Abstract
Psychosis in Alzheimer disease differentiates a subgroup with more rapid decline, is heritable, and aggregates within families, suggesting a distinct neurobiology. Evidence indicates that greater impairments of cerebral cortical synapses, particularly in dorsolateral prefrontal cortex, may contribute to the pathogenesis of psychosis in AD phenotype. Soluble β-amyloid induces loss of dendritic spine synapses through impairment of long term potentiation. In contrast, the Rho GEF kalirin is an essential mediator of spine maintenance and growth in cerebral cortex. We therefore hypothesized that psychosis in AD would be associated with increased soluble β-amyloid and reduced expression of kalirin in the cortex. We tested this hypothesis in postmortem cortical gray matter extracts from fifty-two AD subjects with and without psychosis. In subjects with psychosis, the β-amyloid1-42/β-amyloid1-40 ratio was increased, due primarily to reduced soluble β-amyloid1-40, and kalirin-7, -9, and -12 were reduced. These findings suggest that increased cortical β-amyloid1-42/β-amyloid1-40 ratio and decreased kalirin expression may both contribute to the pathogenesis of psychosis in AD.
Keywords: β-amyloid, kalirin, psychosis, Alzheimer disease
1. Introduction
The emergence of psychosis in individuals with late-onset Alzheimer disease (AD) is an indicator of a more severely progressive form of the disease. Psychotic symptoms, delusions and hallucinations, are frequent in AD, with a prevalence of upwards of 40% (Ropacki and Jeste, 2005). Individuals with AD and psychosis (AD+P) decline more rapidly on measures of cognition and function, and are more likely to be institutionalized (Lopez et al., 1999, Scarmeas et al., 2005). AD+P demonstrates familial aggregation (Sweet et al., 2010, Sweet et al., 2002a), and the estimated heritability of any occurrence of psychotic symptoms in AD is 30%, increasing to 61% for multiple/recurrent symptoms (Bacanu et al., 2005). The familial and heritable nature of AD+P strongly suggests that it develops from a distinct neurobiological origin.
Findings from neuroimaging studies and postmortem studies have pointed to increased synaptic disruption in the neocortex, but not medial temporal cortex, as underpinning the development of psychosis in AD. Structural assessment with magnetic resonance imaging identified reduced gray matter density in frontal and parietal gyri in AD+P subjects in comparison to AD subjects without psychosis (AD-P) (Bruen et al., 2008). Studies of cerebral perfusion with single photon emission computed tomography show hypoperfusion of frontal and parietal lobes (Kotrla et al., 1995), frontal regions (Staff et al., 1999), and dorsolateral frontal and parietal regions (Mega et al., 2000) in AD+P versus AD-P. A positron emission tomography study identified hypometabolism in the frontal lobe of AD+P subjects, compared to AD-P (Sultzer et al., 1995). A magnetic resonance spectroscopy study of synaptic disruption in postmortem tissue identified an excess of membrane breakdown products in several neocortical regions, including the dorsolateral prefrontal cortex (DLPFC), in AD+P (Sweet et al., 2002b). These findings indicate that AD+P is associated with deficits across multiple neocortical regions, however evidence consistently points to frontal regions and the DLPFC in particular.
Evidence of greater neocortical synaptic impairments in AD+P subjects is also consistent with clinical observations that these individuals exhibit a steeper trajectory of cognitive decline than individuals with AD-P (Emanuel et al., 2011, Paulsen et al., 2000, Scarmeas et al., 2005). Loss of synapses is the most robust correlate of degree of cognitive impairment among AD subjects (DeKosky and Scheff, 1990, Scheff and Price, 2006, Terry et al., 1991, Walsh and Selkoe, 2004). The non-specific presynaptic protein synaptophysin, and the intracortical excitatory bouton selective protein vesicular glutamate transporter VGLUT1, are both reduced in AD neocortex and correlated with cognitive decline (Counts et al., 2006, Kashani et al., 2008, Terry et al., 1991). Similarly, dendritic spines, the postsynaptic components of the majority of synapses in the cortex (Rakic et al., 1986), and the dendritic spine associated proteins synaptopodin and drebrin, are reduced in neocortex of subjects with AD, and correlated with cognitive impairment (Counts et al., 2006, Grutzendler et al., 2007, Reddy et al., 2005).
Soluble Aβ oligomers have emerged as the most likely cause of dendritic spine deficits observed in AD. Normally, enhanced synaptic efficacy, dendritic spine enlargement, and spine persistence are correlated phenomena mediated by long-term potentiation (LTP) (Matsuzaki, 2007). Soluble, oligomeric Aβ isolated from AD brains inhibits LTP and induces spine loss (and as a consequence the synapses onto them) in rodent hippocampus, while insoluble plaque cores do not have the same effects unless solubilized first (Shankar et al., 2008). Studies of transgenic mouse models of AD have demonstrated that well before plaque deposition, increased soluble (non-fibrilllar) Aβ concentration is associated with changes in dendritic length and shape (Wu et al., 2004), reductions in synaptic density (Mucke et al., 2000), and impaired synaptic transmission (Hsia et al., 1999). Application of naturally secreted Aβ oligomers inhibits hippocampal LTP in vivo (Walsh et al., 2002) and in vitro (Wang et al., 2002), and induces dendritic spine loss in vitro (Shankar et al., 2007).
The excess synaptic disruption in AD+P could be driven simply by increased concentrations of soluble Aβ leading to greater inhibition of LTP and resultant spine loss. Alternatively, reductions in proteins which serve to mediate the effects of LTP on dendritic spines could independently lead to excess spine and synapse loss in AD+P. One such mediator is kalirin, a GDP/GTP exchange factor (GEF) which activates the Rho family of GTP binding proteins (Alam et al., 1997, Cerione and Zheng, 1996). Four major isoforms generated through alternative splicing of the kalirin gene are expressed in adult CNS (kalirin-5, -7, -9, -12) (Johnson et al., 2000). In the cortex, kalirin is necessary for LTP-induced dendritic spine enlargement and controls expression of AMPA receptors at the synapse (Xie et al., 2007). In the hippocampus however, other GEFs that are not highly expressed in the cortex may substitute for kalirin, as kalirin knockout mice have reduced dendritic spine densities in the cortex but not hippocampus (Cahill et al., 2009). Kalirin mRNA and protein expression is lower in AD hippocampus compared to cognitively normal controls (Youn et al., 2007a, Youn et al., 2007b). However, kalirin expression in the neocortex of AD subjects, and in relationship to psychosis status of AD subjects, has not been evaluated. Considering the integral function kalirin has in activity-dependent mechanisms of spine enlargement and glutamatergic transmission, reduction in kalirin could play a role in rendering synapses to be more vulnerable in AD+P.
We therefore undertook to evaluate soluble Aβ and kalirin expression in the cerebral cortex of subjects with AD+P in comparison to AD-P subjects. We hypothesized that susceptibility to AD+P may result from a deficit in kalirin, from an increase in Aβ, or their combined effect; more Aβ drive acting on vulnerable synapses may lead to greater loss of synapses in AD+P.
2. Method
2.1. Subjects
Fifty-two subjects (Table 1) underwent neurologic, neuropsychologic, and psychiatric diagnostic evaluations at successive time points as part of their participation in the Clinical Core of the Alzheimer Disease Research Center (ADRC), with methods previously described (Sweet et al., 2001, Sweet et al., 2000).
Table 1.
Descriptive information of the subjects involved in the study.
| Variable | AD - P | AD + P | p-value |
|---|---|---|---|
| Age, years | 80.6 ± 8.7 | 80.7 ± 7.9 | 0.936 |
| Age of onset, years | 69.5 ± 9.4 | 71.0 ± 9.0 | 0.564 |
| Duration of illness, years | 11.0 ± 4.5 | 9.7 ± 5.0 | 0.339 |
| Gender | 0.634 | ||
| Male | 11 (50) | 13 (43) | |
| Female | 11 (50) | 17 (57) | |
| Post mortem interval, hours | 4.9 ± 1.6 | 5.7 ± 2.5 | 0.171 |
| α-synuclein | 0.600 | ||
| Positive | 9 (41) | 15 (50) | |
| Negative | 13 (59) | 15 (50) | |
| Braak | 0.332 | ||
| 3 | 4 (18) | 1 (3) | |
| 4 | 3 (14) | 4 (13) | |
| 5 | 4 (18) | 8 (27) | |
| 6 | 11 (50) | 17 (57) |
Mean values ± SD or number of subjects with percentage of group in parentheses. Groups did not differ with regard to any of the variables listed.
The presence or absence of delusions and hallucinations were indicated as part of semi-structured examinations conducted by research psychiatrists and rated on the CERAD Behavioral Rating Scale (Tariot et al., 1995). Delusions were defined as a false belief, not attributable to membership in a social or cultural group, based on incorrect inference about external reality. Hallucinations were defined as sensory perceptions for which there was no basis in reality. Psychosis was defined as the presence of any hallucination or delusion. No patient had a history of schizophrenia, schizoaffective disorder, or other idiopathic psychosis.
Diagnosis of definite AD was made at the time of postmortem exam through regional sampling and semiquantitative scoring of neuritic plaques and neurofibrillary tangles, per CERAD diagnostic criteria and neuropathology protocol (Mirra et al., 1991). This procedure has been described in detail elsewhere (Sweet et al., 2000, Sweet et al., 2002b). The presence or absence of any α-synuclein aggregates was rated as positive or negative, respectively. Semiquantitative rating of cerebral amyloid angiopathy (CAA) severity was also assessed at autopsy (Sweet et al., 2004). Dates of death ranged from November, 1999 to December, 2003.
All samples were obtained through the brain tissue bank of the ADRC at the University of Pittsburgh. At the time of brain removal, postmortem interval (PMI) was recorded and the brain was divided in the midsagittal plane. A series of gray matter samples were dissected from the superior frontal gyrus (DLPFC), inferior parietal cortex (IP), superior temporal gyrus (STG) and occipital cortex (OC) at autopsy and frozen at −80°C.
2.2. Measurement of Soluble Aβ by an ELISA
Gray matter samples from the DLPFC, STG, IP, and OC were homogenized on ice in phosphate-buffered saline (PBS; 150 mg/mL) and rehomogenized in tissue homogenization buffer (250 mM sucrose, 20 mM Tris base, and 10 μL/mL Sigma P8340 protease inhibitor cocktail [Sigma-Aldrich, St. Louis, Missouri, USA]). Aβ1-40 and Aβ1-42 peptide concentrations were quantified in diethylamine (DEA)-soluble Aβ fractions as described previously (Ikonomovic et al., 2008). The DEA-soluble fraction was prepared by centrifuging the homogenate aliquot at 135,000 × g at 4°C for 1 hour and neutralizing the supernatant with 0.5 M Tris-Cl. The Aβ concentrations were assayed using a colorimetric TMB-based ELISA (Invitrogen, Carlsbad, California) read at 450 nm, with a capture antibody specific for the NH2 terminus of human Aβ (amino acids 1–16) and detection antibodies specific for the neoepitope at either the 40- or the 42-amino acid end of Aβ. Values were determined from standard curves using synthetic Aβ peptide (Invitrogen, Carlsbad, California) and are expressed as picomoles per gram wet brain tissue.
2.3. Western blot anaylsis of kalirin protein levels
Samples from one AD+P and one AD-P subject were not available to be included in the kalirin assay, resulting in a final N of 50 subjects. To minimize any effects of interassay variability on our primary comparison of AD+P and AD-P subjects, subjects were grouped into sets of four (“quads”), stratified on psychosis presence. Within quads subjects were matched to the extent possible on age and Braak staging (Braak and Braak, 1991). Gray matter from DLPFC tissue samples was homogenized and sonicated in ice cold SDS extraction buffer (0.125 M Tris-HCl (pH 7), 2% SDS, and 10% glycerol), followed by centrifugation at 16,100 g for 10 minutes. Total protein was extracted using SDS extraction buffer at 70°C. Protein concentration was estimated using a bicinchoninic acid assay (BCA™ Protein Assay Pierce # 23225). Quads were run together and assayed in triplicate. The final protein concentration utilized for each sample was the mean of the triplicate runs.
Two quads were examined per run, and each run consisted of 4 gels. Protein (25 μg) was aliquoted in 1x LI-COR Protein Loading Buffer (Li-Cor #928-40004 Licor Inc. Lincoln, Nebraska, USA), loaded on 4–20% SDS-PAGE gradient gels (Thermo Scientific #26224 Thermo Scientific, Rockford, Illinois, USA), and separated for 2 hours at room temperature in 1X SDS running buffer (Pierce 20X Tris Hepes SDS Buffer #28368) at 75 V. Samples were then transferred to polyvinylidene fluoride membranes (PVDF; Millipore Immobilon-FL PVDF #PFL00010) in 1x Tris Glycine Blotting Buffer (Pierce #28363) at 85 V, for 50 minutes at 4°C. Membranes were incubated for 1 hour in Odyssey LiCor Blocking Buffer (LiCor #927-4000) diluted 1:1 in 1x TBS. The membrane was incubated overnight in primary antibodies directed against the spectrin domain of kalirin (rabbit anti-kalirin spectrin, Millipore # 07-122) diluted 1:500, mouse anti-β-tubulin (Millipore #05-661) diluted 1:30,000, in Pierce SuperBlock blocking buffer (Pierce #37353) with 0.1% Tween 20 (Sigma # P7949 Sigma-Aldrich, St. Louis, Missouri, USA). Specificity of the kalirin antibody is shown in Supplemental Figure 1. Membranes were then incubated in LiCor IRDye secondary antibodies (Li-Cor: goat anti-rabbit 800 nm #926-32211; goat anti-mouse 680 nm #926-68020) 1:10,000 in Odyssey Licor Blocking Buffer (Li-Cor # 927-4000) diluted 1:1 with TBS (0.1% Tween 20 + 0.02% SDS). Blots were scanned while wet and bands detected using a Li-Cor Odyssey Infrared Scanner set at a resolution of 42 μm and the highest image quality. Images were quantified using MCID Core Version 7.0 (InterFocus Imaging Ltd., Linton, Cambridge, UK). The peak for each of the 4 isoforms of kalirin and β-tubulin on the output histograms were independently aligned to a single point on the distance axis for all lanes from all blots. Once aligned, a band definition encompassing the full range of each band was applied to all lanes from all blots in the study on the histogram for each protein Supplemental Figure 2. The integrated intensity (mean intensity × number of pixels) was acquired for each protein.
2.4. Statistical Analysis
Demographic, clinical, and pathology variables were compared between groups using chi square tests, ANOVAs, and t-tests where appropriate. When employing linear models and analysis of variance, which are based on the normal distribution, the natural logarithm transformation is standardly used with non-normally distributed data. Although diagnosis groups and quads were matched as closely as possible, they could not be matched perfectly; therefore, potentially relevant biologic variables were included as covariates in all models because they may explain some variation.
2.4.1. Soluble Aϐ
The response variable for soluble Aβ analyses was the natural logarithm of the concentration values (pmol/g tissue) for Aβ1-40 and Aβ1-42. Since the regions were within subjects, the response variables were initially treated as repeated measures. A repeated measures analysis of covariance (RM-ANCOVA) was used to evaluate group differences, with sex, age, PMI, α-synuclein positivity, and Braak stage included as covariates. The numbers of tissue samples with detectable protein levels were not equal across regions, so RM-ANCOVAs did not include data points from subjects that were missing data in any of the regions. To further clarify the regional differences and to incorporate the additional data, follow-up ANCOVA analyses were performed on the regions individually.
2.4.2. Kalirin
Analyses for the expression amounts of kalirin-5, kalirin-7, kalirin-9, and kalirin-12 were conducted independently. The response variable for each of the analyses was the natural logarithm of the ratio of each kalirin isoform expression level to the corresponding expression level of β-tubulin, which did not differ between groups (F1, 30.001 = 0.432, p = 0.516). Four measurements of this ratio were made for each isoform, within each subject (runs with gel artifacts were excluded). Linear mixed models were used for the analyses, with fixed effects as follows: psychotic or not psychotic, sex, age, PMI, Braak stage, and assay run. Aβ1-40 and Aβ1-42 protein concentrations from the DLPFC were also included as fixed effects.
Secondary models were developed including data from a reference group of 4 neuropathologically normal control subjects (3 male, 1 female; 76 ± 14.5 years of age; 4.3 ± 0.5 hours PMI) included to provide directional information for our primary analyses rather than for hypothesis testing.
All tests were two-tailed with α = 0.05.
3. Results
AD+P and AD-P did not differ with regard to sex ( , p = 0.634), α-synuclein score ( , p = 0.600), neuritic plaque rating ( , p = 0.356), or Braak score ( , p = 0.332). The groups also did not differ with regard to age (F1, 50 = 0.007, p = 0.936) or PMI (F1, 50 = 1.933, p = 0.171).
3.1 Soluble Aβ
3.1.1. Aϐ1-40
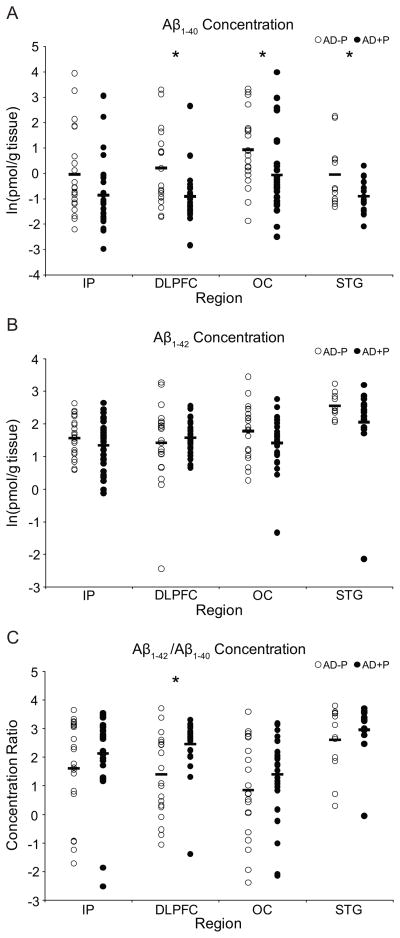
Concentrations of Aβ1-40 were lower in AD+P subjects (Figure 1a, F1, 12 = 8.426, p = 0.013). Aβ1-40 concentrations were not influenced by age (p = 0.614), sex (p = 0.083), PMI (p = 0.743), Braak stage (p = 0.465), or α-synuclein positivity (p = 0.738). Analyses of individual regions found that Aβ1-40 concentrations were reduced by 50% in the DLPFC (F1, 45 = 9.770, p = 0.003), 39% in OC (F1, 47 = 5.394, p = 0.025), 84% in STG (F1, 25 = 5.185, p = 0.032), and were nearly significantly lower in the IP (69%, F1, 49 = 3.903, p = 0.054).
Figure 1.
a. Log-transformed concentrations of Aβ1-40 in the inferior parietal cortex (IP), superior frontal gyrus (DLPFC), occipital cortex (OC), and superior temporal gyrus (STG). b. Log-transformed concentrations of Aβ1-42 in the IP, DLPFC, OC, and STG. c. Log transformations of the concentration ratios of Aβ1-42 and Aβ1-40 in the IP, DLPFC, OC, and STG. Markers represent values or ratios for individual subjects; horizontal bars represent mean values or ratios for each group. * indicates p < 0.05.
Since Aβ1-40 is the predominant species in vascular amyloid deposits (Joachim et al., 1988, Suzuki et al., 1994), we compared CAA ratings (none, mild, moderate, severe) between AD+P and AD-P. CAA ratings did not differ between the groups, , p = 0.144.
3.1.2. Aϐ1-42
Concentrations of Aβ1-42 were not significantly different between AD+P and AD-P subjects (Figure 1b, F1, 13 = 1.479, p = 0.245). Aβ1-42 concentrations were not influenced by age (p = 0.427), sex (p = 0.289), PMI (p = 0.820), Braak stage (p = 0.358), or α-synuclein positivity (p = 0.963). Individual analyses verified that AD+P and AD-P groups had comparable levels of Aβ1-42concentration in the IP (F1, 48 = 1.253, p = 0.269), DLPFC (F1, 47 = 0.391, p = 0.535), OC (F1, 46 = 2.349, p = 0.132), or STG (F1, 25 = 1.867, p = 0.184).
3.1.3. Aϐ1-42/Aϐ1-40 Ratio
The ratios of Aβ1-42/Aβ1-40 were nearly significantly different between AD+P and AD-P (Figure 1c, F1, 12 = 4.396, p = 0.058) in the repeated measures analysis. Ratios were not influenced by age (p = 0.654), sex (p = 0.111), PMI (p = 0.812), α-synuclein positivity (p = 0.854), or Braak stage (p = 0.181). Individual analyses found that the ratio was significantly higher in AD+P DLPFC (F1, 44 = 9.123, p = 0.004), though not in the IP (F1, 48 = 1.364, p = 0.249), the OC (F1, 46 = 1.509, p = 0.226), or the STG (F1, 25 = 0.768, p = 0.389).
3.2 Kalirin isoform expression in the DLPFC
3.2.1. Kalirin-5
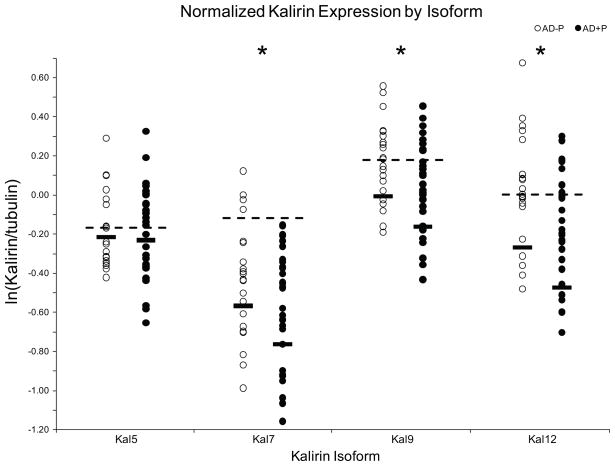
Kalirin expression levels are presented in Figure 2. Kalirin-5 was not significantly changed in AD+P subjects (F1, 28.120 = 0.365, p = 0.550). Expression was not influenced by age (p = 0.353), sex (p = 0.575), PMI (p = 0.591), Braak stage (p = 0.23), Aβ1-40 concentration (p = 0.308), or Aβ1-42 concentration (p = 0.549).
Figure 2.
Log-transformed normalized expression of Kalirin isoforms in the superior frontal gyrus (DLPFC). Markers represent mean values for individual subjects; black horizontal bars represent estimated marginal mean values for each group. Dashed lines represent estimated marginal mean values for control subjects. Marginal means are from models not including Aβ. * indicates p < 0.05.
3.2.2. Kalirin-7
Kalirin-7 was significantly lower in AD+P subjects (11.6%, F1, 27.758 = 6.963, p = 0.013). Expression was not influenced by sex (p = 0.223), PMI (p = 0.785), Aβ1-40 concentration (p = 0.710), or Aβ1-42 concentration (p = 0.253). Age (p = 0.024, Supplemental Figure 3) and Braak stage (p = 0.023, Supplemental Figure 4) did have significant effects, with a slight increase with increasing age and a decrease with increasing Braak score.
3.2.3. Kalirin-9
Kalirin-9 was significantly lower in AD+P subjects (12.1%, F1, 21.588 = 5.923, p = 0.024). Sex (p = 0.946), PMI (p = 0.735), Braak stage (p = 0.197), Aβ1-40 concentration (p = 0.339), and Aβ1-42 (p = 0.439) did not have significant effects. Age had a nearly significant effect (p = 0.056), showing a slight increase with increasing age.
3.2.4. Kalirin-12
Kalirin-12 was significantly lower in AD+P subjects (17.8%, F1, 27.056 = 6.516, p = 0.017). Sex (p = 0.985), PMI (p = 0.953), Braak stage (p = 0.108), Aβ1-40 concentration (p = 0.304), and Aβ1-42 (p = 0.502) did not produce significant effects. Effect of age was significant (p = 0.012), with a slight increase with increasing age.
4. Discussion
In the present study, we explored a model of AD pathology and synaptic vulnerability in AD+P. We found an increased ratio of Aβ1-42/Aβ1-40 in the DLPFC in AD+P, driven largely by reduced concentrations of Aβ1-40 peptide. Although we identified a significantly increased ratio only in the DLPFC in our study, we did detect lower Aβ1-40 across the regions we studied, and a nonsignificant increase in the Aβ1-42/Aβ1-40 ratio in these regions (Figure 1c). In addition to these findings, we found that kalirin-7, -9, and -12 isoforms were also expressed at lower levels in the DLPFC in AD+P.
4.1 Lower Aβ1-40 may enhance the toxicity of Aβ1-42
Emerging data is illuminating the relative contributions of soluble Aβ1-40 and Aβ1-42 to synaptic pathology in AD. Although Aβ1-40 has been shown to produce harmful effects in vitro and in vivo, and it has been shown that, similar to Aβ1-42 species, increased concentrations of Aβ1-40 forms correlate with impaired cognition (Näslund et al., 2000) in the same brain regions examined in the present study. However Aβ1-40 forms do not produce insoluble fibrils as readily as Aβ1-42, and this peptide is not as potent a synaptotoxin as Aβ1-42. Recent studies indicate that Aβ1-40 may even serve to ameliorate the effects of Aβ1-42. PSD-95 protein was reduced in cell culture after application of both Aβ1-40 (Roselli et al., 2005) and Aβ1-42 (Almeida et al., 2005). ICV-administered Aβ1-40 reduced the duration of LTP over time in the rat hippocampus (Cullen et al., 1997); however, Aβ1-42 had a comparable effect at only 2.5% of the Aβ1-40 concentration (0.01 nM vs 0.4 nM). Aβ1-40 has been shown to inhibit the oligomerization of Aβ1-42 into more toxic species by sequestering it into stable mixed tetramers (Murray et al., 2009). In the Swedish APP mutation mouse model, an increased Aβ1-42/Aβ1-40 ratio was identified in conjunction with impairments in LTP and reduced hippocampal spine density, before overt plaque deposition or increase in overall amyloid levels (Jacobsen et al., 2006). Conversely, increased expression of Aβ1-40 relative to Aβ1-42 abolished the premature death rate normally observed in Aβ1-42-overexpressing transgenic mice (Kim et al., 2007). Taken as a whole, these data suggest that the net effect of lower Aβ1-40 and an increased ratio of Aβ1-42/Aβ1-40 in the DLPFC may be overall enhancement of Aβ toxicity in AD+P. This is consistent with the observations by Kuperstein and colleagues (2010) who reported that the Aβ1-42/Aβ1-40 ratio is more critical for neurotoxicity compared to the individual peptide concentration, and that even slight increases in this ratio promote synaptotoxic oligomer species (Kuperstein et al., 2010).
Increasing evidence indicates that it is soluble and not insoluble forms of Aβ that primarily contribute to synapse impairment and loss in AD (Selkoe, 2002) and that plaques composed of insoluble Aβ may serve as deposits or reservoirs of soluble oligomers (Koffie et al., 2009). However, several lines of evidence indicate that our findings of reduced soluble Aβ1-40 do not simply result as an artifact of increased aggregation into plaques. First, the individuals in our cohort all had similarly high plaque burdens. Additionally, it is Aβ1-42, not Aβ1-40, that is the principal alloform present in plaques (Gravina et al., 1995). Finally, previous studies have not identified an association between psychosis in AD and Aβ plaque pathology (Farber et al., 2000, Sweet et al., 2000).
4.2 Lower kalirin may confer a synaptic vulnerability in AD+P
Our findings that kalirin-7, -9, and -12 proteins are lower in AD+P than in AD-P subjects are consistent with the interpretation of an increased synaptic vulnerability and subsequent synaptotoxicity in AD+P. An important question is whether kalirin downregulation is a consequence of Aβ-driven synapse loss, or contributes separately to synaptic pathology in AD+P. We found reductions in kalirin-7, kalirin-9, and kalirin-12 in our subjects, but not in kalirin-5. Kalirin isoforms are present in post-synaptic density fractions of rodent and human cortex (Deo et al., 2011, Penzes et al., 2000); thus, the relative preservation of kalirin-5 in our AD+P subjects suggests that lower kalirin-7, kalirin-9, and kalirin-12 is unlikely to be due to confounding by greater spine loss in AD+P. Similarly, the reductions in expression of kalirin-7, kalirin-9, and kalirin-12 persisted after controlling for Aβ concentrations in our analyses, suggesting they did not arise solely as a consequence of Aβ toxicity, but rather may make independent pathogenic contributions to this deficit.
Kalirin plays an integral role in dendritic spine growth, morphogenesis, and activity-dependent plasticity. NMDA receptor activation leads to kalirin-7 phosphorylation, which is necessary for activity-dependent spine targeting of AMPA receptors and Rac1-dependent spine enlargement (Xie et al., 2007). A Rac1 substrate, p21-activated kinases (PAK), is critical to regulation of actin assembly in dendritic spines (Penzes et al., 2003, Zhao et al., 2006), implicating a direct downstream effect of reduced kalirin on cytoskeleton remodeling in spines (Penzes et al., 2011, Penzes and VanLeeuwen, 2011). Both kalirin-9 and kalirin-12 are involved in dendritic development (May et al., 2002), although the functions of kalirin-9 and kalirin-12 in synaptic signaling and the structural integrity of dendritic spines are still unclear. Kalirin KO mice, in which all kalirin species are eliminated, have reduced cortical spine density, impaired glutamatergic transmission, and associated working memory deficits (Cahill et al., 2009). Aβ1-42 oligomer-mediated dendritic spine loss occurs, at least in part, in an NMDA receptor-dependent manner (Shankar et al., 2007). It is thus plausible that the deleterious effects of Aβ on dendritic spines, coupled with a synaptic vulnerability conferred by less kalirin, may produce additive or synergistic synaptotoxic effects in AD+P.
A significant strength of this study was our cohort of subjects. The individuals were followed and evaluated during the progression of their disease, allowing for an extensive characterization of late-life behavior and cognition with corresponding postmortem analysis. Subjects were matched on a range of variables, including age, illness duration, PMI, Braak stage, and neuritic plaque severity so as to ensure that the identified postmortem correlates of psychosis were not due to having sampled AD+P subjects with more advanced pathologies than AD-P subjects, but rather to a discrete pathogenic process. Importantly, despite matching on these measures, MMSE scores were lower in the AD+P group (t50 = 2.418, p = 0.019), indicating a more severe trajectory of decline in the AD+P subjects. Since synapse loss is strongly correlated with cognitive decline in AD (DeKosky and Scheff, 1990, Scheff and Price, 2006, Terry et al., 1991, Walsh and Selkoe, 2004), this finding lends further support to our proposition of kalirin-mediated synaptic vulnerability in AD+P.
Similar to AD+P, presentation of Dementia with Lewy Bodies (DLB) may include the presence of hallucinations and delusions (McKeith et al., 2004). Though our cohort excluded individuals with isolated DLB, our subjects were matched on the presence or absence of α-synuclein aggregates to control for any potential contribution of comorbid DLB pathology in these AD cases. However, since this was an examination of postmortem tissue, it was impossible to account for all possible variables that may have influenced the findings. We corrected for the strongest and most obvious influences, such as the variables just mentioned both through subject groupings and in the statistical analyses.
5. Conclusion
We found reduced concentrations of soluble Aβ1-40, an increased ratio of soluble Aβ1-42/Aβ1-40 and reduced kalirin-7, -9, and -12 protein expression in AD+P. These findings suggest that decreased Aβ1-40 may be an underlying factor in the development of psychosis in AD. Lower Aβ1-40 may serve to enhance the deleterious effects of Aβ1-42, promoting the dendritic spine loss that is exaggerated in AD+P. Lower kalirin expression may further enhance this effect, with spines more sensitive to Aβ and less responsive to activity-dependent maintenance and growth. While our study did not directly establish a link between soluble Aβ and kalirin, we did describe a profile of protein expression in a large cohort of AD subjects with and without psychosis that were matched on a series of demographic, clinical, and pathologic variables. These findings provide a foundation for identifying a novel pathway in the pathogenesis of AD+P; an increased Aβ1-42/Aβ1-40 ratio driven by lower Aβ1-40, coupled with reduced kalirin, isolates additive and potentially related processes that may underlie the enhanced synaptic disruption in AD+P.
Supplementary Material
Supplemental Figure 1. Western blot demonstrating specificity of the anti-Kalirin-spectrin antibody. Bands corresponding to predicted molecular weights of Kal5, Kal7, Kal9 and Kal12 are identifiable in gray matter extracts from wild type mouse, non-human primate, and human, but not in extracts from Kalirin knockout mice (Deo et al., 2011).
Supplemental Figure 2. a. Representative western blot of Kalirin protein expression in Alzheimer disease with psychosis (AD+P) and without psychosis (AD-P). Band below Kal7 is nonspecific. b. Histograms with band intensity for each of that Kalirin isoforms and tubulin.
Supplemental Figure 3. Scatter plots of the natural log ratio of Kalirin to tubulin expression versus age, for each isoform. Markers represent individuals, empty markers signify Alzheimer disease without psychosis (AD-P) and solid markers signify Alzheimer disease with psychosis (AD+P).
Supplemental Figure 4. Scatter plots of the natural log ratio of Kalirin to tubulin expression versus Braak stage, for each isoform. Markers represent individuals, empty markers signify Alzheimer disease without psychosis (AD-P) and solid markers signify Alzheimer disease with psychosis (AD+P).
Acknowledgments
Funding: This work was supported by the Veterans Health Administration [5I01BX000452 to R.A.S]; and the National Institute on Aging [5P01AG014449 to M.D.I, 5P50AG005133 to O.L.L., 5R01AG027224 to R.A.S.]. P.S.M. is supported by the National Institute of Mental Health [5T32MH019986].
We wish to thank the staff of the Alzheimer Disease Research Center and the Translational Neuroscience Program at the University of Pittsburgh. We also wish to thank Mrs. Mary Brady for assistance with the figures. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute of Mental Health, or the National Institutes of Health.
Abbreviations
- AD
Alzheimer disease
- AD+P
Alzheimer disease with psychosis
- AD-P
Alzheimer disease without psychosis
- DLPFC
dorsolateral prefrontal cortex
- Aβ
β-amyloid
- LTP
long-term potentiation
- CAA
cerebral amyloid angiopathy
- PMI
postmortem interval
- SF
superior frontal gyrus
- IP
inferior parietal cortex
- STG
superior temporal gyrus
- OC
occipital cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam MR, Johnson RC, Darlington DN, Hand TA, Mains RE, Eipper BA. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine -amidating monooxygenase, an integral membrane peptide-processing enzyme. J Biol Chem. 1997;272(19):12667–12675. doi: 10.1074/jbc.272.19.12667. [DOI] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20(2):187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13(7):624–627. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131(9):2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD, Disterhoft JF. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106(31):13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione RA, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8(2):216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65(6):592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by amyloid precursor protein fragments. Neuroreport. 1997;8(15):3213–3217. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Deo AJ, Cahill ME, Li S, Goldszer I, Henteleff R, VanLeeuwen JE, Rafalovich I, Gao R, Stachowski EK, Sampson AR. Increased Expression of Kalirin-9 in the Auditory Cortex of Schizophrenia Subjects: Its Role in Dendritic Pathology. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.11.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel JE, Lopez OL, Houck PR, Becker JT, Weamer EA, DeMichele-Sweet MAA, Kuller L, Sweet RA. Trajectory of Cognitive Decline as a Predictor of Psychosis in Early Alzheimer Disease in the Cardiovascular Health Study. Am J Geriatr Psychiatry. 2011;19(2):160–168. doi: 10.1097/JGP.0b013e3181e446c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB, Rubin EH, Newcomer JW, Kinscherf DA, Miller JP, Morris JC, Olney JW, McKeel DW., Jr Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry. 2000;57(12):1165–1173. doi: 10.1001/archpsyc.57.12.1165. [DOI] [PubMed] [Google Scholar]
- Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Younkin LH, Suzuki N, Younkin SG. Amyloid beta protein (A beta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43) J Biol Chem. 1995;270(13):7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Helmin K, Tsai J, Gan WB. Various Dendritic Abnormalities Are Associated with Fibrillar Amyloid Deposits in Alzheimer’s Disease. Ann N Y Acad Sci. 2007;1097(1):30–39. doi: 10.1196/annals.1379.003. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103(13):5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim CL, Duffy LK, Morris JH, Selkoe DJ. Protein chemical and immunocytochemical studies of meningovascular [beta]-amyloid protein in Alzheimer’s disease and normal aging. Brain Res. 1988;474(1):100–111. doi: 10.1016/0006-8993(88)90673-7. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5 -and 3 -ends along with an internal translational initiation site. J Biol Chem. 2000;275(25):19324–19333. doi: 10.1074/jbc.M000676200. [DOI] [PubMed] [Google Scholar]
- Kashani A, Lepicard, Poirel O, Videau C, David J, Fallet-Bianco C, Simon A, Delacourte A, Giros B, Epelbaum J. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol Aging. 2008;29(11):1619–1630. doi: 10.1016/j.neurobiolaging.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27(3):627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM. Oligomeric amyloid associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106(10):4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrla KJ, Chacko RC, Harper RG, Jhingran S, Doody R. SPECT findings on psychosis in Alzheimer’s disease. Am J Psychiatry. 1995;152(10):1470–1475. doi: 10.1176/ajp.152.10.1470. [DOI] [PubMed] [Google Scholar]
- Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V. Neurotoxicity of Alzheimer’s disease A peptides is induced by small changes in the A 42 to A 40 ratio. The EMBO journal. 2010;29(19):3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Wisniewski SR, Becker JT, Boller F, DeKosky ST. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Arch Neurol. 1999;56(10):1266–1272. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M. Factors critical for the plasticity of dendritic spines and memory storage. Neurosci Res. 2007;57(1):1–9. doi: 10.1016/j.neures.2006.09.017. [DOI] [PubMed] [Google Scholar]
- May V, Schiller MR, Eipper BA, Mains RE. Kalirin Dbl-homology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J Neurosci. 2002;22(16):6980–6990. doi: 10.1523/JNEUROSCI.22-16-06980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H. Dementia with Lewy bodies. Lancet Neurol. 2004;3(1):19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69(2):167–171. doi: 10.1136/jnnp.69.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Van Belle G, Berg L. The consortium to establish a registry for Alzheimer’s disease (CERAD) Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of A 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Bernstein SL, Nyugen V, Condron MM, Teplow DB, Bowers MT. Amyloid protein: Aβ40 inhibits Aβ42 oligomerization. J Am Chem Soc. 2009;131(18):6316–6317. doi: 10.1021/ja8092604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid -peptide in the brain and cognitive decline. JAMA: The Journal of the American Medical Association. 2000;283(12):1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Salmon D, Thal L, Romero R, Weisstein–Jenkins C, Galasko D, Hofstetter C, Thomas R, Grant I, Jeste D. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965–1971. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37(2):263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J Biol Chem. 2000;275(9):6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- Penzes P, VanLeeuwen JE. Impaired regulation of synaptic actin cytoskeleton in Alzheimer’s disease. Brain Res Rev. 2011;67(1–2):184–192. doi: 10.1016/j.brainresrev.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Jr, Kaye JWW, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7(2):103–118. doi: 10.3233/jad-2005-7203. [DOI] [PubMed] [Google Scholar]
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162(11):2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, Morabito M, Almeida O. Soluble -amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J Neurosci. 2005;25(48):11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, Sarazin M, Devanand D, Honig L, Marder K. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid- protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27(11):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA. Amyloid- protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff RT, Shanks MF, Macintosh L, Pestell SJ, Gemmell HG, Venneri A. Delusions in Alzheimer’s disease: spet evidence of right hemispheric dysfunction. Cortex. 1999;35(4):549–560. doi: 10.1016/s0010-9452(08)70818-9. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, Mahler ME, Mandelkern MA, Cummings JL, Van Gorp WG, Hinkin CH, Berisford MA. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1995;7(4):476–484. doi: 10.1176/jnp.7.4.476. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Iwatsubo T, Odaka A, Ishibashi Y, Kitada C, Ihara Y. High tissue content of soluble beta 1-40 is linked to cerebral amyloid angiopathy. Am J Pathol. 1994;145(2):452–460. [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Bennett DA, Graff-Radford NR, Mayeux R. Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain. 2010;133(4):1155–1162. doi: 10.1093/brain/awq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Butters MA, Mulsant BH, Pollock BG, Lewis DA, Lopez OL, DeKosky ST, Reynolds CF. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29(12):2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Healy MT, Wisniewski SR, Henteleff R, Pollock BG, Lewis DA, DeKosky ST. Alterations of striatal dopamine receptor binding in Alzheimer disease are associated with Lewy body pathology and antemortem psychosis. Arch Neurol. 2001;58(3):466–472. doi: 10.1001/archneur.58.3.466. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Lopez OL, Klunk WE, Wisniewski SR, Kaufer DI, Healy MT, DeKosky ST. Psychotic symptoms in Alzheimer’s disease are not associated with more severe neuropathologic features. Int Psychogeriatr. 2000;12(4):547–558. doi: 10.1017/s1041610200006657. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Devlin B, Lopez OL, DeKosky ST. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002a;58(6):907–911. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Panchalingam K, Pettegrew JW, McClure RJ, Hamilton RL, Lopez OL, Kaufer DI, DeKosky ST, Klunk WE. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002b;23(4):547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, Blazina L, Teri L, Rubin E, Mortimer JA. The behavior rating scale for dementia of the consortium to establish a registry for Alzheimer’s disease. The behavioral pathology committee of the consortium to establish a registry for Alzheimer’s disease. Am J Psychiatry. 1995;152(9):1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44(1):181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA. Soluble oligomers of [beta] amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924(2):133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chawla F, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE. Selective vulnerability of dentate granule cells prior to amyloid deposition in PDAPP mice: digital morphometric analyses. Proc Natl Acad Sci U S A. 2004;101(18):7141–7146. doi: 10.1073/pnas.0402147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56(4):640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HS, Jeoung MK, Koo YB, Ji H, Markesbery WR, Ji I, Ji TH. Kalirin is under-expressed in Alzheimer’s disease hippocampus. J Alzheimers Dis. 2007a;11(3):385–397. doi: 10.3233/jad-2007-11314. [DOI] [PubMed] [Google Scholar]
- Youn HS, Ji I, Ji HP, Markesbery WR, Ji TH. Under-expression of kalirin-7 increases iNOS activity in cultured cells and correlates to elevated iNOS activity in Alzheimer’s disease hippocampus. J Alzheimers Dis. 2007b;12(3):271–281. doi: 10.3233/jad-2007-12309. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nature. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Western blot demonstrating specificity of the anti-Kalirin-spectrin antibody. Bands corresponding to predicted molecular weights of Kal5, Kal7, Kal9 and Kal12 are identifiable in gray matter extracts from wild type mouse, non-human primate, and human, but not in extracts from Kalirin knockout mice (Deo et al., 2011).
Supplemental Figure 2. a. Representative western blot of Kalirin protein expression in Alzheimer disease with psychosis (AD+P) and without psychosis (AD-P). Band below Kal7 is nonspecific. b. Histograms with band intensity for each of that Kalirin isoforms and tubulin.
Supplemental Figure 3. Scatter plots of the natural log ratio of Kalirin to tubulin expression versus age, for each isoform. Markers represent individuals, empty markers signify Alzheimer disease without psychosis (AD-P) and solid markers signify Alzheimer disease with psychosis (AD+P).
Supplemental Figure 4. Scatter plots of the natural log ratio of Kalirin to tubulin expression versus Braak stage, for each isoform. Markers represent individuals, empty markers signify Alzheimer disease without psychosis (AD-P) and solid markers signify Alzheimer disease with psychosis (AD+P).