Abstract
Objective
To investigate the relationship of urinary biomarkers (UBM) and established measures of renal function (EMRF) to the histological findings with lupus nephritis (LN); and to test whether certain combinations of the above mentioned laboratory measures are diagnostic of specific histological features of LN.
Methods
Urine samples of 76 patients were collected within 2 months of a kidney biopsy and assayed for the UBM: lipocalin-like prostaglandin-D synthetase (LPGDS), α1-acid-glycoprotein (AAG), transferrin (TF), ceruloplasmin (CP), neutrophil-gelatinase associated lipocalin (NGAL), and monocyte chemotactic factor 1 (MCP1). Using non-parametric analyses, UBM and EMRF levels were compared to histological features seen with LN: mesangial expansion, capillary proliferation, crescent formation, necrosis, wire loops, fibrosis, tubular atrophy, and epimembranous deposits. The area under the receiver operating characteristic (AUC) curve was calculated to predict LN activity, chronicity or membranous LN.
Results
There was a differential increase of the UBM that formed a pattern reflective of specific histological features seen with active LN. The combination of MCP1, AAG, CP plus protein:creatinine ratio were excellent in predicting LN activity (AUC=0.85). NGAL together with creatinine clearance plus MCP1 was an excellent (AUC=0.83) and MCP1, AAG, creatinine clearance plus C4 (AUC=0.75) a good diagnostic test of LN chronicity and membranous LN, respectively.
Conclusions
Select UBM are associated with specific tissue changes observed with LN activity and chronicity. Especially in combination with select EMRF, UBM are well-suited to non-invasively quantify LN activity, LN chronicity, and the presence of membranous LN.
Key Indexing Terms: SLE, lupus nephritis, kidney biopsy, biomarker
INTRODUCTION
Systemic Lupus Erythematosus (SLE) is a multi-system inflammatory autoimmune disease, and renal involvement is one of the main determinants of poor prognosis (1). The histological features seen on kidney biopsy constitute the current criterion standard for the diagnosis of lupus nephritis (LN) and are used to guide LN treatment. Kidney biopsies provide a direct assessment of the presence and severity of acute changes due to active LN and give insight into the chronicity of LN (2). Obtaining kidney biopsies is necessary because traditional measures of LN such as blood pressure, proteinuria, urine sediment, complement components C3 and C4, and glomerular filtration rates (GFR) are considered too inaccurate to reliably discriminate between the acute inflammatory changes that are amenable to immunosuppressive therapy and the chronic degenerative changes that will not improve despite control of SLE activity.
Using proteomic techniques, we identified previously novel urinary biomarkers (UBM) of LN. These include transferrin (TF), ceruloplasmin (CP), α-1-acid-glycoprotein (AAG; also known as orosomucoid), lipocalin-type prostaglandin-D synthetase (LPGDS), monocyte chemotactive factor 1 (MCP1; also known as chemokine ligand 2), and neutrophil gelatinase associated lipocalin (NGAL) (3–5). We have shown that these UBM correlate with and are responsive to clinical measures of LN activity, and that some UBM are even suited to predict future LN flares (6–8). The relationship of these UBM to specific histological features of LN, however, has not been examined and was the focus of this study.
The objectives were to (1) study the relationship of the UBM and traditional laboratory measures of LN to histological findings seen on kidney biopsy in both children and adults with LN; and (2) test whether certain combinations of the above mentioned laboratory measures are diagnostic for specific histological features of LN.
MATERIALS & METHODS
Patients
Children and adults diagnosed with SLE (9) who required a kidney biopsy as part of standard of care therapy were included in this study, if a random spot urine sample was available that was collected within 60 days of the kidney biopsy. On the day of the urine sample collection, information about patient demographics, medications, and disease activity was collected. Key laboratory measures were obtained, including complement C3 and C4 levels, anti-dsDNA antibodies (present/absent), amount of proteinuria as estimated by the protein to creatinine ratio (P/C ratio) in a random or 24-hour urine sample, serum creatinine, and glomerular filtration rate (GFR) as estimated by age-appropriate calculation of the creatinine clearance (10, 11).
The renal domain score of the Systemic Lupus Disease Activity Index (SLEDAIR; range 0 – 16; 0 = inactive LN) served as the clinical measure of LN activity (12). The Systemic Lupus International Collaborating Clinics/ American College of Rheumatology Damage Index items addressing kidney damage (SDI-R; range 0 – 3; 0 = no LN damage) were recorded as a clinical measure of kidney damage in patients with LN (13).
Kidney Histology
The histological characteristics of each kidney biopsy, as per report from the local pathologists, were reviewed in a blinded fashion by one expert nephropathologist (DW), as per the International Society of Nephrology/ Renal Pathology (ISN/RPS) Classification (14).
The following histological features reflective of active inflammation with LN were recorded: mesangial proliferation, endocapillary karyorrhexis (also: fibrinoid necrosis); cellular crescents; capillary proliferation, subendothelial deposits identifiable by light microscopy (also: wire-loops). We also noted features representing LN chronicity or degenerative damage. These included glomerular sclerosis (segmental or global), fibrosis including fibrous adhesions and fibrous crescents, as well as tubular atrophy.
Almost all studies in LN employ a previously developed scoring system to quantify the amount of overall LN activity and overall LN chronicity as is present in the kidney biopsy specimen (15). The features of activity and chronicity listed above were categorized as follows: 0 (no lesions), 1 (lesions in up to 25% of glomeruli), 2 (lesions in 25–50% of glomeruli) or 3 (lesions in >50% of glomeruli). Using these numeric values, a Biopsy Activity Index (BAI) score (range 0 – 24) and a Biopsy Chronicity Index (BCI) score (range 0 – 12) can be calculated, where higher scores represent higher LN activity or chronicity, respectively.
Epimembranous deposits, although not included in the BAI or the BCI scores, were also recorded. Depending on the findings of active inflammation, and chronic changes observed on kidney biopsy, LN is classified in six categories. Pronounced predominance of epimembranous deposits is compatible with Class 5 of LN.
The ISN/RPS Classification, the BAI and the BCI have all been validated for use in adults and children with LN (16, 17). Risk factors for poor LN outcome include BAI scores of 7 or higher and BCI scores of 4 or higher (16, 18–25).
Urinary Biomarker Assays
Urine samples were frozen at −80 degree celsius prior to batch processing. We measured urinary concentrations of TF and L-PGDS by immunonephelometry (Dade Behring BNII Prospect, Marburg, Germany). Urinary CP was quantified by ELISA (Human Ceruloplasmin ELISA Quantitation Kit; Assaypro, St.Charles, MO, USA). Intra and inter-assay coefficients of variation of these assays (%CV) were 3.4% and 2.5% for TF, 2.3% and 6.5% for L-PGDS, and 4.1% and 7.1% for CP, respectively. Likewise, urinary AAG (5.0% and 8.5%) was measured using an ELISA kit (Human Orosomucoid ELISA Quantitation Kit; Genway Biotech, Inc., San Diego, CA, USA). MCP1 levels were also measured by ELISA (R&D Systems, Minneapolis, MN, USA). The respective intra-assay and inter-assay CV was 5.0% and 5.1%. We used all these commercial ELISA kits as per manufacturers’ instructions, while NGAL was measured as previously reported by our group (6, 7). Intra and inter-assay CV of the NGAL assay were 5.0% and 5.1%, respectively.
Concentrations of the UBM (in ng/ml for AAG, NGAL, CP and MCP1 and in mg/dl for TF and L-PDGS) were standardized for urinary creatinine levels (in mg/mL). Laboratory personnel measuring the UBM were blinded to the clinical and histological information.
Statistical analysis
We inspected the central tendency, dispersion, and skewness of the UBM and traditional markers of LN (C3, C4, GFR, P/C ratio) and found them not to fit well into normal distributions. Therefore, medians and interquartile ranges (IQR) were calculated as measures of central tendency for continuous variables, while categorical variables were summarized by frequency (in percentages). We used Spearman correlation coefficients to examine the strength of the association between numerical variables and Wilcoxon rank sum test to assess for statistically significant differences between types of histological features and UGM and traditional renal markers, respectively.
Because of the skewness, we log-transformed the concentrations of the UBM and traditional measures of LN prior to considering them in univariate and multivariate logistic regression modeling to determine relevant predictors of key LN features that are associated with poor prognosis (BAI score ≥ 7, BCI score ≥ 4) or that may require differential therapy (i.e. ISN/RPS Class 5 LN) (26).
We also calculated the relative change of the median and IQR of the laboratory measures with the presence vs. absence of a histological feature or a particular LN outcome (ISN/RPS Class 5 LN; BAI score ≥ 7; BCI score ≥ 4). Hence, values of 100% signify that the UBM (or traditional measure of LN) is present in the same amount with the presence vs. absence of a histological features or a particular LN outcome. Values >100% represent scenarios where the laboratory measure increases, and values < 100% where they decrease with the presence of the histological feature or a particular LN outcomes compared to its absence.
Included in the multivariate logistic models were all candidate biomarkers, and traditional biomarkers measures of LN with p-value of <0.15 on univariate analysis.
As published by our group in the past (5), the diagnostic accuracy of eachbiomarker and biomarker combination was assessed by the receiver operating characteristic (ROC) curve analysis, and the corresponding area under the curve (AUC, range 0 – 1) was calculated. The accuracy of the biomarker and biomarker combinations in predicting LN histology features was considered outstanding, excellent, good, fair, and poor if the AUC was in the range of 0.9 –1.0,0.81–0.90, 0.71–0.80, 0.61– 0.70, and 0.50–0.60, respectively.
The sensitivity and specificity to predict LN outcomes (presence of ISN/RPS Class 5 LN; BAI score ≥ 7; BCI score ≥ 4) were determined for particular cut-off values of each biomarker combination, generally that for sensitivities around 75%.
Furthermore, we tested whether biomarker concentrations and specific kidney biopsy features systematically changed with patient age and explored whether the lag time between urine collection and kidney biopsy conduct was important for the association between UBM and histological features seen with LN.
Statistical analyses were done using SAS version 9.2 software (SAS, Cary, NC, USA). P-values < 0.025 were considered statistically significant. The study was approved by the Institutional Review Boards and Ethics Review Committees of the participating centers.
RESULTS
Patient Characteristics & Features of Kidney Biopsy
A total of 76 patients with a median age of 23 years (range: 9 – 51 years) was included in the study, and 26 patients were 18 years or younger (Table 1). At the time of urine collection, almost all patients were treated with glucocorticosteroids, many with immunosuppressive medications, and the median SLEDAI-R score was 8 (range: 0 – 16). Elevated levels of anti-dsDNA antibodies were present in 75% (49/65) of the patients with available information. Only three patients had renal damage as per the SDI-R.
Table 1.
Patient Demographics, medications and renal status at the time of the urine collection
| Number of patients with available information |
n of N (%) | Median | Interquartile range |
|||
|---|---|---|---|---|---|---|
| Disease-onset | Childhood-onset LN | 76 | 28 (37%) | |||
| Adult-onset LN | 48 (63%) | |||||
| Females | 64 (84%) | |||||
| Race | Black | 76 | 35 (46%) | |||
| White | 33 (43%) | |||||
| Other+ | 8 (11)† | |||||
| Medications | Oral prednisone | 76 | 73 (96%) | |||
| Pulse methylprednisolone | 33 (43%) | |||||
| Mycophenolate mofetil | 23 (30%) | |||||
| Azathioprine | 3 (4%) | |||||
| Cyclophosphamide | 13 (17%) | |||||
| Methotrexate | 4 (5%) | |||||
| Angiotensin blocking agent | 41 (54%) | |||||
| LN Status | GFR < 60 ml/min/m2 | 76 | 14 (18%) | |||
| Protein:creatinine ratio > 0.5 | 76 | 68 (89%) | ||||
| Renal SDI score > 0 | 22 | 3 (14%) | 0 – 2 | |||
| Renal SLEDAI score | 76 | 8 | 0 – 16 | |||
| Presence of double-stranded-dsDNA | 65 | 49 (75%) | ||||
| Timing of urine collection | Time interval to biopsy* | 76 | +3.5 days* | −60 to +60 | ||
| >30 days before biopsy | 5 (7%) | |||||
| >30 days after biopsy | 76 | 17 (22%) | ||||
| ISN/RPS class& | Class 2 | 76 | 6 (8%) | |||
| Class 3 | 12 (16%) | |||||
| Class 4 | 29 (38%) | |||||
| Class 5 | 29 (38%) | |||||
| Histological Features present | Mesangial expansion | 76 | 73 | |||
| Capillary proliferation | 38 | |||||
| Cellular crescents | 22 | |||||
| Fibrinoid necrosis | 22 | |||||
| Wire -loops | 21 | |||||
| Fibrosis | 53 | |||||
| Tubular atrophy | 58 | |||||
| Epimembranous deposits | 33 | |||||
| BAI score‡ | 76 | 3 | 0 – 15 | |||
| BCI scoreΔ | 2 | 0 – 9 | ||||
Positive value indicates that the urine was collected after the kidney biopsy
American Indian 1; Asian 3; Mixed racial 5;
There were no patients with Class 1 or Class 6 LN
Biopsy Activity Index; range 0 – 24; 0 = inactive LN
Biopsy Chronicity Index; range 0 – 12; 0 = LN without chronic changes
The median time interval between kidney biopsy and urine sample collection was 3.5 days, and for 50% (38/76) of the patients the urine sample was collected prior to or on the day of the kidney biopsy. The histological diagnoses included proliferative LN (Class 3 or 4) in 50% (41/ 76), and ISN/RPS Class 5 LN in 38% (29/76) of the patients. Epimembranous deposits (ISN/RPS Class 5 or together with Class 3 or 4 LN) were observed in 43% (33/76) of the biopsies.
As expected, histological features were seen often concomitantly in the same kidney biopsy specimen. Generally, features reflective of active inflammation were clustered as were those representing LN chronicity. For example, in kidney biopsies with capillary proliferation, there was often (85%) moderate mesangial proliferation. Among patients with tubular atrophy, 95% also had fibrotic changes of the renal tissue (Table 2).
Table 2.
Relationship between Histological Features on Kidney Biopsy†
| Histological | Features | N | Capillary proliferation |
Cellular crescents |
Fibrinoid necrosis |
Wire-loops | Fibrosis | Tubular atrophy |
Epimembranous deposits |
BAI Score ≥ 7 |
BCI Score ≥ 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mesangial proliferation | Moderate | 27 | 85% | 44% | 52% | 62% | 78% | 74% | 37% | 41% | 14% |
| No / Mild | 49 | 31% | 20% | 16% | 8% | 65% | 78% | 47% | 2% | 14% | |
| p-value | <0.0001 | - | 0.0016 | <0.0001 | - | - | - | <0.0001 | - | ||
| Capillary proliferation | Yes | 38 | 53% | 39% | 49% | 79% | 76% | 34% | 32% | 13% | |
| No | 38 | 5% | 18% | 5% | 61% | 76% | 53% | - | 16% | ||
| p-value | <0.0001 | - | <0.0001 | - | - | - | <0.0001 | - | |||
| Cellular crescents | Yes | 22 | 68% | 55% | 77% | 73% | 32% | 41% | 9% | ||
| No | 54 | 13% | 15% | 67% | 78% | 48% | 6% | 17% | |||
| p-value | <0.0001 | <0.0001 | - | - | - | 0.0004 | - | ||||
| Fibrinoid necrosis | Yes | 22 | 57% | 68% | 64% | 23% | 41% | 9% | |||
| No | 54 | 15% | 71% | 82% | 52% | 6% | 17% | ||||
| p-value | 0.0005 | _ | - | 0.02 | - | - | |||||
| Wire-loops | Yes | 20 | 80% | 65% | 20% | 60% | 15% | ||||
| No | 55 | 67% | 80% | 53% | 0 | 15% | |||||
| p-value | - | - | 0.02 | 0.0001 | - | ||||||
| Fibrosis | Yes | 53 | 95% | 42% | 17% | 21% | |||||
| No | 23 | 39% | 48% | 13% | - | ||||||
| p-value | 0.0001 | - | - | - | |||||||
| Tubular atrophy | Yes | 58 | 47% | 12% | 19% | ||||||
| No | 18 | 33% | 28% | - | |||||||
| p-value | - | - | - | ||||||||
| Epimembra-nous deposits | Yes | 33 | 9% | 12% | |||||||
| No | 43 | 21% | 16% | ||||||||
| p-value | - | - | |||||||||
| Biopsy Activity Score | ≥7 | 12 | 17% | ||||||||
| <7 | 64 | 14% | |||||||||
| p-value | - | ||||||||||
Only p-values < 0.025 are stated
Associations of Clinical and Laboratory Measures with Histological Features
The age of the patients was significantly associated with serum creatinine (r = 0.27; p< 0.017) and the BCI score ( r= − 0.45; p< 0.0001) but not with any of the UBM levels or other traditional measures of LN.
The concentrations of all the UBM were at least weakly correlated ( r≥ |0.2|) with each other. An exception was TF which was strongly correlated with CP (r = 0.74; p < 0.0001) and AAG (r = 0.61; p = 0.005). Among traditional measures of LN, the only strong correlation (r = 0.79) was, as expected, between the GFR and serum creatinine levels. Notably, levels of C3, C4 and the P/C-ratio were unrelated (r<|0.2|).
This suggests that concentrations of the UBM do not simply increase in the urine due to increased proteinuria and supports the notion that the UBM provide additional information about LN over and above the traditional measures of LN.
Association of SLEDAI Renal Domain Score with LN histology
There were statistically significantly correlations of the SLEDAI-R scores with concentrations of NGAL (r= − 0.39; p< 0.0007), MCP1 (r= 0.23; p< 0.07), CP (r= 0.23; p< 0.05), AAG (r= 0.35; p< 0.003) and L-PGDS (r= 0.28; p< 0.016), respectively. Serum creatinine (r= 0.35; p< 0.002), the P/C-ratio (r= 0.40; p< 0.0004), and C3 levels (r= − 0.34; p <0.0043) were also correlated with SLEDAI-R scores. Conversely, urine concentrations of TF, C4 and the GFR were unrelated to the SLEDAI-R scores (r < |0.2|). BAI scores were weakly correlated with the SLEDAI-R (r= 0.29; p < 0.01)
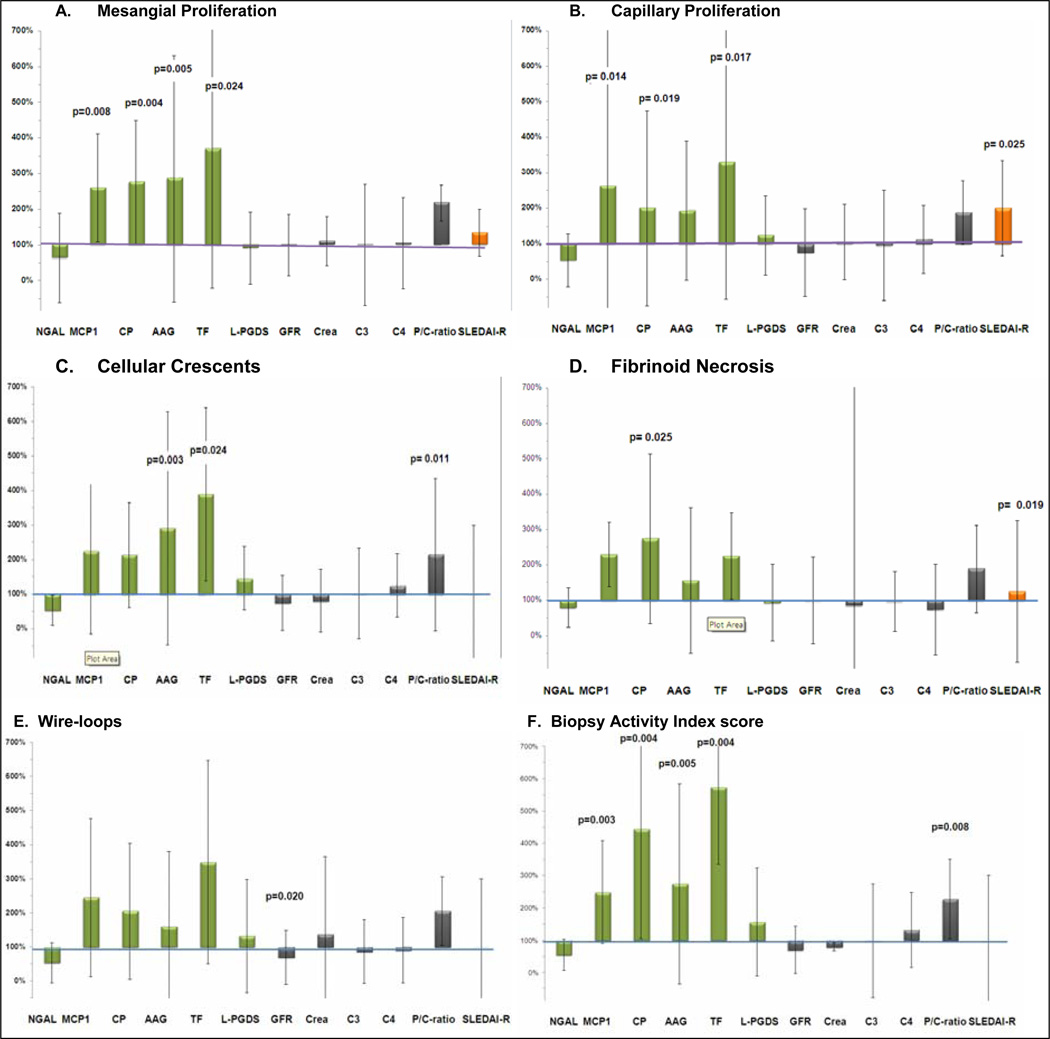
Relative changes of the biomarkers with histological features of LN activity
The relative excretion of the UBM and traditional measures of LN with histological features of LN is shown in Figure 1. There was a relative increased excretion of some UBM with mesangial proliferation (Panel A), capillary proliferation (Panel B), cellular crescents (Panel C), and fibrinoid necrosis (Panel D). There was a trend towards more pronounced relative increases of the UBM with the presence of wire-loops but none of these changes reached statistical significance (Figure 1, Panels E). With the exception of the P/C ratio, there were no statistically significant differences in the levels of traditional measure of LN with any of the histological measures of LN activity. The levels of MCP1, CP, TF, AAG, and the P/C ratio all discriminated between low versus high BAI scores (<7 vs. ≥7) (Figure 1, Panels F). Of note, urinary L-PGDS and NGAL were not differentially associated with any of the histological features under consideration. The same was true of the levels of complement C3 and C4.
Figure 1.
For the 76 patients considered in the study, relative changes of the median levels with the presence vs. absence of specific histological features associated with LN activity are shown (whiskers are percentage change of the IQR) of the biomarkers: NGAL, MCP1, CP, AAG, TF, L-PGDS; serum creatinine, GFR; complement C3 and C4, P/C ratio; and clinical disease activity as measured by the SLEDAI-R score. Y-axis: Values of 100% signify that there is no difference of the biomarker with the presence vs. absence of the histological feature under consideration, while values of < 100% (> 100%) imply that the levels of the biomarker decrease (increase) with the presence of the histological features.
Panel A: MCP1, CP, AAG and TF significantly increase with mesangial proliferation;
Panel B: MCP1, CP and TF significantly increase with capillary proliferation;
Panel C: AAG and TF and the P/C ratio increase with cellular crescents;
Panel D: CP and the SLEDAI-R significantly increase with fibrinoid necrosis;
Panel E: Especially AAG and TF increase with wire-loops but changes do not reach statistical significance at p < 0.025.
Panel F: MCP1, CP, AAP, TF and the P/C ratio significantly increase with high Biopsy Activity Index Scores (BAI).
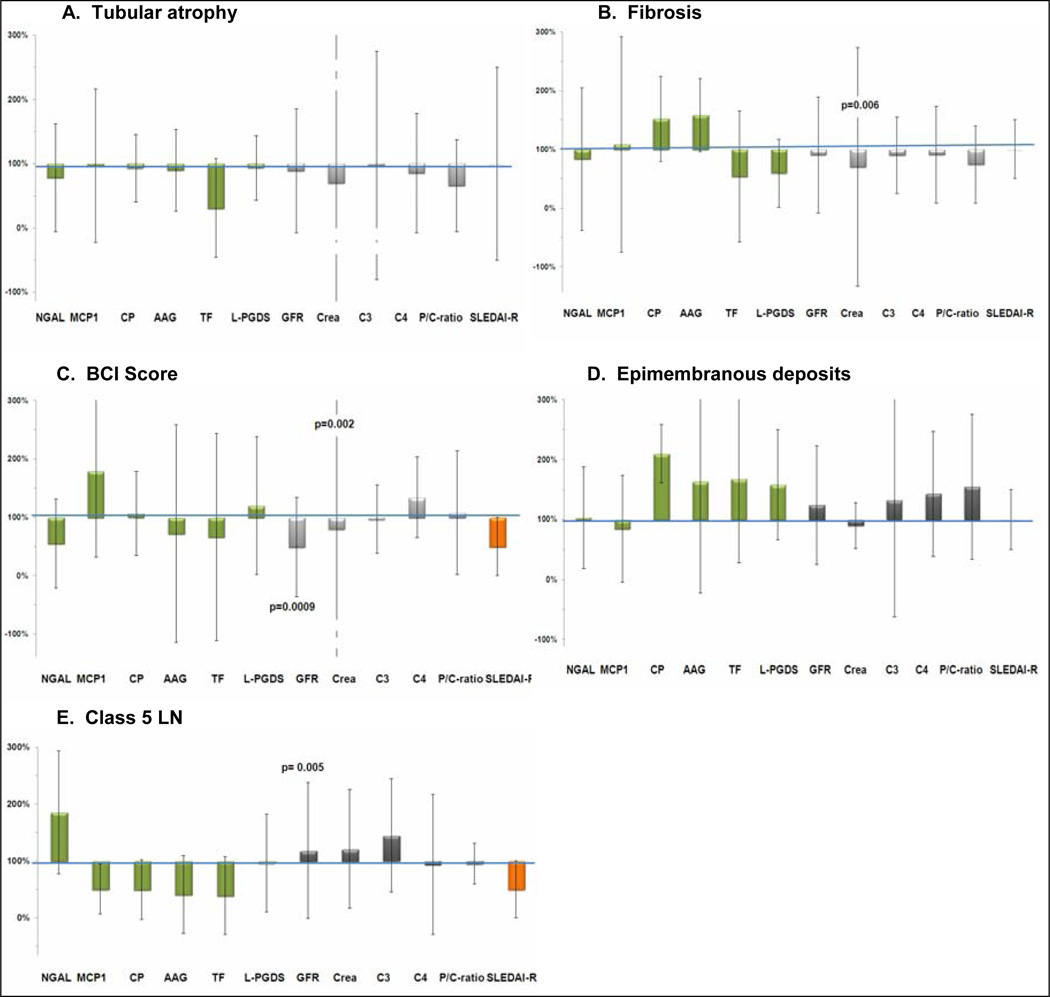
Relative changes of the laboratory measures with histological features of LN chronicity and epimembranous deposits
The levels of the UBM differed especially with features of active LN but not with features of LN chronicity (Figure 2, Panel A – C). Only the GFR and the serum creatinine levels importantly differed with LN chronicity features and distinguished between high vs. low BCI scores (≥ 4 vs. < 4).
Figure 2.
In Panels A – C, relative changes of the median levels (whiskers are percentage change of the inter-quartile ranges) of the biomarkers with the presence versus absence of histological feature or scores associated with LN chronicity are shown. Details on the biomarkers and the interpretation of the values of the y-axis are provided in the legend of figure 1.
Panel A: None of the biomarkers changes significantly with tubular atrophy;
Panel B: Only the serum creatinine increases significantly with fibrosis;
Panel C: Only the serum creatinine and the GFR increase significantly with high Biopsy Chronicity Index Scores (BCI);
Panel D: None of the biomarkers changes significantly with of epimembranous deposits;
Panel E: Only the GFR increases significantly with ISN/RPS Class 5 LN
None of the UBM or traditional measures of LN differed significantly with the presence vs. absence of epimembranous deposits. However, there was a trend towards the UBM showing larger relative differences in urinary excretion with the presence vs. absence of epimembranous deposits. Only the GFR differed with Class 5 as compared to Class 2 – 4 LN significantly [median (IQR) in ml/min/1.73m2: 125 (54) vs. 85 (54)].
Univariate and multivariate logistic modeling to predict LN outcomes
In univariate logistic regression, we assessed the diagnostic accuracy (AUC) of each of the biomarkers for key LN features, i.e. BAI scores ≥ 7, BCI scores ≥ 4 and the presence of ISN/RPS Class 5 LN. Good to excellent accuracy to diagnose patients with high BAI-scores were present only for AAG, TF (both AUC = 0.76), CP (AUC = 0.79), MCP1 (AUC = 0.82), and the P/C ratio (AUC= 0.76). Excellent predictors of high BCI scores were the GFR and serum creatinine (both AUC = 0.82). Univariate analysis did not reveal a single UBM or traditional measure of LN that was at least a good diagnostic measure (AUC ≥ 0.7) of Class 5 LN. Lastly, the SLEDAI-R scores were a poor proxy measure of BAI scores (AUC = 0.5).
The results of multivariate modeling to predict key LN outcomes (high BAI score, high BCI score, Class 5 LN) were summarized in Table 3. A combination of four different biomarkers each was excellent for diagnosing high BAI scores (AUC = 0.85) as the combination of NGAL, GFR and MCP1 for diagnosing high BCI scores (AUC = 0.83). Combinations of five biomarkers yielded a good diagnostic test for ISN/RPS Class 5 LN (AUC = 0.75).
Table 3.
Prediction of Key Biopsy Features with Lupus Nephritis
| Model Outcome Variable | Predictor Variables | Area under the ROC Curve‡ (95% confidence interval) |
Sensitivity* | Specificity |
|---|---|---|---|---|
| Biopsy Activity Score ≥ 7 | MCP1, CP, AAG, P/C ratio | 0.85 (0.69–1.0) |
72% | 66% |
| Biopsy Chronicity Score ≥ 4 | NGAL, GFR, MCP1 | 0.83 (0.67 – 0.93) |
73% | 67% |
| Membranous Lupus Nephritis (Class 5) | MCP1, GFR, AAG, TF, C4 | 0.75 (0.62–0.86) |
75% | 48% |
The area under the ROC (receiver operating characteristic curve) ranges between 0 – 1
Clinically relevant point on ROC with sensitivity of at least 70%
Exclusion of Urine Samples Collected after the Kidney Biopsy
Although we did not have access to recent changes of medications just prior or after the study visit, we hypothesized that LN therapy was intensified after the results of the kidney biopsy had become available. When only considering patients from whom urine samples were collected no later than the day of the kidney biopsy (n= 38), urinary NGAL concentrations were significantly lower with the presence of cellular crescents [median (IQR) in present vs. absent: 8.74 (44.9) vs. 48.5 (64.3); p< 0.0033], and LPGDS concentrations were significantly lower with the presence of tubular atrophy [median (interquartile range) in present vs. absent: 695 (591) vs. 928 (1021); p< 0.0234]. There were no significant relative changes between LN features and any of the other UBM or traditional measures of LN when only considering this subset of 38 patients. The patterns of relative biomarker excretion in relation to LN histology, including those observed in the subanalysis are summarized in Figure 2, Panel F.
DISCUSSION
We examined recently discovered UBM and traditional measures of LN and found especially individual UBM related to specific histological findings representing LN activity. The combination of MCP1, CP, AAG and the P/C was excellent in estimating histological LN activity. NGAL together with GFR and MCP1 were excellent diagnostic tests of LN chronicity. Combinations of biomarkers provided good markers for the presence of Class 5 LN.
Our previous research supports that the UBM correlate with and are responsive to change in clinical measures of LN activity (3, 7). These observations are confirmed by the findings of this study where the UBM were associated with histological features of LN activity. Surprisingly, urinary NGAL and L-PGDS were not differentially associated with features of LN activity in our entire cohort of patients with urine samples collected within 2 months of a kidney biopsy.
We previously reported L-PGDS to be a biomarker of LN activity as measured, among others, by the SLEDAI-R (3). The significance of this protein for inflammatory processes with LN has been confirmed in animal studies (27). Our study found L-PGDS to be weakly associated with clinical measures of LN activity (SLEDAI-R), other UBM and traditional measures of LN but not with a specific histological feature of LN. This might be due to the observation that elevated levels of L-PGDS are reflective of increased permeability of injured glomerular capillary walls (28), a feature not directly visible in histological standard stains of kidney biopsies. Alternatively, as is suggested by the findings of our subanalysis where L-PGDS excretion was 46% higher in patients with tubular atrophy, it may represent an early biomarker or one that rapidly declines with immunosuppressive therapy.
NGAL is an early and predictive urinary biomarker which is rapidly induced by active inflammation with LN, and promptly declines with therapy (5). Thus, when we excluded patients whose urine sample was collected after the kidney biopsy, i.e. already on intensive treatment for LN, and instead considered only the remaining patients (n=38), we found NGAL to be much lower in patients with cellular crescents, an important histological feature of active proliferative LN.
The biologic function of NGAL is still under investigation (29). In the acute setting, the biological role of NGAL appears to be a protective anti-apoptotic mechanism that limits tubule cell damage and enhances proliferation (30). Hence, the low NGAL levels found in patients with cellular crescents may represent a failure to protect from structural changes typically associated with active LN. Besides its role in LN activity, NGAL is also associated with LN damage (6). The importance of NGAL as a biomarker of LN chronicity is supported by its role as a predictor of high BCI scores in this study. Urinary NGAL is also elevated in adults with chronic kidney disease, in whom NGAL is inversely correlated with GFR and positively correlated with tubular atrophy (31). The increased production of NGAL in this chronic context likely constitutes a pathophysiological pathway that leads to progressive renal failure (32).
MCP1 has long been known to be a predictive biomarker of LN flares and LN severity (4). Lupus-prone MRL-lpr/lpr and MCP1 knockout mice exhibit significantly lower proteinuria and prolonged survival (33), indicating a role for MCP1 in LN pathogenesis, in addition to its demonstrated capacity as a urinary biomarker for LN (4). The findings of our study are in line with these previous reports because MCP1 was differentially excreted with features of LN activity and high BAI scores. Multivariate models that predict the presence of membranous LN (ISN/RPS Class 5) included MCP1 as an important predictor, supporting previous observations in idiopathic membranous nephropathy of high urinary MCP1 and high expression of MCP1, especially in the tubular epithelial cells (34).
AAG was markedly increased in the urine of patients with mesangial proliferation and crescents. This finding is line with AAG being a known marker of LN activity whose urinary levels are also elevated with other inflammatory kidney diseases. AAG is produced in epithelial cells and is thought to play an important role in regulating the dynamic properties of the glomerular capillary wall by reducing the permeability towards macromolecules such as albumin (35).
We found TF to be associated with mesangial and capillary proliferation and cellular crescent formation, an observation that is congruent with previous reports from IgA nephropathy (36). Physiologically, recycled and absorbed iron is delivered to the main iron-transporting protein in blood, transferrin. Some TF normally enters the glomerular filtrate, but it is retrieved by specific receptor-mediated uptake in the kidney tubular system (37). Thus, tubular injury will lead to increased urinary TF concentrations.
Similarly, urinary concentrations of CP were higher, especially with mesangial or capillary proliferation, crescent formation and fibrinoid necrosis. We reported CP, an oxidative stress-related protein, to be a biomarker of LN activity in the past (3). CP has been associated with tissue remodeling in the kidney after renal tubular injury as can be observed with LN (38).
Individually, neither the UBM nor the traditional measures of LN are suited to determine whether there are epimembranous deposits. Likely because the various histological features of LN often are seen together in the same histological specimen, the GFR was only found to importantly differ with the presence vs. absence of LN Class 5 but not of epimembranous deposits. We speculate that this is also the reason for the differences in trends of the other LN measures in patients with LN Class 5 compared to those patients whose kidney biopsies showed some epimembranous deposits but who did not have LN Class 5.
Although combinations of the biomarkers included in this study yielded excellent diagnostic tests for LN activity and chronicity, the presented analyses also suggest that additional markers are needed to provide the highly accurate (AUC > 0.9) diagnostic tests that are urgently needed by clinicians to help guide LN therapy.
Our study must be seen in the light of certain limitations. Given the diverse medication regimens used, the multiplicity of distinct kidney biopsy features and their considerable overlap in a given patient, our study findings will need to be confirmed in a larger cohort. Nonetheless, the association of novel as well as traditional biomarkers of LN with specific histological features bears the expectation that accurate longitudinal non-invasive measurement of LN activity and chronicity is feasible. If confirmed this will allow for a more effective and personalized monitoring of LN and its therapy. The availability of standardized clinical platforms for the reliable measurement of the urinary biomarkers will enable the testing of this hypothesis in the near future (39).
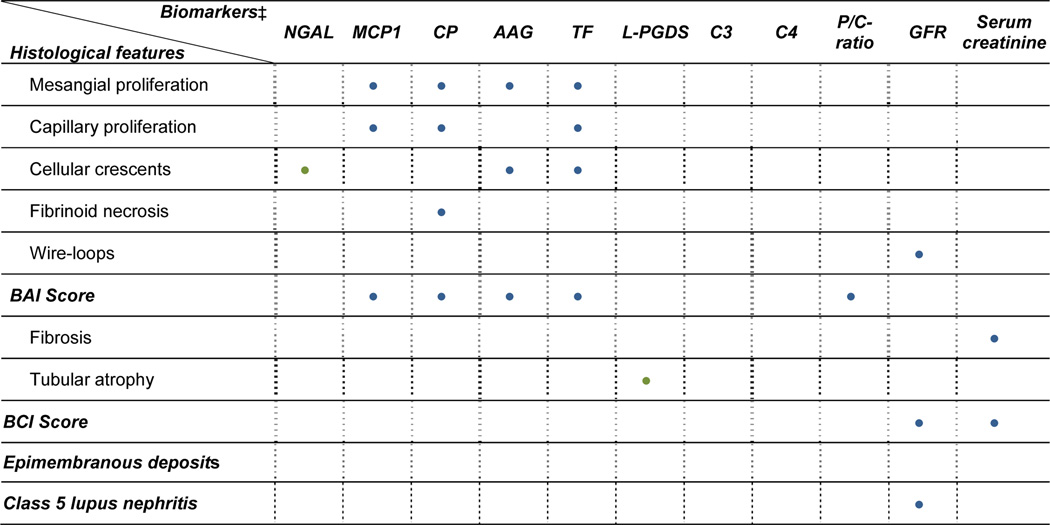
Figure 3.
A summary of significant changes with the presence vs. absence of histological features as are shown in Figure 1 and in Panel A – C of Figure 2. BLUE dots represent changes seen in urine samples that were collected within 2 months of the kidney biopsy (n=76), while GREEN dots represent additional significant differences if only urine samples collected prior to the kidney biopsy are considered (n= 38). The novel urine biomarkers are differentially excreted with histological changes of LN activity but not with membranous changes or LN chronicity. The GFR, serum creatinine and the P/C ratio do not allow for the differentiation between active, chronic or membranous changes of LN.
Acknowledgement
Drs. Elizabeth B. Brooks, Kathleen Haines, Lori Tucker, Marisa S. Klein-Gitelman, Judyann Olson, Karen Onel, Kathleen M. O'Neil, Alison Neal, and Lena Das for the collection of samples and clinical data; Aimee Baker, Lukasz Itert and Shannen Nelson for data management. Dr. Susan Thompson and Ms. Lorie Luyrik for management of the biological samples.
Funding
Drs. Brunner and Devarajan are supported by a grant from the Alliance for Lupus Research and the CCHMC Translational Research Initiative; Dr. Devarajan is supported by grants from the NIH/NIDDK (RO1-DK53289) and Department of Defense (PR064328). Dr. Brunner is supported by NIH/NIAMS U01AR059509 and P60AR47784. Sample storage was supported by P30AR047363. Dr. Petri and Dr. Kiani are supported by Hopkins Lupus Cohort (NIH AR 43727) and by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR). Dr. Rovin is supported by NIH/NIDDK DK074661, DK077331.
Footnotes
Conflict of Interest Statement
Dr. Devarajan is a co-inventor on NGAL patents for the diagnosis of acute kidney injury.
Dr. Devarajan and Brunner are co-inventors on patents covering biomarker panels for the diagnosis of lupus nephritis.
LITERATURE
- 1.Faurschou M, Starklint H, Halberg P, et al. Prognostic factors in lupus nephritis: diagnostic and therapeutic delay increases the risk of terminal renal failure. J Rheumatol. 2006 Aug;33(8):1563–1569. [PubMed] [Google Scholar]
- 2.Wu T, Xie C, Wang HW, et al. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J Immunol. 2007 Nov 15;179(10):7166–7175. doi: 10.4049/jimmunol.179.10.7166. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki M, Wiers K, Brooks EB, et al. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr Res. 2009 May;65(5):530–536. doi: 10.1203/PDR.0b013e31819e4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rovin BH, Song H, Birmingham DJ, et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005 Feb;16(2):467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 5.Hinze CH, Suzuki M, Klein-Gitelman M, et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 2009 Sep;60(9):2772–2781. doi: 10.1002/art.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner HI, Mueller M, Rutherford C, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006 Jul 25;54(8):2577–2584. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Wiers KM, Klein-Gitelman MS, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol. 2008 Mar;23(3):403–412. doi: 10.1007/s00467-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 8.Rovin BH. The chemokine network in systemic lupus erythematous nephritis. Front Biosci. 2008;13:904–922. doi: 10.2741/2731. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976 Aug;58(2):259–263. [PubMed] [Google Scholar]
- 11.Kasitanon N, Fine DM, Haas M, et al. Estimating renal function in lupus nephritis: comparison of the Modification of Diet in Renal Disease and Cockcroft Gault equations. Lupus. 2007;16(11):887–895. doi: 10.1177/0961203307084167. [DOI] [PubMed] [Google Scholar]
- 12.Ibanez D, Gladman DD, Urowitz MB. Adjusted mean Systemic Lupus Erythematosus Disease Activity Index-2K is a predictor of outcome in SLE. J Rheumatol. 2005 May;32(5):824–827. [PubMed] [Google Scholar]
- 13.Gladman DD, Goldsmith CH, Urowitz MB, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000;27(2):373–376. [PubMed] [Google Scholar]
- 14.Weening JJ, D'Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004 Feb;15(2):241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 15.Austin HA, 3rd, Muenz LR, Joyce KM, et al. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984 Apr;25(4):689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 16.Zappitelli M, Duffy CM, Bernard C, et al. Evaluation of activity, chronicity and tubulointerstitial indices for childhood lupus nephritis. Pediatr Nephrol. 2008 Jan;23(1):83–91. doi: 10.1007/s00467-007-0619-7. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu N, Kuroiwa T, Ikeuchi H, et al. Revised classification of lupus nephritis is valuable in predicting renal outcome with an indication of the proportion of glomeruli affected by chronic lesions. Rheumatology (Oxford) 2008 May;47(5):702–707. doi: 10.1093/rheumatology/ken019. [DOI] [PubMed] [Google Scholar]
- 18.Cortes-Hernandez J, Ordi-Ros J, Labrador M, et al. Predictors of poor renal outcome in patients with lupus nephritis treated with combined pulses of cyclophosphamide and methylprednisolone. Lupus. 2003;12(4):287–296. doi: 10.1191/0961203303lu340oa. [DOI] [PubMed] [Google Scholar]
- 19.Marks SD, Sebire NJ, Pilkington C, et al. Clinicopathological correlations of paediatric lupus nephritis. Pediatr Nephrol. 2007 Jan;22(1):77–83. doi: 10.1007/s00467-006-0296-y. [DOI] [PubMed] [Google Scholar]
- 20.Zappitelli M, Duffy C, Bernard C, et al. Clinicopathological study of the WHO classification in childhood lupus nephritis. Pediatr Nephrol. 2004 May;19(5):503–510. doi: 10.1007/s00467-004-1419-y. [DOI] [PubMed] [Google Scholar]
- 21.Lee BS, Cho HY, Kim EJ, et al. Clinical outcomes of childhood lupus nephritis: a single center's experience. Pediatr Nephrol. 2007 Feb;22(2):222–231. doi: 10.1007/s00467-006-0286-0. [DOI] [PubMed] [Google Scholar]
- 22.Demircin G, Oner A, Erdogan O, et al. Long-term efficacy and safety of quadruple therapy in childhood diffuse proliferative lupus nephritis. Ren Fail. 2008;30(6):603–609. doi: 10.1080/08860220802132171. [DOI] [PubMed] [Google Scholar]
- 23.Vachvanichsanong P, Dissaneewate P, McNeil E. Diffuse proliferative glomerulonephritis does not determine the worst outcome in childhood onset lupus nephritis: a 23-year experience in a single centre. Nephrology Dialysis Transplantation. 2009 doi: 10.1093/ndt/gfp173. [DOI] [PubMed] [Google Scholar]
- 24.Hagelberg S, Lee Y, Bargman J, et al. Longterm followup of childhood lupus nephritis. J Rheumatol. 2002 Dec;29(12):2635–2642. [PubMed] [Google Scholar]
- 25.Hersh AO, von Scheven E, Yazdany J, et al. Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2009 Jan 15;61(1):13–20. doi: 10.1002/art.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok CC. Membranous nephropathy in systemic lupus erythematosus: a therapeutic enigma. Nat Rev Nephrol. 2009 Apr;5(4):212–220. doi: 10.1038/nrneph.2009.14. [DOI] [PubMed] [Google Scholar]
- 27.Wu T, Fu Y, Brekken D, et al. Urine proteome scans uncover total urinary protease, prostaglandin D synthase, serum amyloid P, superoxide dismutase as potential markers of lupus nephritis. J Immunol. 2010 Feb 15;184(4):2183–2193. doi: 10.4049/jimmunol.0900292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa M, Hirawa N, Tsuchida T, et al. Urinary excretions of lipocalin-type prostaglandin D2 synthase predict the development of proteinuria and renal injury in OLETF rats. Nephrol Dial Transplant. 2006 Apr;21(4):924–934. doi: 10.1093/ndt/gfk009. [DOI] [PubMed] [Google Scholar]
- 29.Pawar RD, Pitashny M, Gindea S, et al. Neutrophil gelatinase associated lipocalin is instrumental in the pathogenesis of antibody-mediated nephritis. Arthritis Rheum. 2011 Nov 14; doi: 10.1002/art.33485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra J, Mori K, Ma Q, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004 Dec;15(12):3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 31.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009 Feb;4(2):337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viau A, El Karoui K, Laouari D, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010 Nov 1;120(11):4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasegawa H, Kohno M, Sasaki M, et al. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum. 2003 Sep;48(9):2555–2566. doi: 10.1002/art.11231. [DOI] [PubMed] [Google Scholar]
- 34.Mezzano SA, Droguett MA, Burgos ME, et al. Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy. Kidney Int. 2000 Jan;57(1):147–158. doi: 10.1046/j.1523-1755.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnsson E, Haraldsson B. Addition of purified orosomucoid preserves the glomerular permeability for albumin in isolated perfused rat kidneys. Acta Physiol Scand. 1993 Jan;147(1):1–8. doi: 10.1111/j.1748-1716.1993.tb09466.x. [DOI] [PubMed] [Google Scholar]
- 36.Moura IC, Benhamou M, Launay P, et al. The glomerular response to IgA deposition in IgA nephropathy. Semin Nephrol. 2008 Jan;28(1):88–95. doi: 10.1016/j.semnephrol.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, Meyron-Holtz E, Rouault TA. Renal iron metabolism: transferrin iron delivery and the role of iron regulatory proteins. J Am Soc Nephrol. 2007 Feb;18(2):401–406. doi: 10.1681/ASN.2006080908. [DOI] [PubMed] [Google Scholar]
- 38.Kondo C, Minowa Y, Uehara T, et al. Identification of genomic biomarkers for concurrent diagnosis of drug-induced renal tubular injury using a large-scale toxicogenomics database. Toxicology. 2009 Nov 9;265(1–2):15–26. doi: 10.1016/j.tox.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008 May;3(3):665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]