlation has been shown between EGFR immunoreactivity and both IL-8 and MUC5AC mucin staining in asthmatic subjects [45]. EGFR signaling, particularly in the airway epithelium, plays an important role in mediating HDM-induced AHR and airway smooth muscle remodeling in a murine model [46].
It has been shown using HDM preparations that contain both TLR2 and TLR4 activity, which signal through MyD88, that mice deficient in TLR2 generate a robust allergic phenotype [9]. Conversely, Tlr4 knockout mice were protected and HDM-induced eosinophilia, Th2 responses and AHR were attenuated, suggesting these features were triggered through a convergent TLR4–MyD88 pathway. In MyD88-deficient mice, lymph node hyperplasia and mDC migration to the mediastinal lymph nodes is suppressed, an effect not observed in TLR2- or TLR4-deficient mice, suggesting that the allergic response is not initiated simply through a linear TLR4–MyD88 pathway. TLR2 ligands within the HDM extract promote a Th17-like response independent of TLR4. TLR2 phosphorylation by c-Src signals recruitment and activation of PI3K and PLCγ to affect Ca2+ release from intracellular stores via IP3 receptors. This is required for TLR2-dependent NF-κB activation and subsequent chemokine expression leading to lymphocyte recruitment to the lung and activation of the mucin gene MUC-2, which results in increased mucus production in the airways [47], a hallmark of asthma. In addition, Ca2+-dependent proteases (calpains) are activated; these enzymes cleave the transmembrane proteins occludin and e-cadherin on epithelial cells promoting transmigration of leukocytes [48]. Thus, the balance of danger signals or the activation of different combinations of TLRs might promote distinct immune phenotypes. Both protease and endotoxin components of HDM can affect the physical and immunological barrier function of the airway epithelium. Development of HDM-induced AHR and the immunopathological features of asthma have been shown to be dependent on the TLR-MyD88 axis in acute allergen exposure protocols in mouse models [7,9]. It is yet to be determined whether blocking TLR signaling in chronic models of HDM-induced allergic airways disease is sufficient to prevent pathology.
Lipid-binding proteins and allergenicity
Allergens can be enzymes, structural proteins or ligand-binding proteins that often show specific binding affinity for lipids [11]. More than 50% of defined major allergens are lipid-binding proteins [49], and intrinsic adjuvant activity provided by bound lipids might well underlie the allergenicity of these proteins. Concentrated in HDM fecal pellets, Der p 2 and Der f 2 have the highest rates of skin test positivity compared to other mite allergens in HDM allergic patients [50]. They belong to the MD-2-related lipid-recognition domain family of proteins [51]. Der p 2 facilitates aggregation of TLR4, which is needed for receptor activation to promote TLR4 signaling, and can reconstitute LPS-driven TLR4 activation in the absence of the TLR4 co-receptor MD-2, which is required for LPS recognition [52]. This is of special importance because airway epithelial cells express TLR4 but little or no MD-2 [53]. Thus, Der p 2 has auto-adjuvant activity [54].
Der p 7 and Der p 14 elicit strong IgE antibody and T cell responses in patients with mite allergy [55–57]. Der p 14 is a member of the apolipophorin-like group 14 allergens, which are lipid-binding proteins and likely to be major constituents of the lipid bodies and transport particles of the haemolymph [55]. Interestingly, although lipid particles have been associated with the ability to induce Th1 responses, entrapment of antigen in small lipid particles can act as a Th2 adjuvant. Like Der p 2, the Der p 14 peptide predominantly promotes release of IL-13 and IL-4 Th2 cytokines from peripheral blood mononuclear cells of allergic donors. Der p 7 is another ligand-binding protein with specific affinity for lipopeptide polymyxin B, a bacterially-derived lipid product [58]. Der p 7 is closely related to another protein of the TLR4 signaling pathway, LPS-binding protein. The intrinsic adjuvant activity of lipid-binding proteins and their lipid cargo might be a general mechanism underlying allergenicity. The finding that groups 2, 7 and 14 mite allergens are similar to proteins within the TLR pathway further strengthens the connections between dust mites, innate immunity and allergy [11,49,54,58].
β-Glucans
HDMs carry fungal spores on their exoskeleton and fungi are indigenous gut inhabitants of HDM [59]. The C-type lectin receptors dectin-1 and -2 are expressed on myeloid cells, where they function as classical PRRs by binding β-glucans found in fungal and bacterial cell walls, linking innate and adaptive immune responses. Exposure of airway epithelial cells in vitro to HDM results in β-glucan-dependent secretion of CCL20 [60], a chemokine for immature DCs. HDM extract is a specific stimulus for CCL20 secretion and levels of this cytokine are not affected by treatment of cells with purified Der p 1, chitin or LPS, or other allergen extracts, including cockroach and ragweed. β-Glucan stimulation of dectin-2 also stimulates non-IgE-dependent production of proinflammatory lipid mediators from bone marrow-derived dendritic cells, thus identifying the dectin2–FcRγ–Syk-cysteinyl leukotriene axis as a further mechanism by which HDM can activate innate immune cells to promote allergic inflammation [16,61]. In helminth and fungal models of disease, β-glucans activate the transcription of the proinflammatory cytokine IL-1β through dectin-1 and the associated Syk tyrosine kinase pathway and subsequent NALP3 inflammasome activation [62,63]. Thus, the dectin pathway represents another mechanism by which HDM exposure might elicit adaptive and innate immune responses.
Chitin
Chitin, the main component of the cell wall of fungi, provides structural rigidity to the exoskeleton of crustaceans, insects, helminthes and mites. It is a potent multifaceted adjuvant and can regulate both innate and adaptive Th2, Th1 and Th17 immune responses [64]. It acts as a recognition element for tissue infiltration by innate cells and induces the accumulation of IL-4-expressing eosinophils and basophils in mice [65]. Exogenous chitin can stimulate macrophages by interacting with different cell surface receptors, including TLR2 and dectin-1, providing another pathway by which HDM can elicit activation of the immune system. Although humans do not possess chitin, elevated levels of the chitinase AMCase and the chitinase-like protein YKL-40 have been detected in human asthmatics [66,67]. YKL-40 is an important effector molecule in the development of aeroallergen-induced adaptive Th2 inflammation and mediates IL-13-induced pulmonary inflammation and fibrosis [64]. The allergens Der p 15 and Der p 18 are chitinases that show a high frequency of binding to IgE in human sera, detected in up to 70% of HDM allergic subjects, identifying them as potentially important HDM allergens [68].
Epithelial-derived cytokines promote HDM allergenicity
It is increasingly clear that airway epithelial cells are central participants in innate and adaptive immune responses as well as mucosal inflammation [69,70]. The epithelium-derived cytokines IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) are able to elicit eosinophilia, Th2 cytokines and airway hyper-reactivity directly [71–73] (Figure 3). Elevated pulmonary levels of these cytokines have been demonstrated in response to acute HDM challenge in mice [6,19] and their expression is dependent on TLR4 activation [19]. Biopsies of patients with asthma show elevated levels of IL25 and IL17RB (its receptor) transcripts [74,75] and increased numbers of cells containing TSLP transcripts [76] as compared to normal controls. Additionally, asthmatics have a baseline increase in serum ST2 levels, the IL-33 receptor, as compared to normal controls, and there is a marked increase in these levels during asthma exacerbations. These data support a role for these mediators in human disease. The recently described innate lymphoid helper cells, NHCs [77], MPPtype2 [78], Ih2 [79] and nuocytes [80], are all activated by IL-25 and/or IL-33. Although these cell types, which are capable of promoting Th2 cell-dependent immunity and/or inflammation, have thus far been described only in the gut in nematode infection models, it is tempting to speculate that these cells have important roles at other mucosal sites, particularly the lung, in the initiation and propagation of Th2-type immunity.
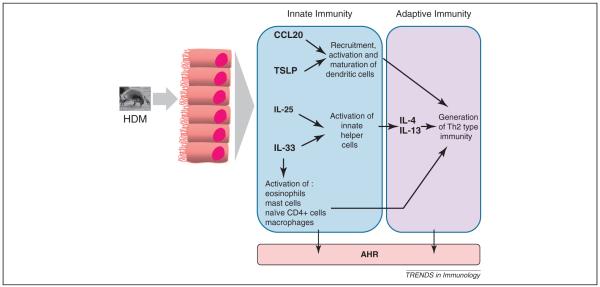
Figure 3.
HDM-induced release of innate epithelial cytokines. Following exposure to HDM, the innate epithelial-derived cytokines TSLP, IL-33 IL-25 and CCL20 are released. Both innate and adaptive arms of the immune response are ultimately activated, resulting in immune-mediated pathology and airway hyper-reactivity.
Epithelial sensing of HDM
Damage-associated molecular patterns (DAMPs), such as ATP and uric acid, are vital danger signals that alert the immune system to tissue damage. Both of these DAMPs are triggered by HDM, and are increased in the airways of asthmatic subjects following allergen exposure [81,82]. Uric acid has been shown to be necessary for Th2-type inflammation, eosinophilia and AHR in HDM-induced allergic airways disease in mice. Mechanistically, HDM-induced uric acid induces Th2 immunity by triggering Syk/PI3K δ-dependent DC activation [82], suggesting it has an important role in allergic inflammation. ATP contributes to disease pathogenesis via signaling at purinergic receptors, expressed at the epithelial surface. Der p 1-mediated stimulation of eosinophils and DCs derived from HDM allergic subjects results in up-regulation of the P2Y2 receptor, which is not observed in non-atopic controls and is accompanied by a stronger chemotactic response of cells to ATP [83]. P2Y2 is expressed also on the apical surface of airway epithelial and goblet cells. ATP via complex Ca2+ and diacylglycerol-regulated mechanisms can increase EGFR activation indirectly leading to airway mucin production and neutrophil recruitment (via interleukin-8 production) [45]. EGFR immunoreactivity has been implicated in epithelial repair and is correlated with asthma severity and with indices of airway remodeling [84].
The NALP3 inflammasome is an intracellular complex that regulates release of proinflammatory cytokines such as IL-1β, in response to exogenous pathogen-associated molecular patterns and endogenous danger signals. In addition to β-glucans, ATP amplifies NALP3 inflammasome activation acting via the P2X7 purinergic receptor [85]. In keratinocytes, HDM stimulates assembly of the inflammasome [86]; however, recent experiments in P2rx7−/− and Nlrp3−/− mice suggest that allergic airway inflammation mediated by HDM exposure in the lung does not rely exclusively on the NALP3 inflammasome [82].
Treatment of HDM-induced asthma; immunotherapy
Asthma control can be achieved by treatment with inhaled corticosteroids, which improves symptoms and inhibits exacerbations in a proportion of patients but is not curative. Allergen-specific immunotherapy redirects inappropriate immune responses in atopic patients and has been shown to be safe and effective for the treatment of IgE-mediated disease [87]. HDM immunotherapy reduces the asthma symptom score and medication requirements and improves bronchial hyper-responsiveness in HDM-allergic asthmatics [88]. Tolerance is increased, as determined by lowered PD20 and decreased HDM-specific IgE values to baseline concomitant with an increase in the effect of components blocking IgE function [89]. Mechanistically, immunotherapy is thought to affect IL-10- and TGF-β-secreting regulatory T cells that are associated with switching of allergen-specific B cells towards IgG4 and suppression of IgE production [90,91]. The importance of DCs in initiating Th2 inflammatory responses is well known; however, it has been proposed that DCs also have a pivotal role in maintaining tolerance to allergens. Tolorogenic DCs can prime T cells to differentiate into Tr1 cells that produce high levels of IL-10, low levels of IL-2 and no IL-4, thus promoting tolerance rather than immunity [92].
The last 10 years has seen substantial growth in alternative models of immunotherapy. Using knowledge of molecular, immunological and biological characteristics of allergens, recombinant allergens can be produced to reduce allergenic activity [93]. For example, creating point mutations in the IgE-binding site [94], fusing allergens to delete B cell epitopes while preserving T cell epitopes [95] and DNA shuffling to maintain T cell epitopes while decreasing allergenicity [96] can create hypoallergenic extracts. Another strategy is to package HDM with TLR agonists into virus-like particles to activate the innate immune system. In this instance, the TLR agonist acts as an immune adjuvant in the presence of allergen, and packaging within virus-like particles improves uptake by antigen-presenting cells and decreases adverse reactions in allergic patients [97,98]. Allergoids are produced by chemical modification of the allergen to preserve T cell epitopes while reducing IgE epitopes [99] and in HDM allergic patients, allergoid immunotherapy reduces symptoms [100] and corticosteroid medication [101,102]. Finally, peptides of allergen-specific T-cell epitopes have been designed which, because of their small size, have reduced ability to cross-link allergen-specific IgE on mast cells, thus inducing immunologic tolerance while decreasing allergenicity [103,104].
Concluding remarks
During allergic responses in the airway, it is clear that properties of the allergen dictate features of the immune response and therefore ensuing pathology. The complexity of HDM induces a multifaceted immune response involving both the innate and adaptive arms of the immune system, activated by enzymatic protease activity and ligand binding to C-type lectin, protease-activated and Toll-like receptors at mucosal surfaces in the lung. This is in contrast to studies with the biochemically simple surrogate allergen OVA that results in a robust Th2-driven eosinophilic response but requires the adjuvant aluminum hydroxide and sensitization is initiated in the periphery rather than within the lung. The lack of translation of the multitude of OVA model studies into patient benefit has been frustrating for scientists, clinicians and patients. However, studies of the in vivo immune response to complex allergens such as HDM in the lung will continue to improve our understanding of epithelial immune and inflammatory responses. HDM-selective protease inhibitors have been proposed as potential therapeutics and inhibitors of the innate epithelium-derived cytokines IL-25, IL-33 or TSLP, or their receptors might offer potential new treatment options. The recognition and understanding of the contribution of innate and adaptive pathways might well lead to the development of new strategies for therapeutic intervention that will play a role in the future treatment of asthma and other allergic diseases.
References
- 1.von Mutius E. Gene-environment interactions in asthma. J. Allergy Clin. Immunol. 2009;123:3–11. doi: 10.1016/j.jaci.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 2.Gavino AC, et al. Atopic dermatitis, patch testing, and house dust mites: a brief review. Dermatitis. 2008;19:121–128. [PubMed] [Google Scholar]
- 3.Kemp AS. Allergic rhinitis. Paediatr. Respir. Rev. 2009;10:63–68. doi: 10.1016/j.prrv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Nelson RP, Jr, et al. Allergen-specific IgE levels and mite allergen exposure in children with acute asthma first seen in an emergency department and in nonasthmatic control subjects. J. Allergy Clin. Immunol. 1996;98:258–263. doi: 10.1016/s0091-6749(96)70148-3. [DOI] [PubMed] [Google Scholar]
- 5.Gregory LG, et al. Inhaled house dust mite induces pulmonary T helper 2 cytokine production. Clin. Exp. Allergy. 2009;39:1597–1610. doi: 10.1111/j.1365-2222.2009.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregory LG, et al. Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am. J. Respir. Crit. Care Med. 2010;182:143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammad H, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JR, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 2004;169:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 9.Phipps S, et al. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am. J. Respir. Crit. Care Med. 2009;179:883–893. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 10.Jin C, et al. Particulate allergens potentiate allergic asthma in mice through sustained IgE-mediated mast cell activation. J. Clin. Invest. 2011;121:941–955. doi: 10.1172/JCI43584. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 11.Chapman MD, et al. Nomenclature and structural biology of allergens. J. Allergy Clin. Immunol. 2007;119:414–420. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Pomes A. Allergen structures and biologic functions: the cutting edge of allergy research. Curr. Allergy Asthma Rep. 2008;8:425–432. doi: 10.1007/s11882-008-0082-y. [DOI] [PubMed] [Google Scholar]
- 13.Fahlbusch B, et al. The effect of storage on allergen and microbial agent levels in frozen house dust. Allergy. 2003;58:150–153. doi: 10.1034/j.1398-9995.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva CA, et al. Chitin particles are multifaceted immune adjuvants. Am. J. Respir. Crit. Care Med. 2010;182:1482–1491. doi: 10.1164/rccm.200912-1877OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Bronswijk JE, Sinha RN. Pyroglyphid mites (Acari) and house dust allergy. J. Allergy. 1971;47:31–52. [PubMed] [Google Scholar]
- 16.Barrett NA, et al. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 18.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Hammad H, et al. Inflammatory dendritic cells–not basophils– are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat. Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat. Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 23.Kroeger KM, et al. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J. Leukoc. Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman MD, et al. Proteases as Th2 adjuvants. Curr. Allergy Asthma Rep. 2007;7:363–367. doi: 10.1007/s11882-007-0055-6. [DOI] [PubMed] [Google Scholar]
- 25.Stewart GA, et al. Immunobiology of the serine protease allergens from house dust mites. Am. J. Ind. Med. 1994;25:105–107. doi: 10.1002/ajim.4700250128. [DOI] [PubMed] [Google Scholar]
- 26.Wan H, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan H, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin. Exp. Allergy. 2001;31:279–294. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhure UN, et al. Lung epithelial permeability and inhaled furosemide: added dimensions in asthmatics. Ann. Nucl. Med. 2009;23:549–557. doi: 10.1007/s12149-009-0275-z. [DOI] [PubMed] [Google Scholar]
- 29.Turi GJ, et al. The effects of inhaled house dust mite on airway barrier function and sensitivity to inhaled methacholine in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L1185. doi: 10.1152/ajplung.00271.2010. [DOI] [PubMed] [Google Scholar]
- 30.Post S, et al. Different biochemical properties of house dust mite induce divergent epithelial and inflammatory responses. Am. J. Resp. Crit. Care Med. 2010;181:A1444. [Google Scholar]
- 31.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 32.Adams MN, et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 2011;13:248–282. doi: 10.1016/j.pharmthera.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Knight DA, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J. Allergy Clin. Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 34.Akers IA, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L1193. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- 35.Hauck RW, et al. alpha-Thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am. J. Physiol. 1999;277:L222. doi: 10.1152/ajplung.1999.277.1.L22. [DOI] [PubMed] [Google Scholar]
- 36.Miglino N, et al. House dust mite extract down-regulates C/EBP{alpha} in asthmatic bronchial smooth muscle cells. Eur. Respir. J. 2011;38:50–58. doi: 10.1183/09031936.00068010. [DOI] [PubMed] [Google Scholar]
- 37.Siracusa MC, et al. New paradigms in basophil development, regulation and function. Immunol. Cell Biol. 2010;88:275–284. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 38.Lee SE, et al. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med. J. 2010;51:808–822. doi: 10.3349/ymj.2010.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartl D, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat. Med. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 40.Simpson A, Martinez FD. The role of lipopolysaccharide in the development of atopy in humans. Clin. Exp. Allergy. 2010;40:209–223. doi: 10.1111/j.1365-2222.2009.03391.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Z, et al. Immunomodulating effects of endotoxin in mouse models of allergic asthma. Clin. Exp. Allergy. 2010;40:536–546. doi: 10.1111/j.1365-2222.2010.03477.x. [DOI] [PubMed] [Google Scholar]
- 42.Valerio CR, et al. Bacterial 16S ribosomal DNA in house dust mite cultures. J. Allergy Clin. Immunol. 2005;116:1296–1300. doi: 10.1016/j.jaci.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 43.Hongjia L, et al. House dust mite regulate the lung inflammation of asthmatic mice through TLR4 pathway in airway epithelial cells. Cell Biochem. Funct. 2010;28:597–603. doi: 10.1002/cbf.1697. [DOI] [PubMed] [Google Scholar]
- 44.Koff JL, et al. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L11068. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 45.Burgel PR, Nadel JA. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur. Respir. J. 2008;32:1068–1081. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- 46.Le Cras TD, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L414–L421. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chun J, Prince A. Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. J. Leukoc. Biol. 2009;86:1135–1144. doi: 10.1189/jlb.0209072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chun J, Prince A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe. 2009;5:47–58. doi: 10.1016/j.chom.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas WR, et al. Structural biology of allergens. Curr. Allergy Asthma Rep. 2005;5:388–393. doi: 10.1007/s11882-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 50.Kidon MI, et al. Mite component-specific IgE repertoire and phenotypes of allergic disease in childhood: the tropical perspective. Pediatr. Allergy Immunol. 2011;22:202–210. doi: 10.1111/j.1399-3038.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 51.Inohara N, Nunez G. ML – a conserved domain involved in innate immunity and lipid metabolism. Trends Biochem. Sci. 2002;27:219–221. doi: 10.1016/s0968-0004(02)02084-4. [DOI] [PubMed] [Google Scholar]
- 52.Carpenter S, O’Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem. J. 2009;422:1–10. doi: 10.1042/BJ20090616. [DOI] [PubMed] [Google Scholar]
- 53.Jia HP, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L4428. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 54.Trompette A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Epton MJ, et al. Sensitisation to the lipid-binding apolipophorin allergen Der p 14 and the peptide Mag-1. Int. Arch. Allergy Immunol. 2001;124:57–60. doi: 10.1159/000053668. [DOI] [PubMed] [Google Scholar]
- 56.Shen HD, et al. IgE and monoclonal antibody binding by the mite allergen Der p 7. Clin. Exp. Allergy. 1996;26:308–315. [PubMed] [Google Scholar]
- 57.Thomas WR, Hales BJ. T and B cell responses to HDM allergens and antigens. Immunol. Res. 2007;37:187–199. doi: 10.1007/BF02697369. [DOI] [PubMed] [Google Scholar]
- 58.Mueller GA, et al. The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins. J. Allergy Clin. Immunol. 2010;125:909–917. doi: 10.1016/j.jaci.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hay DB, et al. How relevant are house dust mite-fungal interactions in laboratory culture to the natural dust system? Exp. Appl. Acarol. 1992;16:37–47. doi: 10.1007/BF01201491. [DOI] [PubMed] [Google Scholar]
- 60.Nathan AT, et al. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J. Allergy Clin. Immunol. 2009;123:612–618. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrett NA, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kankkunen P, et al. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 2010;184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 63.Ritter M, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee CG, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chupp GL, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Z, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 68.O’Neil SE, et al. The chitinase allergens Der p 15 and Der p 18 from Dermatophagoides pteronyssinus. Clin. Exp. Allergy. 2006;36:831–839. doi: 10.1111/j.1365-2222.2006.02497.x. [DOI] [PubMed] [Google Scholar]
- 69.Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet. 2010;376:835–843. doi: 10.1016/S0140-6736(10)61226-3. [DOI] [PubMed] [Google Scholar]
- 70.Saenz SA, et al. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angkasekwinai P, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kondo Y, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 73.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 74.Letuve S, et al. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J. Allergy Clin. Immunol. 2006;117:590–596. doi: 10.1016/j.jaci.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 75.Wang YH, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ying S, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 77.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 78.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Idzko M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 82.Kool M, et al. An Unexpected Role for Uric Acid as an Inducer of T Helper 2 Cell Immunity to Inhaled Antigens and Inflammatory Mediator of Allergic Asthma. Immunity. 2001;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 83.Muller T, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 84.Puddicombe SM, et al. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 85.Petrilli V, et al. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Dai X, et al. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J. Allergy Clin. Immunol. 2011;127:806–814. doi: 10.1016/j.jaci.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Focke M, et al. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin. Exp. Allergy. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 88.Abramson MJ, et al. Injection allergen immunotherapy for asthma. Cochrane Database Syst. Rev. 2010;8 doi: 10.1002/14651858.CD001186.pub2. CD001186. [DOI] [PubMed] [Google Scholar]
- 89.Blumberga G, et al. SQ-standardized house dust mite immunotherapy as an immunomodulatory treatment in patients with asthma. Allergy. 2011;66:178–185. doi: 10.1111/j.1398-9995.2010.02451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frew AJ. Allergen immunotherapy. J. Allergy Clin. Immunol. 2010;125:S306–S313. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 91.James LK, Durham SR. Update on mechanisms of allergen injection immunotherapy. Clin. Exp. Allergy. 2008;38:1074–1088. doi: 10.1111/j.1365-2222.2008.02976.x. [DOI] [PubMed] [Google Scholar]
- 92.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J. Allergy Clin. Immunol. 2009;123:735–746. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 93.Valenta R, et al. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–783. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 94.Swoboda I, et al. Mutants of the major ryegrass pollen allergen, Lol p 5, with reduced IgE-binding capacity: candidates for grass pollen-specific immunotherapy. Eur. J. Immunol. 2002;32:270–280. doi: 10.1002/1521-4141(200201)32:1<270::AID-IMMU270>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 95.Kussebi F, et al. A major allergen gene-fusion protein for potential usage in allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2005;115:323–329. doi: 10.1016/j.jaci.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 96.Gafvelin G, et al. Hypoallergens for allergen-specific immunotherapy by directed molecular evolution of mite group 2 allergens. J. Biol. Chem. 2007;282:3778–3787. doi: 10.1074/jbc.M607938200. [DOI] [PubMed] [Google Scholar]
- 97.Kundig TM, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J. Allergy Clin. Immunol. 2006;117:1470–1476. doi: 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 98.Senti G, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin. Exp. Allergy. 2009;39:562–570. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 99.Casale TB, Stokes JR. Future forms of immunotherapy. J. Allergy Clin. Immunol. 2011;127:8–15. doi: 10.1016/j.jaci.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 100.Chapman MD, et al. Antibody response following prolonged hyposensitization with Dermatophagoides pteronyssinus extract. Int. Arch. Allergy Appl. Immunol. 1980;61:431–440. doi: 10.1159/000232471. [DOI] [PubMed] [Google Scholar]
- 101.Mosges R, et al. Carbamylated monomeric allergoids as a therapeutic option for sublingual immunotherapy of dust mite- and grass pollen-induced allergic rhinoconjunctivitis: a systematic review of published trials with a meta-analysis of treatment using Lais tablets. Acta Dermatovenerol. Alp. Panonica Adriat. 2010;19:3–10. [PubMed] [Google Scholar]
- 102.Pfaar O, et al. Safety of a depigmented, polymerized vaccine for the treatment of allergic rhinoconjunctivitis and allergic asthma. Am. J. Rhinol. Allergy. 2010;24:220–225. doi: 10.2500/ajra.2010.24.3437. [DOI] [PubMed] [Google Scholar]
- 103.Campbell JD, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J. Exp. Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Larche M. Immunotherapy with allergen peptides. Allergy Asthma Clin. Immunol. 2007;3:53–59. doi: 10.1186/1710-1492-3-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]