Abstract
The purpose of this study was to evaluate whether immune responses interfered with gene therapy rescue using subretinally delivered recombinant adeno-associated viral vector serotype 2 carrying the RPE65 cDNA gene driven by the human RPE65 promoter (rAAV2.hRPE65p.hRPE65) in the second eye of RPE65−/− dogs that had previously been treated in a similar manner in the other eye. Bilateral subretinal injection was performed in nine dogs with the second eye treated 85–180 days after the first. Electroretinography (ERG) and vision testing showed rescue in 16 of 18 treated eyes, with no significant difference between first and second treated eyes. A serum neutralizing antibody (NAb) response to rAAV2 was detected in all treated animals, but this did not prevent or reduce the effectiveness of rescue in the second treated eye. We conclude that successful rescue using subretinal rAAV2.hRPE65p.hRPE65 gene therapy in the second eye is not precluded by prior gene therapy in the contralateral eye of the RPE65−/− dog. This finding has important implications for the treatment of human LCA type II patients.
Keywords: rAAV2, Leber congenital amaurosis, RPE65, canine model, immune response, repeated injection
INTRODUCTION
Leber congenital amaurosis is a severe early-onset inherited form of retinal degeneration that shows genetic heterogeneity. The estimated prevalence of LCA in the North American population is 1: 81 000.1 The condition is characterized by severe visual impairment in dim light, typically progressing to complete blindness in the second decade of life. LCA type II results from mutations in the RPE65 gene, and accounts for 10–15% of LCA cases.2,3 RPE65 encodes a protein that forms an essential component of the visual cycle and is expressed within the retinal pigment epithelium.3 The visual cycle is responsible for the supply of the chromophore, 11-cis-retinal, to the photoreceptor cells for combination with the rod and cone opsins to form the visual pigments. RPE65 is an isomerohydrolase that converts esters of vitamin A to 11-cis-retinol for subsequent oxidation to 11-cis-retinal prior to transport to the photoreceptors. A spontaneous 4 basepair deletion in RPE65 in the Briard breed of dog results in a premature stop codon and an absence of RPE65 gene product, resulting in a very similar phenotype to LCA type II.4 Affected dogs have markedly reduced vision and an abnormal electroretinogram with greatly elevated threshold of responses.4,5 The similarities between the human and canine disease resulting from RPE65 mutations, make the RPE65−/− Briard a valuable large animal model for LCA type II.
Dramatic restoration of vision with gene therapy was first reported in the canine RPE65−/− model of LCA.6 A number rod studies have shown and cone photoreceptor rescue using rAAV vectors to deliver a normal copy of the RPE65 gene via a subretinal injection in the RPE65 mutant Briard.6-13 On the basis of the great success of the canine trials, phase I/II clinical trials of rAAV-RPE65 gene replacement therapy in human LCA patients have started with the first reported results showing great promise.14-16 Thus far in all human patients only one eye has been treated. A critical aspect of the management of LCA type II individuals will be the ability to achieve rescue in the second eye. There are concerns that immune responses to the viral capsid and transgene may limit rescue achieved by repeated administration.
Immune responses following rAAV-mediated gene delivery have been analyzed in several detailed studies in animal models, but have generated some contradictory reports and remain inconclusive, with immune responses appearing to depend on the route of administration, vector dose and species differences.17 There are conflicting reports on the success of repeated gene therapy in nonocular tissues. In some studies, readministration of rAAV at later time points was less successful than the initial administration because of neutralizing antibodies (NAb) to the viral capsid proteins.18-21 Serotype switching and transient immunosuppression have been used to try and overcome this obstacle.19,20 Other studies have reported additional transduction events and successful transgene expression after readministration of rAAV, despite the presence of serum NAb to the vector.22,23 In ocular tissues, the site of vector administration is reported to impact on the degree of immune interference with subsequent rAAV administration.24 A study evaluating bilateral intravitreal rAAV2 injections 1 month apart in C57BL/6J mice found that the second eye had very poor reporter gene expression compared with the first treated eye.25 In contrast, the immune privilege of the subretinal space appears to allow for successful readministration. Subretinal injection of rAAV to the contralateral eyes of two previously treated monkeys achieved reporter gene expression at an equivalent level to that seen in the first treated eye.26 Similarly in the rd12 mouse, administration of rAAV-RPE65 to the subretinal space of the second eye also achieved rescue similar to that obtained in the first eye.27 In addition, prior intravitreal injection of the first eye did not interfere with the degree of reporter gene expression achieved by subretinal injection in the second eye of C57BL/6J mice.28
Short duration of rAAV-mediated transgene expression in a number of clinical trials has been linked to the very high level of pre-exposure to wild type AAV in the human population.29 However, a much more limited systemic response to vector has been observed when rAAV was administered to the brain, which is an immune privileged site, with no anti-vector antibodies detected in the CNS itself and only a minority of patients developing circulating NAb.30 The eye is also a site of immune privilege, and thus is a good target for gene delivery. Three clinical trials for LCA II have been initiated, in which one eye of each patient has been treated with rAAV2. All three trials have shown the vector to be well tolerated, with no evidence of antibody responses against the RPE65 transgene product in any patient, and only limited transient NAb responses against the rAAV2 capsid in a minority of patients.14,15
Establishing if repeat subretinal injection of rAAV-RPE65 can achieve rescue in the second eye of RPE65−/− dogs is required prior to including treatment of the second eye in human clinical trial protocols. Previous studies have reported bilateral subretinal injections in RPE65−/− dogs but these have mostly been performed concurrently. Acland et al.8 reported bilateral subretinal rAAV2-RPE65 injections in nine dogs, of these only one dog (BR29 in Table 1) had the second eye injected at a later date and outcome for this eye was not shown. Bennicelli et al.11 reported bilateral subretinal injections of rAAV-RPE65 in two dogs, but both eyes were treated at the same time. In this study, we sought to investigate the rescue achieved in the second eye by gene replacement therapy in RPE65−/− dogs that had previously had the same vector construct administered by subretinal injection in the contralateral eye. We report that successful rescue in the second treated eye of RPE65−/− dogs occurs at a level comparable with that achieved in the first eye. This finding has important implications for the treatment of human LCA II patients.
Table 1. Overview of all dogs treated and details of subretinal injections.
| Dog | Sex | Eye injected |
Age at injection (days) |
Titer (vgml−1) |
Volume (μl) |
Total dose (vg) |
Proportion of tapetal fundus injected |
Post-injection ocular changes |
|---|---|---|---|---|---|---|---|---|
| 1 | F | First (a) | 179 | 1011 | 250 | 2.5×1010 | 0.11 | None |
| Second (b) | 271 | 1011 | 500 | 5×1010 | 0.43 | None | ||
| 2 | F | First (a) | 172 | 1011 | 250 | 2.5×1010 | 0.44 | None |
| Second (b) | 263 | 1011 | 500 | 5×1010 | 0.37 | Tapetal hyper-reflectivity | ||
| 3 | M | First (a) | 179 | 1011 | 250 | 2.5×1010 | 0.45 | None |
| Second (b) | 271 | 1011 | 500 | 5×1010 | 0.48 | Mild tapetal hyper-reflectivity | ||
| 4 | F | First (a) | 172 | 1011 | 250 | 2.5×1010 | 0.06 | Majority choroidal injection |
| Second (b) | 263 | 1011 | 500 | 5×1010 | 0.43 | None | ||
| 5 | M | First (a) | 137 | 1011 | 250 | 2.5×1010 | 0.53 | Mild tapetal hyper-reflectivity |
| Second (b) | 221 | 1011 | 500 | 5×1010 | 0.49 | None | ||
| 6 | M | First (a) | 137 | 1011 | 250 | 2.5×1010 | 0.55 | None |
| Second (b) | 221 | 1011 | 500 | 5×1010 | 0.62 | None | ||
| 7 | M | First (a) | 574 | 1012 | 500 | 5×1011 | 0.52 | Mild tapetal hyper-reflectivity |
| Second (b) | 755 | 1012 | 250 | 2.5×1011 | 0.33 | None | ||
| 8 | M | First (a) | 176 | 1012 | 500 | 5×1011 | 0.17 | None |
| Second (b) | 359 | 1012 | 250 | 2.5×1011 | 0.24 | Cataract | ||
| 9 | M | First (a) | 176 | 1012 | 500 | 5×1011 | 0.46 | None |
| Second (b) | 357 | 1012 | 250 | 2.5×1011 | 0.44 | None |
RESULTS
Subretinal injection of RAAV2/2.hRPE65 in RPE65−/− crossbred dogs
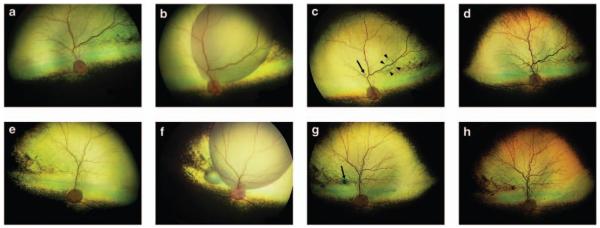
Both eyes of nine RPE65−/− crossbred dogs were subretinally injected with rAAV2.hRPE65p.hRPE65, with the second eye treated 85–180 days after the first eye (Table 1). The age of the dogs at the time of treatment ranged from 137 to 755 days. The injections were made in the superior fundus of both eyes and the mean proportion of the subretinal bleb in relation to tapetal area was 40% (range 6–62%). The retinal detachments created by the subretinal injections typically resolved over the subsequent few days. All eyes had complete retinal reattachment on indirect ophthalmoscopic examination by 1 week after injection, with the exception of eye 6a that still had a small subretinal bleb remaining at that time point (Figure 1c). The small retinotomy created by the cannula sealed in all cases with complete reattachment of the retina at the injection site. The injection site was often visible as a small-pigmented scar (Figures 1c and g). A ‘high-water’ mark indicating the edge of the detachment could be detected on careful fundoscopic examination.
Figure 1.
Wide-angle fundus images (RetCam II, Clarity Medical Systems) of first and second subretinally injected eyes of a RPE65−/− dog (dog 6). The right eye (OD) was injected first and the left eye (OS) was injected 90 days later. (a) OD pre-injection, (b) OD immediately after subretinal injection, (c) OD 1-week post-injection. Note that in this eye, a small region of subretinal fluid remains (indicated by arrowheads in c), (d) OD 1-year post-injection, (e) OS pre-injection, (f) OS immediately after subretinal injection, (g) OS 1-week post-injection, (h) OS 1-year post-injection. The scars resulting at the injection site are indicated with arrows in (c, g).
Evaluation for ocular inflammation
Eyes were examined for ocular inflammatory responses throughout the study. As anticipated following the subretinal injection, mild inflammation, as indicated by slightly lowered intraocular pressure (compared with pre-injection levels) and very mild aqueous flare (ranging from trace flare to 1 on a scale of 1–4), was appreciated on biomicroscopic examination for up to 7 days. The tract of the cannula through the vitreous could be observed. However, no vitreal changes that would indicate an inflammatory reaction were detected in any of the eyes. There was no difference in the post-surgical inflammatory response between first and second injected eyes and in all cases this was a very mild response, which in our experience is typical for that seen in dogs following this sort of surgical intervention. Ophthalmoscopically detectable fundic changes over the period of the study were minimal in 14 of 18 treated eyes. Four eyes showed changes in tapetal reflectivity. In three eyes (3b, 5a, 7a) small foci of tapetal hyper-reflectivity were scattered across the area of the created subretinal bleb. In one eye (2b), a larger area of tapetal hyper-reflectivity developed, covering the majority of the treated fundus, consistent with retinal thinning as described in a previous report.10
Evaluation of retinal function
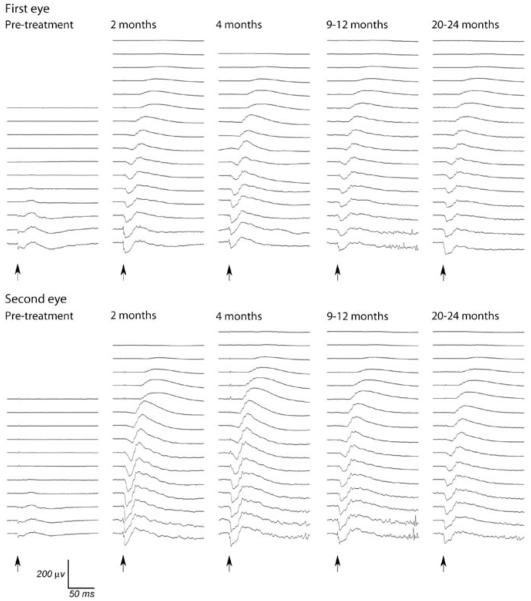
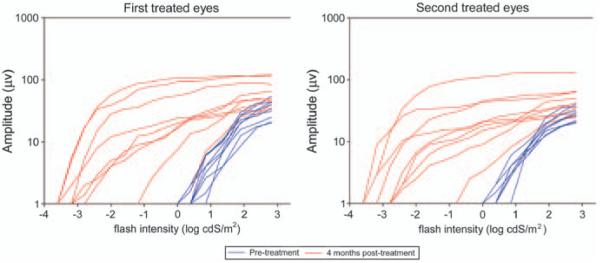
Retinal function was assessed with full-field flash electroretinography (ERG). Rod and cone photoreceptor rescue was observed for all treated eyes with the exception of eyes 4a (very small retinal bleb with majority of vector administered choroidally because of poor injection) and 2b (as anticipated because of aforementioned changes). Representative dark-adapted ERG tracings and light-adapted cone (33 Hz) flicker responses measured before and after treatment are shown in Figures 2 and 3. Dark-adapted a- and b-wave intensity–response curves were generated. These showed that there was an increased a- and b-wave amplitude and lower response threshold for post-injection ERGs relative to pre-treatment recordings in 16 of the 18 eyes. These improvements in ERG waveforms were comparable between first and second injected eyes (Figure 4). As a measure of predominately rod rescue, the amplitude of the dark-adapted b-wave at a flash intensity of 0.0 log cdSm −2 was selected for further analysis. This flash intensity was below the response threshold for all untreated RPE65−/− dogs. Cone function was assessed by the amplitude of the 33 Hz flicker response. This response was either unrecordable or of a very low-amplitude response for all eyes before injection. Using these parameters, recovery of rod and cone photoreceptor function was observed at all time points post-injection for all eyes except 2b and 4a. There was no significant difference in the degree of rod or cone rescue between first and second treated eyes at any time point (Tables 2 and 3). A power analysis was performed to indicate the magnitude of difference in the mean b-wave amplitude at 0.0 log cdSm−2 and the mean cone flicker amplitudes that given the sample size and variability would have reached significance (at P<0.05). These values were 13.3 and 2.5 μV, respectively.
Figure 2.
Dark-adapted ERGs from first and second subretinally injected eyes of dog 6. The second injected eye was treated 90 days after the first eye. Flash intensities from bottom to top were 2.82, 2.38, 1.9, 1.36, 0.85, 0.39, 0.0, −0.39, −0.79, −1.19, −1.6, −2.0, −2.41, −2.79, −3.18, −3.6 log cdSm−2. Arrows indicate timing of flashes.
Figure 3.
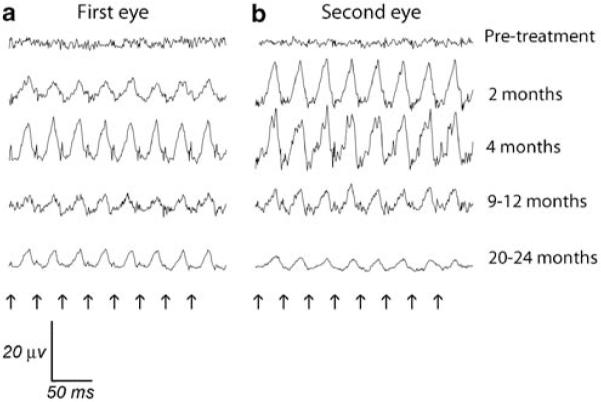
Cone flicker responses from first and second injected eyes of dog 6. Flicker frequency was 33 Hz, at a light intensity of 0.39 log cd m−2. (a) First injected eye, (b) Second injected eye, treated 90 days after the first eye. Note that there was a marked reduction in flicker amplitudes from the peak response by the 20–24 months time point. Arrows indicate timing of flashes.
Figure 4.
Montage of dark-adapted b-wave log intensity response curves for all treated eyes. Pre-injection intensity:response curves are shown in blue, 4 months post-injection are shown in red.
Table 2. ERG results for individual dogs comparing pre-treatment and 4 months post-treatment for first and second injected eyes.
| Dog | Eye |
Rod a-wave pre-injection (μV) |
Rod a-wave 4 months post-injection (μV) |
Rod b-wave pre-injection (μV) |
Rod b-wave 4 months post-injection (μV) |
Cone flicker pre-injection (μV) |
Cone flicker 4 months post-injection (μV) |
|---|---|---|---|---|---|---|---|
| 1 | First (a) | 0 | 2 | 0 | 7 | 0 | 4 |
| Second (b) | 0 | 4 | 0 | 21 | 0 | 4 | |
| 2 | First (a) | 0 | 28 | 0 | 94 | 0 | 9 |
| Second (b) | 0 | 1 | 0 | 3 | 0 | 0 | |
| 3 | First (a) | 0 | 4 | 0 | 20 | 0 | 5 |
| Second (b) | 0 | 3 | 0 | 15 | 0 | 2 | |
| 4 | First (a) | 0 | 0 | 0 | 0 | 0 | 0 |
| Second (b) | 0 | 4 | 0 | 9 | 0 | 3 | |
| 5 | First (a) | 0 | 5 | 0 | 16 | 2 | 8 |
| Second (b) | 0 | 19 | 0 | 45 | 0 | 7 | |
| 6 | First (a) | 0 | 22 | 0 | 56 | 0 | 4 |
| Second (b) | 0 | 44 | 0 | 111 | 0 | 14 | |
| 7 | First (a) | 0 | 10 | 0 | 24 | 0 | 3 |
| Second (b) | 0 | 11 | 0 | 21 | 0 | 3 | |
| 8 | First (a) | 0 | 8 | 0 | 18 | 0 | 4 |
| Second (b) | 0 | 5 | 0 | 18 | 0 | 2 | |
| 9 | First (a) | 0 | 50 | 0 | 107 | 0 | 15 |
| Second (b) | 0 | 24 | 0 | 43 | 0 | 5 | |
| Normalsa | 46 (±18) | 124 (±38) | 21 (±9) | ||||
Abbreviation: ERG, electroretinography.
ERG results from 18 RPE65+/+ eyes of dogs of similar breeding (mean±s.d.).
Rod responses recorded at 0.0 log cdSm−2, cone responses recorded from 33Hz flicker.
Table 3. Mean (a) dark-adapted b-wave amplitude (μV) at 0 log cdSm−2 and (b) light-adapted 33Hz flicker results for all dogs pre- and post-injection, with P-values comparing amplitude of response for first and second treated eyes.
| Eye |
Pre- injection |
2 months |
4 months |
9–12 months |
20–24 months |
P-value (pre vs 4months) |
P-value (2months vs 4months) |
P-value (9-12months vs 20–24months) |
P-value (4months vs 20–24months) |
|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||
| First (a) | 0 | 25 | 38 | 36 | 27 | <0.001* | 0.122 | 0.633 | 0.235 |
| Second (b) | 0 | 30 | 32 | 30 | 25 | <0.001* | 0.573 | 0.364 | 0.348 |
| P-value (1st vs 2nd) | 0.748 | 0.722 | 0.814 | 0.887 | |||||
| (b) | |||||||||
| First (a) | 0 | 4.6 | 5.8 | 5.9 | 3.0 | <0.001* | 0.214 | 0.519 | 0.10 |
| Second (b) | 0 | 4.5 | 4.4 | 4.0 | 1.9 | <0.001* | 0.322 | 0.157 | 0.041 |
| P-value (1st vs 2nd) | 0.954 | 0.474 | 0.303 | 0.350 |
Significant difference.
The mean post-injection amplitudes of rod and cone responses did not change significantly over the period of the study (Table 3). However, there was a trend towards an increase in b-wave amplitude between the 2- and 4-month ERGs, and a decline between the 9–12- and 20–24-month ERGs. This observation was consistent between the two eyes. When the effect of age at injection, total viral dose, injection volume, vector concentration, subretinal bleb size and NAb response on rod and cone rescue was assessed, only the subretinal bleb size correlated significantly with the amplitude of rod and cone ERG responses (Table 4).
Table 4. Impact of variables affecting mean rod and cone rescue at 4 months (P-values displayed).
| Variable (P-value) |
Rod b (0 log cdSm−2) |
Cone (33Hz) flicker |
|---|---|---|
| Age at injection | 0.438 | 0.229 |
| Total dose of vector for both first and second injected eyes |
0.564 | 0.601 |
| Total dose of vector—first injected eyes | 0.42 | 0.56 |
| Total dose of vector—second injected eyes | 0.69 | 0.64 |
| Volume of injection | 0.653 | 0.843 |
| Concentration (vpml−1) | 0.511 | 0.565 |
| Subretinal bleb size | 0.024* | 0.0034* |
| Peak NAb response | 0.161 | 0.123 |
| Change in NAb levels (before and after injection) | 0.146 | 0.102 |
| NAb level at time of second injection with ERG outcome in second eye |
0.718 | 0.909 |
Abbreviations: ERG, electroretinography; NAb, neutralizing antibody.
Significant correlation.
Evaluation of vision
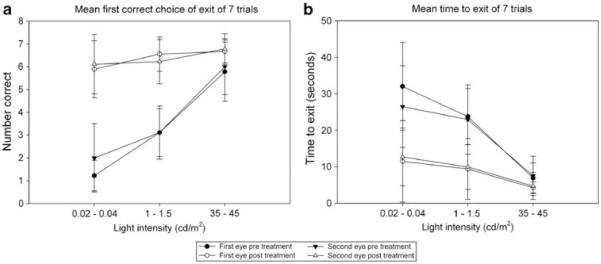
A vision-testing apparatus that assessed the dog’s ability to see an open exit tunnel was used to quantitatively measure visual function under varying light levels as previously described.31 Visual function was evaluated at normal room light and two lower light intensities, by recording the time to exit the device and the first exit tunnel entered. A significant improvement in visual function relative to pre-treatment values was seen at the two lower light intensities for both parameters evaluated (Figure 5). Importantly, there was no significant difference in visual function between first and second treated eyes (Table 5).
Figure 5.
Graphical representation of mean vision-testing outcomes for both outcomes measured with the vision-testing device. Pre-injection values are compared with post-injection for first and second subretinally injected eyes. Testing was performed at three light intensities: 0.02–0.04, 1.0–1.5 and 35–45 cd m−2 for all trials. (a) Mean time to exiting the vision-testing apparatus is shown, averaged from seven trials per eye (b) mean outcome for the first correct choice of four exit tunnels is displayed as number correct of seven trials. Error bars=s.d.
Table 5. P-values for the vision testing assessments.
| P-values | Time to exit (7 trials) | First correct choice of exit (7 trials) | ||||
|---|---|---|---|---|---|---|
| Light intensity (cdm−2) | 35–45 | 1.0–1.5 | 0.02–0.04 | 35–45 | 1.0–1.5 | 0.02–0.04 |
| P—pre vs post (1st eye) | 0.063 | <0.001* | 0.002* | 0.052 | <0.001* | <0.001* |
| P—pre vs post (2nd eye) | 0.025* | 0.001* | <0.001* | 0.043* | <0.001* | <0.001* |
| P—1st vs 2nd eye (pre) | 0.797 | 0.848 | 0.327 | 0.714 | 1.0 | 0.174 |
| P—1st vs 2nd eye (post) | 0.739 | 0.982 | 0.877 | 0.694 | 0.525 | 0.716 |
Significant difference.
Immunology
Evaluation for serum NAb to rAAV2 capsid showed that prior to gene delivery, 1 out of 9 dogs (dog 5) had detectable circulating NAb against rAAV2. Four weeks after vector delivery, all dogs had substantially increased titers of NAb that declined by 12 months, but remained above pre-treatment levels. The NAb titer increased again in 7 out of 9 dogs after the second delivery of vector and peaked at a higher level after the second injection compared with the first injection. A large variation was observed in the NAb titers between dogs; 4 weeks after the first injection the NAb titer ranged from 1/100 to 1/32 000 and 4 weeks after the second injection titers ranged from 1/125 to 1/35 000 (Table 6). Aqueous humor collected from the second injected eye at the time of injection did not have detectable levels of NAbs (data not shown). Notably, higher serum Nab titers to rAAV2 capsid were observed in dog 4 after the first injection and again after the second injection relative to the other dogs. The response after the first injection correlated with choroidal administration of vector construct in this eye (eye 4a). Enzyme-linked immunosorbent assay performed on serum to evaluate for potential humoral immune responses directed to RPE65 showed no detectable change in antibody response specific to RPE65 in any dog after first or second subretinal injection of rAAV2 (data not shown). An analysis was performed on the ERG results of the second injected eyes to see if there was a correlation in ERG outcome and the NAb level at the time of the second injection. There was no correlation between the second eye b-wave at 0.0 log cdSm−2 and the NAb at the time of injection (P=0.718), nor between cone flicker outcome at 4 months and NAb at the time of injection (P=0.909). The analysis was repeated following adjustment of the ERG amplitudes for the size of the bleb and again there was no significant correlation.
Table 6. rAAV2 serum NAb titers pre- and post-injection for first and second injected eyes.
| Dog | Eye injected | Titer pre-injection | Titer 4 weeks post-injection |
|---|---|---|---|
| 1 | First (a) | 1 in 2 | 1 in 300 |
| Second (b) | 1 in 150 | 1 in 125 | |
| 2 | First (a) | 1 in 2 | 1 in 100 |
| Second (b) | 1 in 150 | 1 in 8500 | |
| 3 | First (a) | 1 in 2 | 1 in 2000 |
| Second (b) | 1 in 800 | 1 in 800 | |
| 4 | First (a) | 1 in 2 | 1 in 32 000 |
| Second (b) | 1 in 8500 | 1 in 35 000 | |
| 5 | First (a) | 1 in 150 | 1 in 5000 |
| Second (b) | 1 in 2000 | 1 in 8000 | |
| 6 | First (a) | 1 in 3 | 1 in 3000 |
| Second (b) | 1 in 1000 | 1 in 5000 | |
| 7 | First (a) | 1 in 2 | 1 in 200 |
| Second (b) | 1 in 25 | 1 in 25 000 | |
| 8 | First (a) | 1 in 2 | 1 in 3500 |
| Second (b) | 1 in 200 | 1 in 3500 | |
| 9 | First (a) | 1 in 2 | 1 in 750 |
| Second (b) | 1 in 1000 | 1 in 7500 |
Abbreviation: NAb, neutralizing antibody.
DISCUSSION
In this study, we sought to establish whether successful rescue could be achieved in the second eye of RPE65−/− dogs following previous gene therapy treatment of the fellow eye. We found that rescue achieved in the second eye was comparable to that in the first treated eye, with no evidence of interference of RPE transduction by immune reaction to the first treatment. These findings have significant implications for treatment of human Leber congenital amaurosis type II patients.
Consistent with other studies evaluating immune responses to rAAV, we saw elevated serum NAb titers after all injections. After injection of the first eye, the maximum titer observed was 1/32 000 in the dog 4. In eye 4a, the majority of vector was injected choroidally resulting in a very small subretinal bleb (6% of tapetal fundus). The NAb titers in this animal were much higher than all other animals and as the choroid is not protected by the blood retinal barrier this finding is not surprising. The highest NAb titer after exclusively subretinal administration was 1/5000. The titers declined by 12 months post-injection but remained higher than baseline pre-injection NAb levels. The serum NAb response was higher after the second injection than after the first injection, similar to a prime-boost effect, but this did not appear to affect the rescue of the second injected eye compared with the first. NAb were not detected in the aqueous of the second eye at the time of injection, indicating that the serum NAb did not cross the blood–aqueous barrier in the untreated eye. The NAb response showed no significant correlation with the degree of mean rod or cone rescue achieved. It is of interest that eye 4a, which received vector choroidally, had a lack of detectable ERG and vision rescue. The lack of rescue in this eye may be accounted for by the very small subretinal bleb that was achieved, but we cannot rule out an effect of the presence of a high NAb titer. Although both rod and cone photoreceptor rescue was seen in the second injected eye of this dog (4b), it was observed that the amplitude and threshold of ERG responses for eye 4b were lower than anticipated by the size of the subretinal bleb achieved in this eye. It is possible that the lower than anticipated rescue seen in eye 4b is a function of the higher systemic NAb titer seen after the first injection. However, with only one dog with this complication any correlation between the high systemic antibody level at the time of injection of the second eye and outcome cannot be proven.
In addition to the importance of route of administration, trials of repeated AAV gene therapy in other organ systems have shown that the vector dose is important in determining the degree of immune response and success of transgene expression. Consistent with this, we have previously observed transgene expression in both eyes of Rd12 mice when a lower concentration (1 × 1011 vg ml−1) of vector construct was used, whereas a higher concentration (5 × 1011 vg ml −1) resulted in higher NAb titers and variable transgene expression in the second eye.27 In the larger canine eye, we have achieved successful photoreceptor rescue at both high and low concentrations (1 × 1011 and 1 × 1012 vg ml−1) in both first and second treated eyes without evidence of any significant variation in NAb response. Similarly, administration of a larger total dose of vector did not have a major effect on immune responses or degree of rescue in the first or second treated eyes. This variation may reflect the higher relative dose of viral particles in the smaller murine eye or species-specific immune responses.
Importantly, we observed no ocular or systemic adverse effects after successful subretinal gene delivery. The mild post-injection inflammatory response seen in all injected eyes was typical for that seen after this sort of intervention in dogs. Aqueocentesis itself in the dog induces a mild inflammatory response and has been used in canine studies to test efficacy of anti-inflammatory medications.32 The mild inflammation resolved completely, and there was no indication of any ongoing inflammatory reaction in any eye. Despite choroidal administration of vector and subsequently elevated serum NAb titers, eye 4a did not show evidence of an inflammatory response greater or more persistent that that in other eyes. In this eye, an anticipated degree of post-operative aqueous flare was noted, comparable with that observed in other treated eyes. Small foci of tapetal hyper-reflectivity were observed in 3 of 18 injected eyes, consistent with focal retinal thinning as described in a previous report.10 The presence of the tapetum in the dog allows for detection of retinal thinning by ophthalmoscopic examination more readily than in species with no tapetum. Previous studies have documented the histologic appearance of lesions resulting from subretinal injections in dogs and include damage at the injection site, retinal thinning and focal defects in reattachment resulting in retinal ‘ripples’.10,11,33 The small areas of tapetal hyper-reflectivity that we detected in 3 of the 18 eyes did not preclude successful recovery of rod and cone function in these eyes. Reasons for the more diffuse tapetal hyper-reflectivity and presumed retinal thinning in the injected area of eye 2b are not clear but may reflect the potential detrimental effects of the creation of a retinal detachment. Certainly, this finding correlated with failed recovery of rod and cone ERG responses in this eye.
Improved rod and cone function was seen in 16 of 18 injected eyes, this success rate is comparable with that reported in previous studies evaluating unilateral injections.6-13 However, the amplitudes of rescued cone responses were somewhat variable between eyes. Consistent with this finding Acland et al.8 describe rod rescue in 23 of 26 eyes, but saw cone rescue in only 8 of these 23 eyes using more stringent criteria for rescue. In considering these outcomes, previous biochemical and immunochemistry data show that only the area of the subretinal bleb regains functional RPE65 expression and subsequent 11-cis-retinal production.8 Similarly, PCR results have shown that viral DNA only persists in the neural retina, RPE and choroid of the injected area.6 Concordant with these reports, we saw a significant effect of subretinal bleb size on the amplitude and threshold of dark-adapted rod ERG responses, and on the amplitude of cone (33 Hz) flicker responses. In fact, subretinal bleb size explained the majority of variability observed in ERG responses between treated eyes in this study. Although we sought to create a consistently sized subretinal bleb located over the area centralis, the formed blebs varied somewhat in size and location, consistent with a previous description.10 The area centralis has the highest cone density and corresponds to the human macula, although it has a lower cone density and does not have a rod-free region.34 The location and size of the subretinal bleb likely has a significant role in determining measurable cone photoreceptor rescue.
Improved rod and cone ERG responses were observed for the duration of the study. However, although the mean rod responses at 4 months and those at 20–24 months were not statistically different, there was a statistically significant decline in cone flicker responses between the two time points. These findings were consistent between the first and second injected eyes. These results are similar to those reported by Narfstrom et al.,12 who describe a peak rod ERG response at 3–6 months post-injection and a peak cone ERG response at 18 months post-injection, both rod and cone responses declining thereafter. In contrast, Acland et al.8 followed 2 eyes out to 3 years and did not report a significant decline in rod or cone function. Bennicelli et al.11 report a decline in response from initial ERG at 5 weeks to the second ERG at 3 months, but also described one treated eye followed out to 3 years whose rod ERG varied by <10%. The cause for this apparent variability in the maintenance of ERG responses in the longer term is not clear and its potential significance to human clinical trials of gene replacement therapy for LCA type II remains to be determined.
We have demonstrated functional rescue of the ERG and improved visual outcomes in the RPE65−/− dog injected subretinally with rAAV2.hRPE65p.hRPE65 into both eyes, 85–180 days apart. This demonstrates that vector of the same serotype and expressing the same transgene can be effectively readministered to treat the contralateral eye of previously treated RPE65−/− dogs without evidence of an untoward effect of the therapy. These results support the inclusion of treatment of the second eye in current clinical trials of gene therapy in LCA type II patients.
MATERIALS AND METHODS
Subjects
Nine RPE65−/− dogs were used (Table 1), from a colony maintained at Michigan State University Comparative Ophthalmology Laboratory. All animals were housed under 12:12 h light–dark cycles and cared for in compliance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. Procedures performed were approved by Michigan State University’s Institutional Animal Care and Use Committee.
rAAV2/2 construct and subretinal injection
A recombinant rAAV2/2 vector was used, containing the human RPE65 cDNA coding sequence driven by the human RPE65 promoter (rAAV.hR-PE65p.hRPE65), flanked by AAV2 ITRs encapsidated in an AAV2 shell. Viral vector was produced by Targeted Genetics (Seattle, WA, USA), with the use of a B50 packaging cell line.35 The rAAV titers were determined by dot blot, generating vector concentrations of 1 × 1011 and 1 × 1012vg ml −1. Subretinal injections were performed as previously described.36 An aqueous paracentesis of 100 μl was performed immediately before the injection, and the sample was stored at −80°C for the immunological studies. Two different volumes of injection were administered 250 and 500 μl (Table 1). Dogs were treated with oral prednisone 0.5 mg kg−1 daily 1 week before vector administration. Immediately after subretinal injection, all dogs received a subconjunctival injection of 2 mg dexamethasone solution and 8 mg gentamicin. Oral prednisone was administered post-operatively at 1 mg kg−1 daily for week 1, 0.5 mg kg−1 for week 2, 0.25 mg kg−1 for week 3 and 0.125 mg kg−1 for week 4.
Ophthalmic evaluation and fundic imaging
To monitor for any resultant ocular inflammation, complete ophthalmic examination was performed, including slit lamp biomicroscopy (model SL14; Kowa, Tokyo, Japan), indirect ophthalmoscopy (Welch Allyn, Skaneateles Falls, NY, USA) and fundus photography (RetCam II, Clarity Medical Systems, Pleasanton, CA, USA). Examination was performed pre-injection and then after injection every other day for the first week, twice weekly for the first 2 months, then monthly. Wide-angle digital fundus images captured immediately post-subretinal injection were used to calculate the proportion of the tapetal fundus injected, with measurements performed using Photoshop CS4 (Adobe, San Jose, CA, USA). The extent of the tapetum was similar between all dogs; therefore this method of measuring the subretinal bleb was comparable between eyes.
Electroretinography
ERG recordings were performed pre-injection, and 2, 4 and then between 9-12 months after injection for all treated eyes. A final ERG was recorded at 20-24 months post-injection for 15 of the 18 eyes. ERGs were recorded under inhalant isoflurane anesthesia as previously described, except ERG-Jet corneal contact lens electrodes were used.37 Briefly, globes were positioned in primary gaze using stay sutures of 4–0 silk (Ethicon, Inc., Piscataway, NJ, USA), and the pupils were dilated with 1% tropicamide (Mydriacyl, Alcon Laboratories, Honolulu, HI, USA) and 10% phenylephrine hydrochloride (AK-Dilate, Akorn Inc., Buffalo Grove, IL, USA). Full-field flash ERGs were recorded using ERG-Jet lenses (Microponent, Le Cret-du-Locie, Switzerland) and the UTAS-E 3000 electrophysiology unit with a Ganzfeld (LKC Technologies Inc.; Gaithersburg, MD, USA). Band pass filter cutoff was set at 0.5-500 Hz. Dark-adapted ERG responses were recorded following 1 h of dark adaptation, from a series of 16 white flash stimuli (−3.6 −3.18, −2.79, −2.41, −2.0, −1.6, −1.19, −0.79, −0.39, 0.00, 0.39, 0.85, 1.36, 1.9, 2.38 and 2.82 log cdSm −2). Interstimulus and intervals were increased from 1 s at low intensities to 360 s at the highest intensity to avoid light adapting the rods.37 Following exposure to a background light of 30 cd m−2 for 10 min, cone-mediated flicker responses were recorded at 33 Hz (0.39 log cdSm−2).
For the assessment of rod responses, the dark-adapted b-wave amplitude at 0.0 log cdSm−2 was used. This flash intensity was below the response threshold for all pre-treatment RPE65−/− eyes. Dark-adapted ERGs were assessed for threshold of response and shape of waveform. Dark-adapted b-wave intensity:response curves were plotted. The a- and b-wave amplitudes were measured for each averaged response. The a-wave amplitude was measured from the onset of light stimulus to the trough of the first-negative wave; b-wave amplitude from the trough of the first-negative wave to the peak of the first-positive wave. To evaluate cone responses, the amplitudes of light-adapted 33 Hz flicker responses were analyzed. Flicker responses were chosen rather than single flash light-adapted responses because of the concern that rods not supplied with 11-cis retinal have markedly reduced sensitivities and may still retain some recordable function even in the presence of a background light that saturates normal canine rods. The origin of the sometimes relatively large amplitude response to bright flash stimuli in untreated RPE65−/− dogs is not established. For the 33 Hz flicker responses, amplitude (trough to peak) was measured.
Vision testing
Vision testing was performed using a vision-testing device as previously described.31 Evaluation was performed twice, before injection and 70–255 days post-injection for all dogs. Each injected eye was individually assessed by placement of an eye mask over the contralateral eye. Vision was tested by seven repeated trials at three different light intensities (0.02–0.04, 1.0–1.5 and 35–45 cd m−2). Average time to exit the device and the number of first correct choices of exit tunnel were recorded.
Detection of neutralizing antibodies to rAAV
Serum was collected pre-injection, every 2 weeks until 28 weeks post-injection, then at 9 and 12 months after injection of each eye. Aqueous humor was collected from all eyes immediately after the first injection. Aqueous and serum were stored at −80°C. To determine NAb titers, serial dilutions of serum were prepared in triplicate and 1 × 108 vg rAAV.CMV.GFP was added to each sample. Plates were incubated at 37°C for 1 h, then the contents of the wells were added to 96-well plates of 293T cells containing 2.5 × 104 cells per well. Plates were incubated for 48 h, and then the number of GFP-positive cells per well was counted using an inverted fluorescence microscope. The titer of NAb was defined as the highest dilution that produced 50% fluorescence compared with the rAAV2-positive media only control.
Detection of total IgG and IgM to recombinant RPE65
Aqueous and serum was collected and stored as for detection of NAb to rAAV. In all, 96-well Maxisorp microtitre plates (NUNC, Roskilde, Denmark) were coated recombinant human RPE65 (gift from Professor Martin Warren, University of Kent, UK) (1 in 5000 dilution in 100 μl phosphate-buffered saline (PBS)) overnight at room temperature, then washed with PBS+0.05% Tween-20 (PBS-T). Plates were blocked with 1% BSA in PBS for ≥1 h at room temperature, then washed and samples (1:100 or 1:200 dilution) were applied. Plates were incubated at room temperature for 1.5 h and washed 3× with PBS-T. Bound Canine IgG or IgM was detected with sheep-anti-canine IgG-HRP or goat-anti-canine IgM-HRP (AdB Serotec, Kidlington, UK) for 1.5 h at room temperature, then washed 3× with PBS-T and color was developed with TMB substrate (Pharmingen, Oxford, UK) and quenched with 1 n HCL. Absorbance at 450 nm was quantified using a plate reader (E Max, Molecular Devices, Wokingham, UK).
Statistical analysis
The ERG results and vision-testing outcomes data were analyzed using PASW Statistics 17.0 (SPSS Inc., Chicago, IL, USA). Independent samples t-tests were used to test for differences between average pre- and post-injection outcomes as well as to assess for any difference between first and second eye injected at all time points. Analysis was performed on 33 Hz flicker responses and b-wave amplitudes at 0 log cdSm−2. Power analysis calculations were performed using the program G*Power, version 3.1.2 (Dusseldorf, Germany). For correlation between age, bleb size, viral dose and ERG outcomes, two-tailed Pearson analysis was performed to assess the relationship between the variables. For vision-testing outcomes, mean time to exit, and the mean number of correct exits, independent samples t-tests were again used to test for differences between pre- and post-injection outcomes as well to assess for any difference between first and second eye injected. Independent samples t-tests were chosen above more complex tests because of the relatively low sample size of this study. Data were considered significant at P<0.05.
ACKNOWLEDGEMENTS
This work was supported by the British Retinitis Pigmentosa Society, The Midwest Eye Banks and Transplantation Center Research Program and Michigan State University College of Veterinary Medicine Purebred Dog Endowment Fund. JWB is a Wellcome Trust Advanced Fellow. RRA and JWB are investigators at The NIHR Centre for Ophthalmology at UCl and Moorfields Eye Hospital. We acknowledge Janice Querubin for providing veterinary technician expertise in all aspects of handling and caring for the dogs and Cheri Johnson for assistance with canine reproduction.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Stone EM. Leber congenital amaurosis—a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144:791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 3.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 4.Veske A, Nilsson SE, Narfstrom K, Gal A. Retinal dystrophy of Swedish briard/briardbeagle dogs is due to a 4-bp deletion in RPE65. Genomics. 1999;57:57–61. doi: 10.1006/geno.1999.5754. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre GD, Baldwin V, Pearce-Kelling S, Narfstrom K, Ray K, Acland GM. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. [PubMed] [Google Scholar]
- 6.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 7.Narfstrom K, Katz ML, Ford M, Redmond TM, Rakoczy E, Bragadottir R. In vivo gene therapy in young and adult RPE65−/− dogs produces long-term visual improvement. J Hered. 2003;94:31–37. doi: 10.1093/jhered/esg015. [DOI] [PubMed] [Google Scholar]
- 8.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narfstrom K, Bragadottir R, Redmond TM, Rakoczy PE, van VT, Bruun A. Functional and structural evaluation after AAV.RPE 65 gene transfer in the canine model of Leber’s congenital amaurosis. Adv Exp Med Biol. 2003;533:423–430. doi: 10.1007/978-1-4615-0067-4_54. [DOI] [PubMed] [Google Scholar]
- 10.Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14:292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 11.Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, et al. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narfstrom K, Vaegan, Katz M, Bragadottir R, Rakoczy EP, Seeliger M. Assessment of structure and function over a 3-year period after gene transfer in RPE65−/− dogs. Doc Ophthalmol. 2005;111:39–48. doi: 10.1007/s10633-005-3159-0. [DOI] [PubMed] [Google Scholar]
- 13.Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, et al. Functional and structural recovery of the retina after Gene Therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44:1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- 14.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of Gene Therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 15.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauswirth W, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Phase I Trial of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaiss AK, Muruve DA. Immune responses to adeno-associated virus vectors. Curr Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- 18.Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW, Miller AD. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halbert CL, Standaert TA, Wilson CB, Miller AD. Successful readministration of adenoassociated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- 22.Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D, et al. Repeated adenoassociated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125:509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 23.Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand V, Duffy B, Yang Z, Dejneka NS, Maguire AM, Bennett J. A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Mol Ther. 2002;5:125–132. doi: 10.1006/mthe.2002.0525. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- 26.Anand V, Chirmule N, Fersh M, Maguire AM, Bennett J. Additional transduction events after subretinal readministration of recombinant adeno-associated virus. Hum Gene Ther. 2000;11:449–457. doi: 10.1089/10430340050015914. [DOI] [PubMed] [Google Scholar]
- 27.Barker SE, Broderick CA, Robbie SJ, Duran Y, Natkunarajah M, Buch P, et al. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J Gene Med. 2009;11:486–497. doi: 10.1002/jgm.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Kong F, Li X, Dai X, Liu X, Zheng Q, et al. Gene Therapy following subretinal AAV5 vector delivery is not affected by a previous intravitreal AAV5 vector administration in the partner eye. Mol Vis. 2009;15:267–275. [PMC free article] [PubMed] [Google Scholar]
- 29.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 30.McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- 31.Gearhart PM, Gearhart CC, Petersen-Jones SM. A novel method for objective vision testing in canine models of inherited retinal disease. Invest Ophthalmol Vis Sci. 2008;49:3568–3576. doi: 10.1167/iovs.07-0625. [DOI] [PubMed] [Google Scholar]
- 32.Gilmour MA, Lehenbauer TW. Comparison of tepoxalin, carprofen, and meloxicam for reducing intraocular inflammation in dogs. Am J Vet Res. 2009;70:902–907. doi: 10.2460/ajvr.70.7.902. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13:1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Mowat FM, Petersen-Jones SM, Williamson H, Williams DL, Luthert PJ, Ali RR, et al. Topographical characterization of cone photoreceptors and the area centralis of the canine retina. Mol Vis. 2008;14:2518–2527. [PMC free article] [PubMed] [Google Scholar]
- 35.Gao GP, Qu G, Faust LZ, Engdahl RK, Xiao W, Hughes JV, et al. High-titer adenoassociated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 36.Petersen-Jones SM, Bartoe JT, Fischer AJ, Scott M, Boye SL, Chiodo V, et al. AAV retinal transduction in a large animal model species: comparison of a self-complementary AAV2/5 with a single-stranded AAV2/5 vector. Mol Vis. 2009;15:1835–1842. [PMC free article] [PubMed] [Google Scholar]
- 37.Tuntivanich N, Pittler SJ, Fischer AJ, Omar G, Kiupel M, Weber AJ, et al. Characterization of a canine model of autosomal recessive retinitis pigmentosa due to a PDE6A mutation. Invest Ophthalmol Vis Sci. 2009;50:801–813. doi: 10.1167/iovs.08-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]