Abstract
Sphingosine-1-phosphate (S1P) is lipid messenger involved in the regulation of embryonic development, immune system functions, and many other physiological processes. However the mechanisms of S1P transport across cellular membranes remain poorly understood with several ATP-binding cassette family members and the spinster 2 (Spns2) member of the major facilitator superfamily known to mediate S1P transport in cell culture. Spns2 was also shown to control S1P activities in zebrafish in vivo and to play a critical role in zebrafish cardiovascular development. However the in vivo roles of Spns2 in mammals and its involvement in the different S1P-dependent physiological processes have not been investigated. Here we characterized Spns2-null mouse line carrying the Spns2tm1a(KOMP)Wtsi allele (Spns2tm1a). The Spns2tm1a/tm1a animals were viable, indicating a divergence in Spns2 function from its zebrafish orthologue. However the immunological phenotype of the Spns2tm1a/tm1a mice closely mimicked the phenotypes of partial S1P deficiency and impaired S1P-dependent lymphocyte trafficking, with a depletion of lymphocytes in circulation, an increase in mature single-positive T cells in the thymus, and a selective reduction in mature B cells in the spleen and bone marrow. Spns2 activity in the non-hematopoietic cells was critical for normal lymphocyte development and localization. Overall Spns2tm1a/tm1a resulted in impaired humoral immune responses to immunization. This work thus demonstrated a physiological role for Spns2 in mammalian immune system functions but not in cardiovascular development. Other components of the S1P signaling network are investigated as drug targets for immunosuppressive therapy, but the selective action of Spns2 may present an advantage in this regard.
Keywords: Spns2 (spinster 2), sphingosine-1-phosphate (S1P), lymphocyte egress
Introduction
Lipid messenger sphingosine-1-phosphate (S1P) is essential for normal embryonic development and the functions of the cardiovascular and immune systems (1); and components of the S1P signaling network are widely investigated as drug targets for suppression of transplant rejection, autoimmunity and sepsis (2). In the extracellular environment S1P acts through five G-protein coupled receptors (S1P1-5) expressed on a variety of cell types (reviewed in (3, 4)). In particular the loss of S1P receptor 1 (S1P1) results in embryonic lethality with abnormal development of the cardiovascular system, while a lymphocyte-specific loss of S1P1 causes impaired exit of mature T cells out of the thymus (5, 6), B cells out of the bone marrow (7, 8), as well as a severe defect in lymphocyte egress from secondary lymphoid organs during their physiological recirculation (9, 10). Receptor S1P1 is also required for normal localization of marginal zone B cells (11), B1 cells (12), plasma cells (13), and gut intra-epithelial T lymphocytes (14), while receptor S1P5 controls NK cell migration (15). Intracellular activities of S1P as a second-messenger molecule are broadly linked to cell survival and growth (16), and in the immune system intracellular S1P promotes inflammatory and antimicrobial activities of mast cells, macrophages, and neutrophils (17-22). In particular S1P is produced downstream of FcεR receptor cross-linking and stimulates mast cell degranulation (23, 24). It is also produced downstream of TNFα-receptor (TNFR) signaling, required for TRAF2-dependent RIP1 activation, NFκB signaling (25) and inflammatory cytokine production (26). These activities of S1P have been implicated in the pathologies of allergic and inflammatory disorders (27, 28).
The activities of S1P as a chemokine and a second-messenger are critically dependent on the S1P concentrations in the different cellular compartments, the tissue environment and the circulation, and these are established through the controlled rates of S1P production, degradation, and transport. S1P is synthesized by the sphingosine kinases SphK1 and SphK2 (29-31), and degraded by the S1P lyase and S1P phosphatases (1, 32). Combined loss of SphK1 and SphK2 in mice results in embryonic lethality with abnormal cardiovascular development, while the loss of S1P lyase causes a milder phenotype with impaired lymphocyte egress from lymphoid organs. In contrast, the mechanisms of S1P transport across cellular membranes remain poorly understood. Several proteins of the ATP-binding cassette (ABC) transporter superfamily can mediate S1P secretion in cell culture, for example ABCC1 in mast cells (33), ABCA1 in red blood cells (34), and another family member in platelets (35). Expression of the cystic fibrosis transmembrane conductance regulator (CFTR) on epithelial cell lines was also shown to contribute to S1P transport (36). Yet knockout mice for these proteins do not phenocopy the knockouts of known S1P signaling network components, and plasma S1P levels in the mice lacking ABCA1 and ABCC1 are unaltered (37). Some S1P is also produced extracellularly, by the extracellular SphK1 enzyme released from the vascular endothelium (38).
Spns2 (spinster homologue 2) is a member of the major facilitator superfamily of transmembrane proteins (39). In recent studies, zebrafish Spns2 was shown to mediate S1P secretion, and the loss of Spns2 activity resulted in lethal defects in cardiovascular development (40, 41), similar to the phenotypes of the SphK1−/−SphK2−/− and S1P1−/− mouse lines (31, 42). The human Spns2 protein was also shown to mediate the secretion of S1P as well as of S1P-receptor agonist and immunosuppressive drug phospho-FTY720 (43). Furthermore, human Spns2 expression could rescue the developmental defects in zebrafish embryos (40). However, the in vivo functions of Spns2 in the mammalian system have not been investigated.
In the current work we characterized an Spns2-targeted mouse line Spns2tm1a/tm1a generated by the Wellcome Trust Sanger Institute as part of the International Knockout Mouse Consortium (IKMC), and demonstrate the requirement for Spns2 for the normal lymphocyte localization and mammalian immune system function but not the other S1P-mediated functions such as embryonic viability and cardiovascular development.
Materials and Methods
Gene Targeting and Mouse Production
The mouse strain carrying the Spns2tm1a(KOMP)Wtsi allele was generated by blastocyst injection of ESC clone EPD0090_5_B04 obtained from the KOMP resource (44). Prior to microinjection, the identity of the targeted ES cells was verified by 5′ long-range PCR using a primer external to the targeting vector. Chimeric mice were bred to C57BL/6-Tyrc-Brd and germline transmission was verified by quantitative PCR (qPCR) to detect the neo transgene included in the mutant allele (single insertion event), as well as by loss-of-wild type allele (LOA) qPCR (correct targeted locus) in the F1 heterozygous mice. The presence of the downstream loxP site was verified by PCR. The C57BL/6N-HprtTg(CMV-cre)Brd/Wtsi and C57BL/6N-Gt(ROSA)26Sortm1(FLP1)Dym/Wtsi transgenic lines with systemic expression of Cre and Flp recombinases were previously described (45, 46). The Spns2tm1b(KOMP)Wtsi allele was generated by crossing the tm1a allele to the C57BL/6N-HprtTg(CMV-Cre)Brd allele to delete exon 3 and the neo cassette between the LoxP sites, and the Cre allele was bred out of the colony before study. The Spns2tm1c(KOMP)Wtsi allele was generated by breeding to C57BL/6N-Gt(ROSA)26Sortm1(FLP1)Dym/Wtsi (Rosa26Fki) allele expressing Flp recombinase ubiquitously, to delete the inserted cassette but retaining exon 3 flanked by LoxP sites. All the studies were performed on a C57BL/6 genetic background. The mice were maintained in specific pathogen-free conditions, and matched by age and sex within experiments. The care and use of all mice was in accordance with UK Home Office regulations, UK Animals Scientific Procedures Act 1986.
RNA isolation and qPCR
For the comparisons of Spns2 transcript levels in wild type and Spns2tm1a/tm1a tissues (Figure 1B) RT-qPCR was performed using an “RNA-to-Ct One Step” kit (Applied Biosystems) in a 10μl reaction with 1μl of total RNA (20-500ng depending on tissue type). A TaqMan assay (Mm01249325) spanning the exons flanking the splice acceptor site of the construct was used in a multiplex reaction with a GAPDH endogenous control to normalize for variations between the amounts of input RNA (Applied Biosystems), and amplified in triplicate using a Viia7 qPCR machine (Applied Biosystems). Analysis was performed using the Viia7 1.1 analysis software and the ΔΔCt relative quantification module. For the comparisons of Spns2-transcript levels across different tissues of wild type mice (Figure 5A), RNA was isolated using the RNeasy Plus Mini Kit (Qiagen) and reverse-transcribed with the QuantiTect Reverse Transcription Kit (Qiagen). qPCRs were performed using the QuantiTect SYBR Green PCR Kit (Qiagen), using Spns2 QuantiTect Primer Assay (Qiagen) and primers Actb_Fw CTAAGGCCAACCGTGAAAAG, Actb_Rv ACCAGAGGCATACAGGGACA (Sigma-Aldrich). The Spns2 primers spanned the boundaries of exons 5 to 7 of the Spns2 coding transcript ENSMUST00000045303. The data was acquired on the StepOnePlus™ Real-Time PCR system (Applied Biosystems), and analyzed using the ΔΔCt method.
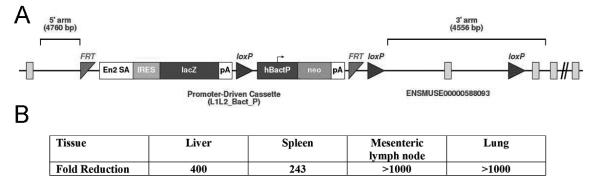
Figure 1. Spns2 gene targeting.
(A) Structure of the Spns2m1a(KOMP)WTSI (Spns2tm1a) allele. (B) Average fold reduction in Spns2-transcript levels in Spns2tm1a/tm1a relative to wild type tissues (liver, spleen, lymph nodes and lung), analyzed by qRT-PCR using primers spanning the junction of exons 2-3 of the Spns2 coding transcript ENSMUST00000045303.
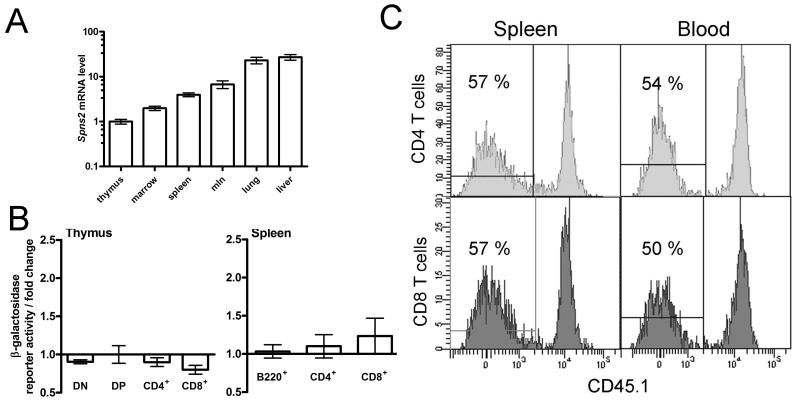
Figure 5. Analysis of Spns2 expression.
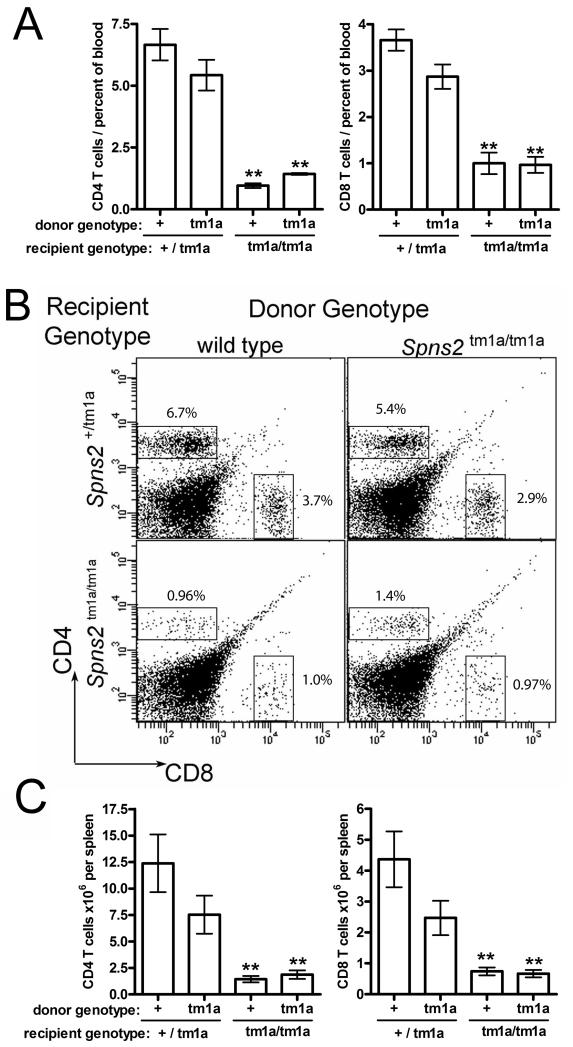
(A) qRT-PCR analysis of the Spns2 transcript levels in the tissues of wild type mice. Analysis using primers spanning exon junctions 5-7 of Spns2 coding transcript ENSMUST00000045303, data analyzed using the ΔΔC method with β-actin as the housekeeping control; Spns2 transcript levels in the thymus were assigned the arbitrary value of one and the levels in other tissues expressed relative to it. (B) β-galactosidase reporter activity in thymocytes, and splenic B and T cells, as a measure Spns2 gene expression. The measurements were done in Spns2+/tm1a cells and are presented as fold change relative to the background β-galactosidase activity in the same cell type in wild type mice. The cells were gated as: CD4−CD8− for double negative thymocytes (DN), CD4+CD8+ for double positive thymocytes (DP), and B220+ for splenic B cells. Bars represent means ± SEM from ≥3 mice per group. (C) Mixed bone marrow chimeras, demonstrating that the defects in the development and localization of Spns2tm1a/tm1a T cells are not due to a cell-intrinsic requirement for Spns2. Lethally-irradiated Spns2+/+Rag1−/− recipients were reconstituted with a 50:50 mix of wild type CD45.1+-marked and Spns2tm1a/tm1a bone marrow. Flow cytometry histograms of CD4 and CD8 T cells in the spleen and blood at 8 weeks following reconstitution are shown, percentage of C45.1- Spns2tm1a/tm1a T cells in each plot is indicated.
Flow cytometry
Cell suspensions of mouse tissues were prepared in RPMI-1640 with 2% (v/v) fetal calf serum (Sigma-Aldrich), 100 μg/ml streptomycin, 100 U/ml penicillin (all from Invitrogen). Blood was collected into heparin-coated tubes (Kabe Labotechnik) by cardiac puncture and erythrocytes lysed using PharmaLyseTM (BD Biosciences). The cells were stained in PBS with 2% fetal calf serum (Sigma-Aldrich) and 0.2% (w/v) sodium azide (Sigma-Aldrich) for 20 minutes on ice, with the following antibodies. Fluorescein-conjugated antibodies were against CD4 (clone L3T4), CD8 (53-6.7), CD11b (M1/70), CD21 (7G6), CD86 (GL1), and B220 (RA3-6B2, all from BD Pharmingen). Phycoerythrin-conjugated antibodies were against CD8 (clone 53-6.7), CD19 (1D3), CD69 (H1.2F3), CD80 (16-10A1), and IgM (R6-60.2, all from BD Pharmingen). Allophycocyanin conjugated antibodies were against CD4 (RM4-5), CD8 (53-6.7), and CD44 (IM7, all from BD Pharmingen). Allophycocyanin Cy7 antibodies were against CD8 (53-6.7), CD11b (M1/70, both from BioLegend), and B220 (RA3-6B2, from BD Pharmingen). Peridinin chlorophyll A protein (PerCP) conjugated anti-CD45.1 (A20, BioLegend), Alexa Fluor 647 conjugated anti-IgD (clone 11-26, eBioscience), and Phycoerythrin Cy7 anti-CD23 (B3B4, eBioscience) were also used. Flow cytometric measurements of β-galactosidase activity were performed using FluoReporter LacZ Flow Cytometry Kits (Invitrogen, Molecular Probes). The cells were stained for appropriate combinations of cell-surface lineage markers, before loading with fluorescein di-β-D-galactopyranoside (FDG) and analysis by flow cytometry. The data was acquired on BD FACS Aria or LSRII flow cytometers, and analyzed with FACS Diva Software.
ELISA
For the measurements of antibody levels, mouse blood was collected by tail-bleed or cardiac puncture, and serum prepared and stored at −20°C. For antigen-specific antibody measurements in mouse serum Nunc Maxisorp plates were coated overnight at 4°C with 2 mg/mL of tetanus toxoid C (TetC) in 0.1M Na2HPO4 pH 9.0, blocked with 3% (w/v) bovine serum albumin (BSA) in PBS for 1 hour, and incubated with 5-fold serial dilutions of mouse serum in PBS with 1% BSA for 1 hour. The plates were developed with anti-mouse IgG, IgG1 or IgG2a horseradish peroxidase conjugated antibodies (BD Pharmingen), followed by the OPD Substrate Tablets (o-phenylenediamine, Sigma-Aldrich) dissolved in water. Cytokine ELISA on cell culture supernatants was performed using anti-mouse TNF-α coating antibody clone 1F3F3D4 and biotin-conjugated detection antibody clone XT3/XT22, followed by avidin horseradish peroxidase (all from eBioscience), and the TMB Liquid Substrate System (Sigma-Aldrich). Absorbances were measured using the BioRad 680 MicroPlate Reader (BioRad).
Measurements of S1P levels and activity
For the measurements of S1P levels, mouse blood was collected from the retro-orbital sinus. S1P levels in the plasma were measured using the ELISA-based S1P assay kit (Echelon Biosciences) according to the manufacturer’s protocol. Assays of S1P activity in mouse plasma used the S1P1 Redistribution Assay (Thermo Scientific) according to the manufacturer’s instructions. Briefly, the assay measured S1P-induced internalization of S1P1 receptor, when mouse plasma is added at different dilutions to the U2OS cells stably expressing GFP-tagged S1P1. Internalization of S1P1-GFP was quantified using the spot detection algorithm and the ‘spot total area per object’ function on Cellomics ArrayScan VTI system. S1P (Cayman Chemicals) was used to prepare the positive controls.
Mouse immunization
Recipient mice were immunized by intranasal inhalation of 30μL volume of PBS containing 10mg Tet-c (tetanus-toxin fragment C recombinant protein, a gift from Omar Qazi, Imperial College) combined with 1mg heat-labile toxin (LT) of Escherichia coli (gift of Rino Rappuoli, Chiron) adjuvant. Mice were boosted on days 7, 21 and 37. Serum samples were collected on days 36 and 40. Detection of TetC specific antibodies from sera was performed by ELISA as described above.
Mouse bone marrow transfer experiments
Recipient animals were irradiated with 2 doses of 4.5 Gy, 3 hours apart, and injected intravenously with 3×106 donor bone marrow cells. The mice were maintained on clindamycin (250mg/l) in drinking water for 2 week, and analyzed 8 weeks after reconstitution. In one experiment Spns2tm1a/tm1a and Spns2+/tm1a recipients were reconstituted either with wild type CD45.1+-marked or with Spns2tm1a/tm1a donor bone marrow. In a separate study Spns2+/+Rag1−/− recipients were reconstituted with wild type CD45.1+-marked bone marrow and Spns2tm1a/tm1a bone marrow, either separately or together mixed in 1:1 ratio. Using CD45.1+-marked wild type donor bone marrow we confirmed that this protocol results in a complete replacement of the hematopoietic system of the recipients, so that >95% of the hematopoietic progenitors, bone marrow cells of all hematopoietic lineages, as well as cells in the thymus were donor-derived (data not shown).
Tissue culture
Bone marrow derived macrophages (BMDMs) were generated by culturing mouse bone marrow for 6 days in high-glucose DMEM (Invitrogen) supplemented with 20% (v/v) fetal calf serum (Sigma-Aldrich), 25% (v/v) L-conditioned media (supernatant of cell line L-929), 2mM L-glutamine, 1mM sodium pyruvate, 100 μg/ml streptomycin, 100 U/ml penicillin (all from Invitrogen). The cells were maintained at 37°C and 5% CO2 in a humidified incubator. For stimulation the cells were re-plated at 1×106/ml, and treated with 100 ng/ml LPS (Sigma-Aldrich), 25 ng/ml IFNγ (R&D Systems), and/or 25 ng/ml TNFα (eBioscience) over a 48 hour time course.
Tissue staining for β-galactosidase activity
Mice were fixed by cardiac perfusion with 4% (w/v) paraformaldehyde. Following dissection, the tissues were fixed in 4% paraformaldehyde for further 30 minutes, rinsed in PBS, and stained in 0.1% (w/v) X-gal solution (bromo-chloro-indolyl-galactopyranoside, Invitrogen) for up to 48 hours. After an additional overnight fixation in 4% paraformaldehyde, the tissues were cleared with 50% (v/v) glycerol and transferred to 70% glycerol for long-term storage. Images were taken using a Leica MZ16A microscope and Imagic software.
Statistical analyses
Statistical comparisons were performed with Prism 4.0 Software (GraphPad Inc.), using two-tailed Student’s t-test or non-parametric Mann Whitney test for comparisons of two data sets, and ANOVA for multiple comparisons.
Results
Spns2 gene targeting and mouse production
Spns2 gene targeting was carried out as part of the International Knockout Mouse Consortium, in the JM8 embryonic stem cell line on a C57BL/6N genetic background (44). The targeted Spns2tm1a(KOMP)WTSI allele carried a gene-trap DNA-cassette, inserted into the second intron of the gene, consisting of a splice acceptor site, an internal ribosome entry site (IRES) and a β-galactosidase reporter, followed by a neomycin resistance marker expressed from an independent β-actin promoter (Figure 1A, Genbank file available at www.knockoutmouse.org). The use of the splice acceptor site in the cassette is predicted to generate a truncated non-functional transcript encoding the first 145 out of 549 amino acids of the Spns2 protein, including only one out of the eleven predicted Spns2 transmembrane α-helices, (prediction using Swiss EMBNet www.ch.embnet.org/software/TMPRED_form.html). qRT-PCR analysis of Spns2tm1a/tm1a mouse tissues (liver, lung, spleen, and mesenteric lymph nodes) confirmed that the targeted allele effectively disrupted the production of Spns2-coding mRNA, with 200-1000 fold reduction in the levels of the transcript containing the junction of exons 2-3 of Spns2 mRNA ENSMUST00000045303 in the different tissues analyzed (Figure 1B). The locations of the LoxP and Frt sites are designed to allow the conversion of the allele to a conditional configuration in future studies (44).
The Spns2tm1a/tm1a mice were profiled using a series of high-throughput phenotype screens, under the scope of the Sanger Institute Mouse Genetics Project, with the full information available at http://www.sanger.ac.uk/mouseportal/search?query=spns2. The Spns2tm1a/tm1a animals showed no significant increase in the levels of embryonic mortality in strong contrast to the previously characterized embryonic lethal SphK1−/−SphK2−/− and S1P1−/− mouse lines (31, 42). The Spns2tm1a/tm1a mice also showed no gross dysmorphology, apart from abnormal eye pigmentation and opacity, and were able to breed normally. In particular these mice demonstrated no alterations in heart weight or histology at 16 weeks of age, in strong contrast to Spns2−/− zebrafish that exhibited defects in embryonic heart development (cardia bifida) (40, 41), and SphK1−/−SphK2−/− mice that demonstrated poor embryonic development of the dorsal aorta (31). Abnormalities in the immune phenotype of the Spns2tm1a/tm1a line were analyzed in this work, while the phenotypes of the ear and eye will be reported elsewhere (Chen J., Steel K.P., manuscript in preparation).
Abnormal T and B lymphocyte development and localization in Spns2tm1a/tm1a mice
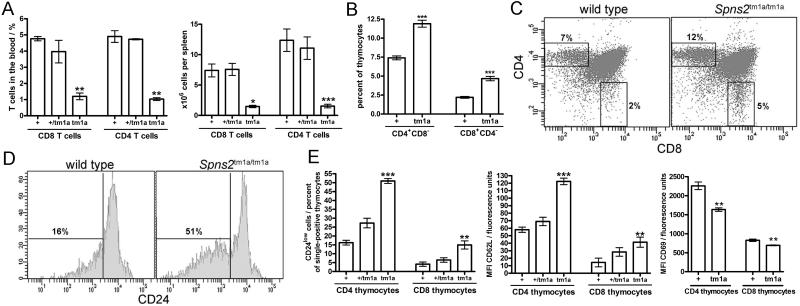
To establish the impact of Spns2tm1a/tm1a on lymphocyte development and localization, the blood and lymphoid organs of Spns2tm1a/tm1a mice were analyzed. We observed a reduction in the overall leukocyte counts in Spns2tm1a/tm1a mouse blood (3.38 × 103/μl in Spns2tm1a/tm1a versus 6.63 ×103/μl in the wild type mice, Supplementary Figure S1A), while erythrocyte and platelet counts were not affected (data not shown). The percentage of CD4 and CD8 lineage T cells in the blood, and the absolute numbers of CD4 and CD8 T cells in the spleen of Spns2tm1a/tm1a mice were significantly reduced (Figure 2A, ~ 4 fold). This was accompanied by a moderate increase in the proportion of mature CD4 and CD8 single-positive T cells in the thymus (Figure 2B-C), with the CD4+CD8− cells constituting 12±0.9% of thymocytes in Spns2tm1a/tm1a as compared with 7±0.5% in wild type mice (mean ± S.D.). The thymic CD4+CD8− and CD4−CD8+ Spns2tm1a/tm1a T cells expressed lower levels of CD24 and CD69, and higher levels of CD62L, indicating their more mature status (Figure 2D-E). These features closely resemble the phenotypes of other mouse lines with defects in S1P production, turnover, or sensing (5, 6, 32, 47), indicating an accumulation of mature T cells in the thymus and suggesting a defect in T cell recruitment from the thymus into the circulation. There were no abnormalities in the numbers of the earlier thymocyte subsets, including CD4−CD8− double negative thymocytes (DN1-DN4, differentiated by CD44 and CD25 expression) and CD4+CD8+ double-positive thymocytes (data not shown).
Figure 2. T cell abnormalities in the Spns2tm1a/tm1a mice.
(A) Percentage of CD4 and CD8 T cells in the blood, and numbers of CD4 and CD8 T cells in the spleen of wild type, Spns2+/tm1a, and Spns2tm1a/tm1a mice (+, +/tm1a, and tm1a, respectively). (B) Percentage of CD4+ and CD8+ single-positive cells in the thymus of wild type, Spns2+/tm1a, and Spns2tm1a/tm1a mice (+, +/tm1a, and tm1a, respectively). (C) Representative flow cytometry plots of wild type and Spns2tm1a/tm1a thymocytes stained for CD4 and CD8; average percentage of cells within the CD4+ and CD8+ single-positive gates is indicated. (D) Representative flow cytometry histograms indicating CD24 expression on CD4+ single-positive thymocytes in wild type and Spns2tm1a/tm1a mice. (E) Percentage of CD24low cells within the CD4+ and CD8+ single-positive thymocyte gates; and the expression of CD62L and CD69 on CD4+ and CD8+ single-positive thymocytes in wild type (+), Spns2+/tm1a (+/tm1a) and Spns2tm1a/tm1a (tm1a) mice. Bars represent means ± SEM; MFI, mean fluorescence intensity; *p<0.05, **p<0.01, *** p<0.001 using ANOVA with Bonferroni’s post-hoc test or t-test; data from 3-4 mice per group and reproducible in two independent experiments.
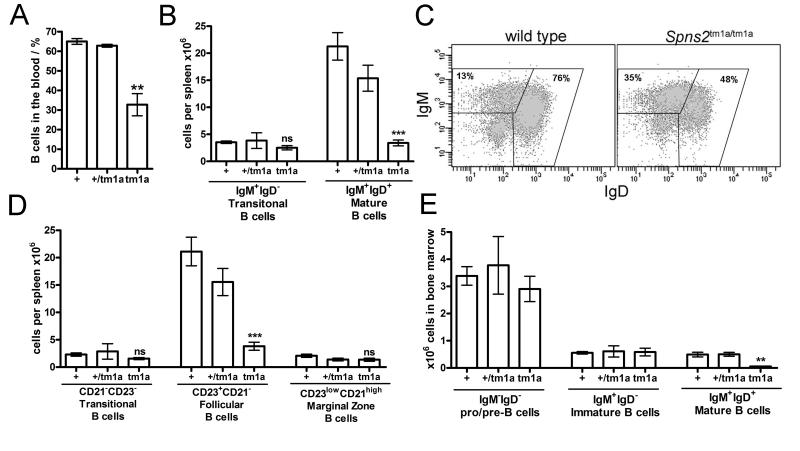
There was also a significant reduction in the number of re-circulating B cells in Spns2tm1a/tm1a mice (CD45+CD19+, Figure 3A). This was accompanied by a reduction in the follicular B cell population in the spleen (gated as B220+IgM+IgD+ or B220+CD23+CD21lo, Figures 3B-D) and the mature B cell population in the bone marrow (B220+IgM+IgD+, Figure 3E). In contrast no abnormalities in earlier B cell developmental subsets were observed, with normal numbers of pro/pre-B cells (B220+IgM−IgD−) and immature B cells (B220+IgM+IgD−) in the bone marrow and transitional B cells (B220+CD23loCD21lo) in the spleen of the Spns2tm1a/tm1a mice (Figure 3D-E). Overall, this phenotype resembles the loss of re-circulating and mature follicular B cell population previously reported in other mouse lines with impaired S1P production or sensing (5, 32, 47). We also observed a reduction in the numbers of B1 cells in the peritoneal cavity of Spns2tm1a/tm1a mice (B220lowIgMhighIgDlow, data not shown), consistent with the role of S1P in the control of their trafficking (12).
Figure 3. Reduction in the numbers of re-circulating B cells in the blood and mature B cells in the spleen and bone marrow of Spns2tm1a/tm1a mice.
(A) Reduction in the percentage of B cells (gated as CD45+CD19+) in the blood of Spns2tm1a/tm1a mice. (B) Reduction in the absolute number of mature B cells (gated as B220+IgM+IgD+) in the spleen of Spns2tm1a/tm1a mice. (C) Representative flow cytometry plots of splenocytes, stained for B220, IgM and IgD, and gated on the B220+ B cell population. Gates indicate IgM+IgD− transitional and IgM+IgD+ mature B cells; the average percentage of cells within each gate for all mice in the group is indicated. (D) Reduction in the numbers of mature follicular B cells in the spleen of Spns2tm1a/tm1a mice confirmed by B220+CD21−CD23+ staining. (E) Reduction in mature B cells (B220+IgM+IgD+) in the bone marrow of Spns2tm1a/tm1a mice. All bars represent means ± SEM; **p<0.01, *** p<0.001 using ANOVA with Bonferroni’s post-hoc test; data from 3 mice per group and reproducible in two independent experiments; bone marrow cell counts are per one tibia and femur.
Reduced antibody responses to immunization in Spns2tm1a/tm1a mice
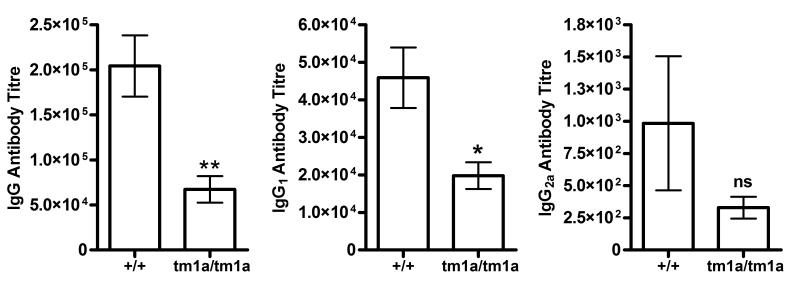
To establish whether the defects in lymphocyte development and localization seen in the Spns2tm1a/tm1a mice led to impaired antibody responses to immune challenge, the mice were immunized intra-nasally with TetC (tetanus-toxin fragment C recombinant protein), boosted at days 7 and 21, and analyzed for antigen specific antibody titers in the serum at day 36. Significant reductions in antigen-specific total-IgG and IgG1 were seen (Figure 4), indicating impaired humoral immunity in the Spns2tm1a/tm1a mouse line. The mice were subsequently given a tertiary boost, and serum antibody levels measured again three days later (day 40), confirming the reduction in antigen-specific total-IgG and IgG in Spns2tm1a/tm1a mice (data not shown). The levels of IgG2a were, however, not significantly reduced (Figure 4), and this might have been due to the low levels and high variation in IgG2a production in both the wild type and Spns2tm1a/tm1a groups.
Figure 4. Impaired humoral immune response in the Spns2tm1a/tm1a mouse line.
Wild type (+/+) and Spns2tm1a/tm1a (tm1a/tm1a) mice were immunized intra-nasally with TetC, boosted at days 7 and 21, and analyzed for antigen specific antibody titers in the serum at day 36. Bars represent means ± SEM; *p<0.05, ** p<0.01, ns- non-significant, using Student’s t-test; data from 6 mice per group.
Normal macrophage responses to inflammatory stimuli in Spns2tm1a/tm1a mice
S1P functions not only as an chemoattractant in the extracellular environment, but also as an intracellular messenger required for the normal signaling downstream of TNFR and Toll-like receptors (TLRs) (25); furthermore inhibition of S1P production is protective in animal models of endotoxemia and sepsis (27). To establish whether Spns2tm1a/tm1a disrupted normal cellular responses to TLR and TNFR stimulation, bone marrow derived macrophages (BMDM), generated from Spns2tm1a/tm1a and wild type control mice, were stimulated with lipopolysaccharide (LPS, 100 ng/ml), interferon γ (IFNγ, 25 ng/ml), and/or TNFα (25 ng/ml), and analyzed for induction of CD80 and CD86 activation markers and secretion of inflammatory cytokines. No differences in the responses of Spns2tm1a/tm1a and wild type cells were observed (Supplemental Figure S1B). This suggests that Spns2 does not affect intracellular S1P functions in macrophages downstream of TLR or TNFR stimulation. This is in contrast to SphK1, the knockdown of which in similar systems was previously shown to reduce monocyte and macrophage responses to TLR and TNFR stimulation (26).
Cell-intrinsic activity of Spns2 is dispensable for lymphocyte development and localization
Many cell types have been shown to produce and release S1P, including erythrocytes, lymphatic and vascular endothelial cells, platelets, and mast cells (33, 35, 47-51). To assess Spns2 expression in a broad range of mammalian cell types, a range of tissues and organs from Spns2+/tm1a mice were profiled for the activity of the β-galactosidase reporter expressed from the endogenous Spns2-promoter in mice carrying the Spns2tm1a allele (52). The studies were done as part of the Sanger Mouse Genetics Project high-throughput phenotyping, and the data on the β-galactosidase reporter activity in 39 organs and tissues of Spns2+/tm1a mice is summarized in Supplemental Table 1, with images available at: www.sanger.ac.uk/mouseportal/phenotyping/MBNZ/adult-lac-z-expression. The expression of the Spns2 gene was further confirmed in a selection of tissues using wild type tissues and qRT-PCR. Overall it was demonstrated that there were high Spns2 transcript levels in the liver and lung, lower levels in the lymph nodes, spleen, and bone marrow, and low but detectable levels in the thymus (Figure 5A).
To establish whether Spns2 was expressed in the lymphocyte populations affected by the Spns2tm1a/tm1a phenotype, Spns2-promoter-driven β-galactosidase reporter activity was analyzed in Spns2+/tm1a lymphocytes using flow cytometry with a fluorescent β-galactosidase substrate. The data showed no significant β-galactosidase activity over the background level in the different subpopulations of thymocytes or in splenic T cells (Figure 5B), indicating that Spns2 gene is not expressed in these cell types. The Spns2 gene was also not expressed in splenic B cells (Figure 5B). As a positive control, high levels of β-galactosidase reporter activity were detected in hematopoietic cells of another mouse line Mysm1+/tm1a (53).
The requirement for Spns2 expression and activity in lymphocytes for their normal development and localization was further tested using bone marrow chimeras. Lethally-irradiated Spns2+/+Rag1−/− recipients were reconstituted with a 50:50 mix of CD45.1+-marked wild type and Spns2tm1a/tm1a bone marrow and analyzed at 8 weeks after reconstitution. No significant differences in the development and localization of wild type and Spns2tm1a/tm1a lymphocytes were observed in this study. For example, approximately 50% of CD4 and CD8 T cells in the blood and spleen of the chimeric mice were CD45.1−ve Spns2tm1a/tm1a (Figure 5C). This demonstrated that Spns2tm1a/tm1a lymphocytes could develop and migrate normally when placed in a wild type environment, and therefore that Spns2 expression in lymphocytes was dispensable for their normal development and localization. Additionally, an adoptive intra-venous transfer of 107 Spns2-wild type GFP-expressing splenocytes into either wild type or Spns2tm1a/tm1a recipients, demonstrated a significant reduction in the transferred CD4 T cells in the blood of Spns2tm1a/tm1a as compared to wild type mice at 48 hours, suggesting the role of Spns2tm1a/tm1a environment in affecting CD4 T cell localization, however no reduction in CD8 T cells and B cells was observed (data not shown).
Spns2 activity in the non-hematopoietic stromal cells is essential for normal immune function
The requirement for Spns2 expression and activity on different cell types for normal lymphocyte development and trafficking was investigated further using bone marrow chimeras. Lethally-irradiated recipients of Spns2tm1a/tm1a and Spns2+/tm1a genotypes were reconstituted either with wild type (CD45.1+) or with Spns2tm1a/tm1a (CD45.1−) bone marrow, and analyzed by flow cytometry at 8 weeks after the reconstitution. The results indicated that the Spns2 genotype of the non-hematopoietic cells was of primary importance for normal lymphocyte development in the chimeras. Thus when the wild type hematopoietic system was reconstituted into Spns2tm1a/tm1a hosts, the numbers of T cells were depleted in the blood and spleen, to the same extent as in Spns2tm1a/tm1a mice reconstituted with Spns2tm1a/tm1a bone marrow (Figure 6 A-C). Similarly, mature B cells were depleted to an equal extent in both groups of mice, in the blood, spleen, as well as the bone marrow (Supplemental Figure S2). In contrast, in the chimeric mice with selective loss of Spns2-function in the hematopoietic compartment, there was a trend towards decreased lymphocyte numbers but this did not reach statistical significance (Figure 6, Figure S3), further indicating that Spns2 is primarily functional in the non-hematopoietic cells. Overall these data suggested that Spns2 expression and function on the cells of the non-hematopoietic stroma had a primary role in the maintenance of normal lymphocyte development and immune system function.
Figure 6. Bone marrow chimeras experiments indicate that Spns2 expression and function in the non-hematopoietic cells of the stoma is required for normal lymphocyte development.
Lethally irradiated (2 × 4.5Gy) recipients of Spns2tm1a/tm1a and Spns2+/tm1a genotypes were reconstituted either with wild type (+) or with Spns2tm1a/tm1a (tm1a) donor bone marrow, and the numbers of lymphocyte subsets were analyzed by flow cytometry at 8 weeks following reconstitution. (A-B) Percentage of CD4 and CD8 T cells in the blood of the chimeric mice of the four groups. (B) Representative flow cytometry plots of the blood of the chimeric mice, stained for CD4 and CD8; average percentages of cells within the gated populations are indicated. (C) Absolute numbers of CD4 and CD8 T cells in the spleen of the chimeric mice of the four groups. Bars represent means ± SEM from ≥3 mice per group; statistical comparisons using ANOVA with Bonferroni’s post-hoc test to compare each dataset to the control group; **p<0.01.
No significant reduction in the plasma S1P levels in Spns2tm1a/tm1a mice
S1P levels in the plasma of Spns2tm1a/tm1a and wild type mice were measured using two methods, an ELISA-based S1P assay (Echelon Biosciences, Figure 7A), and the S1P1 Redistribution Assay that measures S1P-induced internalization of S1P1 receptor (Thermo Scientific, Figure 7B-C). In the latter assay, the plasma of wild type and Spns2tm1a/tm1a mice was added at different dilutions to U2OS cells expressing GFP-tagged S1P1 receptor and the internalization of S1P1-GFP was quantified using a Cellomics Array-Scan VTI high-throughput cell imaging system. No significant differences in S1P concentrations or activity were observed between Spns2tm1a/tm1a and wild type mice in either assay (Figure 7A, C). This indicated that Spns2-independent mechanisms exist for maintaining overall S1P levels in the blood of Spns2tm1a/tm1a mice, but did not rule out the possibility that S1P levels are reduced in certain localized environments and that this was responsible for the altered lymphocyte distribution and immune function in Spns2tm1a/tm1a mice. Normal viability and lack of gross developmental defects in the Spns2tm1a/tm1a mice are also consistent with localized rather than systemic defects in S1P export. S1P concentrations in the lysates of spleen and thymus tissues were below the limit of detection of the Echelon Biosciences ELISA assay for both wild type and Spns2tm1a/tm1a mice (<0.06μM, data not shown).
Figure 7. No significant alterations in the levels of S1P in the plasma of Spns2tm1a/tm1a mice.
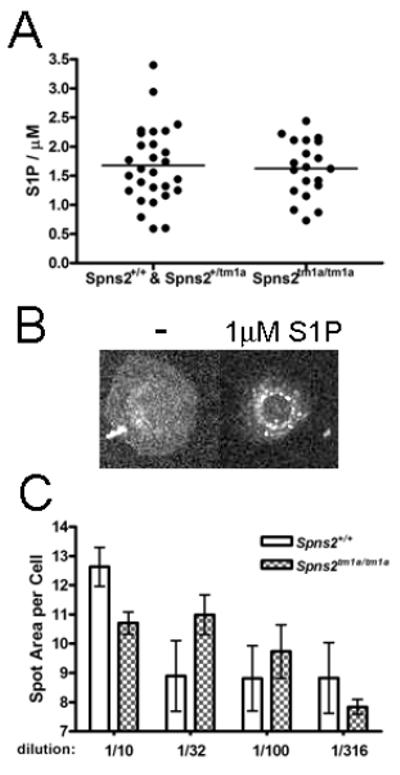
(A) S1P concentration in plasma measured using the ELISA-based S1P assay kit (from Echelon Biosciences). (B) Sample images of U2OS cells expressing GFP-tagged S1P1 receptor (Thermo Scientific) following exposure to media containing either no S1P or 1μM S1P. The data was acquired using the Cellomics cell imaging system, and shows internalization of S1P1 receptor in the cells exposed to S1P. (C) Cellomics-system based quantification of S1P1-GFP receptor internalization in U2OS cells exposed to wild type or Spns2tm1a/tm1a plasma at different dilutions; quantification performed using the spot detection algorithm and the ‘spot total area per object’ function. Bars represent means ± SEM from 4 mice per group; the differences between wild type and Spns2tm1a/tm1a samples are not statistically significant.
Characterization of Spns2-knockout Spns2tm1b/tm1b mice
To further confirm that the Spns2tm1a/tm1a mice were phenotypically equivalent to Spns2 knockout animals, the Spns2tm1a/tm1a line was crossed to the C57BL/6N-HprtTg(CMV-cre)Brd/Wtsi transgenic line with systemic expression of Cre-recombinase (45). This resulted in germline excision of asymmetric exon 3 of the Spns2 gene (Supplemental Figure S3A), causing a frame-shift in the Spns2-transcript, and is therefore predicted to result in a full loss Spns2 protein expression. Additionally the removal of the β-actin promoter, contained in the neomycin cassette, controlled for any possible side-effects of this cassette on the phenotype. The resulting allele structure was designated Spns2tm1b(KOMP)Wtsi; and the homozygous Spns2tm1b/tm1b mice were viable with no significant increase in embryonic mortality. Flow cytometry analysis of lymphoid organs demonstrated a reduction in CD4 and CD8 T cells, and mature B cells in the spleen of Spns2tm1b/tm1b (Supplemental Figure S3B), comparable to Spns2tm1a/tm1a mice (Figures 1A, 2A). There was also an increase in the proportion of CD4+CD8− and CD4−CD8+ T cells in the thymus of Spns2tm1b/tm1b mice, and these cells expressed higher levels of CD62L and lower levels of CD24 indicating their more mature status (Supplemental Figure S3B), as seen previously in Spns2tm1a/tm1a mice (Figure 1B-E). Overall this confirmed that Spns2tm1a/tm1a phenotype is equivalent to the Spns2-knockout Spns2tm1b/tm1b.
Additionally, to further confirm that the Spns2tm1a/tm1a phenotype resulted from the gene-trap cassette in the Spns2-locus, the Spns2tm1a/tm1a animals were crossed to a transgenic line with systemic expression of Flp-recombinase C57BL/6N-Gt(ROSA)26Sortm1(FLP1)Dym/Wtsi (46), causing germline excision of the gene-trap cassette (Supplemental Figure S3A). The resulting allele was designated Spns2tm1c(KOMP)Wtsi, and the Spns2tm1c/tm1c mice showed a rescue of lymphocyte numbers in the spleen and bone marrow and thymus (Supplemental Figure S3C and data not shown), confirming that the immune phenotype of the Spns2tm1a/tm1a line was caused by the Spns2tm1a-gene trap cassette.
Discussion
In this study we have characterized an Spns2-targetted mouse line and demonstrated that Spns2 is required for normal lymphocyte development and localization, and for normal humoral immune response to immunization. Overall the changes in lymphocyte subpopulations in Spns2tm1a/tm1a and Spns2tm1b/tm1b mice closely mimicked the phenotypes of partial S1P deficiency and impaired S1P-dependent lymphocyte trafficking, including the depletion of lymphocytes in circulation, increase in the mature single-positive T cells in the thymus, and a selective reduction in the mature B cell population in the spleen and bone marrow (5, 6, 10, 32, 47). Although we did not detect a reduction in S1P levels in Spns2tm1a/tma1 mouse plasma, the phenotypic data presented here together with the previous in vitro demonstrations that human Spns2 can transport S1P and S1P-mimic FTY720 (40, 43), suggest that the Spns2tm1a/tm1a phenotype may arise from localized disruptions in S1P concentrations at certain restricted physiological locations.
Critically this work indicates that Spns2 functions are limiting for lymphocyte trafficking with some degree of specificity, as the viability and lack of developmental defects in Spns2tm1a/tm1a mice contrasts with lethality and defects in cardiovascular and neural development in Spns2-mutant zebrafish (40, 41). Importantly this is not due to different requirements for S1P-production between the two species, as knockout mice lacking sphingosine kinases SphK1−/−SphK2−/− or S1P-receptor S1P1−/− are also embryonic lethal with abnormal cardiovascular development (31, 42). In comparison, Spns2tm1a/tm1a mice more closely mimic the phenotypes of partially reduced S1P production, such as the single-null knockouts for SphK1 or SphK2 (54-56), or the knockout for S1P lyase with normal S1P production but disrupted S1P concentration gradients (57). Overall this indicates that sufficient S1P levels are maintained in correct anatomical and cellular locations in the Spns2tm1a/tm1a mice to allow normal embryonic development, and suggests significant divergence in the expression and functions of Spns2 between mouse and zebrafish. We can speculate that alternative mechanisms of S1P release may operate during embryonic development in mouse but not in zebrafish species. These may include other transporters that have been shown to pump S1P in vitro in mammalian cells, such as ABCC1 and ABCA1 (33, 34), or extracellular S1P production by secreted SphK1 enzyme (38), or even the two Spns2 paralogs Spns1 and Spns3.
This work further demonstrated that Spns2 activity in the non-hematopoietic cells of the stroma is of key importance for normal immune system function. In contrast, previous studies showed that plasma S1P levels in the mouse are maintained by hematopoietic cells such as erythrocytes (47), and ABC-family transporters have been implicated in mediating S1P export from erythrocytes (33) and platelets (35). This suggests that the primary role of Spns2 is to maintain appropriate S1P concentrations at other in vivo locations, consistent with the unaltered S1P levels in the plasma of Spns2tm1a/tm1a mice. Lymph is one of the sites were analysis of S1P concentrations would be particularly interesting (47), as S1P in the lymph was shown to be derived from non-hematopoietic cells, in particular the lymphatic endothelium (48). Overall this warrants further investigation of Spns2 expression in different non-hematopoietic cell types and tissues, including lymphatic endothelium (47, 48).
S1P also acts as an intracellular messenger; and in mast cells and antigen presenting cells increased S1P production is associated with cell activation, degranulation, and inflammatory cytokine production (18, 20, 23, 24). However, the current work indicates that in contrast to the SphK1-knockdown cells , Spns2tm1a/tm1a macrophages respond normally to TNFR and TLR stimulation. This indicates that Spns2 likely does not impact on intracellular S1P levels, at least in this cell type. Whether the activity of Spns2 and other S1P transporters can affect intracellular S1P levels in other cell types, by altering S1P secretion or uptake, remains to be addressed in future research.
Components of the S1P signaling pathways are targets for therapies aimed at treating autoimmunity, transplant rejection, inflammatory diseases, and cancer (2). S1P receptor agonist FTY720 is the most advanced of such therapies and was recently approved for the treatment of multiple sclerosis (58), while other pharmaceutical agents are under development and have shown efficacy in animal models of inflammatory diseases, sepsis, and cancer (28, 59, 60). Knowledge of the mechanisms regulating S1P concentrations in vivo in a mammal is essential for the future development of such pharmaceutical agents and may lead to better targeted therapies. For example, S1P-targeting therapies for the treatment of autoimmunity and transplant rejection aim to achieve immunosuppression without inhibiting other S1P activities. S1P-targeting cancer therapies suppress vascularization, cell growth and migration, but ideally aim to maintain full immune system function. In contrast therapies for systemic inflammatory disorders primarily aim to suppress inflammation and intravascular coagulation, while retaining the protective activities of S1P on endothelial barrier integrity (61, 62). Given the diverse roles of S1P in many physiological processes, understanding of the mechanisms regulating its bioavailability in different tissues and conditions is essential for the development of such therapies. Transporter proteins have proven highly effective drug targets in other areas, particularly neuropharmacology (63). The demonstration that Spns2-deficiency selectively impaired lymphocyte functions and antigen-specific immune responses, without affecting vascular and neural development, highlights Spns2 as a possible drug target with potential for the treatment of autoimmunity and transplant rejection.
Supplementary Material
Acknowledgements
Sanger Mouse Genetics Project members are Allan Bradley, Ramiro Ramirez-Solis, David J. Adams, Jacqueline K. White, Niels C. Adams, Karen Steel, Bill Skarnes, Gordon Dougan, the Mouse Informatics Group including David Melvin, David Gannon, Mark Griffiths, Christian Kipp, Arthur Evans, Simon Holroyd, the Phenotyping/Histology/Infection Groups including Caroline Barnes, Emma Cambridge, Damian Carragher, Simon Clare, Kay Clarke, Hayley Protheroe, Jeanne Estabel, Anna-Karin Gerdin, Yvette Hooks, Natalia Igosheva, Ozama Ismail, Leanne Kane, Natasha Karp, David Tino Lafont, Mark Lucas, Simon Maguire, Katherine McGill, Lynda Mottram, Lee Mulderrig, Christine Podrini, Hayley Protheroe, Laura Roberson, Grace Salsbury, Daniel Sanger, Mark Sanderson, Carl Shannon, David Sunter, Elizabeth Tuck, Valerie Vancollie, Genotyping: Debarati Bhattacharjee, Diane Gleeson, Matt Hardy, Edward Ryder, Sapna Vyas, and Mouse Production: James Bussell, Joanna Bottomley, Ellen Brown, Evelyn Grau, Richard Houghton, Helen Kundi, Alla Madich, Danielle Mayhew, Tom Metcalf, Stuart Newman, Laila Pearson, Caroline Sinclair, Hannah Wardle-Jones, Mike Woods. We gratefully acknowledge the contribution of the Sanger Institute Research Support Facility. We thank J. Pass for generating the tm1b and tm1c alleles of Spns2.
1Sources of Funding: This work was funded by the Wellcome Trust (grant no. 098051), and Genome Canada. A.N. was a recipient of fellowships from the Canadian Institutes for Health Research and the Michael Smith Foundation. REWH is the recipient of a Canada Research Chair in Health and Genomics.
Abbreviations
- ABC
ATP-binding cassette
- BMDM
bone marrow derived macrophage
- CFTR
cystic fibrosis transmembrane conductance regulator
- FTY720
2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol, hydrochloride
- TetC
tetanus-toxin fragment C recombinant protein
- S1P
sphingosine-1-phosphate
- S1P1
S1P receptor 1
- SphK
sphingosine kinase
- Spns2
spinster homologue 2
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
While this manuscript was under review another manuscript has been released that substantially agrees with many of the findings presented here (64).
References
- 1.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japtok L, Kleuser B. The role of sphingosine-1-phosphate receptor modulators in the prevention of transplant rejection and autoimmune diseases. Curr Opin Investig Drugs. 2009;10:1183–1194. [PubMed] [Google Scholar]
- 3.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 4.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 6.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 7.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira JP, Cyster JG, Xu Y. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One. 5:e9277. doi: 10.1371/journal.pone.0009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 10.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 11.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr., Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunisawa J, Kurashima Y, Gohda M, Higuchi M, Ishikawa I, Miura F, Ogahara I, Kiyono H. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood. 2007;109:3749–3756. doi: 10.1182/blood-2006-08-041582. [DOI] [PubMed] [Google Scholar]
- 13.Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, Cyster JG. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. 2006;203:2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunisawa J, Kurashima Y, Higuchi M, Gohda M, Ishikawa I, Ogahara I, Kim N, Shimizu M, Kiyono H. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J Exp Med. 2007;204:2335–2348. doi: 10.1084/jem.20062446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 16.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivera A. Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model. Prostaglandins Other Lipid Mediat. 2008;86:1–11. doi: 10.1016/j.prostaglandins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florey O, Haskard DO. Sphingosine 1-phosphate enhances Fc gamma receptor-mediated neutrophil activation and recruitment under flow conditions. J Immunol. 2009;183:2330–2336. doi: 10.4049/jimmunol.0901019. [DOI] [PubMed] [Google Scholar]
- 19.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Prakash H, Luth A, Grinkina N, Holzer D, Wadgaonkar R, Gonzalez AP, Anes E, Kleuser B. Sphingosine kinase-1 (SphK-1) regulates Mycobacterium smegmatis infection in macrophages. PLoS One. 2010;5:e10657. doi: 10.1371/journal.pone.0010657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlasenko LP, Melendez AJ. A critical role for sphingosine kinase in anaphylatoxin-induced neutropenia, peritonitis, and cytokine production in vivo. J Immunol. 2005;174:6456–6461. doi: 10.4049/jimmunol.174.10.6456. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim FB, Pang SJ, Melendez AJ. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J Biol Chem. 2004;279:44802–44811. doi: 10.1074/jbc.M403977200. [DOI] [PubMed] [Google Scholar]
- 23.Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 24.Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after Fc epsilon receptor I triggering. J Exp Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFalpha in primary human monocytes. J Cell Physiol. 2006;208:109–115. doi: 10.1002/jcp.20646. [DOI] [PubMed] [Google Scholar]
- 27.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 28.Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, McInnes IB, Melendez AJ, Leung BP. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181:8010–8017. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 29.Pitson SM, D’Andrea RJ, Vandeleur L, Moretti PA, Xia P, Gamble JR, Vadas MA, Wattenberg BW. Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem J. 2000;350(Pt 2):429–441. [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 31.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 33.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. Faseb J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA, Obeid LM. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 37.Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 39.Saier MH, Jr., Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, Jack DL, Jahn PS, Lew K, Liu J, Pao SS, Paulsen IT, Tseng TT, Virk PS. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- 40.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 41.Osborne N, Brand-Arzamendi K, Ober EA, Jin SW, Verkade H, Holtzman NG, Yelon D, Stainier DY. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr Biol. 2008;18:1882–1888. doi: 10.1016/j.cub.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011 doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skarnes W, Rosen B, West A, Koutsourakis M, Bushell W, Iyer V, Cox T, Jackson D, Severin J, Biggs P, Thomas M, Mujica A, Harrow J, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A. A conditional knockout resource for genome-wide analysis of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su H, Mills AA, Wang X, Bradley A. A targeted X-linked CMV-Cre line. Genesis. 2002;32:187–188. doi: 10.1002/gene.10043. [DOI] [PubMed] [Google Scholar]
- 46.Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- 47.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 48.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. S11–14. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 51.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. Faseb J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 52.Adams NC, Gale NW. Mammalian and Avian Transgenesis - New Approaches. Springer; 2006. High Resolution Gene Expression Analysis Using Genetically Inserted Reporter Genes; pp. 131–173. [Google Scholar]
- 53.Nijnik A, Clare S, Hale C, Yusa K, Everitt AR, Raisen CR, Mottram L, Podrini C, Lucas M, Estabel J, Goulding D, Adams N, Ramirez-Solis R, White JK, Hancock REW, Dougan G. The Critical Role of Histone H2A-deubiquitinase Mysm1 in Haematopoiesis and Lymphocyte Differentiation. Blood. 2011 doi: 10.1182/blood-2011-05-352666. [DOI] [PubMed] [Google Scholar]
- 54.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 55.Kharel Y, Lee S, Snyder AH, Sheasley-O’neill SL, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Pearson-White S, Lynch KR. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 56.Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 57.Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ, Sun W, Swaffield J, Oravecz T. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 59.Milstien S, Spiegel S. Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell. 2006;9:148–150. doi: 10.1016/j.ccr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 61.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 63.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 64.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. The Journal of clinical investigation. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.