Abstract
Objective
Stress is associated with increased intake of palatable foods and with weight gain, particularly in overweight women. Stress, food, and body mass index (BMI) have been separately shown to impact amygdala activity. However, it is not known whether stress influences amygdala responses to palatable foods, and whether this response is associated with chronic stress or BMI.
Design
Fourteen overweight and obese women participated in a functional magnetic resonance imaging (fMRI) scan as they consumed a palatable milkshake during script-driven autobiographical guided imagery of stressful and neutral-relaxing scenarios.
Results
We report that a network including insula, somatomotor mouth area, ventral striatum, and thalamus responds to milkshake receipt, but none of these areas are impacted by stress. In contrast, while the left amygdala responds to milkshake irrespective of condition, the right amygdala responds to milkshake only under stressful conditions. Moreover, this right amygdala response is positively associated with basal cortisol levels, an objective measure of chronic stress. We also found a positive relationship between BMI and stress related increased response to milkshake in the orbitofrontal cortex.
Conclusions
These results demonstrate that acute stress potentiates response to food in the right amygdala and orbitofrontal cortex as a function of chronic stress and body weight, respectively. This suggests that the influence of acute stress in potentiating amygdala and OFC responses to food is dependent upon individual factors like BMI and chronic stress. We conclude that BMI and chronic stress play a significant role in brain response to food and in stress-related eating.
Keywords: Stress potentiates brain response to food in obese with chronic stress, Risk factors for obesity, Obesity and the brain, neuroimaging
Introduction
Sixty-eight percent of Americans are now overweight or obese1. Despite efforts by academia, industry and government, the incidence of obesity continues to rise unabated. In 2008 the costs estimated to be related to obesity neared 148 billion2. One important component of tackling this recalcitrant problem is identifying factors that promote overeating. Stress influences eating behavior3 and this effect is associated with weight gain4. In the current study we tested whether an acute stressor could potentiate brain response to a palatable and energy dense food in overweight women.
Prior work clearly demonstrates that acute stress can increase food intake, particularly of high-fat and high-sugar foods3,5. In humans, acute and chronic stress has been associated with weight gain6,7, total feeding, sweet food consumption8,9, and a high-fat diet10. However, eating in response to stress varies according to the type of stressor and the behavioral and physiological characteristics of the individual11. Those most at risk for stress-induced overeating and weight gain include females and overweight individuals12; in particular, high BMI individuals show a stronger association between chronic stress and weight gain than low BMI individuals who experience similar degrees of stress6. This suggests that overweight individuals may be particularly vulnerable to the influence of stress on increased food intake. Consistent with this possibility, stress-related eating (defined as trying to make oneself feel better by eating or drinking in a stressful situation) is significantly associated with obesity in women13.
The neurobiological underpinnings of the influence of stress on eating are currently not well understood. However, the neural correlates of food reward have been described in humans14–18, and there is evidence that some of these areas are associated with future weight gain19–22. Of potential interest is the amygdala, since it plays a key role in non-homeostatic feeding and is influenced by measures of acute and chronic stress. More specifically, stress affects amygdala neuronal firing rate23, can elicit changes in synaptic structure within the amygdala24 and leads to lasting increases in the firing rate of amygdala neurons25. Such effects come about even after a single exposure to a stressor, and can last long after that stressor is experienced24. In humans, the amygdala responds to stressful movie clips26, as well as the act and anticipation of stressful public speaking27,28. Amygdala activity is also related to individual differences in stress responses; increased amygdala activity to a stressor positively correlates with increased stress-evoked blood pressure response29, while decreased amygdala response to stress correlates with lower cortisol and lower subjective stress responses30.
Animal work has also highlighted a role for the amygdala in eating in the absence of hunger31, which is a hallmark of stress-induced eating8. In 1983 Weingarten showed that cues that had predicted the delivery of food during hunger could later elicit feeding in sated rats32. This demonstrated that learned environmental cues could stimulate feeding in the absence of hunger. Subsequent studies have shown that this “cue-potentiated feeding” is blocked by lesions to the basolateral nucleus of the rodent amygdala31. This indicates that the amygdala is critical for orchestrating the process by which food cues acquire the ability to over-ride homeostatic mechanisms to promote intake. Although less is understood about the role of the human amygdala in feeding, functional neuroimaging studies have demonstrated that the amygdala of lean healthy individuals responds to food cues14,16–18,33,34, is sensitive to the devaluation of food cues by satiation16,33,35,36, and to the “revaluation” of food cues by the infusion of ghrelin in sated individuals37. Amygdala response is also heightened to food pictures in high vs. low BMI subjects38. Moreover, amygdala response to consumption of a milkshake in subjects who are neither hungry nor full22 - but not in fasted subjects21 -predicts future weight gain, thus linking response in the amygdala to eating in the absence of hunger in humans.
Since stress influences amygdala functioning, and since the amygdala is implicated in overeating, we reasoned that stress might promote overeating by impacting amygdala responses to food. We were also interested in the influence of stress on response in the midbrain and medial orbitofrontal cortex. These regions have been implicated in food reward39 and their response to milkshake predicts milkshake intake following scanning40. We therefore reasoned that acute stress may also influence intake by affecting response in these regions. To test these hypotheses we used fMRI to measure brain response during the consumption of a high fat and sweet palatable milkshake while overweight women imagined personalized neutral-relaxing and stressful scenarios via an autobiographical script-driven guided imagery method41. This method has been shown to reliably induce stress41,42 and amygdala activation43. On a separate day we also measured morning basal cortisol levels as an objective physiological measure of allostatic load44. Morning cortisol levels show good intra-individual stability across time45 and serve as a useful biological correlate of chronic stress46. We predicted that response to milkshake in the amygdala, medial OFC and midbrain would be greater during the stress vs. the neutral-relaxing condition, and that these responses would correlate with cortisol levels. We also reasoned that the influence of acute stress may be greater in those with evidence of chronic stress and those with higher BMI’s.
Materials and Methods
Subjects
Sixteen women between the age of 18 and 45, with a BMI greater than 25, were recruited and provided informed consent to participate in this research approved by the Yale University Human Investigation Committee. Exclusion factors included any non-removable metal on the body, currently or recently taking major medications such as antidepressants, claustrophobia, food allergies, diabetes, any history of psychiatric disorder or drug abuse and current active medical illness. Subjects completed an interview in which they were questioned about recent stressful life events and an fMRI training session (see below). Subjects who were unable to recall stressful life events or reported being uncomfortable with the scanning environment were excluded (n = 2). Subjects (n=2) who expressed significant discomfort with needles were excluded from the baseline cortisol measurement. The main fMRI analyses are therefore based upon the data from fourteen subjects (all women, 11 right handed, age 26.9 ± 7.1, BMI 29.8 ± 5.3), while the baseline cortisol measurements (mean: 29.7 ug/dl ± 13.36) are based upon data from twelve subjects (all women, 9 right handed, age 27.8 ± 7.7, BMI 28.3 ± 2.6).
Stimuli
Stimuli were a chocolate milkshake drink and a tasteless rinse solution, delivered via gustometer to a gustatory manifold that rested just above the subject’s tongue. This procedure has been used in previous studies15 and is described in detail in SI. During each real and mock run subjects received the milkshake solution as a 1-cc bolus over 3 sec, followed by a 1-cc bolus rinse of tasteless solution (also delivered over 3 sec). Baseline events consisted of a 1-cc bolus of tasteless solution, not followed by a rinse.
Sessions
All subjects participated in three separate sessions prior to the fMRI scan: In session one, they received initial training in which they were exposed to scanning conditions and procedures in a mock scanner and also completed an imagery script development procedure, wherein they were interviewed in detail about 3 stressful and 3 neutral-relaxing situations in their life, based on techniques previously validated77,78. The information from these interviews was used to develop 6 personalized stories that were used to induce a stress or neutral-relaxing condition during the scanning session. Session two was a baseline cortisol measurement session, and in session three, subjects were trained in the mental imagery and relaxation techniques for use during the fMRI scan. See SI for detailed descriptions of each session.
fMRI scanning session
The final session consisted of the fMRI scan. Subjects were instructed to refrain from eating or drinking for an hour before the scan, and told that they should arrive for scanning neither hungry nor full. Prior to scanning, subjects rated their hunger and fullness on visual analog scales (VAS) titled “How hungry (full) are you right now?” and rated sips of the milkshake and tasteless solutions for pleasantness, familiarity, and wanting. These ratings were repeated after the completion of the scan.
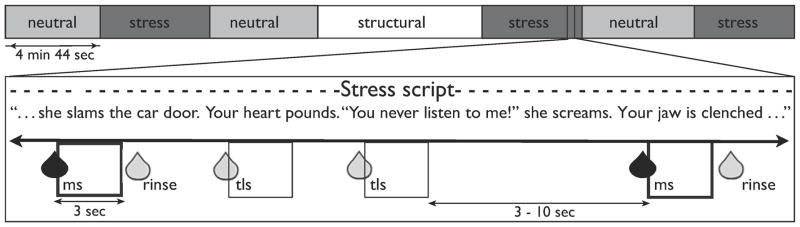
We used a 3T Trio scanner by Siemens to collect functional and anatomical images. Echoplanar imaging was used to measure the BOLD signal as an indication of brain activation. During each of 6 4-min, 44-sec BOLD runs, subjects listened to a recording of one of their personalized imagery induction scripts, so that the entire run was either under the stress condition (S) or under the neutral-relaxing condition (N). During each imagery run, subjects also received several 1-mL deliveries of either milkshake (ms) or tasteless solution (tls) (Fig 1). This resulted in 4 different events of interest: milkshake under stress (msS), tasteless under stress (tlsS), milkshake under neutral-relaxing (msN), and tasteless under neutral-relaxing (tlsN). Prior to and immediately after each run, subjects verbally rated their anxiety levels on a 10-point Likert scale. For details of fMRI image acquisition, see SI.
Figure 1. Scanning paradigm.
Each subject underwent 6 4min, 44sec runs and 1 structural scan. For each run, they listened to the personalized stress (shown in dark grey) or neutral script (shown in light grey) of the situation being described and were asked to imagine the scenario “as if it were happening right now”. While they imagined, they received 1-mL sips of chocolate milkshake (followed 3 to 10 seconds later by 1mL of tasteless rinse) and 1-mL sips of tasteless solution at random intervals of 3 to 10 seconds. Milkshake events of interest are shown as dark-edged boxes, tasteless events of interest are shown as thin-edged boxes. Rinses following milkshake events were not modeled as events of interest.
Data analysis
The neuroimaging data were pre and post-processed using SPM5 (Welcome Department of Cognitive Neurology, London, UK) using standard procedures79–81, including time acquisition correction, realignment, normalization (resulting in a voxel size of 4 × 4 × 4 mm for functional images and 1 × 1 × 1 mm for structural images), and smoothing with a 6 mm kernel, see SI. Analyses were based on random effects models in order to account for inter-subject variability82. Parameter estimate images from each stimulus in each condition were entered into second-level analyses using 1-sample t-tests, to test for significant differences in response83. Note that effects refer to the Statistical Parametric Maps (SPM’s) of each stimulus (milkshake or tasteless) in each condition (stress or neutral-relaxing). Unpredicted peaks were considered significant at p<0.05, FDR-corrected across the entire brain at the voxel level. For predicted peaks, we used a region-of-interest (ROI) approach, in which we used WFU pickatlas84 to create masks of predicted ROI’s, see SI. Peaks within these masks were considered significant at p<.05, FDR-corrected across the ROI. BMI and basal cortisol levels were entered into models as regressors to evaluate the influence of these factors on events and contrasts of interest. To ensure that our results were not skewed by the inclusion of one subject with BMI >45, we additionally ran all analyses excluding that subject and found that all results remained significant. Data presented here represent the full group of subjects.
Results
Group Demographics & Questionnaires
All subjects were overweight or obese at the time of scanning (BMI 25.8 to 46.2) (Table S1). Dutch Eating Behavior Questionnaire (DEBQ) scores and Three Factor Eating Questionnaire (TFEQ) scores are reported in Table S1.
Ratings
As intended, subjective ratings indicated that participants were neither hungry nor full at the time of scanning (Table S1). The tasteless solution was rated as neutral and the milkshakes as pleasant and wanted (Table S1). Paired-samples t-tests indicated that the milkshakes were rated as significantly more pleasant; t(22)=6.68, (p=0.000) and more wanted; t(21)=2.71, (p=0.003) than the tasteless solution. Analysis of anxiety ratings revealed increased anxiety after vs. before stress-condition scans but not after neutral-condition scans, and greater post-scan anxiety scores after stressful vs. neutral scans (see SI).
Neuroimaging
Main effect of stimulus
To isolate areas responding to milkshake vs. tasteless irrespective of condition, we contrasted (milkshake under stress (msS) + milkshake under neutral-relaxing (msN)) − (tasteless under stress (tlsS) + tasteless under neutral-relaxing (tlsN)). Consistent with our previous research14,15, large significant clusters of activation were observed bilaterally that spanned the somatomotor mouth area (SMMA) (64, −8, 36, z=5.56, p<0.001; −60, −16, 32, z=4.87, p<0.001), insula (40, −8, 12, z=3.98, p=0.001; −44, −12, 12, z=4.06, p=0.001), ventral striatum/dorsal amygdala (−28, −8, 4, z=4.18, p=0.001; 32, −20, −4, z=3.91, p=0.002), and thalamus (8, −20, 12, z=3.09, p=0.011; −12, −24, 0, z=2.93, p=0.016) (Fig. 2; all fMRI results included in Table 2). These and all following p-values are False-Discovery Rate (fdr)-corrected either across the whole brain (unpredicted peaks) or a region of interest (predicted peaks; see methods for details). No areas responded preferentially to tasteless vs. milkshake irrespective of condition.
Figure 2. Main effect of milkshake.
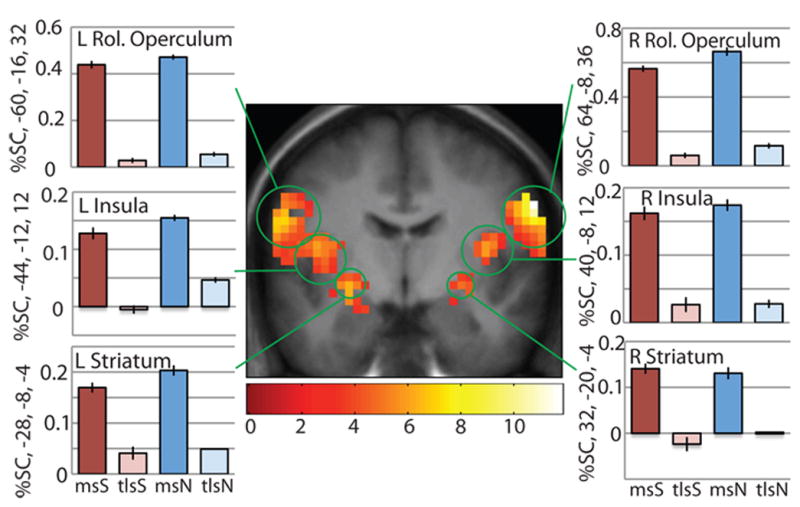
An extensive feeding network including bilateral Rolandic operculum/somatomotor mouth area, insula, and striatum bilaterally responds to milkshake more than tasteless regardless of stress condition. T-map is thresholded at p<0.005 and k>3 voxels. For this and successive pictures, bar graphs show activity in peak voxel within circled brain region in response to each stimulus in percent signal change, averaged over subjects. Error bars represent 2 SEM. Activations are significant at p<0.05 FDR-corrected across regions of interest. The bar graphs reflect percent signal change data fitted to the canonical HRF, extracted using the RFXplots toolbox. Not shown are significant activations in the thalamus.
Condition-specific effects of stimulus
Significant responses in the left amygdala to milkshake-tasteless were observed in both the neutral-relaxing and stress conditions; (msN-tlsN): −28, −4, −12, z=3.89, p=0.003; (msS-tlsS): −24, 0, −16, z=3.22, p=0.014. In contrast, response in the right amygdala to milkshake-tasteless was only observed during stress imagery (msS-tlsS); 24, −8, −12, z=2.90, p=0.014 (Fig. 3).
Figure 3. Interaction between stress & milkshake.
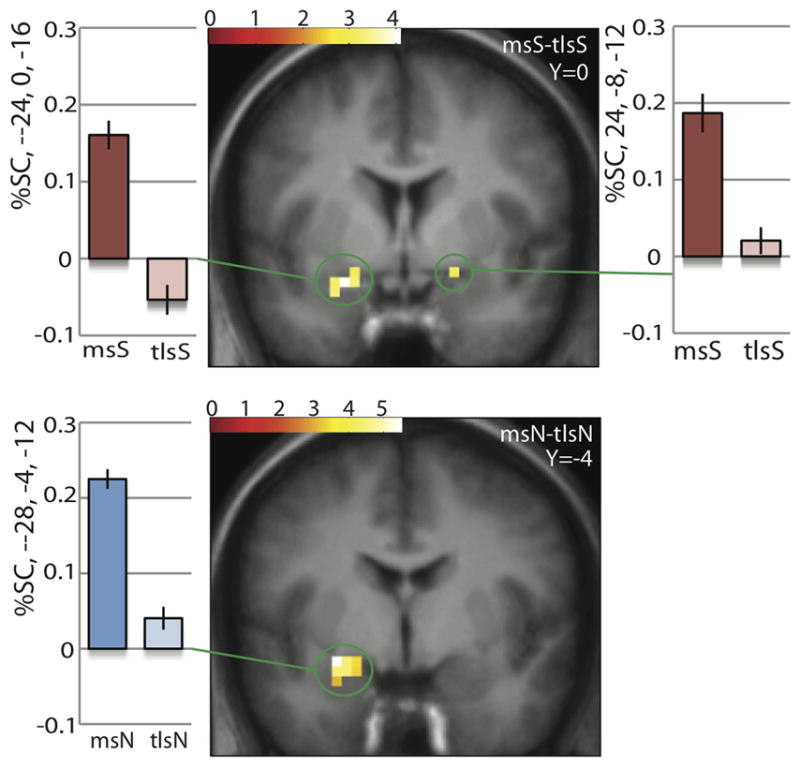
Under stress, left and right amygdala are more active to milkshake vs. tasteless. Both activations are significant at pFDR=0.014 across the amygdala ROI. Under neutral, only left amygdala is more active to milkshake vs tasteless. Activation is significant at pFDR=0.003 across the amygdala ROI. No significant differences in activation were found in the right amygdala under neutral.
Main effect of condition
The combined response to milkshake + tasteless did not significantly differ as a function of stress vs. neutral-relaxing imagery conditions.
Stimulus by Condition Interaction
To investigate interactions between stimulus and condition, we used the contrast of (msS-tlsS) – (msN-tlsN) and its inverse, (msN-tlsN) – (msS-tlsS). We found a trend for the predicted effect in the amygdala with greater response to milkshake vs. tasteless in stress vs. neutral-relaxing condition (8, −8, 16, z=2.75, p=.051).
Regression with basal cortisol
To test whether brain responses were associated with chronic stress we regressed basal morning cortisol levels against whole brain response. We identified a significant and selective positive association between basal cortisol measurements and right amygdala response to milkshake vs. tasteless under the stress condition (msS-tlsS; 20, −4, −20, z=2.90, p=0.044; Fig. 4a). No other regions displayed this relationship. We also found no significant relationships between basal cortisol level and response to milkshake - tasteless under the neutral-relaxing condition (msN-tlsN). Post-hoc tests showed the strength of this correlation in the peak amygdala voxel to be r2 = 0.62 during the stress condition and r2 = 0.12 during the neutral-relaxing condition. Cortisol was not correlated with BMI (r2=0.041, p=0.53).
Figure 4. Relationship between brain response to milkshake vs. tasteless and other variables.
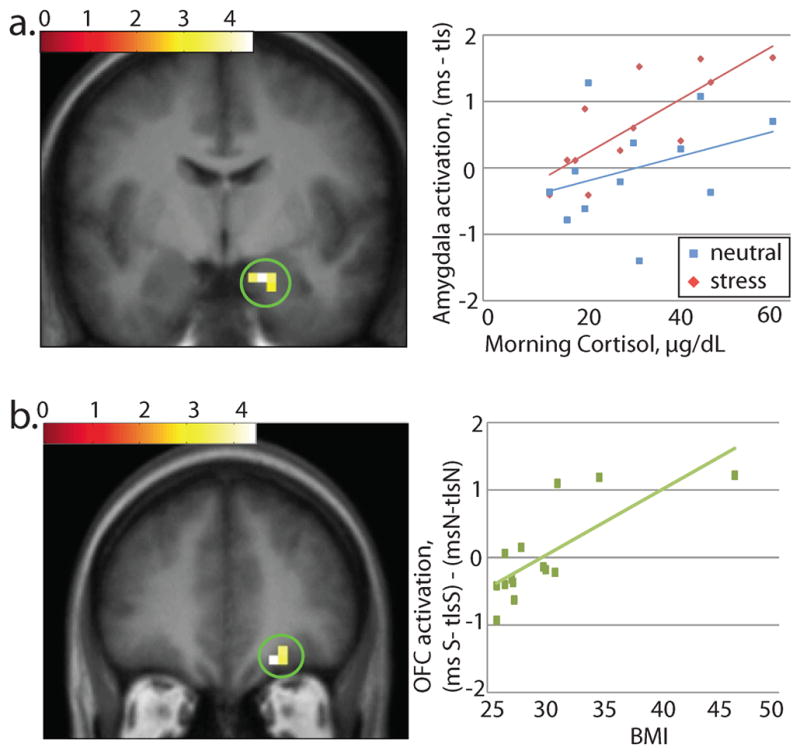
a. Basal cortisol measurements across subjects showed a relationship with brain response to milkshake vs. tasteless in the stress condition, but no relationship with response to the same contrast in the neutral-relaxing condition, in right amygdala (20, −4, −20). Post-hoc tests showed the strength of this correlation in the peak voxel to be r2 = 0.62 in stress and r2 = 0.12 in neutral-relaxing. Effect is significant at p=0.044 across the amygdala ROI.
b. In the right OFC (20, 40, −16), brain response to milkshake vs. tasteless in the stress vs. the neutral-relaxing condition was correlated with BMI with a strength of r2 = .059. Effect is significant at p=.043 across the midbrain & OFC ROI.
Regression with BMI
To determine if BMI influenced the effect of stress on brain response to milkshake, we introduced BMI as a covariate in the contrast of milkshake vs. tasteless under stress vs. neutral-relaxing ((msS-tlsS)-(msN-tlsN). Within our midbrain and OFC areas of interest, we found a small but significant positive correlation in the right medial orbitofrontal cortex (20, 40, −16; z=3.19; p=.043; Fig. 4b). This correlation remained significant when this analysis was run excluding the highest BMI subject.
Discussion
In the current work we set out to determine whether overweight women who are at risk for stress-induced weight gain6 show an increased response to milkshake consumption in the amygdala, a region known to be associated with hyperphagia47, nonhomeostatic eating48, increased response to palatable foods as a function of BMI38,49, and future weight gain22; as well as the OFC and midbrain because response to milkshake in these regions predicts subsequent milkshake intake40. We also tested whether such responses would be influenced by BMI or morning cortisol levels as a marker of chronic stress45,46,50. We found that compared to consuming a neutral tasteless solution, consuming a pleasant milkshake results in unilateral left amygdala activation during the neutral-relaxing condition and bilateral amygdala activation during the stress condition. This stress-evoked response to milkshake in the right amygdala correlated positively with basal cortisol levels. In contrast, no correlations were observed between brain response to milkshake and cortisol levels during the neutral-relaxing condition. Taken together, these findings suggest that overweight women with high chronic stress, as measured by cortisol levels, are more vulnerable to the acute effects of stress on amygdala response to milkshake. We also observed a positive correlation between BMI and the influence of the acute stressor on orbitofrontal response to milkshake. This is in keeping with the proposal that overweight individuals are vulnerable to stress-induced increases in food intake and with the possibility that the mechanism by which this occurs involves the OFC.
Brain response during the consumption of palatable food
Consistent with prior work14,15,22,51, we found activity in the somatomotor mouth area, insula, ventral striatum, thalamus, and amygdala, in response to the consumption of milkshake compared to the consumption of the control solution. Except for the response in the right amygdala, these responses occurred irrespective of acute stress or neutral-relaxing condition and were unrelated to morning cortisol, suggesting that response to palatable food in these regions is not influenced by acute or chronic stress. In keeping with the fact that our subjects were all overweight, many of these regions have been shown to respond differentially as a function of adiposity or risk for weight gain38,49,52–55. Thus, the neural responses we observed to milkshake are consistent with prior reports of brain response to palatable food in overweight individuals.
Response to milkshake in the right amygdala is associated with stress
Since the amygdala has been independently implicated in stress24,29 and in nonhomeostatic feeding48, we reasoned that acute stress may influence food intake in overweight women by influencing amygdala response to food. Of particular relevance to the current investigation is the fact that a prior study showed that amygdala response to milkshake consumption correlates positively with future weight gain22 in participants who were neither hungry nor full, but not in subjects who had fasted and were hungry21. This suggests that the amygdala plays a role in promoting eating in the absence of hunger, which is consistent with animal work31,48. If so, then acute stress may influence eating by modulating amygdala response. The current findings provide some support for this possibility. We found a trend towards greater amygdala response to milkshake while subjects experienced acute stress. Further investigation indicated that variation in greater amygdala response during stress was influenced by morning cortisol levels. Thus, individuals with higher allostatic load as measured by morning cortisol levels, showed greater stress-induced right amygdala response to milkshake that was not seen in the neutral condition. These data suggest that acute stress may influence intake by modulating amygdala response specifically in individuals with higher chronic stress and allostatic load.
Morning basal cortisol levels have been shown to be related to chronic stress, including worries load and social stress45, prolonged job strain50, and subclinical depressive symptomatology56. Thus, morning basal cortisol levels provide a useful approximation of allostatic load, or the cumulative effect of the wear and tear of stress on the body and brain57. Furthermore, cortisol levels are also related to feeding in humans. High cortisol levels are related to increased high-fat food consumption44,57, increased caloric intake and weight gain58, and higher BMI’s59. Higher basal cortisol and enhanced cortisol responses to stress have been related to obesity60 and disordered eating61–63. It is also likely that chronic life stressors may contribute more to future weight gain than acute stressors, and this chronic stress effect may be most pronounced in individuals with high cortisol reactivity 4,64. In the current sample, subjects had a range of basal morning cortisol levels ranging from low to high levels suggesting variation in chronic stress levels in these otherwise healthy individuals. Consistent with the previous work that chronic stress may contribute to stress-related food intake and also influence brain response to food, we show that the impact of acute stress on right amygdala response to food is greater with increasing levels of morning cortisol.
Interestingly, the effect of stress on brain response to milkshake and its relationship with cortisol were present only in the right amygdala. Although reports are mixed and indefinite on whether amygdala response to negative affect is lateralized65, it has been suggested that stress and negative affect primarily affect the right amygdala66. Animal studies suggest that the right amygdala is involved in the memory of aversive experiences67, and that stimulating the right amygdala has anxiogenic effects68. In humans, unconscious processing of negative affect takes place primarily in the right amygdala69 and depressive patients show increased right amygdala volume70. Without a right amygdala, startle response to aversive events decreases71, as does recognition of fear in facial expressions72. Finally, in a study using personalized imagery scripts, the right (but not left) amygdala is activated by stressful scripts recalling past traumatic events73. Here, we show that stress exacerbates the right, but not left, amygdala response to a food, in keeping with the idea of right amygdala specialization for response to stress.
Also consistent with the hypothesis that acute stress may influence brain response to promote eating in vulnerable individuals, we found a positive relationship between BMI and the influence of acute stress on OFC response to milkshake. Critically, this effect was observed in the exact region of OFC where response to milkshake predicts subsequent milkshake intake40. Unfortunately, we did not have the power in the current study to determine if there is an interaction between BMI and chronic stress exposure. However, given the current results this possibility seems like an important focus for future research. Finally, acute stress has also been shown to decrease amygdala response during a menu selection task74. More specifically, after an overnight fast, subjects completed either a solvable (rest condition) or unsolvable (stress condition) math test, followed by an fMRI scan during which they chose the foods they would eat immediately post-scan. In the stress compared to the rest condition, subjects showed decreased activity in the amygdala, as well as the putamen, OFC, cingulate cortex, and hippocampus. Whether chronic stress exposure influences this response is unknown. However, the finding suggests that the direction of the influence of acute stress on response in the amygdala may vary as function of task. It is also possible that internal state may influence the effect of stress on amygdala response to milkshake consumption. Bohon and colleagues used fMRI to measure whole brain response to the taste of milkshake, while manipulating mood with music exposure. In one condition, dirges were played at half time to produce a negative mood state and in the other condition, cheerier, upbeat music was played to produce a positive mood state. They found that activity in anterior cingulate cortex, pallidum, and thalamus were increased in response to milkshake under negative vs. positive mood75. They did not find differential amygdala response as a function of mood state. One important distinction between these studies and the current experiment is the internal state of the subjects. Whereas these studies scanned hungry, fasted subjects, our participants were scanned while neither hungry nor full. We chose to scan subjects in this state because we were interested in the influence of acute stress on eating in the absence of hunger. The amygdala is highly responsive to food stimuli when hungry33,76. Therefore it is possible that ceiling effects, related to the influence of hunger on amygdala response may account for the failure of previous studies to observe differential effects in the amygdala dependent on mood or stress.
In summary, our results indicate that chronic stress and BMI are key individual difference factors that influence acute stress effects on brain response in the amygdala and OFC to palatable foods. The observed association between basal cortisol and acute stress in the amygdala suggests an important connection between physiologically measured allostatic load and the impact of stress on brain response to food. The positive association between BMI and the impact of stress in the OFC implicates overeating and/or adiposity in potentiating the effects of acute stress. It is important to note that the current findings are limited by small sample size. In future work with a larger sample size, it will be important to examine how these two vulnerabilities interact and possibly potentiate the influence of acute stress on brain response to palatable and energy dense foods.
Supplementary Material
Table 1.
Effect of Milkshake-Tasteless, collapsed across groups
| Brain region | x | y | z | pFDR | z-value | t | k |
|---|---|---|---|---|---|---|---|
| R SMMA | 64 | −8 | 36 | 0.000 | 5.66 | 11.77 | 202 |
| *middorsal insula | 40 | −8 | 12 | 0.001 | 3.98 | 5.72 | |
| L SMMA | −60 | −16 | 32 | 0.000 | 4.87 | 8.52 | 341 |
| *middorsal insula | −44 | −12 | 12 | 0.001 | 4.06 | 5.95 | |
| *amygdala/striatum | −28 | −8 | −4 | 0.001 | 4.18 | 6.26 | |
| R Striatum | 32 | −20 | −4 | 0.002 | 3.91 | 5.56 | 25 |
| R Thalamus | 8 | −20 | 12 | 0.011 | 3.09 | 3.85 | 10 |
| L Thalamus | −12 | −24 | 0 | 0.016 | 2.93 | 3.58 | 5 |
| (msS-tlsS) | |||||||
| L Amygdala | −24 | 0 | −16 | 0.014 | 3.22 | 4.09 | 13 |
| R Amygdala | 24 | −8 | −12 | 0.014 | 2.90 | 3.52 | 3 |
| (msN-tlsN) | |||||||
| L Amygdala | −28 | −4 | −12 | 0.003 | 3.89 | 5.51 | 12 |
| Correlation between basal cortisol and activation to (msS-tlsS) but not (msN-tlsN) | |||||||
| R Amygdala | 20 | −4 | −20 | 0.044 | 2.90 | 4 | |
| Correlation between BMI and activation to (msS-tlsS)-(msN-tlsN) | |||||||
| R OFC | 20 | 40 | −16 | .043 | 3.19 | 4.11 | 6 |
Acknowledgments
Thanks to Sandra Stankovic and Dr. Keri Bergquist for assistance in study design and Dr. Marga Veldhuizen for assistance in data processing. This work was funded by the National Institutes of Health (NIH) R01DK085579 and U54 DA022292 pilot project awarded to DMS, as well as the NIH Roadmap for Medical Research Common Fund grants UL1-RR024139 (Yale Clinical and Translational Science Award), UL1-DE019586 and the PL1-DA024859 awarded to RS, and NIH NRSA F31-DC010557-01 awarded to KR.
Footnotes
Conflict of Interest
The authors declare that they have no competing financial conflicts of interest.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual Medical Spending Attributable To Obesity: Payer-And Service-Specific Estimates. Health Affairs. 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: A new view of “comfort food”. Proc Natl Acad Sci. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epel ES, Lapidus R, McEwen BS, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 5.Dallman MF, Pecoraro N, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain, Behavior, and Immunity. 2005;19(4):275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial Stress and Change in Weight Among US Adults. Am J Epidemiol. 2009;170(2):181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner EJ, Chandola T, Marmot MG. Prospective Effect of Job Strain on General and Central Obesity in the Whitehall II Study. Am J Epidemiol. 2007;165(7):828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- 8.Rutters F, Nieuwenhuizen AG, Lemmens SGT, Born JM, Westerterp-Plantenga MS. Acute Stress-related Changes in Eating in the Absence of Hunger. Obesity. 2008;17(1):72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- 9.Ely DR, Dapper V, Marasca J, et al. Effect of Restraint Stress on Feeding Behavior of Rats. Physiol & Behav. 1997;61(3):395–398. doi: 10.1016/s0031-9384(96)00450-7. [DOI] [PubMed] [Google Scholar]
- 10.Ng DM, Jeffery RW. Relationships Between Perceived Stress and Health Behaviors in a Sample of Working Adults. Health Psychology. 2003;22(6):638–642. doi: 10.1037/0278-6133.22.6.638. [DOI] [PubMed] [Google Scholar]
- 11.Adam TC, Epel ES. Stress, eating and the reward system. Physiol & Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Greeno CG, Wing RR. Stress-Induced Eating. Psychological Bulletin. 1994;115(3):444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- 13.Laitinen J, Ek E, Sovio U. Stress-Related Eating and Drinking Behavior and Body Mass Index and Predictors of This Behavior. Preventive Medicine. 2002;34(1):29–39. doi: 10.1006/pmed.2001.0948. [DOI] [PubMed] [Google Scholar]
- 14.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable Substrates for Anticipatory and Consummatory Food Chemosensation. Neuron. 2008;57(5):786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felsted JA, Ren X, Chouinard-Decorte F, Small DM. Genetically Determined Differences in Brain Response to a Primary Food Reward. J Neurosci. 2010;30(7):2428–2432. doi: 10.1523/JNEUROSCI.5483-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottfried JA, O’Doherty J, Dolan RJ. Encoding Predictive Reward Value in Human Amygdala and Orbitofrontal Cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 17.Beaver JD, Lawrence AD, van Ditzhuijzen J, et al. Individual Differences in Reward Drive Predict Neural Responses to Images of Food. J Neurosci. 2006;26(19):5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small DM, Gerber JC, Mak YE, Hummel T. Differential Neural Responses Evoked by Orthonasal versus Retronasal Odorant Perception in Humans. Neuron. 2005;47(4):593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Stice E, Yokum S, Blum K, Bohon C. Weight Gain Is Associated with Reduced Striatal Response to Palatable Food. J Neurosci. 2010;30(39):13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokum S, Ng J, Stice E. Attentional Bias to Food Images Associated With Elevated Weight and Future Weight Gain: An fMRI Study. [Accessed June 24, 2011];Obesity. 2011 doi: 10.1038/oby.2011.168. Available at: http://dx.doi.org/10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed]
- 21.Stice E, Spoor S, Bohon C, Small DM. Relation Between Obesity and Blunted Striatal Response to Food Is Moderated by TaqIA A1 Allele. Science. 2008;322(5900):449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouinard-Decorte F, Felsted J, Small DM. Increased amygdala response and decreased influence of internal state on amygdala response to food in overweight compared to healthy weight individuals. Appetite. 2010;54(3):639. [Google Scholar]
- 23.Duvarci S, Pare D. Glucocorticoids Enhance the Excitability of Principal Basolateral Amygdala Neurons. J Neurosci. 2007;27(16):4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelletier JG, Likhtik E, Filali M, Paré D. Lasting increases in basolateral amygdala activity after emotional arousal: Implications for facilitated consolidation of emotional memories. Learning & Memory. 2005;12(2):96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cousijn H, Rijpkema M, Qin S, et al. Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci. 2010;107(21):9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral Blood Flow in Subjects With Social Phobia During Stressful Speaking Tasks: A PET Study. Am J Psychiatry. 2001;158(8):1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 28.Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiat. 2002;52(11):1113–1119. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- 29.Gianaros PJ, Sheu LK, Matthews KA, et al. Individual Differences in Stressor-Evoked Blood Pressure Reactivity Vary with Activation, Volume, and Functional Connectivity of the Amygdala. J Neurosci. 2008;28(4):990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SE, Burklund LJ, Eisenberger NI, et al. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- 31.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol & Behav. 2002;76(1):117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 32.Weingarten H. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220(4595):431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 33.LaBar KS, Gitelman DR, Parrish TB, et al. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 34.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural Responses during Anticipation of a Primary Taste Reward. Neuron. 2002;33(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 35.Mohanty A, Gitelman DR, Small DM, Mesulam MM. The Spatial Attention Network Interacts with Limbic and Monoaminergic Systems to Modulate Motivation-Induced Attention Shifts. Cerebral Cortex. 2008;18(11):2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Doherty J, Rolls ET, Francis S, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 11(4):893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- 37.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin Modulates Brain Activity in Areas that Control Appetitive Behavior. Cell Metabolism. 2008;7(5):400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Stoeckel LE, Weller RE, Cook EW, III, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Small DM. Toward an Understanding of the Brain Substrates of Reward in Humans. Neuron. 2002;33(5):668–671. doi: 10.1016/s0896-6273(02)00620-7. [DOI] [PubMed] [Google Scholar]
- 40.Nolan-Poupart S, Veldhuizen MG. Midbrain and medial orbital cortex response to milkshake predicts ad lib milkshake intake. Quebec CIty; In press. [Google Scholar]
- 41.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 43.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiat. 2005;57(8):832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 44.McEwen BS, Stellar E. Stress and the Individual: Mechanisms Leading to Disease. Arch Intern Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- 45.Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- 46.Schulz P, Kirschbaum C, Pruessner JC, Hellhammer DH. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress and Health. 1998;14(2):91–97. [Google Scholar]
- 47.Holsen LM, Zarcone JR, Brooks WM, et al. Neural Mechanisms Underlying Hyperphagia in Prader-Willi Syndrome. Obesity. 2006;14(6):1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-Hypothalamic Circuit Allows Learned Cues to Override Satiety and Promote Eating. J Neurosci. 2002;22(19):8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng J, Stice E, Yokum S, Bohon C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite. 2011;57(1):65–72. doi: 10.1016/j.appet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steptoe A, Cropley M, Griffith J, Kirschbaum C. Job Strain and Anger Expression Predict Early Morning Elevations in Salivary Cortisol. Psychosom Med. 2000;62(2):286–292. doi: 10.1097/00006842-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 51.Marciani L, Pfeiffer JC, Hort J, et al. Improved methods for fMRI studies of combined taste and aroma stimuli. J Neurosci Meth. 2006;158(2):186–194. doi: 10.1016/j.jneumeth.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 52.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain. 1997;120(9):1675–1684. doi: 10.1093/brain/120.9.1675. [DOI] [PubMed] [Google Scholar]
- 54.Martin LE, Holsen LM, Chambers RJ, et al. Neural Mechanisms Associated With Food Motivation in Obese and Healthy Weight Adults. Obesity. 2009;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 55.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. NeuroImage. 2010;50(4):1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-Reported Depressive Symptoms and Stress Levels in Healthy Young Men: Associations With the Cortisol Response to Awakening. Psychosom Med. 2003;65(1):92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 57.Mcewen BS. Protection and Damage from Acute and Chronic Stress: Allostasis and Allostatic Overload and Relevance to the Pathophysiology of Psychiatric Disorders. Ann NY Acad Sci. 2004;1032(1):1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 58.Tataranni PA, Larson DE, Snitker S, et al. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol-Endoc M. 1996;271(2):E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 59.Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes. 2000;24(Suppl 2):S80–S85. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- 60.Mårin P, Darin N, Amemiya T, et al. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41(8):882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 61.Koo-Loeb JH, Costello N, Light KC, Girdler SS. Women With Eating Disorder Tendencies Display Altered Cardiovascular, Neuroendocrine, and Psychosocial Profiles. Psychosom Med. 2000;62(4):539–548. doi: 10.1097/00006842-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Abell TL, Malagelada JR, Lucas AR, et al. Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology. 1987;93(5):958–965. doi: 10.1016/0016-5085(87)90557-9. [DOI] [PubMed] [Google Scholar]
- 63.Gluck ME, Geliebter A, Lorence M. Cortisol Stress Response Is Positively Correlated with Central Obesity in Obese Women with Binge Eating Disorder (BED) before and after Cognitive-Behavioral Treatment. Ann NY Acad Sci. 2004;1032(1):202–207. doi: 10.1196/annals.1314.021. [DOI] [PubMed] [Google Scholar]
- 64.Torres S, Nowson C. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiat. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 66.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cog Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 67.Coleman-Mesches K, McGaugh JL. Differential involvement of the right and left amygdalae in expression of memory for aversively motivated training. Brain Research. 1995;670(1):75–81. doi: 10.1016/0006-8993(94)01272-j. [DOI] [PubMed] [Google Scholar]
- 68.Adamec RE, Morgan HD. The effect of kindling of different nuclei in the left and right amygdala on anxiety in the rat. Physiol & Behav. 1994;55(1):1–12. doi: 10.1016/0031-9384(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 69.Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bremner JD, Narayan M, Anderson ER, et al. Hippocampal Volume Reduction in Major Depression. Am J Psychiatry. 2000;157(1):115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 71.Angrilli A, Mauri A, Palomba D, et al. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119(6):1991–2004. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- 72.Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15(3):396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- 73.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 74.Born JM, Lemmens SGT, Rutters F, et al. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes. 2009;34(1):172–181. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- 75.Bohon C, Stice E, Spoor S. Female emotional eaters show abnormalities in consummatory and anticipatory food reward: A functional magnetic resonance imaging study. Int J Eat Disord. 2009;42(3):210–221. doi: 10.1002/eat.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hinton EC, Parkinson JA, Holland AJ, et al. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20(5):1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 77.Sinha R, Lovallo W, Parsons O. Cardiovascular differentiation of emotions. Psychosom Med. 1992;54(4):422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Miller GA, Levin DN, Kozak MJ, et al. Individual differences in imagery and the psychophysiology of emotion. Cognition Emotion. 1987;1(4):367. [Google Scholar]
- 79.Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to Detect Taste in a Tasteless Solution: Modulation of Early Gustatory Cortex by Attention to Taste. [Accessed August 24, 2010];Chem Senses. 2007 doi: 10.1093/chemse/bjm025. Available at: http://chemse.oxfordjournals.org/cgi/content/abstract/bjm025v1. [DOI] [PubMed]
- 80.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- 81.Worsley KJ, Friston KJ. Analysis of fMRI Time-Series Revisited--Again. NeuroImage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 82.Strange B, Portas C, Dolan RJ, Holmes AP, Friston KJ. Random effects analysis for event-related fMRI. NeuroImage. 1999;9:1053–1089. [Google Scholar]
- 83.Nichols T, Brett M, Anderson J, Wager T, Poline J-P. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.