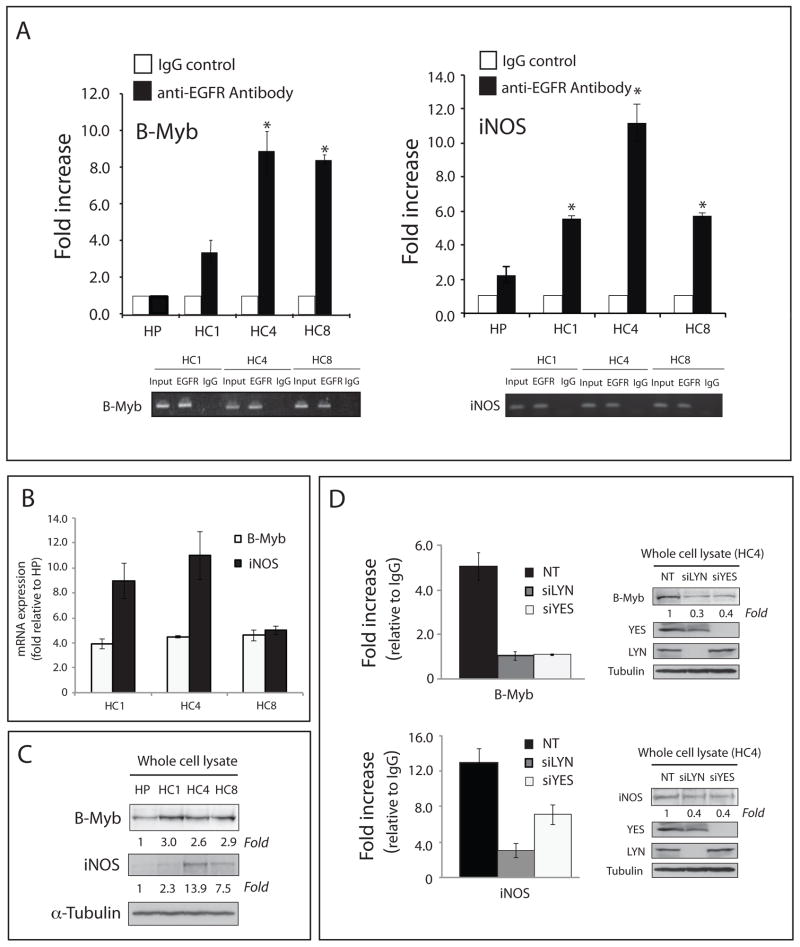
Figure 5. Yes and Lyn influence the binding of EGFR complexes to the B-Myb and iNOS promoter regions.
(A) EGFR regulated gene promoter regions are more strongly associated with EGFR in CtxR clones (HC1, HC4, and HC8) as compared to CtxS cells (HP). EGFR-ChIP and subsequent qPCR from the ChIP sample for the presence of B-Myb and iNOS promoter sequences. Data points are represented as mean ± SEM (n = 3). *p< 0.05. qPCR specificity for the B-Myb and iNOS promoter regions was also confirmed by agarose gel electrophoresis of semi-quantitative PCR products. (B) B-Myb and iNOS mRNA levels were significantly up-regulated in CtxR cells (HC1, HC4, and HC8) as compared to the CtxS cell line (HP) by qPCR. The mRNA expression of B-Myb and iNOS in HP, HC1, HC4 and HC8 were determined by qPCR. Data points are represented as mean ± SEM (n = 3). (C) B-Myb and iNOS protein levels were increased in CtxR cells (HC1, HC4, and HC8) as compared to the CtxS cell line (HP) by immunoblot analysis. Cells were harvested and protein lysates were fractionated on SDS-PAGE followed by immunoblotting for the indicated proteins. α-tubulin was used as a loading control. (D) Loss of Yes or Lyn prevents EGFR association with B-Myb and iNOS promoters, and corresponds with a decrease in protein expression. EGFR-ChIP and subsequent qPCR from the ChIP sample for the presence of B-Myb and iNOS promoter sequences. The non-targeting siRNA (NT) was used as a control. B-Myb and iNOS protein levels were decreased in HC4 after siYES or siLYN treatment by immunoblot analysis. Cells were harvested after treatment with siLYN or siYES for 72 hr and protein lysates were fractionated on SDS-PAGE followed by immunoblotting for the indicated proteins. α-tubulin was used as a loading control.