Abstract
Bone destruction is a hallmark of multiple myeloma and affects more than 80% of patients. However, current therapy is unable to completely cure and/or prevent bone lesions. Although it is accepted that myeloma cells mediate bone destruction by inhibition of osteoblasts and activation of osteoclasts, the underlying mechanism is still poorly understood. This study demonstrates that constitutive activation of p38 mitogen-activated protein kinase in myeloma cells is responsible for myeloma-induced osteolysis. Our results show that p38 is constitutively activated in most myeloma cell lines and primary myeloma cells from patients. Myeloma cells with high/detectable p38 activity, but not those with low/undetectable p38 activity, injected into SCID or SCID-hu mice caused bone destruction. Inhibition or knockdown of p38 in human myeloma reduced or prevented myeloma-induced osteolytic bone lesions without affecting tumor growth, survival, or homing to bone. Mechanistic studies showed that myeloma cell p38 activity inhibited osteoblastogenesis and bone formation and activated osteoclastogenesis and bone resorption in myeloma-bearing SCID mice. This study elucidates a novel molecular mechanism—sactivation of p38 signaling in myeloma cells—by which myeloma cells induce osteolytic bone lesions and indicates that targeting myeloma cell p38 may be a viable approach to treating or preventing myeloma bone disease.
Keywords: Myeloma, p38 MAPK, Osteolytic bone lesions, Osteoblastogenesis, Osteoclastogenesis
Introduction
Bone destruction is a hallmark of multiple myeloma, which is characterized by accumulation of malignant plasma cells in the bone marrow (1). More than 80% of myeloma patients have osteolytic bone lesions, which can cause pathological fractures, severe bone pain, spinal cord compression, and hypercalcemia, having a severe impact on patients’ quality of life (1). Although several new reagents have been reported to delay myeloma-induced osteolysis, current therapy is unable to completely cure and/or prevent bone lesions (1).
Bone is a dynamic tissue that is constantly being remodeled by bone-resorbing osteoclasts and bone-forming osteoblasts. It is commonly accepted that myeloma cells are responsible for the bone lesions associated with this cancer. Osteolytic lesions are observed clinically only in bones where myeloma cells infiltrate and accumulate (1). Bone destruction results from increased osteoclast formation and activity that occur in close proximity to myeloma cells (1). Furthermore, histomorphometric studies demonstrate that myeloma patients with osteolytic bone lesions have fewer osteoblasts and decreased bone formation (2, 3), suggesting that myeloma cells suppress osteoblasts and inhibit bone formation. However, the underlying mechanisms by which myeloma cells induce osteoblast inhibition and osteoclast activation are unclear.
p38 mitogen-activated protein kinase (MAPK; hereafter, p38) participates in a variety of cellular responses, including inflammatory response, differentiation, proliferation, and survival (4–6). Elevated levels of p38 activity have been found in benign bone diseases (7–10) and in malignant osteolytic tumors, such as multiple myeloma (11–14). Previous studies have shown that activation of p38 signaling is involved in myeloma growth and survival and resistance to chemotherapy drugs. Treatment with p38 inhibitor SCIO-469 has been shown to overcome resistance to proteasome inhibitor bortezomib-induced myeloma cell apoptosis by inhibiting the expression and activity of Hsp27, upregulating levels of p53 and phosphorylation of JNK, and downregulating anti-apoptotic proteins Bcl-X(L) and Mcl-1, all of which lead to increased cleavage of caspases, poly-ADP-ribose polymerase levels, and G2/M arrest (12). Furthermore, a combination of arsenic trioxide and p38 inhibition abolishes interleukin-6 (IL-6)–induced protection of myeloma cell apoptosis, suggesting that arsenic trioxide–induced p38 activation may play an important role in resistance to arsenic trioxide treatment in myeloma patients (15, 16). Recent studies show, furthermore, that p38α, but not other isoforms, is essential to regulation of arthritic bone loss. Others show that RANKL-induced osteoclast differentiation requires activation of the p38 signaling pathway in osteoclast progenitors (17). The p38 signaling pathway in bone marrow stromal cells mediates inflammatory cytokine-induced RANKL expression and secretion (10, 18), and myeloma cells enhance bone marrow stromal cell secretion of IL-6 and RANKL by upregulating p38 signaling in stromal cells through p62 sequestosome-1 (19). Inhibition of p38 by specific inhibitors, such as VX-745 (11) or SB202190, or by small interfering RNAs reduces either bone marrow stromal cell secretion of IL-6 and VEGF (14) or osteoblast secretion of DKK-1 (20). Moreover, treatment with a p38 inhibitor reduces tumor burden, prevents osteolytic bone lesions, and prolongs survival in the 5T2MM and 5T33MM mouse models of myeloma (21). These findings suggest that activation of p38 in the bone marrow microenvironment may be involved in the activation of osteoclastogenesis and bone resorption in myeloma.
In this study, we demonstrate for the first time that constitutive activation of p38 in myeloma cells, but not in bone marrow stromal cells, induces osteolysis. Our results show that p38 is constitutively active in myeloma cells, both myeloma-derived cell lines and primary myeloma cells from patients. Injection of myeloma cells with high/detectable p38 activity into mice not only established myeloma but also induced bone destruction, whereas myeloma cells with undetectable p38 activity only established myeloma. Disruption of p38 activity in myeloma cells by a specific inhibitor or short hairpin RNA (shRNAs) abrogated myeloma-induced bone lesions. Our results also show that p38 activity in myeloma cells induces osteolytic bone lesions by inhibiting osteoblastogenesis and activating osteoclastogenesis. These findings indicate that inhibition of p38 signaling in myeloma cells may be a novel therapeutic approach to preventing or treating myeloma-induced bone disease.
Materials and Methods
Tumor cell lines and primary myeloma cells
Myeloma cell line ARP-1 was established at the Arkansas Cancer Research Center from bone marrow aspirates of patients with multiple myeloma. Cell line MM.1S was kindly provided by Dr. Steven Rosen, Northwestern University (Chicago, IL). Other myeloma cell lines were purchased from American Type Culture Collection (Rockville, MD). All myeloma cell lines were cultured in RPMI-1640 medium supplemented with 10% FBS (Invitrogen, Carlsbad, CA). Primary myeloma cells were isolated from patient bone marrow aspirates collected during routine clinic visits. CD138+ myeloma cells were isolated by magnetic bead sorting (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Plamids and reagents
shRNAs for p38 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and packed into retroviral vector pSIREN-RetroQ (BD Biosciences Clontech, Mountain View, CA). Retroviral infections were performed according to the manufacturer’s instructions. p38 MAPK-specific inhibitors were purchased from Axon Medchem BV (Groningen, the Netherlands).
Mouse models, radiography, μ-computed tomography, histology, and bone histomorphometry
CB.17 SCID mice were purchased from Harlan (Indianapolis, IN). SCID-hu mice were established from CB.17 SCID mice, as previously described (22). Xenografted primary myeloma-SCID-hu mouse model mimics the in vivo situation of patients with myeloma by harboring primary myeloma cells in a human bone marrow microenvironment where they cause lytic bone lesions (23–26). All mice were maintained in American Association of Laboratory Animal Care–accredited facilities, and the studies were approved by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center. 1 × 106 tumor cells were inoculated intravenously to six- to 8-week-old SCID mice or 1× 106 CD138+ myeloma cells, isolated from myeloma patients, were injected into the implanted human fetal bones (Advanced Bioscience Resources, CA) of SCID-hu mice (23–26). Serum was collected from mice daily during the treatment and tested for myeloma-secreted M-proteins (human Ig) or their light chains by ELISA. To measure size of lytic bone lesions, radiographs were scanned with a Faxitron X-ray cabinet (Faxitron X-ray, Lincolnshire, IL). We also scanned the trabecular bone of the distal femur by μ–computed tomography (μ-CT-40, Scanco Medical, Wayne, PA). For histologic and bone histomorphometric analyses, mice were killed and their tibias fixed in 10% neutral-buffered formalin for 18 hours. Sections of paraffin-embedded tissues were stained with hematoxylin and eosin and for tartrate-resistant acid phosphatase (TRAP) activities by using a leukocyte acid phosphatase staining kit (Sigma, St Louis, MO) according to the manufacturer’s instructions. The number of TRAP-positive, multinuclear (>3) osteoclasts per millimeter of bone at the bone-tumor interface was calculated using a computerized image analysis system. To detect osteoblasts at the bone-tumor interface, the sections were stained with toluidine blue by using standard protocols. Number of osteoblasts and bone formation activity were determined by the computerized image analysis system.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections of bone marrow biopsies from myeloma patients, tissue arrays containing bone marrow biopsy specimens from patients with multiple myeloma and healthy donors, and bones from tumor-inoculated SCID or SCID-hu mice were deparaffinized, as previously described. Malignant plasma cells were identified by morphologic assessment. Expression of phosphorylated p38 (pp38), nonphosphorylated p38, and CD138 was detected by using specific antibodies. Slides were stained with chromagen 3,3-diaminobenzidine/H2O2 (DAKO, Carpinteria, CA) and then counterstained with hematoxylin. All slides were observed with light microscopy, and images were captured with a SPOT RT camera (Diagnostic Instruments, Burlingame, CA).
Western blotting
Cells were harvested and lysed with lysis buffer. Cell lysates were subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted with antibodies against phosphorylated or nonphosphorylated kinases, including p38, ERK, MKK3/6, AF-2, and MAPKPK-2 (Cell Signaling Technology, Inc., Beverly, MA). The membrane was stripped and reprobed with anti-β-actin antibody (Sigma) to ensure equal protein loading.
Myeloma cell proliferation and apoptosis
The proliferation of myeloma cells were determined by MTT assay with Cell Proliferation Assay kit (Promega). The fraction of apoptotic cells was determined by staining with FITC-conjugated Annexin-V and propidium iodide (PI), and analyzed by flow cytometry. Both assays were performed according to manufacturer’s instructions.
Statistical analysis
All data are shown as means ± SD. The Student t-test was used to compare various experimental groups; significance was set at P less than .05.
Results
p38 is constitutively active in myeloma cells
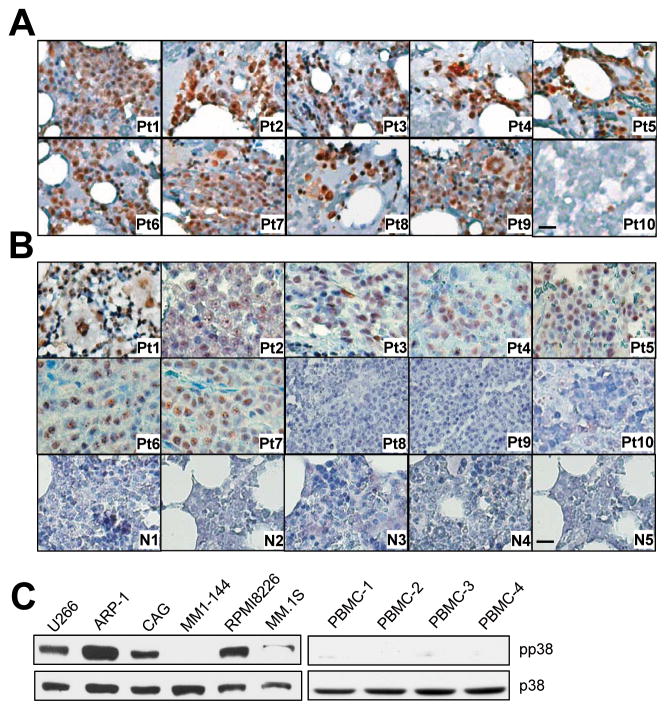
Constitutive activation of p38 has been found in various malignant tumors (11, 12, 14). We wonder whether p38 is active in myeloma cell. By using immunohistochemistry staining, we examined the expression of pp38 in bone marrow biopsy specimens from randomly selected patients with newly diagnosed myeloma. Myeloma cells from nine of the 10 patients were pp38 positive (Figure 1A). Similar results were obtained from the staining of a tissue array containing bone marrow samples from 10 myeloma patients and 11 healthy donors. Seven of the 10 myeloma samples but none of the healthy donor samples stained positive for pp38 (Figure 1B). Western blot analysis detected pp38 in five of six myeloma cell lines (Figure 1C). These results confirm that p38 is constitutively activated in the majority of primary myeloma cells and established cell lines.
Figure 1.
Constitutive activation of p38 in myeloma cells. Representative images of immunohistochemical staining for pp38 in (A) bone marrow biopsy specimens of 10 randomly selected patients with newly diagnosed myeloma (Pt1–Pt10) and (B) a tissue array containing bone marrow samples of 10 myeloma patients (Pt1–Pt10) and 11 healthy donors. (Only five samples, N1–N5; are shown. The other six samples also stained negatively for pp38.) Scale bar, 10 μM. Western blot analysis showing the levels of phosphorylated p38 (pp38) and nonphosphorylated p38 (p38) in (C) six myeloma cell lines and in (D) three normal PBMCs. Representative results of three independent experiments are shown.
Myeloma cells with high p38 activity induce lytic bone lesions in vivo
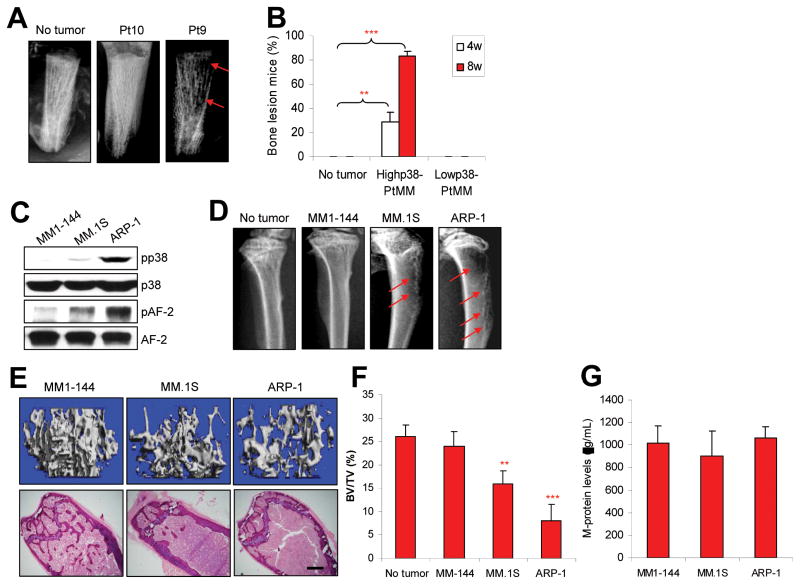
Because over 80% of myeloma patients have osteolytic bone lesions (27), we hypothesized that constitutive activation of p38 in bone-residing myeloma cells might play a critical role in myeloma-induced bone lesions. We used the primary myeloma-SCID-hu xenograft mouse model, which mimics the in vivo situation of patients with myeloma by harboring primary myeloma cells in a human bone marrow microenvironment where they cause lytic bone lesions (23–26), to examine induction of bone lesions by primary myeloma cells with high/detectable or undetectable pp38 expression (determined by immunohistochemical staining of bone marrow biopsies). As shown in Figure 2A, 2B and Supplementary Figure 1, severe bone resorption was observed in 20 of 24 SCID-hu mice injected with primary myeloma cells with high/detectable p38 activity from eight patients, while none of 12 mice injected with primary myeloma cells with undetectable pp38 (from four patients) had visible bone resorption, as detected by radiography at 8 weeks after tumor inoculation. Interestingly, the eight patients with pp38-positive myeloma cells had focal osteolytic bone lesions (determined by magnetic resonance imaging), while the four patients with undetectable pp38 myeloma cells did not (data not shown).
Figure 2.
Myeloma cells with high/detectable p38 activity cause bone lesions in mouse models. (A) Representative radiographic images show lytic bone lesions in the implanted human bones of SCID-hu mice bearing primary myeloma cells from one (patient 10; pt10) of four patients with low or undetectable p38 or one (patient 9; pt9) of eight patients with high/detectable p38 activity. (B) Percentages of mice with bone lesions, detected by radiographs, at 4 and 8 weeks after injection ofprimary myeloma cells with high/detectable p38 activity from eight patients or with undetectable pp38 activity from four patients. (C) Western blot analysis shows the levels of phosphorylated p38 (pp38) and nonphosphorylated p38 (p38) and AF-2 in MM1-144, MM.1S, and ARP-1 myeloma cells. (D) Representative radiographic images show lytic bone lesions in the distal femurs of SCID mice (10 per each cell line) injected with ARP-1, MM.1S, or MM1-144 myeloma cells. Tumor-free mice (no tumor) served as controls. Red arrows indicate osteolytic lesions. (E) Representative μ-CT images (upper panels) and histologic examinations (lower panels) and (F) quantitative analyses show the trabecular bone volume density (BV/TV) in distal femurs of SCID mice bearing ARP-1, MM.1S, or MM1-144 myeloma. ** P < 0.01 to *** P < 0.001, compared with tumor-free mice. Scale bars: 1 mm. (G) ELISA showing no difference in the levels of M-protein in serum of mice injected with ARP-1, MM.1S, or MM1-144 myeloma cells at week 8 after tumor injection. Similar results were obtained at week 4 and week 6 after tumor injection.
Next we examined the ability of human myeloma cell lines to induce bone lesions in SCID mice. Of six human myeloma cell lines examined, only ARP-1, MM.1S, and MM1-144 established myeloma in the murine bone marrow and other organs after intravenous injection (data not shown). Our preliminary results showed that ARP-1 cells expressed high levels of pp38 and phosphorylated AF-2 (pAF-2), the downstream kinase of p38 that is directly activated by pp38. MM.1S cells expressed low but detectable levels of pp38 and pAF-2, whereas MM1-144 expressed no detectable pp38 or pAF-2 (Figure 2C). SCID mice injected intravenously with one of these cell lines were monitored for bone lesions by radiography. At 8 weeks after injection, eight of 10 mice injected with ARP-1 displayed three to five focal lytic pits in the distal femurs; three of 10 mice injected with MM.1S showed similar bone lesions; while none of the 10 mice injected with MM1-144 showed detectable bone lesions (Figure 2D and Supplementary Figure 2), although all mice developed myeloma as detected by level of circulating M-proteins or light chains secreted by the myeloma cells (data not shown). By the time the mice were killed because of development of systemic symptoms (weeks 12–13), all of the mice injected with MM1-144 remained bone-lesion free.
To confirm the lytic bone pits identified by radiography, sections of the distal femurs from the injected mice were subjected to peripheral quantitative μ-CT and histologic examinations. As shown in Figure 2E, distal femurs from mice injected with ARP-1 or MM.1S cells had lower trabecular bone volumes (upper panels) and lower density of trabeculae (lower panels) than mice injected with MM1-144 cells. The quantitative trabecular bone volume data from mice injected with three cell lines are shown in Figure 2F, and tumor-free mice served as control (P < 0.01 to P < 0.001). In addition, after tumor cell injection, mouse serum was collected weekly and the circulating levels of M-protein, secreted by myeloma cells, were measured by ELISA. As shown in Figure 2G, at 8 week after tumor cell injection, no significant difference was found in the levels of circulating M-protein among mice injected with the three cell lines. To exclude the possibility that the tumor cells differed in their ability to home to the bone, we killed mice injected with ARP-1, MM.1S, or MM1-144 cells at different time points and examined tumor infiltration of bone marrow and other tissues. All three cell lines were able to home to the bone marrow and other organs, and no significant difference was noted in the numbers of bone marrow–infiltrating tumor cells among the three cell lines (data not shown). Taken together, these results indicate that myeloma cells, including primary cells and established cell lines, with high or detectable, but not undetectable, p38 activity can induce osteolytic bone lesions in both human and murine bones in vivo.
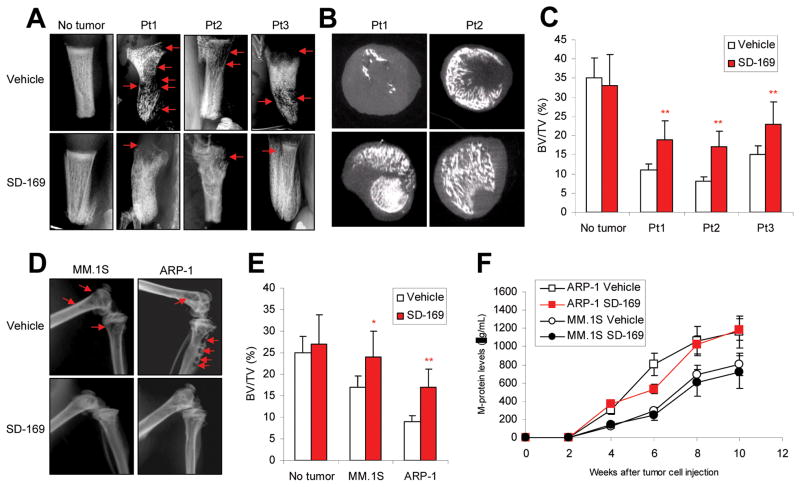
p38 inhibitor reduces osteolytic bone lesions in mice injected with myeloma cells
To confirm the contribution of p38 activation in inducing bone lesions in vivo, a series of experiments were conducted using both human primary myeloma cells and established cell lines. We used a commercially available p38 inhibitor, SD-169, a selective, orally active, and ATP-competitive inhibitor of p38α that lacks activity against a panel of other MAPKs, to disrupt p38 activity in myelomas induced in mice. In one set of experiments, primary myeloma cells with high or detectable levels of pp38 were injected into the implanted human bones of SCID-hu mice. When circulating M-protein levels reached 10 μg/ml, mice were fed daily with SD-169 (10 mg/kg) or equal volume of PBS (vehicle controls) for a total of 30 days. At the end of treatment, the implanted human bones were harvested and examined for lytic bone lesions by radiography and μ-CT scanning. Compared with PBS controls, mice treated with SD-169 had significantly fewer lytic bone lesions (Figure 3A; P < 0.01), and μ-CT scanning detected more trabecular bone in their marrow spaces and higher bone mass densities (Figure 3B). Compared with vehicle control, SD-169 treatment significantly reduced the trabecular bone volumes in primary myeloma-bearing SCID-hu mice (Figure 3C; P < 0.01). Similarly, treatment of SCID mice bearing ARP-1 or MM.1S cells with SD-169 significantly reduced or prevented bone lesions (Figure 3D). The quantitative trabecular bone volume data from mice injected with primary myeloma cells from three patients and treated with SD-169 are shown in Figure 3E (P < 0.01 to P < 0.001; compared with vehicle control). However, SD-169 did not affect tumor burden as measured by level of circulating M-proteins (Figure 3F) or the numbers of infiltrating CD138+ myeloma cells in the bone marrow (Figure 5E). As expected, SD-169 downregulated pp38 in myeloma cells in vitro and in vivo (data not shown). These results strongly suggest that inhibition of p38 activation reduces or prevents myeloma-induced lytic bone lesions in both human and murine bones in vivo.
Figure 3.
Administration of p38 inhibitor reduces myeloma-induced osteolytic bone lesions. In vivo injection of p38 specific inhibitor SD-169 significantly reduced myeloma-induced bone lesions in fetal human bones implanted into SCID-hu mice bearing a primary myeloma xenograft; lesions were detected by (A) radiography and (B) μ-CT scanning. (C) SD-169 also reduced bone lesions in distal femurs of ARP-1- or MM.1S-bearing SCID mice. (D) Tumor burden was measured as circulating human Ig in SCID mice inoculated with ARP-1 or MM.1S cells treated without or with p38 inhibitor SD-169. CD138+ primary myeloma cells were isolated from three myeloma patients (Pt1, Pt2, and Pt3) with high/detectable p38 activity and injected into the implanted human bones of SCID-hu mice. Myeloma ARP-1 or MM.1S cells were injected intravenously into SCID mice (10 per each cell line). When circulating M-protein levels reached 10 μg/ml, mice were fed daily with SD-169 (10 mg/kg) or an equal volume of PBS (vehicle controls) for a total of 30 days. Arrows indicate osteolytic bone lesions.
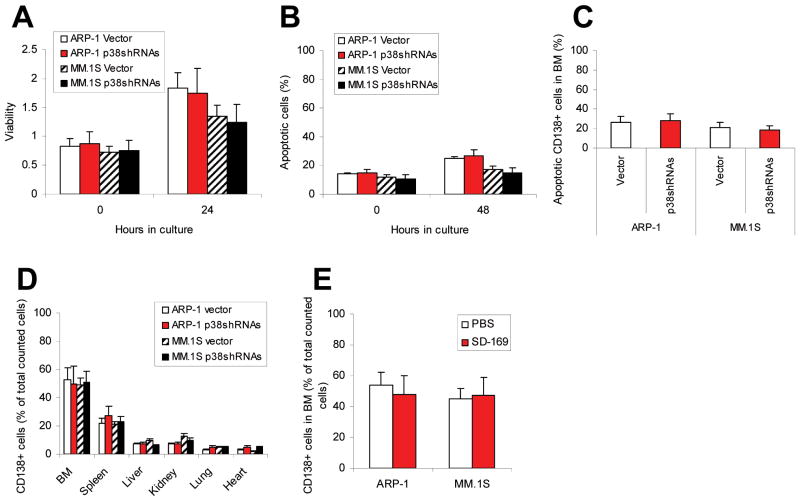
Figure 5.
Disruption of myeloma cell p38 activity has no effect on tumor growth or survival or on the ability of myeloma cells to home to bones. Shown are (A) viability of vector-or p38 shRNA-ARP-1 or -MM.1S cells (p38shRNAs) examined byMTT assayat 0 and 24 hour culture; and (B) percentages of apoptotic vector- or p38 shRNA-ARP-1 or -MM.1S cells examined by Annexin-V binding assay at 0 and 48 hour cultures. Results represent average values from five independent experiments. (C) In situ TUNEL assay results show the percentages of apoptotic CD138+ tumor cells in the bone marrow of SCID mice injected with vector- (vector) or p38 shRNA-ARP-1 or -MM.1S cells. Flow cytometry results show tissue-infiltrating CD138+ human myeloma cells in (D) different organs, including bone marrow (BM), spleen, liver, kidney, heart and lung, of SCID mice injected with vector- (vector) or p38 shRNA-ARP-1 or -MM.1S cells or (E) bone marrow of SCID mice injected with parental ARP-1 or MM.1S cells and treated with p38 inhibitor SD-169 or PBS. In the experiments, myeloma cells were injected intravenously into SCID mice (10 per each cell line). When circulating M-protein levels reached 10 μg/ml, mice were fed daily with SD-169 (10 mg/kg) or an equal volume of PBS (vehicle controls) for a total of 30 days. After treatment, bone marrow cells were flushed out and CD138+ human myeloma cells were identified with flow cytometry. The results represent average values from three independent experiments of five mice per group.
Knockdown of p38 activity in myeloma cells by shRNAs abrogates their ability to cause osteolytic bone lesions in vivo
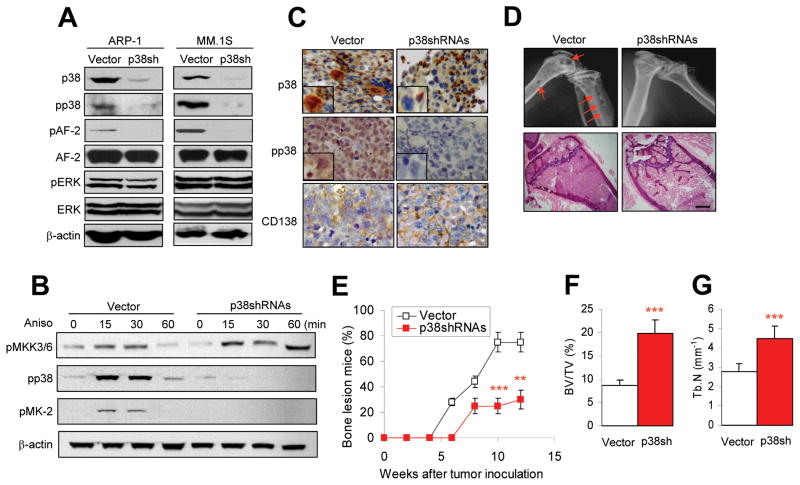
Since in vivo administration of p38 inhibitors blocks p38 activity in cells other than myeloma cells, and primary myeloma cells are difficult to genetically manipulate ex vivo, we applied shRNAs via a retroviral vector to specifically and stably knockdown p38 protein expression in ARP-1 and MM.1S cells. As expected, levels of p38 protein were significantly reduced in the tumor cells infected with p38-shRNA (Figure 4A). Similarly, the levels of pp38 and ppAF-2, but not AF-2, were also significantly downregulated in the infected cells. That the knockdown was specific for p38 was shown by the lack of effect on other MAPKs, such as ERK1/2 (Figure 4A). To determine whether p38 activity could still be activated in p38 shRNA-infected myeloma cells, the tumor cells were treated with anisomycin, a specific activator of p38 (28). Anisomycin upregulated the phosphorylation of p38 and its upstream kinases MKK3/6 (pMKK3/6) and downstream kinase MAPKPK-2 (pMK-2) in vector-infected but not in p38 shRNA-infected myeloma cells, except pMKK3/6 (Figure 4B). These results indicate that p38 shRNAs inhibited the activation of p38 and its downstream kinases, and thus its signaling pathway in the cells.
Figure 4.
Knockdown of tumor cell p38 activity by shRNAs reduces myeloma-induced osteolytic bone lesions. Myeloma cells were stably transfected with retrovirus containing control vector or p38-shRNAs to knock down p38 activity. Western blot analysis shows (A) levels of phosphorylated p38 (pp38), nonphosphorylated p38 (p38), nonphosphorylated or phosphorylated AF-2 (a p38 downstream kinase), and nonphosphorylated or phosphorylated ERK1/2 in vector-(vector) or p38 shRNA-ARP-1 or -MM.1S cells, and (B) levels of phosphorylated MKK3/6 (pMKK3/6, upstream kinase of p38), pp38, and phosphorylated MAPKPK-2 (pMK-2, downstream kinase of p38) in vector- or p38 shRNA-ARP-1 (p38shRNAs) or -MM.1S (data not shown) cells treated with 10 nM of anisomycin (Aniso) at different time points (0, 15, 30, 60 minutes). β-actin protein levels served as loading controls. (C) Immunohistochemical staining shows the expression of p38, pp38, and CD138 (myeloma cell marker) in bone sections of SCID mice injected with vector- (vector) or p38 shRNA-ARP-1 cells. The vector-ARP-1 or p38 shRNA-ARP-1 cells were intravenously injected into SCID mice. Sections of distal femur taken from mice killed 4 weeks after tumor inoculation were immunostained with antibodies against p38, pp38, and CD138. Scale bar, 10 μM. (D) Representative radiographic images (upper panels) show osteolytic lesions in the distal femurs and histologic examinations (lower panels) show the densities of trabecular bones in the bone marrow of SCID mice (10 per group) injected with vector- or p38 shRNA-ARP-1 cells and killed 8 weeks after tumor injection. Arrows indicate osteolytic bone lesions. (E) Percentages of mice with bone lesions, detected by radiographs, are shown at different time intervals after injection with vector- or p38shRNA-ARP-1 cells. Analysis of bone sections by μ-CT scanning shows (F) greater trabecular bone volumes and (G) larger densities inthe distal femurs of SCID mice injected with p38 shRNA-ARP-1 cells than in those injected with vector-ARP-1 cells. **P ≤ 0.01; ***P ≤ 0.001.
To confirm that p38 activity was inhibited in p38 shRNA-ARP-1 or -MM.1S cells in vivo, these cells were injected intravenously into SCID mice. At 6 weeks, when the mice had developed myeloma, they were killed and their femurs removed, sectioned, and examined for p38 and pp38 expression by immunohistochemistry. As shown in Figure 4C, p38 shRNA-ARP-1 cells seldom stained positive for p38 and pp38 as compared with infiltrating parental (data not shown) or vector-ARP-1 cells. Interestingly, knocking down tumor cell p38 also led to downregulated expression of pp38, but not p38 proteins, by surrounding nonmyeloma cells in the bone marrow (Figure 4C). These results confirm that p38 shRNAs successfully disrupted p38 activity and its downstream signaling pathways in the tumor cells in vivo, which may also have had an impact on the p38 activity in the surrounding nontumor cells in the bone marrow microenvironment. To examine the effects of p38 shRNA-ARP-1 cells on osteolysis in vivo, by week 8 (Figure 4D) or a longer period (Figure 4E) after tumor inoculation, furthermore, eight of 10 mice injected with parental (data not shown) or vector-ARP-1 cells had several osteolytic lesions in the distal femurs, whereas only three of 10 of mice injected with p38 shRNA-ARP-1 cells developed osteolytic bone lesions. μ-CT scanning of the distal femurs showed significantly higher trabecular bone volumes (Figure 4F) and higher density of trabeculae (Figure 4G) in mice injected with p38 shRNA-ARP-1 cells than in mice injected with parental (data not shown) or vector-control tumor cells (P < 0.001). Histologic analysis showed that the trabecular bone of the proximal tibial metaphysis in mice injected with control tumor cells had been largely destroyed and the bone marrow cavity had been replaced by tumor cells, whereas in mice injected with p38 shRNA-ARP-1 cells, bone destruction was much less extensive and trabecular bone was relatively intact, even though the bone marrow cavity had also been replaced by tumor cells (Figure 4D, lower panel). Similar results were obtained with MM.1S cells (data not shown).
Disruption of myeloma cell p38 activity has no effects on tumor growth, survival, or homing to bone
Because p38 participates in a variety of cellular responses, we wanted to rule out the possibility that the reduced ability of p38-knockdown tumor cells to cause bone lesions in vivo was the result of inhibited homing to and/or survival of the tumor cells in the bone marrow milieu. First, we examined tumor growth and survival of p38 shRNA-ARP-1 or -MM.1S cells or control cells in vitro. As shown in Figure 5A, cells proliferated in a 24-hour cell culture, but there was no difference between proliferation of ARP-1 or MM.1S cells infected with vector and those infected with p38 shRNA. Similarly, there was no difference between survival of myeloma cells infected with vector and that of cells infected with p38 shRNAs in a 48-hour cell culture (Figure 5B). In our examination of in vivo tumor cell survival, in situ TUNEL assay consistently revealed no difference between numbers of apoptotic myeloma cells in SCID mice killed 4, 8, or 10 weeks (data not shown) after intravenous injection of p38 shRNA-ARP-1 or -MM.1S cells and in those injected with control cells (Figure 5C).
We also examined the ability of p38-knockdown myeloma cells to home to bone. In the experiments, p38 shRNA-infected or control myeloma cells were injected intravenously into SCID mice, which were killed at different time points following inoculation. The numbers of bone marrow– and other tissue-infiltrating CD138+ myeloma cells were quantified by flow cytometry. There was no difference between infiltration by p38 shRNA-ARP-1 or MM.1S cells and by control cells into the bone marrow at 4 weeks (Figure 5D) or at 8 or 10 weeks (data not shown) after tumor inoculation. At week 4, about 50% of bone marrow cells were infiltrating CD138+ human myeloma cells, whereas spleens and other organs contained 20% or fewer than 10%, respectively, of tumor cells. These data clearly show that the numbers of tumor cells residing in the bone marrow did not differ significantly among mice injected with either vector- or p38 shRNA-ARP-1 or -MM.1S cells. Moreover, there was no difference in the numbers of tissue-infiltrating CD138+ myeloma cells found in bone marrow (Figure 5E) or other tissues (data not shown) of myeloma-bearing mice treated with p38 inhibitor SD-169 or control PBS. These results indicate that knocking down p38 activity in myeloma cells did not affect myeloma cell homing to and survival within bone marrow.
Myeloma cell p38 inhibits osteoblastogenesis
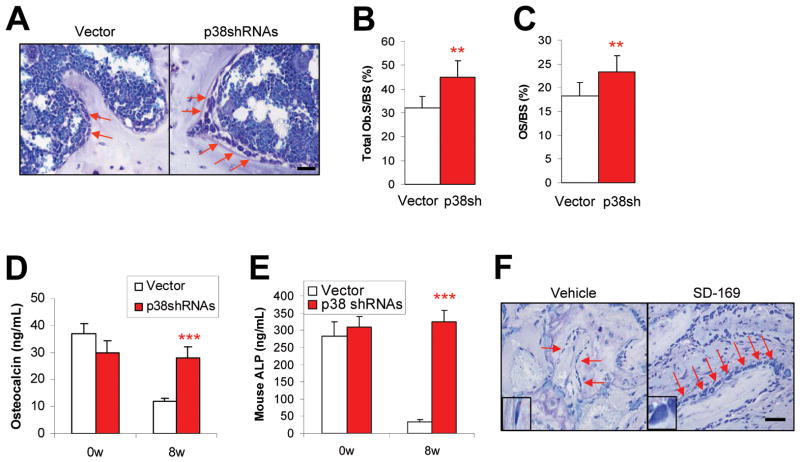
It is well known that bone remodeling is regulated by osteoblast-mediated bone formation and osteoclast-induced bone resorption (29). To dissect the mechanisms underlying osteolytic bone disease in the models, we examined whether myeloma p38-induced lytic bone lesions were the result of decreased bone formation due to inhibited osteoblastogenesis. We compared the numbers of osteoblasts in bone sections and their bone-forming activity in mice injected with vector- or p38 shRNA-ARP-1 or -MM.1S (data not shown) cells. Osteoblasts localized on trabecular bone surface were identified by toluidine blue staining, and the concentrations of osteocalcin (a serum marker for bone formation) and alkaline phosphatase (ALP, a marker for osteoblast activity) in mouse serum were measured. At week 8 after tumor cell injection, toluidine blue staining of bone sections showed a greater number of osteoblasts in mice bearing p38 shRNA-ARP-1 myeloma (Figure 6A and 6B; P < 0.01) than in those injected with control ARP-1 cells. Quantitative histomorphometry of the bone sections indicated a significantly increased production of osteoid in the mice injected with p38 shRNA-ARP-1 cells (Figure 6C; P < 0.01), which reflects an increase in osteoblast activity (30). Mice inoculated with p38 shRNA-ARP-1 cells also had higher levels of circulating osteocalcin (Figure 6D; P < 0.001) and ALP (Figure 6E; P < 0.001) than mice injected with control ARP-1 cells. Similarly, bone sections from ARP-1–xenografted SCID mice (data not shown) or primary myeloma-implanted SCID-hu mice treated with p38 inhibitor SD-169 also had significantly higher numbers of osteoblasts than those from control mice (Figure 6F). These data indicate that myeloma cell p38 activity leads to inhibited osteoblastogenesis, and that inhibiting or knocking down tumor cell p38 activity can largely increase osteoblast numbers and activity in tumor-bearing mice.
Figure 6.
Activated tumor cell p38 inhibits osteoblastogenesis in vivo. Shown are (A) representative images of toluidine blue staining showing the numbers of osteoblasts, and the results of quantitative analysis of osteoblast parameters, including (B) total osteoblast numbers on the surface and (C) osteoid percentage of trabecular bone of SCID mice bearing vector- or p38 shRNA-ARP-1 myeloma. ELISA showed levels of circulating (D) osteocalcin or (E) ALP in mice prior to (week 0; 0w) and 8 weeks (8w) after injection with controls cells or p38 shRNA-ARP-1 cells. (F) Immunohistochemical staining shows the numbers of osteoblasts present at week 8 on the surface of human trabecular bone implanted into primary myeloma-xenografted SCID-hu mice treated without (vehicle) or with p38 inhibitor SD-169. Arrows indicate osteoblasts. Generation of osteoblasts from normal mesenchymal stem cells in osteoblast medium with addition of conditioning medium of vector or p38 shRNA-ARP-1 cells (p38SH). Representative results from four independent experiments are shown. **P ≤ 0.01; ***P ≤ 0.001.
Myeloma cell p38 activates osteoclastogenesis
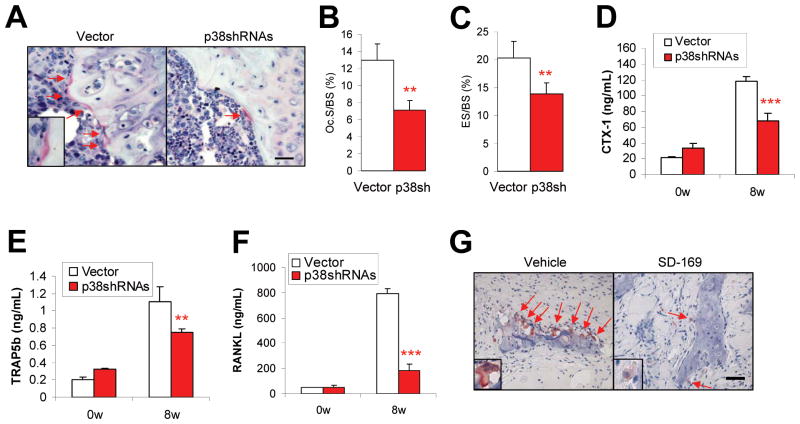
Next we investigated the impact of myeloma cell p38 activity on osteoclastogenesis and bone resorption. We used TRAP staining and computerized image analysis to identify the number, size, and activity of osteoclasts in bone sections from mice killed 8 weeks after injection of ARP-1 or MM.1S (data not shown) cells. TRAP staining showed lower numbers of multinuclear, mature osteoclasts localized on the trabecular bone surface in the bone-tumor interface (Figure 7A and 7B; P < 0.01 to P < 0.001), and fewer eroded lesions that reflect in vivo osteoclast activity (30) (Figure 7C; P < 0.01) in the bone sections of SCID mice bearing p38 shRNA-ARP-1 myeloma than in mice with control ARP-1 myeloma. Moreover, serum analysis showed significantly lower levels of circulating bone resorption marker CTX-1 (Figure 7D), mature osteoclast marker TRAP5b (31); (Figure 7E; P < 0.01), and osteoclast activator RANKL (Figure 7F; P < 0.001) in mice bearing p38 shRNA-ARP-1 myeloma than in those bearing control ARP-1 myeloma. Similarly, bone sections from ARP-1–xenografted SCID mice (data not shown) or primary myeloma-implanted SCID-hu mice treated with p38 inhibitor SD-169 contained fewer osteoclasts than those of control mice (Figure 7G; P < 0.01). These data indicate that myeloma cell p38 activity leads to enhanced osteoclastogenesis, and that inhibiting or knocking down tumor cell p38 activity can largely normalize osteoclast numbers and activity in tumor-bearing mice.
Figure 7.
Activated tumor cell p38 enhances osteoclastogenesis in vivo. Shown are (A) representative images and numbers of TRAP-positive osteoclasts on murine trabecular bone (scale bars in larger windows: 50 μm; in smaller windows: 10 μm), and quantitative analysis of osteoclast parameters such as (B) percentages of osteoclast-mediated erosion of bone surface (ES/BS) and (C) numbers of osteoclasts present on bone surface (Oc. S/BS) in bone sections of SCID mice bearing vector- or p38 shRNA-ARP-1 (p38shRNAs/p38SH) myeloma at week 8 after tumor inoculation. Shown are levels of circulating (D) CTX-1, (E) TRAP5b, and (F) RANKL in mice injected with vector- or p38 shRNA-ARP-1 cells at 0 week (0w) or 8 weeks (8w) after tumor inoculation. (G) Immunohistochemical staining shows the numbers of TRAP-positive osteoclasts present on human trabecular bone surfaces of primary myeloma-bearing SCID-hu mice treated without (vehicle) or with p38 inhibitor SD-169 at week 8 after tumor injection. Arrows indicate osteoclasts. Representative results from four independent experiments are shown. **P ≤ 0.01; ***P ≤ 0.001.
Discussion
Our findings indicate that constitutive activation of p38 in myeloma cells contributes to myeloma-induced bone lesions. We clearly show that activation of the p38 pathway in human myeloma cells, both primary myeloma cells from patients and established cell lines, can cause osteolytic lesions in human and murine bones in vivo in murine myeloma models. Knocking down p38 by shRNA or inhibiting p38 with a specific inhibitor abrogated the ability of myeloma cells to cause bone destruction in vivo without affecting myeloma cell homing to and growth within the bone marrow microenvironment. Mechanistic studies revealed that activated p38 signaling in myeloma cells causes osteoclast activation and bone resorption while, on the other hand, inhibiting osteoblasts and bone formation, both of which result in bone destruction. These results strongly suggest that disruption of p38 signaling in tumor cells might be a novel and effective approach to treating tumor-induced osteolytic bone lesions in myeloma patients.
Previous studies have shown that activation of the p38 signaling pathway in the bone marrow microenvironment is involved in osteoclastogenesis. Recent studies show that induction of c-Fos and NFATc1 during RANKL-induced osteoclast differentiation is mediated by the p38 signaling pathway (32). Administration of proteasome inhibitor bortezomib can inhibit RANKL-induced osteoclast differentiation and bone resorption activity by inhibition of p38, AP-1, and NF-κB activation (33). Moreover, TNFα- or IL-1–induced osteoclast differentiation and bone resorption activity requires p38 activation in the progenitors of osteoclasts (10, 34). Inflammatory cytokines stimulate bone marrow stromal cell production of cytokines or chemokines, which are involved in osteoclastogenesis, via activation of p38 signaling (10, 18, 19). On the other hand, p38 signaling pathway also participates in osteoblastogenesis (35). Greenblatt et al. reported that the p38 signaling pathway is required for normal skeletogenesis in mice, as mice with deletion of any of the p38 signaling pathway member-encoding genes displayed profoundly reduced bone mass and defective osteoblast differentiation (36). Moreover, activation of p38 and ERK cascades is involved in osteoblast differentiation induced by bone morphogenetic protein-2 (BMP-2) (37, 38) or TGF-β1 (39, 40) in vitro. However, other studies have shown that inhibition of p38 and PI3K/p70 S6K signaling pathways increases BMP-2–induced osteoblast differentiation (41). These findings indicate that activation of p38 in the bone marrow microenvironment is essential not only for osteoclastogenesis, but also for osteoblastogenesis.
Myeloma cell–induced osteoclastogenesis activation and osteoblastogenesis inhibition have been shown to be two major mechanisms in the pathogenesis of myeloma-induced bone destruction (2, 3, 42, 43). Recent studies show that myeloma cells upregulate secretion of osteoclast activators and/or osteoblast inhibitors by stimulating p38 activation in bone marrow stromal cells (19). Our results clearly show that activation of p38 in myeloma cells activates p38 signaling in downstream bone marrow cells (Figure 4C), including bone marrow stromal cells and osteoclasts. Knocking down p38 activity in myeloma cells significantly downregulates p38 activity in the bone marrow microenvironment. As a result, myeloma p38 activity is responsible for enhanced osteoclastogenesis and osteoclast-mediated osteolytic bone lesions in vivo. Myeloma p38 activity also inhibits osteoblastogenesis and thus bone formation and repair of damaged bone. These results clearly demonstrate that p38 activation in myeloma cells, but not in the bone marrow microenvironment, plays a critical role in osteoclastogenesis activation and osteoblastogenesis inhibition in myeloma-induced bone destruction. It is necessary to further address the mechanism how p38 activity in myeloma cells regulates osteoclast formation and bone resorption activity and osteoblast differentiation and bone formation function.
Recent studies have shown that treatment with p38 inhibitors can not only enhance chemotherapy-induced myeloma cell apoptosis and overcome drug resistance (11, 15, 44, 45), but also prevent myeloma-induced osteolytic bone lesions and osteoclastogenesis in murine models (21). By using p38 inhibitor SD-169, we confirmed that inhibition of p38 significantly reduced myeloma-induced osteolytic bone lesions and restored bone mass by downregulating osteoclastogenesis and osteoblastogenesis in the xenografted primary myeloma-SCID-hu or myeloma cell line-SCID mouse models. However, in vivo administration of a p38 inhibitor not only blocks p38 activity in myeloma cells but also reduces it activity in the surrounding normal bone marrow cells, including bone marrow stromal cells and the progenitors of osteoclasts and osteoblasts, increasing the difficulty of studying the mechanisms of myeloma-induced bone lesions. By using specific shRNAs of p38 in the present study, we knocked down p38 activity in myeloma cells. We identified p38 activity in myeloma cells as a master initiator and regulator of the process of osteolytic bone lesions, since modulated myeloma cells with reduced p38 activity had less effect on activation of osteoclastogenesis and inhibition of osteoblastogenesis and did not have destructive effects on human or murine bones of myeloma-bearing SCID-hu or SCID mice. In future studies, we will further investigate the underlying mechanisms by which myeloma cell p38 regulates osteoclastogenesis and osteoblastogenesis. Taken together, our results provide a novel and mechanistic insight into myeloma cell–induced bone destruction in vivo, and provide evidence that targeting myeloma cell p38 may be a viable approach to preventing myeloma bone disease.
Supplementary Material
Acknowledgments
We thank Brian Dawson for technical support with μ-CT scanning and analysis. We also thank our departmental Myeloma Tissue Bank for patient samples.
Grant Support
This work was supported by National Cancer Institute R01 grants CA138402 and CA138398 and P50 grant CA142509 (Q. Yi), the Leukemia and Lymphoma Society Translational Research grants, the Multiple Myeloma Research Foundation (Q. Yi), the Commonwealth Foundation for Cancer Research (Q. Yi), National Cancer Institute K99/R00 grant CA137158 (J. Yang), the International Myeloma Foundation (J. Yang), the Lymphoma Research Foundation (J. Yang), the American Society of Hematology (J. Yang), and by funds from the University Cancer Foundation and the Center for Targeted Therapy of The University of Texas MD Anderson Cancer Center (Q. Yi).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary information is available at Leukemia’s website.
References
- 1.Roodman GD. Myeloma bone disease: pathogenesis and treatment. Oncology (Williston Park) 2005 Jul;19(8):983–984. 986. [PubMed] [Google Scholar]
- 2.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003 Dec 25;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 3.Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood. 2006 Dec 15;108(13):3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Hong S, Yang J, Qian J, Zhang X, Shpall E, et al. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients’ monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006 Dec 15;108(13):4071–4077. doi: 10.1182/blood-2006-04-016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006 Mar 15;107(6):2432–2439. doi: 10.1182/blood-2005-06-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Qian J, Yang J, Wang S, Freeman ME, 3rd, Yi Q. Critical roles of Raf/MEK/ERK and PI3K/AKT signaling and inactivation of p38 MAP kinase in the differentiation and survival of monocyte-derived immature dendritic cells. Exp Hematol. 2005 May;33(5):564–572. doi: 10.1016/j.exphem.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wei S, Siegal GP. p38 MAPK as a potential therapeutic target for inflammatory osteolysis. Adv Anat Pathol. 2007 Jan;14(1):42–45. doi: 10.1097/PAP.0b013e31802ef4f2. [DOI] [PubMed] [Google Scholar]
- 8.Mbalaviele G, Anderson G, Jones A, De Ciechi P, Settle S, Mnich S, et al. Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J Pharmacol Exp Ther. 2006 Jun;317(3):1044–1053. doi: 10.1124/jpet.105.100362. [DOI] [PubMed] [Google Scholar]
- 9.Bohm C, Hayer S, Kilian A, Zaiss MM, Finger S, Hess A, et al. The alpha-isoform of p38 MAPK specifically regulates arthritic bone loss. J Immunol. 2009 Nov 1;183(9):5938–5947. doi: 10.4049/jimmunol.0901026. [DOI] [PubMed] [Google Scholar]
- 10.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005 Feb;115(2):282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hideshima T, Akiyama M, Hayashi T, Richardson P, Schlossman R, Chauhan D, et al. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood. 2003 Jan 15;101(2):703–705. doi: 10.1182/blood-2002-06-1874. [DOI] [PubMed] [Google Scholar]
- 12.Hideshima T, Podar K, Chauhan D, Ishitsuka K, Mitsiades C, Tai YT, et al. p38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene. 2004 Nov 18;23(54):8766–8776. doi: 10.1038/sj.onc.1208118. [DOI] [PubMed] [Google Scholar]
- 13.Navas T, Zhou L, Estes M, Haghnazari E, Nguyen AN, Mo Y, et al. Inhibition of p38alpha MAPK disrupts the pathological loop of proinflammatory factor production in the myelodysplastic syndrome bone marrow microenvironment. Leuk Lymphoma. 2008 Oct;49(10):1963–1975. doi: 10.1080/10428190802322919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen AN, Stebbins EG, Henson M, O’Young G, Choi SJ, Quon D, et al. Normalizing the bone marrow microenvironment with p38 inhibitor reduces multiple myeloma cell proliferation and adhesion and suppresses osteoclast formation. Exp Cell Res. 2006 Jun 10;312(10):1909–1923. doi: 10.1016/j.yexcr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Wen J, Cheng HY, Feng Y, Rice L, Liu S, Mo A, et al. P38 MAPK inhibition enhancing ATO-induced cytotoxicity against multiple myeloma cells. Br J Haematol. 2008 Jan;140(2):169–180. doi: 10.1111/j.1365-2141.2007.06895.x. [DOI] [PubMed] [Google Scholar]
- 16.Wen J, Feng Y, Huang W, Chen H, Liao B, Rice L, et al. Enhanced antimyeloma cytotoxicity by the combination of arsenic trioxide and bortezomib is further potentiated by p38 MAPK inhibition. Leuk Res. Jan;34(1):85–92. doi: 10.1016/j.leukres.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003 May 30;305(2):211–214. doi: 10.1016/s0006-291x(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 18.Zwerina J, Hayer S, Redlich K, Bobacz K, Kollias G, Smolen JS, et al. Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum. 2006 Feb;54(2):463–472. doi: 10.1002/art.21626. [DOI] [PubMed] [Google Scholar]
- 19.Hiruma Y, Honjo T, Jelinek DF, Windle JJ, Shin J, Roodman GD, et al. Increased signaling through p62 in the marrow microenvironment increases myeloma cell growth and osteoclast formation. Blood. 2009 May 14;113(20):4894–4902. doi: 10.1182/blood-2008-08-173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, et al. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res. Feb;25(2):200–210. doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderkerken K, Medicherla S, Coulton L, De Raeve H, Willems A, Lawson M, et al. Inhibition of p38alpha mitogen-activated protein kinase prevents the development of osteolytic bone disease, reduces tumor burden, and increases survival in murine models of multiple myeloma. Cancer Res. 2007 May 15;67(10):4572–4577. doi: 10.1158/0008-5472.CAN-06-4361. [DOI] [PubMed] [Google Scholar]
- 22.Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998 Oct 15;92(8):2908–2913. [PubMed] [Google Scholar]
- 23.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007 Mar 1;109(5):2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Cao Y, Hong S, Li H, Qian J, Kwak LW, et al. Human-like mouse models for testing the efficacy and safety of anti-beta2-microglobulin monoclonal antibodies to treat myeloma. Clin Cancer Res. 2009 Feb 1;15(3):951–959. doi: 10.1158/1078-0432.CCR-08-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Qian J, Wezeman M, Wang S, Lin P, Wang M, et al. Targeting beta2-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell. 2006 Oct;10(4):295–307. doi: 10.1016/j.ccr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Wezeman M, Zhang X, Lin P, Wang M, Qian J, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007 Sep;12(3):252–265. doi: 10.1016/j.ccr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009 Mar;23(3):435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 28.Awasthi A, Mathur R, Khan A, Joshi BN, Jain N, Sawant S, et al. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. J Exp Med. 2003 Apr 21;197(8):1037–1043. doi: 10.1084/jem.20022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roodman GD. Treatment strategies for bone disease. Bone Marrow Transplant. 2007 Dec;40(12):1139–1146. doi: 10.1038/sj.bmt.1705802. [DOI] [PubMed] [Google Scholar]
- 30.Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, et al. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009 Apr;15(4):417–424. doi: 10.1038/nm.1945. [DOI] [PubMed] [Google Scholar]
- 31.Yan D, Gurumurthy A, Wright M, Pfeiler TW, Loboa EG, Everett ET. Genetic background influences fluoride’s effects on osteoclastogenesis. Bone. 2007 Dec;41( 6):1036–1044. doi: 10.1016/j.bone.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y, Kim HH. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem Biophys Res Commun. 2006 Dec 8;351(1):99–105. doi: 10.1016/j.bbrc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 33.von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007 Sep;21(9):2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 34.Yun HJ, Lee EG, Lee SI, Chae HJ, Yoo WH. Adrenomedullin inhibits MAPK pathway-dependent rheumatoid synovial fibroblast-mediated osteoclastogenesis by IL-1 and TNF-alpha. Rheumatol Int. 2009 Aug;29(10):1161–1168. doi: 10.1007/s00296-008-0832-0. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki A, Guicheux J, Palmer G, Miura Y, Oiso Y, Bonjour JP, et al. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone. 2002 Jan;30(1):91–98. doi: 10.1016/s8756-3282(01)00660-3. [DOI] [PubMed] [Google Scholar]
- 36.Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest. Jul 1;120(7):2457–2473. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001 May;28(5):491–498. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- 38.Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003 Nov;18(11):2060–2068. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Ma R, Flavell RA, Choi ME. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J Biol Chem. 2002 Dec 6;277(49):47257–47262. doi: 10.1074/jbc.M208573200. [DOI] [PubMed] [Google Scholar]
- 40.Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002 Oct 17;21(47):7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 41.Vinals F, Lopez-Rovira T, Rosa JL, Ventura F. Inhibition of PI3K/p70 S6K and p38 MAPK cascades increases osteoblastic differentiation induced by BMP-2. FEBS Lett. 2002 Jan 2;510(1–2):99–104. doi: 10.1016/s0014-5793(01)03236-7. [DOI] [PubMed] [Google Scholar]
- 42.Esteve FR, Roodman GD. Pathophysiology of myeloma bone disease. Best Pract Res Clin Haematol. 2007 Dec;20(4):613–624. doi: 10.1016/j.beha.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Roodman GD. Bone-breaking cancer treatment. Nat Med. 2007 Jan;13(1):25–26. doi: 10.1038/nm0107-25. [DOI] [PubMed] [Google Scholar]
- 44.Ishitsuka K, Hideshima T, Neri P, Vallet S, Shiraishi N, Okawa Y, et al. p38 mitogen-activated protein kinase inhibitor LY2228820 enhances bortezomib-induced cytotoxicity and inhibits osteoclastogenesis in multiple myeloma; therapeutic implications. Br J Haematol. 2008 May;141(5):598–606. doi: 10.1111/j.1365-2141.2008.07044.x. [DOI] [PubMed] [Google Scholar]
- 45.Wen J, Feng Y, Huang W, Chen H, Liao B, Rice L, et al. Enhanced antimyeloma cytotoxicity by the combination of arsenic trioxide and bortezomib is further potentiated by p38 MAPK inhibition. Leuk Res. 2009 Jul 14; doi: 10.1016/j.leukres.2009.05.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.