Abstract
Cerebrovascular inflammation contributes to diverse central nervous system (CNS) disorders through mechanisms that are incompletely understood. The recruitment of neutrophils to the brain can contribute to neurotoxicity, particularly during acute brain injuries such as cerebral ischaemia, trauma and seizures. However, the regulatory and effector mechanisms that underlie neutrophil-mediated neurotoxicity are poorly understood. Here we show that mouse neutrophils are not inherently toxic to neurons but that trans-endothelial migration across IL-1 stimulated brain endothelium triggers neutrophils to acquire a neurotoxic phenotype that causes rapid death of cultured neurons. Neurotoxicity was induced by addition of transmigrated neutrophils or conditioned medium taken from transmigrated neutrophils to neurons, and was partially mediated by excitotoxic mechanisms and soluble proteins. Transmigrated neutrophils also released de-condensed DNA associated with proteases, which are known as neutrophil extracellular traps (NETs). The blockade of histone-DNA complexes attenuated transmigrated neutrophil-induced neuronal death, whilst the inhibition of key neutrophil proteases in the presence of transmigrated neutrophils rescued neuronal viability. We also show that neutrophil recruitment in the brain is IL-1 dependent and release of proteases and de-condensed DNA from recruited neutrophils in the brain occurs in several in vivo experimental models of neuroinflammation. These data reveal new regulatory and effector mechanisms of neutrophil-mediated neurotoxicity, namely the release of proteases and de-condensed DNA triggered by phenotypic transformation during cerebrovascular transmigration. Such mechanisms have important implications for neuroinflammatory disorders, notably in the development of anti-leukocyte therapies.
Keywords: neutrophil, neurotoxicity, cerebrovascular, neuroinflammation, extracellular trap, de-condensed DNA, cytokine, transmigration
Introduction
Cerebral ischaemia and other central nervous system (CNS) disorders induce a potent central and systemic inflammatory response. A key hallmark of CNS inflammation is the mobilisation and recruitment of inflammatory cells into the brain and breakdown of the blood-brain barrier (BBB), leading to increased neuronal loss (1, 2). Neutrophils appear in the brain within hours of an ischaemic event, adhering to activated blood vessels or migrating to the parenchyma, which is increased under systemic inflammatory conditions (3, 4). However, mechanisms of neutrophil activation and recruitment, and their contribution to neuroinflammation and brain injury are poorly understood.
The recruitment and migration of neutrophils during inflammation and infection is key for the subsequent activation of several inflammatory events (5). Primed neutrophils are able to activate T-cells and secrete potent chemoattractants including leukotrienes (6). Activated neutrophils also produce chemokines, cytokines, proteases and reactive oxygen species (ROS) (6), all of which could be detrimental to the surrounding healthy tissue of the host.
In peripheral tissues, migration of neutrophils to sites of inflammation reportedly changes their phenotype. Transmigration across activated endothelium in vivo is known to induce an increase in ROS production and degranulation of neutrophils (7). Further work investigating in vivo transmigration has shown the involvement of complex intravascular chemotactic gradients, which guide transmigrated neutrophils to the site of sterile injury (8).
We have shown that cerebral ischaemia triggers rapid neutrophil activation and release from the bone marrow (9). The infiltration of activated neutrophils to peripheral tissues is relatively well documented (10, 11), but much less is known as to whether neutrophils undergo phenotypic and functional changes upon their recruitment to the brain.
We have shown that the pro-inflammatory cytokine interleukin-1 (IL-1), a key mediator of neuroinflammation, exacerbates ischaemic damage via neutrophil-dependent mechanisms leading to increased BBB breakdown and subsequent neuronal injury (4, 12). Neutrophils exert toxicity to neuronal cell cultures within 24 to 72 h in vitro (13-15), indicating that these cells are likely to deliver neurotoxic products to the brain upon migration in response to cerebrovascular inflammatory changes in vivo.
It is not known whether cerebrovascular extravasation makes neutrophils acquire a neurotoxic phenotype and if so, whether it happens rapidly enough to contribute to acute brain injury. The aim of this study therefore was to test the hypothesis that IL-1-induced cerebrovascular transmigration triggers neutrophils to acquire a neurotoxic phenotype. We show here that trans-endothelial migration of neutrophils across cerebral endothelium critically alters neutrophil phenotype to a neurotoxic state, and that neurotoxicity of transmigrated neutrophils is mediated via rapid release of a cocktail of active proteases associated with de-condensed DNA, referred to as neutrophil extracellular traps (NETs). NETs contribute to the defence against extracellular bacteria (16), but actions in the brain have not been previously described. Collectively, our data identify novel trigger and effector mechanisms of neutrophil-mediated neurotoxicity and highlight how a key neutrophil anti-microbial strategy can also be damaging to host tissue.
Materials and methods
Animals
Wild type (WT) and IL-1α/β deficient (IL-1α/β−/−) mice, all on a C57BL/6 background, were bred in-house and maintained on a 12 h light/dark cycle. Sprague-Dawley rats were purchased from Charles River (UK). Animal studies were performed under United Kingdom Home Office personal and project licenses, and protocols adhered to the Animals (Scientific Procedures) Act (1986).
Focal cerebral ischaemia and other models of neuroinflammation
Focal cerebral ischaemia was induced by transient (60 min) middle cerebral artery occlusion (MCAo) as described previously (4, 17). After MCAo, mice were subjected to 8 h or 24 h reperfusion. Some animals undergoing MCAo were injected with vehicle or rat recombinant IL-1β (National Institute for Biological Standards and Controls, NIBSC, UK) (intraperitoneal injection, 100 IU, 30 min before MCAo) as described previously (12). Lipopolysaccharide (LPS, 4 μg, Sigma, UK) was injected into the striatum of mice to induce parenchymal inflammation. Intrastriatal co-administration of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA, 15 nM/μl, Tocris, UK) and human IL-1β (5 ng/μl, NIBSC) (2 min infusion at 0.5 μl/min) was carried out as described previously to induce striatal and cortical damage in rats (18-20).
Primary mouse brain endothelial cell culture
Primary cultures of mouse brain endothelial cells (MBEC) were prepared from the brains of 8-12 week old C57BL/6 mice as described previously (21) with the following modifications: Isolated brain vessels were resuspended in maintenance medium consisting of Dulbecco’s modified Eagle medium (DMEM) F-12 (Invitrogen, UK), 10 % plasma-derived serum (PDS)(FirstLink, UK), 10 % fetal calf serum (FCS), 100 μg/ml endothelial cell growth supplement (BD Biosciences, Oxford, UK), 100 μg/ml heparin, 2 mM glutamine, 1 U/ml penicillin and 100 μg/ml streptomycin. For neutrophil migration assays, microvessels were plated onto murine collagen-IV (50 μg/ml, BD Biosciences, UK) -coated 24-well cluster Transwell® inserts (6.5 mm diameter, 3.0 μm pore, Corning, UK). Puromycin (3 μg/ml) was added to the media for three days in vitro. Cultures were grown at 37 °C in a 5 % CO2 humidified atmosphere, and were used after 14 days in vitro, once cells were confluent. Cultures were 95.8±1.6 % positive for platelet endothelial cell adhesion molecule-1 (PECAM-1) and expressed endothelial markers Von Willebrand Factor (vWF) and zona occludens-1 (ZO-1) (not shown).
bEnd.5 mouse brain endothelial cell line cultures
The bEnd.5 mouse brain endothelioma cell line, which closely resembles primary brain endothelium in culture (4) was purchased from the Health Protection Agency Culture Collections (Salisbury, UK). Cells were grown in DMEM (high glucose, 4.5 g/L, Invitrogen, UK), supplemented with 10 % FCS, 1% non-essential amino acid, 2 mM glutamine, 1 U/ml penicillin and 100 μg/ml streptomycin.
Primary cortical neuronal cell cultures
Primary, cortical neuronal cell cultures were prepared from embryonic (day 15) mice as described previously (7). Cells were seeded at 180,000 cells/cm2 and grown in Neurobasal Medium (Invitrogen, UK), 2% B27 minus anti-oxidants (Invitrogen), 5% PDS, 2 mM glutamine, 1 U/ml penicillin and 100 μg/ml streptomycin . Neurons were used between 10-13 days in vitro. Cultures were on average 98 % pure neurons (data not shown).
Collection of naïve neutrophils
Freshly isolated neutrophils (termed naïve neutrophils) were collected from 8 to 12 week-old mice euthanized by CO2 inhalation. Femurs and tibias were removed, and bone marrow flushed using a 25G needle with 1-2 ml neutrophil buffer [PBS without calcium or magnesium with 0.1% low endotoxin BSA (w/v) and 1 mM EDTA] (Sigma, UK). Red blood cells were lysed with 0.2 % (w/v) NaCl, and osmolarity was restored by the addition of 1.2 % (w/v) NaCl. Ly6G-positive neutrophils were separated immunomagnetically by passing the previously labelled cell suspension through an LS column and magnet (Miltenyi, UK). The column was removed from the magnet and the eluted cells were washed and resuspended in serum-free neurobasal medium at 4 × 106 cells/ml. The average purity of neutrophil preparations was 96%, as identified by flow cytometry (see below).
Neutrophil trans-endothelial migration assay
MBEC or bEnd.5 cells grown to confluence on Transwell® inserts were pre-treated for 4 h with rat IL-1β (100 ng/ml; R&D Systems, UK) in the absence or presence of human IL-1 receptor antagonist (IL-1Ra, 100 μg/mL, R&D Systems). Following treatment, cells were washed twice with fresh medium and transferred to fresh tissue culture plates. Purified neutrophil suspensions of 2 × 105 were added to the luminal (top) compartment of each 24-well Transwell®. After an incubation period of 24 h the abluminal transmigrated fraction of neutrophils (termed transmigrated neutrophils) was collected, centrifuged at 400 g for 10 min, and cells were counted using a haemocytometer. Neutrophil transmigration was expressed as fold increase compared to vehicle-treated (control) cultures.
Collection of transmigrated neutrophils
To obtain transmigrated neutrophils in sufficient quantities in order to analyse their phenotypes, we collected neutrophils which had migrated across IL-1β-stimulated brain endothelium grown on larger 6-well format Transwell® inserts (4.7 cm2 area per Transwell®). For this purpose, and due to low yields of MBEC primary cultures, we used the bEnd.5 cell line to support neutrophil transmigration. For this trans-endothelial migration using larger Transwell® inserts, a concentration of IL-1β of 10 ng/ml for 4 h was used. This concentration of IL-1β induced a similar increase in neutrophil transmigration across bEnd.5 cells as observed with 100 ng/ml (Supplementary Fig. 1a) and it would also reduce the possibility of IL-1 carried over after activation. This allowed us to determine the effects of activated versus non-activated endothelial-derived factors on non-migrated neutrophil phenotypes. A purified neutrophil suspension totalling 3.5 × 106 cells was added to the luminal (top) compartment of each 6-well Transwell®. After the specified incubation period the abluminal transmigrated fraction of neutrophils (termed transmigrated neutrophils) was collected, centrifuged at 400 g for 10 min. For the direct addition of neutrophils to neuronal cultures, transmigrated neutrophils were collected from the abluminal compartments 4 h after application of naïve neutrophils to the luminal compartment. All non-migrated neutrophil controls were exposed to bEnd.5 cells which had also been treated previously with vehicle or IL-1β (10 ng/ml) for 4 h, in the abluimnal compartment of the Transwell® insert. Previous studies have shown that a period of greater than 1 h is sufficient to allow neutrophils to respond to endothelial-derived factors (13). In addition, naïve neutrophils were also incubated for 4 h in the presence of conditioned medium obtained from activated endothelium, washed and incubated for 4 h. For the addition of neutrophil conditioned medium to neuronal cultures, transmigrated neutrophils were collected 20 h after addition of naïve neutrophils to the luminal compartment. This time-point allowed collection of sufficient neutrophils for subsequent analysis.
To induce neutrophil transendothelial migration outside the brain in vivo, a model of thioglycollate-induced peritonitis was used. Thioglycollate medium (3%) aged for 1 month was injected into the peritoneal cavity and neutrophils recovered by peritoneal lavage 4h after injection. Contaminating red blood cells were lysed, neutrophils washed and resuspended in neurobasal medium for addition to neurons.
Collection of neutrophil lysates and neutrophil-conditioned medium
To determine the effects of neutrophil secreted factor(s) on neurons, neutrophil-conditioned medium was collected by re-suspending neutrophils in serum-free neurobasal medium at 1.2 × 106 cells/ml, and seeding into tissue culture plates for 3 h. Cells were collected, centrifuged at 400 g for 10 min, and pellets were lysed in lysis buffer containing protease inhibitors. Supernatants were further centrifuged at 10,000 g for 10 min. Lysates and cleared supernatants were aliquoted and stored at −80 °C.
Treatment of neurons with neutrophils or neutrophil conditioned medium
Direct application of neutrophils onto primary neuronal cultures was performed by adding 10 μl of age-matched naive neutrophils, non-migrated neutrophils (using non-activated endothelium, activated endothelium or neutrophils which were also incubated for 4 h in the presence of conditioned medium obtained from activated endothelium, washed and incubated for 4 h) or transmigrated neutrophils (suspended at 4 × 106 cells/ ml) to neurons grown in 96-well plates, corresponding to 120,000 neutrophils/cm2 of neurons. Neutrophil-conditioned medium was added to neuronal cultures at a 1:4 dilution (one quarter of neuronal media was removed and replaced with neutrophil-conditioned medium). Neurons were treated with 10 μM MK-801 (Tocris, UK), 10 μM cathepsin-G inhibitor (Calbiochem, UK), 10 μM elastase IV inhibitor (Calbiochem, UK), 20 μM aprotinin (Sigma, UK), or 12.5 μM metalloproteinase-9 (MMP-9) inhibitor (SB3CT, Enzo, UK) added 20 min prior to treatment with neutrophil-conditioned medium. Neutrophil-conditioned medium was also pre-treated with washed trypsin-agarose beads (1U, 30 min at 37°C) (Sigma, UK), or was heat-inactivated for 30 min at 95°C, prior to application to neurons. PL2-3 monoclonal anti-mouse antibody (anti-H2A-H2B-DNA) or an IgG2a isotype control (22, 23) was pre-incubated (15 μg/ml) with pre-chilled neutrophil-conditioned medium for 10 min before addition to neurons. Neutrophil conditioned medium was also pre-treated in the presence or absence of DNase I (Invitrogen, UK) at concentration of 30U/ml for 30 min at 37°C before being added to neuronal cultures (as described above) for a further incubation of 24 h.
Live cell imaging
Neurons were loaded with 1 μM CellTracker Red (Invitrogen) for 30 min and washed into imaging buffer: 121 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 6 mM NaHCO3, 5.5 mM glucose, 25 mM HEPES, pH 7.3. Neurons were imaged every 30 s in the absence or presence of transmigrated neutrophils (neutrophils added to neuronal cultures at 120,000 cells/cm2) on transmitted light channel and on Alexa 594 red fluorescent channel using the BD Pathway Bioimager (BD Biosciences, UK). Live cell microscopy was performed locally (see http://www.ls.manchester.ac.uk/research/facilities/bioimaging/). All offline analysis of images and movies were processed using ImageJ software (http://rsb.info.gov/ij/). Movies (6 h of footage) are shown at 15 frames/s.
Immunohistochemistry
Tissue processing for immunohistochemistry was performed as described previously (4, 17). Antibodies used were goat anti-ICAM-1 (1:500, R&D Systems), goat anti-VCAM-1 (1:500, R&D Systems, mouse anti-fibronectin, (1:100, Sigma, UK), rabbit anti-glial fibrillary acidic protein (GFAP) 1:500, (Abcam, UK) rabbit anti-neutrophil elastase (1:500, Abcam), chicken anti-protein gene product 9.5 (PGP 9.5) (1:500, Abcam, UK), mouse anti-microtubule associated protein 2 (MAP2) (1:500, Sigma, UK), mouse anti-H2A-H2B-DNA complex (PL2-3) (1:1000, Temple University), rat anti-CD45 (1:500, Serotec) and rabbit anti-granulocyte serum, SJC (1:5000, kindly provided by Drs. Daniel Anthony and Sandra Campbell, University of Oxford, UK). Biotinylated tomato lectin was purchased from Sigma. . Endogenous peroxidase activity was blocked with 0.3 % H2O2 in methanol, and non-specific binding sites were blocked with 10% normal serum (Vector Laboratories, UK). Sections were incubated in primary antibody (diluted in 5 % normal serum in PBS) overnight at 4 °C. For peroxidase-based staining, sections were incubated with anti-rabbit biotinylated secondary IgG (1:200 in PBS; Vector Laboratories) before incubation in Vectastain ABC solution (Vector Laboratories) and development of staining by diaminobenzidine (DAB) reaction (Vector Laboratories). Sections were lightly counterstained with cresyl violet. The number of SJC-positive cortical neutrophils was determined in the hemisphere ipsilateral to MCAo at four coronal levels (1.1, 0.2, -0.5, -1.1 mm relative to bregma), and the mean was calculated. For double-labelling immunofluorescence, following primary antibody incubation, sections were incubated with Alexa Fluor-conjugated secondary antibodies (Invitrogen) (1:1000 in PBS) and mounted with ProLong Gold with or without DAPI counterstain (Invitrogen). Brightfield images were collected on a Zeiss Axioskop upright microscope and captured using Zeiss Axiovision software. Widefield fluorescence images were collected on an Olympus BX51 upright microscope and captured using MetaVue software (Molecular Devices Inc, CA, USA). The images were collected using a Coolsnap HQ camera (Photometrics, AZ, USA) and the raw images were then deconvolved using the Softworx software.
Immunocytochemistry
Neurons cultured on PDL-coated 12 mm glass coverslips were fixed in 4 % paraformaldehyde / 4 % sucrose (w/v), permeabilised with 0.1 % (v/v) Triton-X-100 in PBS, quenched with 0.25 % (w/v) NH4Cl2, and blocked with 5 % (w/v) BSA in PBS. Cultures were immunostained with a combination of the following antibodies; neurofilament (NeuF) (1:500, Millipore, UK); neuronal nucleus (NeuN) (1:200, Chemicon, UK), neutrophil elastase (1:500, Abcam), PL2-3 (1:500, anti-H2A-H2B-DNA complex) and SJC antibody (1:5000. Immunodetection was performed with Alexa Fluor-conjugated secondary antibodies (1:1000; Invitrogen, UK), and cells were mounted with ProLong Gold with or without DAPI counterstain. Widefield fluorescence images were collected on an Olympus BX51 upright microscope and captured using MetaVue software (Molecular Devices Inc, CA, USA). Z-stacked images were acquired on a Delta Vision (Applied Precision, Inc. WA, USA) restoration microscope objective.
Scanning electron microscopy
Transmigrated neutrophils were added to neuronal cultures as described above and fixed with 2.5 % (w/v) glutaraldehyde (Sigma). The fixed cells then underwent three 5 min washes with 0.1 M PBS (Sigma). After the final wash, 1 % (v/v) osmium tetra-oxide (Sigma) in 0.1 M PBS was added to the cells for a minimum of 1 h at 4 °C. The cells were rinsed with 0.1 M PBS and dehydrated with a 15 min incubation in increasing alcohol solutions (70 % and 95 %) and then three further incubations in 100 % alcohol for 10 min. The tissue was dried to the critical drying point and then mounted for 3 min of sputter coating. The samples were then viewed using the Gatan 3View System, (Gatan, UK).
Flow cytometry
Cells were fixed in FACS/FIX buffer [1 % (w/v) PFA and 0.1 % (w/v) low-endotoxin BSA (Sigma, UK)] for 15 min at room temperature. Cells were washed with FACS buffer [0.1 % BSA (w/v) PBS] and pelleted at 400 g for 5 min. Neutrophils were incubated with anti-CD16/CD32 for 30 min at 4 °C (1:200 in FACS buffer, BD biosciences, UK) and then washed and pelleted as described before. Staining was carried out with either anti-Ly6G-allophycocyanin (APC) (1:200, Ebiosciences, UK), anti-CD11b-Alexa Fluor-488 conjugated antibody (1:100, Serotec) or isotype control for 30 min at 4 °C before final wash and centrifugation. Neutrophils were identified by flow cytometry. 96 % of the purified, naïve neutrophil population was CD11b/Ly6G-positive.
For the staining of intracellular phosphorylated proteins, neutrophils were labelled with Ly6G as before, but then permeabilised with PerIII (BD biosciences) for 30 min. Cells were washed and collected by centrifugation at 400 g for 5 min and incubated with anti-phosphorylated nuclear factor kappa-B (P-NFκB)-PE, anti-P-p38-PE or anti-P-Akt-PE (1:25 in FACS buffer, Ebiosciences) for 30 min at 4 °C in the dark. After a final wash and centrifugation, the cells were re-suspended in FACS/FIX buffer and analysed by a Cyan ADP (Beckman Coulter, UK) flow cytometer.
Tissue homogenisation
WT or IL-1α/β−/− mice were anaesthetised 8 h after MCAo, perfused with 0.9% saline, and brain samples were rapidly removed and frozen on dry ice. Frozen cortical samples were homogenised on ice in following buffer:50 mM Tris-HCl pH 7.6, 150 mM NaCl, 5 mM CaCl2, 0.02% NaN3, 1% Triton X-100, and centrifuged (15,000 g, Hettich Mikro 200R, Hettich UK), and protein concentration was determined in supernatants by BCA protein assay (detection range 800 μg-1.55 μg/ml) (Pierce, UK).
ELISA
ICAM-1 and VCAM-1 concentrations in cortical homogenates were quantified by ELISA according to the manufacturer’s instructions (R & D Systems). Samples were read at 450/570 nm on a Biotek plate reader (detection range 8 ng/ml – 13.25 pg/ml; intra-assay variation <3 %) (Biotek, Bedford, UK). Data obtained were analysed using GraphPad Prism 5.0, (GraphPad software for Windows, CA, USA).
Due to the high sensitivity and specificity of PL2-3 antibody (22), a specific histone-DNA complexes ELISA was developed “in-house”. PL2-3 antibody was used to detect H2A-H2B-DNA complex immobilised from neutrophil conditioned medium samples on Nunc (Sigma) microplates. Following incubation with biotinylated horse anti-mouse antibody (Vector laboratories) and streptavidin-HRP (R & D Systems), the reaction was developed with TMB substrate solution (BD Biosciences). Samples were read at 450/570 nm on a Biotek plate reader.
Cytometric bead array (CBA)
Lysates of naive neutrophils, non-migrated neutrophils and transmigrated neutrophils were assayed by CBA analysis (BD Biosciences, UK) for the following 14 inflammatory mediators as follows: macrophage inflammatory protein 1-α (MIP-1), TNF-α, RANTES (CCL5), monocyte chemoattractant protein (MCP), CXCL1, IL-6, IL-1α, IL-1β, IL-17, IL-10, interferon-γ (IFNγ), granulocyte-colony stimulating factor (G-CSF), L-selectin and E-selectin.
Identification of nuclear and mitochondrial DNA by polymerase chain reaction
Neutrophil-conditioned medium prepared from naive or transmigrated neutrophils was assayed for the presence of mitochondrial or nuclear DNA as follows: DNA was precipitated from a fixed volume of conditioned medium using sodium acetate/ethanol protocol before re-suspension in 10 μl of RNAse-free distilled water. To confirm the presence of mitochondrial or nuclear DNA, sections of DNA within unique mitochondrial or nuclear genes were targeted by polymerase chain reaction (PCR) using specific primers (sequence available on request) as follows: As fixed volume of neutrophil-conditioned medium was used for precipitation a fixed volume of 1μl was used for each PCR reaction. Each PCR reaction contained 1mm MgCl2, 1x reaction buffer, 1.2mM dNTPs, 1pmol forward and reverse primer, 2.5 U Taq polymerase (all reagents from Bioline, London, UK) 5 min 94°C; 30 cycles of [30 s 94°C, 30 s 56°C, 30 s 72°C] with a final step of 5 min 72°C, then 4°C for 15 min). Products were detected on a 1.5 % (w/v) agarose gel containing 5μg/ml ethidium bromide (Sigma) at 100V for 45 min, and visualised using ImageQuant LAS 400 (GE Healthcare).
Gel zymography
To determine the presence of active mature MMP-9, neutrophil-conditioned medium was collected from naive or transmigrated neutrophils and subjected to gelatin zymography as described previously (21).
Western blot analysis
Total protein content of bEnd.5 lysates was determined using BCA assay. A fixed protein concentration of each sample was diluted in 5x dissociation buffer [200mM Tris/HCl, pH 6.8, 10 % SDS, 20 % (v/v) glycerol, 10 mM DTT, 0.05 % bromophenol blue]. Samples were heated at 95°C for 5 min prior to being resolved by 12% SDS-polyacrylamide gel electrophoresis. Proteins were then transferred onto a Hybond (polyvinylidene difluoride GE Healthcare, UK) membrane. ICAM-1 and VCAM-1 were then detected using specific antibodies (as mentioned above) diluted in PBS 0.1 % Tween (v/v) and 2 % BSA. All bound antibodies were detected using specific HRP-conjugated secondary antibodies, which were detected using the enhanced chemiluminescence system (GE healthcare, UK). Actin was detected using an HRP-conjugated primary antibody to determine protein loading (Sigma, UK). Images were captured using ImageQuant LAS 400 (GE Healthcare) and saved as 8-bit TIFFs for densitmetric analysis using Northern Eclipse Sofware 6.0, (Empix Imaging, ON, USA).
Cell death assays
LDH assay
Cell death was assayed by measuring the release of lactate dehydrogenase (LDH) into cell culture supernatants using CytoTox96® assay (Promega, UK).
MTT assay
Viability of the neuronal cultures was measured using MTT (5 μg/ml; Sigma), directly into the neuronal culture in the absence or presence of neutrophils. The production of formazan was observed after 2 h incubation. Brightfield micrographs of neurons (pre- and post-addition of MTT) were collected on an Olympus CKX31 cell culture microscope using Motic imaging software.
Trypan blue assay
Non-migrated or transmigrated neutrophils were collected 20 h after isolation or application to bEnd.5 cells and were incubated with a 1:2 dilution of the vital dye Trypan blue (Sigma) and the percentage of Trypan blue-positive cells was determined.
Statistical analysis
Data are expressed as mean (± standard error of the mean, SEM) from a minimum of three independent experiments carried out on separate cultures. Depending on the number of groups within the data set, data were analyzed using either a Student’s t-test (two groups) or one-way ANOVA with Bonferroni's post-hoc test (three or more groups) using GraphPad Prism 5.0, (GraphPad software for Windows, CA, USA). P<0.05 was considered to be statistically significant.
Results
IL-1 mediates cerebrovascular activation and recruitment of neutrophils in vivo and induces cerebrovascular transmigration of neutrophils in vitro
We first used MCAo as a model to induce neuroinflammation, and found that neutrophil migration in the brain was significantly reduced in IL-1α/β−/− mice compared to WT mice (Supplementary Fig. 1a – c). These observations were supported with the altered IL-1-dependent ICAM-1 and VCAM-1 levels in vivo (Supplementary Fig. 1e – g) and in IL-1-treated bEnd.5 cells in vitro (Supplementary Fig. 1h). IL-1β induced the migration of neutrophils (CD11b/Ly6G+, Supplementary Fig. 2a) across primary cultures of MBEC and bEnd.5 cells (Fig. 1a) into the abluminal compartments of Transwell® inserts. Treatment with vehicle had little effect on migration (less than 3 %) across MBECs or bEnd.5 cells. Activation of MBECs by IL-1β caused a 4.5 fold increase in neutrophil migration compared to the vehicle treated control (Fig. 1a), which was blocked by IL-1Ra (Supplementary Fig. 2b). A comparable increase was also seen in bEnd.5 cultures treated with the same concentration of IL-1β (Fig. 1a).
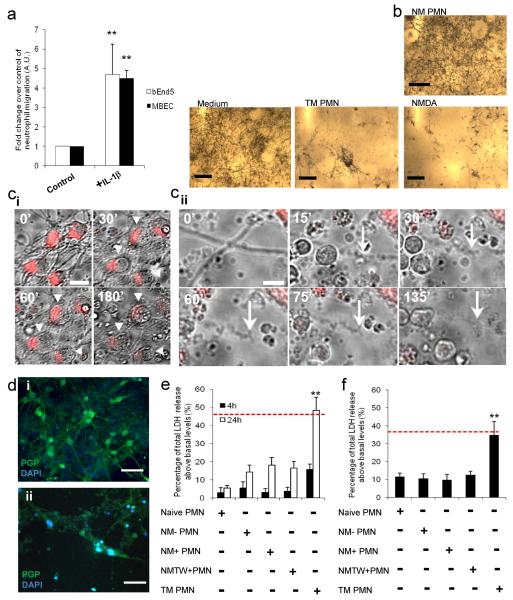
Figure 1. Neutrophils acquire a neurotoxic phenotype after transmigration across activated brain endothelium.
(a) Transmigration of neutrophils across brain endothelioma cells (bEnd.5) and primary murine brain endothelial cells (MBEC) treated with IL-1β (4 h) after 24 h. (b) Brightfield images of neurons exposed to neurobasal medium, non-migrated neutrophils (NM-PMN) or transmigrated neutrophils (TM PMN) (120,000 cells/cm2), or 600 μM NMDA in the presence of MTT (5 μg/ml) for 24 h. Neuronal viability under these conditions is indicated by the presence or absence of MTT crystals. (c) Brightfield and CellTracker Red images of stained neurons (arrowheads) exposed to TM PMN for 0-180 min (i, scale bar, 15 μm, and see movie 1). Brightfield images of a neuronal process (arrow) exposed to TM PMN for 0-135 min (ii, scale bar, 5 μm). (d) Immunofluorescent images of neurons (PGP, green) exposed to (i) naïve PMN or (ii) TM PMN (120,000 PMN /cm2) for 24 h (scale bar, 30 μm). (e) Quantification of total LDH release from neurons 4 h or 24 h after application of naïve neutrophils (PMN), non-migrated neutrophils, which had been incubated with non-activated endothelial cells (NM− PMN), non-migrated neutrophils, which had been incubated with IL-1β-activated brain endothelial cells (NM+ PMN), or incubated with conditioned medium from activated endothelium after washing, (NMTW+ PMN) or transmigrated neutrophils (TM PMN) (120,000 cells/cm2. (f) Effect of conditioned medium (applied at 1:4 dilution) from naive PMN, NM− PMN, NM+ PMN, NM+TW or TM PMN on neuronal viability as assessed by LDH release after 24 h. Bars represent the mean data +/− SEM for a minimum of 3 independent experiments carried out on separate cultures. Red dotted line indicates neuronal death induced by 600μM NMDA. **P<0.01 (one-way ANOVA, with a Bonferroni post hoc test).
Cerebrovascular transmigration induces a neurotoxic phenotype in neutrophils, leading to rapid neuronal death
Transmigrated neutrophils applied to neurons for 3 h induced significant loss of neuronal viability (Fig. 1b), which was comparable to the neurotoxicity induced by treatment with NMDA (600 μM) for 24 h (Fig 1b). Real time CellTracker monitoring of healthy neurons showed that transmigrated neutrophils induced rapid neuronal death (Fig. 1c i and 1c ii). Neuronal cell bodies loaded with CellTracker Red and their processes were visualised every 30 s for 6 h, in the absence or the presence of transmigrated neutrophils (movie 2 and 1, respectively). Neurons exposed to transmigrated neutrophils swelled rapidly (within 180 min), rounded-up and lost CellTracker Red staining intensity (Fig. 1c ii and movie 1). Loss of CellTracker Red labelling was not apparent over a similar time period in untreated neurons (Fig. 1c i and movie 2). Neuronal processes became bead-like and fragmented within 30 min of application of transmigrated neutrophils (Fig. 1c ii and movie 1). Addition of transmigrated neutrophils to neuronal cultures for 24 h induced a marked loss of neuronal cell bodies and processes, as identified by PGP 9.5 staining (Fig. 1d ii).
Neurotoxicity induced by the application of transmigrated neutrophils was confirmed by LDH release where a 3-fold increase in neuronal death was observed after 24 h (Fig. 1e). Application of naive neutrophils or non-migrated neutrophils, which had been incubated with non-activated or IL-1β-activated brain endothelial cells, or incubated with conditioned medium from activated endothelium after washing, did not significantly affect neuronal viability after 24 h incubation (Fig. 1e). Cell death was not due to IL-1β being carried over, since naive neutrophils incubated with neurons in the presence of IL-1β did not induce a significant increase in cell death within the same time-frame (Supplementary Fig. 2d). This increase in LDH release observed within neurons after exposure to transmigrated neutrophils was not due to compromised neutrophil viability, which was determined through trypan blue staining where the viability of transmigrated neutrophils 20 h after isolation was 40 % less than that observed for the naïve neutrophils (Supplementary Fig. 2e).
The neurotoxicity of transmigrated neutrophils was also conveyed through the release of soluble factors from the neutrophils, since conditioned medium from transmigrated neutrophils (applied at 1:4 dilution to the primary neuronal cultures) induced more than a 4-fold increase in neuronal death (overall 30-40 % increase in neuronal LDH release) after 24 h incubation (Fig. 1f). Conditioned medium from naive neutrophils or non-migrated neutrophils induced a reduction in neuronal viability to a much smaller extent, compared to that induced by the conditioned medium from transmigrated neutrophils (Fig. 1f), which was comparable to the neurotoxicity induced by NMDA (600 μM) after 24 h incubation (indicated by red-dotted line). An increase in neurotoxicity was also observed in and the presence of transmigrated neutrophils after migration across IL-1α-stimulated bEnd.5 (Supplementary Fig. 2f). This rapid induction in neuronal death could therefore be solely attributed to the transmigration of neutrophils across activated brain endothelium, as no increase was observed in any of the four non-migrated control groups.
We also investigated whether trans-endothelial migration-induced neutrophil neurotoxicity is unique to activated brain endothelium. To this end, we used a thioglycollate-induced peritonitis model, which also allowed us to assess the role of in vivo neutrophil transmigration on neurotoxicity in vitro. Conditioned medium from neutrophils collected from the peritoneal cavity after thioglycollate injection was neurotoxic and led to increased LDH release and reduced MTT metabolism in neuronal cultures (Supplementary Fig. 2g and 2h).
Cerebrovascular transmigration profoundly alters neutrophil phenotype
Both the morphology of naïve neutrophils and transmigrated neutrophils was determined by immunofluorescence. Compared to naive neutrophils, transmigrated neutrophils were devoid of lobed nuclei (Fig. 2b A, white arrow) and displayed extracellular de-condensed DNA (Fig.2b B, white arrow), which was associated with an increase in the abundance of elastase staining (Fig. 2b B and C). Using immunofluorescence, the loss of neuronal MAP-2 staining observed after 4 h incubation with transmigrated neutrophils was extensive in comparison to the neurons incubated with the naïve controls (Fig. 2c and d). In this instance the neutrophils were also stained with CD45, as well as MMP-9, indicating the presence of the secondary and tertiary granules. A 3-dimensional z-stack was produced from the wide-field fluorescent images, allowing visualisation of the morphology of naïve and transmigrated neutrophils in the presence of the neuronal cultures in a transverse cross section (Fig. 2c ii and 2d ii). The presence of the punctuate MMP-9 staining within the spherical naïve neutrophils can be clearly seen, resting above the neuronal cultures (Fig. 2c ii). This MMP-9 staining was lost in the transmigrated neutrophils, which alongside the altered shape and appearance suggests their degranulation (Fig. 2d ii).
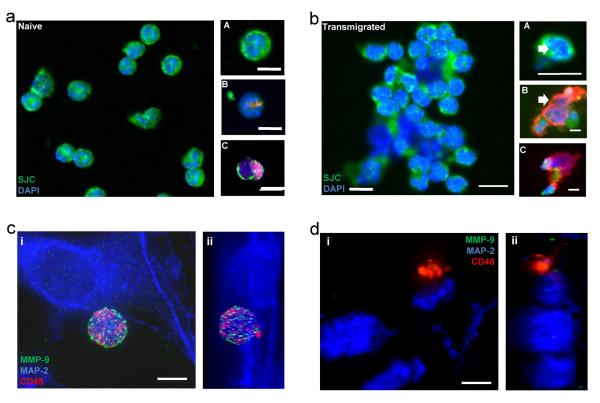
Figure 2. Neutrophils migrated across IL-1β-activated cerebrovascular endothelium show altered phenotype in comparison to naïve controls.
(a) Naïve neutrophils stained with (A) anti-neutrophil serum (SJC, green) and DAPI (blue), (B) SJC (red), DAPI (blue) and PL2-3 (green) or (C) elastase (red), DAPI (blue) and PL2-3 (green) (scale bar, 10 μm). (b) Transmigrated neutrophils stained with (A) SJC (green) and DAPI (blue), (B) SJC (red), DAPI (blue) and PL2-3 (green) or (C) elastase (red), DAPI (blue) and PL2-3 (green) (scale bar, 10 μm). Immunofluorescent images of neurons (MAP2, blue) exposed to (c i) naïve (z-stack c ii) or (d i) transmigrated neutrophils (z-stack d ii) (120,000 neutrophils /cm2) for 4 h. Neutrophils (CD45, red) contain high levels of MMP-9 (c i) (green), which is lost from transmigrated neutrophils added to neurons (d i) (scale bar, 5 μm).
We then determined the inflammatory phenotype of the transmigrated neutrophils in comparison to naïve neutrophils by CBA analysis (Table 1). Transmigrated neutrophils had increased inflammatory profiles, with reduced cell-associated CD62L (L-selectin) indicative of activation-induced CD62L shedding, as well as significantly increased neutrophil CXCL1 and IL-6 expression. We also detected a significant increase (6-fold) in neutrophil cell-associated (but not secreted) inflammatory factors RANTES and MCP-1 (Table 1). These results show that cerebrovascular transmigration profoundly alters neutrophil viability, activation and inflammatory phenotype.
Table 1. Cytokine profile of naïve neutrophil and transmigrated neutrophil lysates as determined using CBA analysis.
Levels of inflammatory mediators in the lysates of naïve neutrophils or transmigrated neutrophils were determined using CBA analysis (pg/mg). Data are the mean +/− SEM from a minimum of 3 independent experiments carried out on separate cultures.
| Corrected pg/mg | ||
|---|---|---|
| naïve | TM | |
| CD62E | 34±12 | 116±34 |
| CD62L | 569±73 | 177±63* |
| IL-1α | 13±3 | 12±4 |
| TNFα | 24±7 | 19±8 |
| MIP-1 | 36±5 | 17±6 |
| G-CSF | 22±4 | 23±41 |
| MCP-1 | 85±12 | 579±110* |
| RANTES | 55±11 | 507±80** |
| IL-6 | 7±2 | 46±14* |
| IL-1β | 30±6 | 16±5 |
| KC | 21±3 | 1159±290* |
| IL-10 | 37±27 | 46±10 |
| IL-17 | 4±1 | 4±12 |
P<0.05 and
P<0.01 (Student’s unpaired T-test).
Flow cytometric analysis demonstrated a rapid activation of transmigrated neutrophils after transmigration (Supplementary Fig. 3a i-iii). The percentage of positive staining for both P-p38 and P-Akt in the transmigrated neutrophils were significantly increased in comparison to the naïve neutrophils and a trend towards increased P-NFκB was observed (Supplementary Fig. 3a i and iii). Activation of P-NFκB, p38 and Akt pathways has been shown to promote survival of activated neutrophils (24-26). These data are consistent with previous studies on non-cerebral endothelium demonstrating that neutrophil transmigration across endothelium prolongs neutrophil viability (27, 28).
Release of neutrophil proteases associated with de-condensed DNA is linked with neuronal death in vitro
Following our previous observation of de-condensed DNA within transmigrated neutrophils (Fig. 2b B), we hypothesised that the neurotoxicity of transmigrated neutrophils was mediated by histone-DNA complexes or neutrophil-derived proteases, which are released upon degranulation and are associated with NETs (29). Through the use of a monoclonal antibody to histone-DNA complexes (PL2-3, H2A-H2B-DNA) (22) a significant reduction in intracellular H2A-H2B-DNA staining in vitro in transmigrated neutrophils was observed when compared to naïve controls (Fig. 3a i, ii and iii), implying that the de-condensed DNA was released upon or after transmigration. By PCR using specific mitochondrial or nuclear gene-primers, we found that the presence of extracellular DNA was also detected in the conditioned medium of transmigrated neutrophils and compared to the conditioned medium of naïve neutrophils (Fig. 3b i and ii). The presence of extracellular de-condensed DNA was confirmed by ELISA using the PL2-3 antibody, where a significant increase in the abundance of de-condensed DNA was detected in the conditioned medium of transmigrated neutrophils in comparison to the naïve conditioned medium (Fig. 3c).
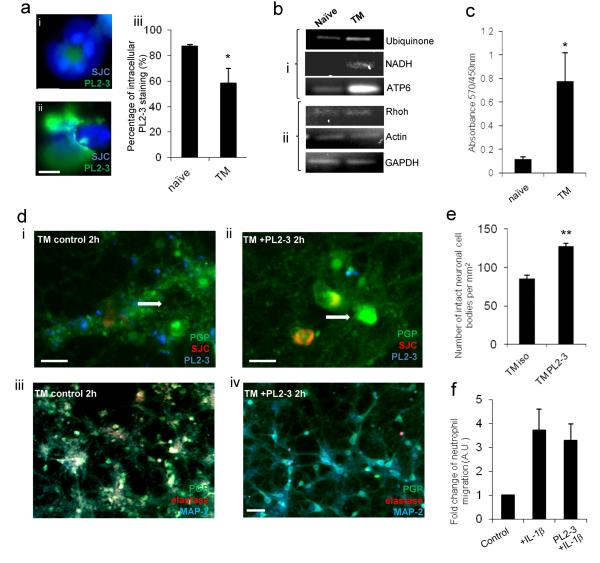
Figure 3. Neutrophil proteases are associated with neutrophil extracellular traps and neuronal death in vitro.
(a) Intracellular PL2-3 staining is significantly reduced in (ii) transmigrated (TM) neutrophils compared to (i) naïve neutrophils (DAPI and PL2-3) (scale bar, 5 μm), (iii) graph showing the percentage of neutrophils present with intracellular PL2-3 staining. (b) Neutrophil-derived mitochondrial DNA is increased in conditioned medium from transmigrated neutrophils but not in that of naïve neutrophils as determined by PCR. (i) mitochondrial and (ii) nuclear DNA was observed in the conditioned medium of transmigrated neutrophils compared to naïve controls. (c) Elevated levels of extracelluar histone-DNA was detected in conditioned medium of naïve neutrophils and transmigrated neutrophils, (d) Neurons were incubated for 2 h and stained with PGP (green), neutrophils were stained with SJC (red) and NETs stained with PL2-3 (blue) (scale bar, 10 μm). (iii) Reduction in neurotoxicity of transmigrated neutrophils in the presence of PL2-3 (15 μg/ml) is seen in neuronal cultures stained with PGP (green) and MAP-2 (red), as compared to the (iv) isotype treated control (scale bars, 30 μm). Maintained structural integrity is shown in cultured neurons (white arrows) after (ii) exposure to transmigrated neutrophils treated with PL2-3 (15 μg/ml) in comparison to (i) neutrophils transmigrated across activated endothelium in the presence of an isotype control (scale bar, 10 μm). (e) Percentage of maintained integrity of neuronal cell bodies after exposure to transmigrated neutrophils treated with PL2-3 (15 μg/ml) in comparison to isotype treated control.(f) Presence of PL2-3 antibody (15 μg/ml) does not affect neutrophil migration across IL-1β-activated brain endothelium after 24 h of migration. Bars in graphs represent the mean +/− SEM from a minimum of 3 independent experiments carried out on separate cultures. *P<0.05 **P<0.01 (Student’s t-test or one-way ANOVA, with a Bonferroni post hoc test or Student’s t-test where appropriate).
Neutrophil proteases contribute to tissue injury during inflammation (30-32) and brain injury after MCAo (21, 33, 34). Extensive work has also shown that neutrophil elastase is associated with NETs (35-37). The monoclonal antibody PL2-3 has also been effective in identifying NETosis and associated inflammation in the periphery (36). After the direct transmigration of neutrophils onto neuronal cultures in the presence of PL2-3, an improvement in neuronal viability was observed using immunofluorescence, where MAP2 staining was retained in comparison to vehicle-treated controls (Fig. 3d iii and iv). The maintained integrity of neuronal cell bodies was also quantified in both the PL2-3-treated and vehicle-treated control after neutrophil transmigration, where a significant increase in cell body integrity was observed in the presence of PL2-3 (Fig. 3e). This could not be attributed to compromised neutrophil migration due to the PL2-3 antibody as the transmigration of neutrophils across IL-1-activated brain endothelium was not affected when the antibody was present (Fig. 3f). We also determined whether the presence of the PL2-3 antibody altered the neurotoxicity induced by the application of conditioned medium from transmigrated neutrophils. LDH assay detected a small, non-significant, but consistent reduction of neuronal death of an average of 16 % after PL2-3 antibody treatment in the presence of conditioned medium of transmigrated neutrophils (Supplementary Fig. 3b). This difference would suggest the impedance of NETosis in the presence of PL2-3 during migration, rather than the inhibition of NET release. These results suggest that the release of de-condensed DNA-protease complexes is likely to be involved in the process of neutrophil activation and protease release, but transmigrated neutrophil-mediated neurotoxicity was not directly induced by histone/DNA complexes. Alongside NET production the degranulation of the transmigrated neutrophils could also contribute to the acquired neutrotoxicity. As previously shown, the loss of MMP-9 staining in transmigrated neutrophils was observed, suggesting degranulation (Fig. 2d). A significant increase in the presence of active MMP-9 was detected in conditioned medium of transmigrated neutrophils in comparison to the naïve control (Supplementary Fig. 3c). As neutrophil proteases are known to be associated with de-condensed DNA, their potential involvement in the acquired neurotoxic phenotype of transmigrated neutrophils was investigated further.
Neutrophil proteases mediate rapid neurotoxicity of transmigrated neutrophils
To evaluate the potential role of the released proteases and other substances in the neurotoxic mechanisms induced by transmigrated neutrophils, we first performed interventions using conditioned medium from transmigrated neutrophils. Pre-treatment of neurons with MK-801(NMDA receptor antagonist) partially, but significantly, reduced the neurotoxicity induced by conditioned medium from transmigrated neutrophils (Fig. 4a). Heat-inactivation or trypsin treatment of conditioned medium from transmigrated neutrophils also inhibited its neurotoxic effect (Fig. 4a), suggesting a proteinaceous neurotoxin.
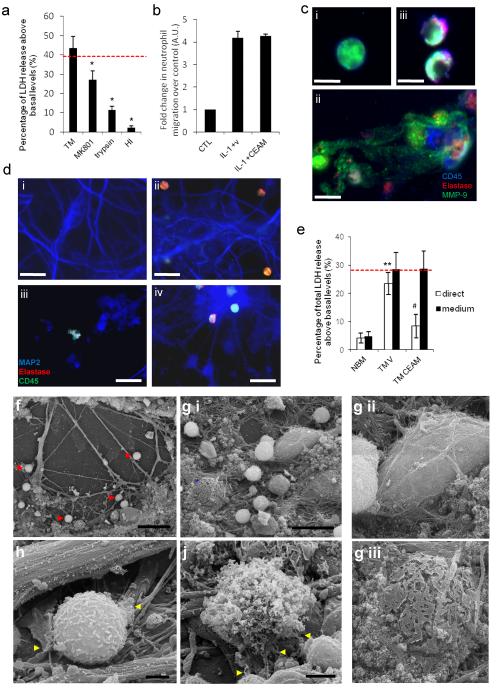
Figure 4. Degranulation and NET release are associated with neurotoxicity in transmigrated neutrophils through neutrophil proteases.
(a) Neuronal LDH release is shown 24 h after treatment with conditioned medium from transmigrated (TM) neutrophils with or without pre-treatment of neurons with MK-801 (10 μM), trypsin or heat-inactivated (HI) CM. (b) The transmigration of neutrophils across IL-1β-activated brain endothelium (bEnd.5) is not significantly altered by the presence of CEAM after 24 h. (c) Neutrophil morphology indicated by presence of elastase (red), CD45 (blue) and MMP-9 (green) using immunofluorescence in (i) naïve neutrophils. (iii) shows an attenuation of degranulation in the presence of CEAM, when compared to the vehicle treated transmigrated neutrophils (ii) (scale bar, 5 μm). (d) Maintained neuronal structural integrity shown in neuronal cultures after exposure to transmigrated neutrophils in the presence of CEAM (iv) in comparison to (iii) neutrophils transmigrated across activated endothelium. Vehicle treated transmigrated neutrophils induced extensive neuronal damage when comparing with naive neutrophils. Neurons were incubated for 4 h and stained with MAP2 indicating neuronal viability (blue); neutrophils were stained with CD45 (green) and elastase (red) (scale bar, 30 μm). (e) Neuronal viability was established using LDH assay after the direct application of naïve and transmigrated neutrophils (white bars) or naïve and transmigrated neutrophils conditioned medium (black bars) in the presence of CEAM or a vehicle control (f) Scanning electron micrograph images of transmigrated neutrophils in primary murine neuronal cultures after 1 h incubation. The presence of neutrophils indicated by red arrows (scale bar 30 μm). (g) (i) Identification and comparison of a healthy neuronal body (white asterisk) with a dying neuronal body (blue asterisk) (scale bar 20 μm). Neuronal bodies shown in a higher magnification on (ii) and (iii). (h) Transmigrated neutrophil in initial phases of degranulation (yellow arrows) surrounding neuronal processes (scale bar 2 μm). (j) Full degranulation of the transmigrated neutrophils, where the granules and globular structures of NETs are clearly visualized (scale bar 2 μm). Bars represent the mean data +/− SEM for a minimum of 3 independent experiments carried out on separate cultures. Red dotted line indicates neuronal death induced by 600μM NMDA. *P<0.05 vs. TM; **P<0.01 TM vs. naïve (NBM); #P<0.05 TM vs. TM CEAM (one-way ANOVA, with Bonferroni's post-hoc test).
Neutrophil-derived proteases are associated with NETs (37), therefore we tested whether MMP-9, cathepsin-G, proteinase-3 or elastase, released from transmigrated neutrophils may be responsible for the observed neurotoxicity. No effect was seen when specific inhibitors against certain proteases were used alone (not shown). Because these proteases exhibit their actions in concert (38), a cocktail of inhibitors against cathepsin G (C), neutrophil elastase (E), proteinase-3 (aprotinin, A) and MMP-9 (SB3CT, M) was also tested (defined as CEAM). CEAM did not affect neutrophil transmigration across IL-1-activated brain endothelium (Fig 4b). Immunofluorescence showed maintained integrity, reduced degranulation and less elastase/MMP-9 staining in transmigrated neutrophils in the presence of CEAM compared to vehicle treated transmigrated neutrophils (Fig. 4c ii and iii). Neutrophils were directly migrated onto neuronal cultures across activated bEnd.5 cells in the presence of vehicle or CEAM for 2 h (Fig. 4d iii and iv). Importantly, neuronal structural integrity appeared to be maintained after the migration of transmigrated neutrophils in the presence of CEAM (Fig 4d iv) in comparison to the vehicle treated transmigrated neutrophils (Fig 4d iii), thus supporting the link between neuronal toxicity and the proteases released from transmigrated neutrophils.
When transmigrated neutrophils were added directly to neurons, the presence of CEAM significantly rescued neuronal viability compared to the vehicle treated transmigrated neutrophil control (Fig. 4e). The presence of CEAM was ineffective when using conditioned medium from transmigrated neutrophils, suggesting that the cascade of events subsequent to full degranulation involving these neutrophil-derived proteases might result in neurotoxicity.
This is supported by the observation that neurotoxicity could be successfully inhibited on site in neuronal cultures whilst maintaining transmigrated neutrophil integrity, but not in conditioned medium from transmigrated neutrophils where it is possible that proteolytic events had already occurred and full degranulation had taken place.
DNase treatment of conditioned medium from transmigrated neutrophils failed to restore neuronal viability, indicating that DNA released from transmigrated neutrophils was not directly responsible for the observed neurotoxicity (Supplementary Fig. 2i). Several other inhibitors (calcium channel blocker nifidipene; broad spectrum caspase inhibitor BOC-1; cysteine protease inhibitor E64; protease activated receptor inhibitor-1; LαAA; EDTA; EGTA; BAPTA to chelate free calcium; apocynin to inhibit NAPDH oxidase; MEK1 inhibitor; the MAC-1 inhibitor clusterin, and the four individual CEAM components) all failed to rescue the neuronal viability in the presence of conditioned medium from transmigrated neutrophils (data not shown).
These data indicate that neuronal viability can be maintained if neutrophil degranulation and release of multiple proteases are prevented in the presence of neurons. Time lapse imaging indicated the presence of transmigrated neutrophils that were moving continually, and degranulated neutrophils that were in close association with neurons (movie 2). Scanning electron microscopy was employed to visualise the transmigrated neutrophils and neurons in greater detail (Fig. 4f-j). Transmigrated neutrophils were associated with the neurons (Fig. 4f), and were attached to intact neuronal cell bodies (Fig. 4g i and ii). In contrast, neuronal death, as evidenced by the presence of disintegrated neuronal cell bodies, was seen in the vicinity of collapsed neutrophils (Fig. 4g i and iii). Degranulation and NET release from transmigrated neutrophils was observed, where the strand-like globular structures of the de-condensed DNA were found protruding from the disintegrating neutrophil cell membrane (Fig. 4h and j).
Neutrophils recruited to the brain in vivo are associated with extracellular proteases and a loss of intracellular de-condensed DNA
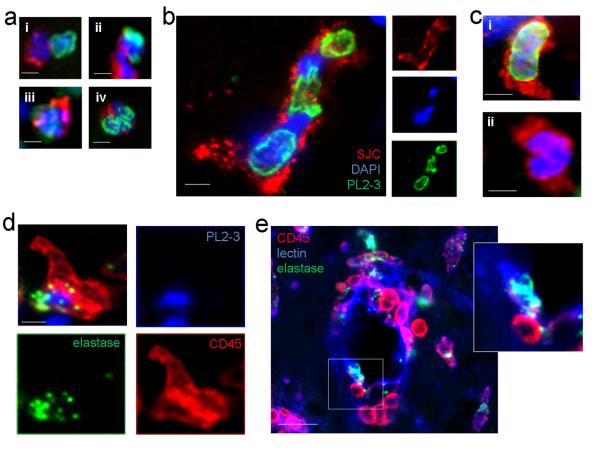
De-condensed DNA associated with neutrophils and loss of intracellular histone-DNA complexes was observed in animal models of neuroinflammation tested in vivo (Fig. 5a) such as after intracerebral LPS administration or MCAo (Fig. 5a i); MCAo in the presence of peripherally-administered IL-1β (Fig. 5a ii); MCAo preceded by systemic infection (Fig. 5a iii); and AMPA/IL-1-induced neurotoxicity (Fig. 5a iv). Within these models of neuroinflammation intracellular PL2-3 staining was observed to be reduced or uneven in close proximity to the nuclear DNA staining, as indicated by DAPI (Fig. 5b). In general, the presence of neutrophil associated de-condensed DNA was reduced in the brain (80% of intracerebral neutrophils PL2-3-negative) (Fig. 5c i and ii) in comparison to meningeal neutrophils (20% PL2-3-negative). This provides further evidence for an altered state of recruited neurophils in vivo. There was no significant difference in neutrophilic intracellular PL2-3 staining between different neuroinflammatory conditions. Quantification revealed extracellular neutrophil-associated DNA (PL2-3) in 4±1% of recruited neutrophils after intracerebral LPS administration, whereas a trend towards more neutrophils with extracellular PL2-3 was observed after AMPA/IL-1-induced neurotoxicity, MCAo in the presence of peripherally-administered IL-1β or after MCAo preceded by systemic infection (9±5%, 11±5% and 15±5%, respectively). Extracellular neutrophil-associated PL2-3 staining was mostly punctuate and appeared in close proximity of neutrophils (Supplementary Figure 4a). Long NET-like structures containing both nuclear DNA and PL2-3 were not observed in the brain. Neutrophil elastase was also determined through immunofluoresence in vivo and was observed to be associated with de-condensed DNA (Fig. 5d). The presence of extracellular elastase associated with recruited neutrophils was observed in vivo, along the blood vessel wall and occasionally in the parenchyma after MCAo (Supplementary Fig. 4b). Further to this, the disintegration of the perivascular basement membrane from the endothelial monolayer after MCAo was also observed in vivo (Supplementary Fig. 4c), indicating that products derived from recruited neutrophils could reach nearby neurons via the compromised BBB. In addition to this, populations of elastase-positive neutrophils were also observed perivascularly inside and outside the endothelial monolayer (Fig. 5e and Supplementary Fig. 4d).
Figure 5. Neutrophil recruitment in acute brain injury is associated with extracellular proteases and loss of intracellular de-condensed DNA in vivo.
(a) Loss of intracellular histone-DNA complexes (PL2-3 staining) in neutrophils identified in the cerebral cortex in vivo in different established models of neuroinflammation (i) after induction of cerebral ischaemia, (ii) after induction of cerebral ischaemia in the presence of peripherally injected IL-1β, (iii) after induction of cerebral ischaemia with following systemic infection with Trichuris muris (3), and (iv) after stereotaxic intrastriatal administration of IL-1 and AMPA in rat (scale bars, 5 μm). (b) In a population of recruited neutrophils in the inflamed brain PL2-3 staining is uneven and de-condensed DNA is observed in close proximity to nuclear DNA (DAPI, blue) (c) (i) Meningeal neutrophils contain high amounts of intracellular histone-DNA complexes (PL2-3) after stereotaxic injection of LPS (scale bar 10 μm). (ii) Neutrophils recruited to the inflamed cerebral cortex contain less de-condensed DNA (PL2-3) after stereotaxic injection of LPS (scale bar 10 μm). (d) Extracellular PL2-3 and elastase are found in the cerebral cortex in vivo after stereotaxic injection of LPS (scale bar, 5 μm). (e) Immunofluorescence showing perivascular inflammatory cells (CD45, red) containing neutrophil elastase (green) adhering to the endothelial monolayer (lectin, blue) in the brain (scale bar, 20 μm).
Discussion
We show here that transmigration through activated cerebrovascular endothelium critically alters neutrophils, leading to a pro-inflammatory, neurotoxic phenotype. Using established in vitro and in vivo models of neuroinflammation, we show the presence of released de-condensed DNA (NETs) associated with key proteases after neutrophil transendothelial migration. Neurotoxicity of neutrophils has been demonstrated previously, but over a 1-3 day period (13, 15), whereas here the transmigration-induced neurotoxicity developed very rapidly (within 30 min). These data therefore identify a novel neuroinflammatory mechanism: the development of rapid neurotoxicity of neutrophils by IL-1-induced cerebrovascular transmigration.
Neutrophil recruitment through endothelial activation is a common observation in inflammatory disease, where the two stage process of neutrophil activation of priming and mobilisation leads to their adherence, rolling and transmigration (6). The stimulation of this priming and recruitment can occur via the presence of pathogen associated molecular patterns (PAMPs) or in the case of sterile injury, damage associated molecular patterns (DAMPs) produced by necrotic and apoptotic cells (5). Previous studies highlighting the importance of neutrophil recruitment in peripheral inflammation have already demonstrated a clear role for NETs through PAMP and DAMP stimulation linking both in vitro and in vivo observations (30-32).
Systemic inflammatory changes in response to cerebral ischaemia also lead to the mobilisation of peripheral inflammatory cells, which takes place in parallel to activation of the cerebrovascular endothelium (1, 17, 21). The release of neutrophils from the bone marrow in response to MCAo is associated with an increase in pro-MMP9 in circulating blood cells within hours (39). Activated neutrophils are recruited and adhere to inflamed blood vessels in the brain in response to neuroinflammation (40, 41). Their extravasation, which is associated with vascular leakage in the brain, was demonstrated with in vivo two photon imaging (42). Intra-striatal injection of LPS or co-injection of AMPA and IL-1 into the striatum provided us additional in vivo paradigms of neuroinflammation that induce neutrophil recruitment, and that are relevant to pathogen- and sterile-induced brain inflammatory conditions. Our in vivo data showed that most recruited neutrophils were associated with blood vessels in the ipsilateral hemisphere after MCAo and in excitotoxic- or endotoxin-induced neuroinflammation. Transmigrated neutrophils contained high levels of pro-inflammatory cytokines (e.g. KC and RANTES) indicating that such cells may contribute further to cerebrovascular activation and leukocyte recruitment to the brain.
The degranulation of neutrophils and the release of NETs containing de-condensed DNA and proteases takes place normally as part of an anti-bacterial defence (16, 37, 43, 44). This might be initiated in vivo to prevent the bacterial invasion of seriously injured tissues. These properties could contribute to the poor outcome observed in stroke patients with systemic inflammation (45, 46). Murine in vivo models also reflect this, as the up-regulation observed in neutrophil recruitment and neutrophil-derived proteases, could possibly account for BBB damage and increased neuronal death (4, 21). Through the release de-condensed DNA and proteases during extravasation, these neurotoxic products could reach neurons through the disrupted basement membrane of the glia limitans and lead to their demise. Additional stimuli received from parenchymal cells or extracellular matrix may also exacerbate the effects of the transmigrated neutrophils. Although neutrophils are not recruited to the brain until several hours after acute brain injury, once trans-endothelial migration takes place, neutrophils can exert rapid toxicity (within 30 min, based on our in vitro data) to neurons. Therefore it is not surprising that powerful mechanisms have evolved to prevent parenchymal infiltration of neutrophils, such as the complex barrier structures of the neurovascular unit or phagocytosis of invading neutrophils by microglia (47, 48).
While our data suggest that the process of transmigration itself is a sufficient stimulus to endow neutrophils with neurotoxic properties, and thus the ability to kill neurons immediately, it does not imply that diapedesis of neutrophils is the sole factor that contributes to their neurotoxicity. Adherence to activated brain endothelium, partial diapedesis or the initiation of transmigration could also contribute to the development of a neurotoxic phenotype in vivo and these mechanisms warrant further investigation. Pro-inflammatory alterations in neutrophils induced by their transmigration may reflect a heightened state of activation, such that they are primed to respond to additional stimuli which may indirectly contribute to further neurotoxicity in vivo. Similarly, the pro-survival effect of transmigration on neutrophils is likely to further exacerbate the neurotoxic potential of neutrophils in the injured brain. Although the release of NETs often occurs in association with neutrophil death, recent data indicate that viable neutrophils can form NETs, which parallels the release of mitochondrial, but not nuclear DNA (35). This is strikingly similar to our findings: release of proteases and mitochondrial DNA in neutrophil conditioned medium and in vivo, but restricted release of nuclear DNA released in the brain. This might indicate that the release of proteases and de-condensed DNA from neutrophils in the brain is an active process and not simply a consequence of neutrophil death. It is not known when the release of proteases and DNA takes place in vivo, but most recruited neutrophils lacked intracellular PL2-3 immunopositivity in the brain. It is also possible that classical NETosis involving the release of nuclear DNA as observed in vitro or in peripheral tissues, is restricted in the brain or such cells are rapidly phagocytosed by resident microglia.
Our data also show that the neurotoxic phenotype of neutrophils after transendothelial migration is not unique to activated brain endothelium, since conditioned medium of neutrophils collected in vivo after thioglycollate-induced peritonitis also exerts toxicity in neuronal cultures. Irrespective of whether neurotoxicity is triggered by peripheral or central transendothelial migration, neutrophils recruited to the brain parenchyma can exert neurotoxicity, which is likely to be further increased by the fact that toxic products could reach nearby neurons from transmigrating neutrophils if the BBB is compromised. Our in vitro data show that neuron-neutrophil contact is not required for neutrophil neurotoxicity. Furthermore, peripherally located transmigrating neutrophils could exert toxicity to neurons outside the brain as well, which has to be further investigated in the context of peripheral neuropathies.
Elastase, cathepsin-G and proteinase-3 are known to be present in neutrophil primary granules, whilst tertiary granules contain amongst other components, MMP-9 (49). Other studies have shown the localisation of NETs with elastase, cathepsin-G and proteinase-3 (36, 44, 50), which would suggest that the release of primary granules and NETs is the last line of defence for a neutrophil before apoptosis (29). The formation of NETs provides a link between the released proteases and de-condensed DNA, where the presence of elastase was identified attached to the NET itself (16, 51). Elastase is involved in the very first stages of nuclear DNA decondensation through its exit from the primary granules directly to the nucleus (50). The fact that CEAM was not protective when added to conditioned medium of transmigrated neutrophils indicates that a cascade of events subsequent to protease and de-condensed DNA release is responsible for neurotoxicity, which has to be investigated in further studies. Similarly, it would require considerable further work to establish the functional link between mitochondrial DNA and protease activation in neutrophils during NETosis, though it is relevant that mitochondrial DNA can activate human neutrophils resulting in the release of proteases such as MMP-9 (52).
Our in vitro imaging data and scanning electron microscopic observations indicated that transmigrated neutrophils are closely associated with neurons. This accords with the fact that inhibition of the proteases present after degranulation is successful only if transmigrated neutrophils are applied directly to neuronal cultures and not when in the presence of just the conditioned medium of transmigrated neutrophils. This implies that proteolytically cleaved products of these proteases are neurotoxic. CEAM provided almost full protection against neurotoxicity when applied in the presence of transmigrated neutrophils indicating that proteases are the main mediators of toxicity in this model. The possibility that the actual presence of neurons triggers the degranulation of neutrophils is not yet known and remains to be determined in future studies.
In conclusion we show that neutrophils which have migrated across an activated cerebrovascular endothelium profoundly change their phenotype and secrete de-condensed DNA and proteases that contribute to neuronal death. IL-1 has a key role in cerebrovascular activation, neutrophil recruitment and transendothelial migration, which contribute to brain inflammation and neuronal death. Inhibition of neutrophil recruitment and extravasation from cerebral vessels by blocking IL-1 actions might therefore be a preferable strategy to inhibiting the various factors released by neutrophils once they have migrated, as it is likely to offer more effective protection against neutrophil-mediated neuronal death, a significant component in ischaemic and other inflammatory brain injuries. These findings therefore further support the development of inhibitors of IL-1 as neuroprotective agents.
Supplementary Material
Acknowledgements
Special thanks go to Hannah Buggey, James Giles and Emily Robinson for in vivo tissue samples, Laura Cooper for immunohistochemistry and Catherine Smedley for her technical assistance. We also thank Joel Pachter (University of Connecticut Health Center, USA) for advice with the preparation of MBEC culture and Drs. Sandra Campbell and Daniel Anthony (University of Oxford, UK) for the SJC antibody. IL-1α/β−/− mice were kindly provided by Professor Yoichiro Iwakura (Institute of Medical Science, University of Tokyo, Japan). The Bioimaging Facility microscopes in the Faculty of Life Sciences used in this study were purchased with grants from BBSRC, Wellcome and the University of Manchester Strategic Fund, and the authors acknowledge the support of Peter March and Steve Marsden within this core facility for their help with the microscopy. The authors would also like to express their gratitude for the assistance of Tobias Starbourg in the Faculty of Life Sciences EM Facility, supported by a Wellcome Trust equipment grant.
1. This work was supported by the Medical Research Council, UK.
2. Abbreviations used in this article
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- BBB
blood-brain barrier
- CBA
cytometric bead array
- DAB
diaminobenzidine
- LDH
lactate dehydrogenase
- MAP2
microtubule associated protein 2
- MBEC
mouse brain endothelial cells
- MCAo
middle cerebral artery occlusion
- MMP-9
metalloproteinase-9
- NET
neutrophil extracellular trap
- NeuF
neurofilament
- NMDA
N-Methyl-D-aspartate
- PGP 9.5
protein gene product 9.5
- ROS
reactive oxygen species
Footnotes
Disclosures. NJR is a non executive director of AstraZenca but this has no relation to the current research.
References
- 1.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 2.Neumann J, Sauerzweig S, Ronicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30:10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 6.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 7.Nourshargh S, Marelli-Berg FM. Transmigration through venular walls: a key regulator of leukocyte phenotype and function. Trends in Immunology. 2005;26:157–165. doi: 10.1016/j.it.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 8.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 9.Denes A, McColl BW, Leow-Dyke SF, Chapman KZ, Humphreys NE, Grencis RK, Allan SM, Rothwell NJ. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J. Cereb. Blood Flow Metab. 2011;31:1036–1050. doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 11.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci U S A. 2004;101:331–336. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen HX, O’Barr TJ, Anderson AJ. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases, reactive oxygen species, and TNF-alpha. J Neurochem. 2007;102:900–912. doi: 10.1111/j.1471-4159.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 15.Shaw SK, Owolabi SA, Bagley J, Morin N, Cheng E, LeBlanc BW, Kim M, Harty P, Waxman SG, Saab CY. Activated polymorphonuclear cells promote injury and excitability of dorsal root ganglia neurons. Exp Neurol. 2008;210:286–294. doi: 10.1016/j.expneurol.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 17.Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allan SM, Parker LC, Collins B, Davies R, Luheshi GN, Rothwell NJ. Cortical cell death induced by IL-1 is mediated via actions in the hypothalamus of the rat. Proc Natl Acad Sci U S A. 2000;97:5580–5585. doi: 10.1073/pnas.090464197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence CB, Allan SM, Rothwell NJ. Interleukin-1beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 20.McCluskey L, Campbell S, Anthony D, Allan SM. Inflammatory responses in the rat brain in response to different methods of intra-cerebral administration. J Neuroimmunol. 2008;194:27–33. doi: 10.1016/j.jneuroim.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010;115:3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- 22.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(−)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]
- 23.Losman MJ, Fasy TM, Novick KE, Monestier M. Relationships among antinuclear antibodies from autoimmune MRL mice reacting with histone H2A-H2B dimers and DNA. Int Immunol. 1993;5:513–523. doi: 10.1093/intimm/5.5.513. [DOI] [PubMed] [Google Scholar]
- 24.Arruda MA, Rossi AG, de Freitas MS, Barja-Fidalgo C, Graca-Souza AV. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-kappaB. J Immunol. 2004;173:2023–2030. doi: 10.4049/jimmunol.173.3.2023. [DOI] [PubMed] [Google Scholar]
- 25.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 26.Sunil VR, Connor AJ, Lavnikova N, Gardner CR, Laskin JD, Laskin DL. Acute endotoxemia prolongs the survival of rat lung neutrophils in response to 12-O-tetradecanoyl-phorbol 13-acetate. J Cell Physiol. 2002;190:382–389. doi: 10.1002/jcp.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGettrick HM, Lord JM, Wang KQ, Rainger GE, Buckley CD, Nash GB. Chemokine- and adhesion-dependent survival of neutrophils after transmigration through cytokine-stimulated endothelium. J Leukoc Biol. 2006;79:779–788. doi: 10.1189/jlb.0605350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson RW, Rotstein OD, Nathens AB, Parodo J, Marshall JC. Neutrophil apoptosis is modulated by endothelial transmigration and adhesion molecule engagement. J Immunol. 1997;158:945–953. [PubMed] [Google Scholar]
- 29.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Berghe TV. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death and Differentiation. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil Extracellular Trap Formation Is Associated with IL-1beta and Autophagy-Related Signaling in Gout. PLoS ONE. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumari R, Willing LB, Patel SD, Baskerville KA, Simpson IA. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem. 2011;119:1029–1040. doi: 10.1111/j.1471-4159.2011.07487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stowe AM, Adair-Kirk TL, Gonzales ER, Perez RS, Shah AR, Park TS, Gidday JM. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis. 2009;35:82–90. doi: 10.1016/j.nbd.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death and Differentiation. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 36.Brinkmann V. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 37.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. Plos Pathogens. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinz A, Jung MC, Jahreis G, Rusciani A, Duca L, Debelle L, Weiss AS, Neubert RH, Schmelzer CE. The action of neutrophil serine proteases on elastin and its precursor. Biochimie. 2011;94:192–202. doi: 10.1016/j.biochi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Denes A, McColl BW, Leow-Dyke SF, Chapman KZ, Humphreys NE, Grencis RK, Allan SM, Rothwell NJ. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab. 2011;31:1036–1050. doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wewer C, Seibt A, Wolburg H, Greune L, Schmidt MA, Berger J, Galla HJ, Quitsch U, Schwerk C, Schroten H, Tenenbaum T. Transcellular migration of neutrophil granulocytes through the blood-cerebrospinal fluid barrier after infection with Streptococcus suis. J Neuroinflammation. 2011;8:51. doi: 10.1186/1742-2094-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Kim S, Ubel P, De Vries R. Pruning the regulatory tree. Nature. 2009;457:534–535. doi: 10.1038/457534a. [DOI] [PubMed] [Google Scholar]
- 43.Pham CTN. Neutrophil serine proteases: specific regulators of inflammation. Nature Reviews Immunology. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 44.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 45.Worthmann H, Tryc AB, Deb M, Goldbecker A, Ma YT, Tountopoulou A, Lichtinghagen R, Weissenborn K. Linking infection and inflammation in acute ischemic stroke. Ann N Y Acad Sci. 2010;1207:116–122. doi: 10.1111/j.1749-6632.2010.05738.x. [DOI] [PubMed] [Google Scholar]
- 46.McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain research reviews. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Denes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppinen RA, Allan SM. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- 49.Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen MH, Bainton DF. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 50.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. Journal of Cell Biology. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. Journal of Cell Biology. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.