Abstract
Expression of IL-33 is elevated in patients with pulmonary diseases, and full-length (not proteolytically processed) IL-33 is the predominant form in the lungs in health and disease. To determine whether activation of IL-33 is needed for functional effects, activities of full-length mouse (flm) and mature mouse (mm) forms of IL-33 were compared in vivo. Replication-deficient adenoviral constructs were used for gene delivery. Both isoforms caused pulmonary infiltration of lymphocytes and neutrophils, whereas mmIL-33 also caused pulmonary eosinophilia and goblet cell hyperplasia, and increased expression of IL-4, IL-5, IL-13, IL-17, MCP-1, and KC. The different effects were not associated with differential release from IL-33-producing cells or by differences in subcellular distributions of IL-33 isoforms. Germline deficiency of the cell surface receptor chain ST2 abrogated the mmIL-33-induced Th2-associated effects (pulmonary eosinophilia, goblet cell hyperplasia, and increased IL-4 and IL-5), yet the lymphocytic infiltration induced by flmIL-33 or mmIL-33 was not fully abrogated by the absence of ST2. The similar effects of IL-33 isoforms were associated with comparable regulation of gene expression, notably matrix metalloproteinases MMP3, MMP10, and MMP13. Thus, full-length IL-33 is functionally active in vivo in an ST2-independent fashion, and its effects are partially different from those of mature IL-33. The different effects of these isoforms, particularly the pro-Th2 effects of mature IL-33, are due to differential utilization of the IL-33 receptor chain ST2, whereas their similar effects result from regulation of gene expression.
Keywords: Interleukin-33, ST2, inflammation, lymphocytes, lung
INTRODUCTION
Interleukin (IL)-33, a member of the IL-1 family of cytokines (1)(2), has recently emerged as a key regulator of inflammatory and immune processes (3)(4). It is also a chromatin-associated protein acting as a transcriptional regulator, making it a dual-function protein that is both a cytokine and a nuclear factor (5), similar to alarmins IL-1α and HMGB1. The key component of the IL-33 receptor (R), T1/ST2 (6), is expressed on a variety of cell types (T lymphocytes, dendritic cells, NK and NKT cells, basophils, eosinophils, and mast cells), and is responsible for this cytokine’s biological activity in these diverse cells (4). T1/ST2 is expressed both as a membrane-associated and as a soluble protein, with activated Th2 cells expressing predominantly the soluble form (7).
The mechanisms of post-synthetic processing of IL-33 remain to be fully understood. A seminal study (6) has suggested that, although the full-length (fl) human (h) and mouse (m) IL-33 are respectively composed of 270 and 266 amino acids, cleavage of flIL-33 with caspase-1 yields “mature” hIL-33 (residues 112–270) and mature mIL-33 (residues 109–266). Whereas maturation of flIL-33 by caspase-1 has been shown in vitro (6), conversion of the IL-33 precursor in cells may occur via digestion by calpain (8). The issue of IL-33 maturation has been further complicated by several findings. The release of IL-33 by macrophages in cell culture was found to be unaffected by caspase-1 deficiency and inhibition of caspase-8 and calpain (9). Moreover, flIL-33 is fully capable of binding to the IL-33R, whereas caspase-3, instead of promoting maturation, inactivates the cytokine function of flIL-33 without affecting its ability to translocate to the nucleus (10). Extensive evidence suggests that caspase cleavage is not required for pro-IL-33 secretion and bioactivity but that caspase-dependent processing actually inactivates IL-33 bioactivity (11)(12)(13). Several previous studies have been performed with recombinant mature IL-33, and the results are not necessarily fully representative of the in vivo biology of IL-33. Consistent with this notion and in contrast to the effects of in vivo treatment with mature IL-33 (6), a recent report on IL-33 transgenic mice showed that the CMV promoter-driven constitutive systemic expression of flmIL-33 leads to pulmonary inflammation with a significant accumulation of lymphocytes, monocytes, and neutrophils, but mild accumulation of eosinophils in the lungs (14).
In this study, we sought to compare the effects of full-length mouse (flm) and mature mouse (mm) IL-33 on mouse lung in vivo. Lung has been selected as the target organ because of the emerging specific roles of IL-33 in pulmonary pathology. Lung tissue normally expresses IL-33 (6), and the levels of this cytokine in the lungs are increased in asthma (15)(16) and influenza virus infection (17), whereas serum levels of IL-33 are elevated in patients with scleroderma lung disease (18). The levels of soluble ST2 protein were elevated in the sera of patients with asthma (19) and acute exacerbation of idiopathic pulmonary fibrosis (20).
MATERIALS AND METHODS
Patients and patient samples
The study was approved by the Institutional Review Board at the University of Maryland. Patients with idiopathic pulmonary fibrosis (IPF) were recruited from the University of Maryland Medical Center. Bronchoalveolar lavage (BAL) samples were obtained from patients with IPF and normal healthy volunteers, and IPF tissue samples were obtained from lung explants or video-assisted thoracoscopic (VATS) biopsy. Normal lung tissue samples were obtained from lungs harvested initially for lung transplantation, but ultimately not used for transplantation purposes.
Adenoviral Constructs
Adenoviral gene delivery was used to express IL-33 in mouse lungs in vivo. Replication-deficient recombinant adenoviruses (AdV) were constructed, manufactured, and validated as in previous reports (21)(22)(23)(24)(25). Briefly, the GenBank sequence NM_001164724.1 for mouse IL-33 was used to artificially synthesize (GenScript, Piscataway, NJ, USA) DNA fragments corresponding to full-length mouse (flm) and mature mouse (mm, amino acids 109–266). Separately, an artificial fusion construct was created consisting of the flmIL-33 coding sequence preceded by the first 78 basepairs of the mouse IL-1ra sequence, which correspond to the first 26 amino acids (the secretory signal peptide) of the mIL-1ra protein (26). The encoded recombinant protein was termed secreted fusion construct (sfc) IL-33. Each of the fragments was subcloned into a shuttle vector and transferred into a recombinant, replication-deficient AdV vector using RAPAD technology (Vira-Quest, North Liberty, IA, USA). The constructs were designed to ensure expression of IL-33 under control of the CMV promoter, and the validity of the constructs was confirmed by direct automated sequencing. The AdVs were purified by two rounds of CsCl-gradient centrifugation, and the resulting purified viruses had a concentration of ~1×1012 particles/ml and an infectious titer of ~4×1010 PFU/ml. All viruses, including control AdV-NULL, which did not encode a cytokine, encoded GFP in their backbone under control of a separate (RSV) promoter. Infectivity of the viruses was confirmed by GFP fluorescence of infected primary pulmonary mouse fibroblasts (27)(28), as well as HEK293, A549, small airway epithelial (SAEC), and TC-1 (transformed mouse lung epithelial) cells in culture (all from American Type Culture Collection, Manassas, VA). Transcription was confirmed by real-time quantitative PCR with primers specific for mIL-33 mRNA. Production of mIL-33 protein was confirmed by ELISA and western blot (R&D Systems, Minneapolis, MN) of cell lysates and supernates.
In vivo and in vitro experiments
Most of the experiments were performed in female C57BL/6 mice aged 10–12 weeks (The Jackson Laboratory, Bar Harbor, ME). ST2-deficient animals on the Balb/c background were produced as previously reported (29), and wild type Balb/c mice were used as controls for the experiments with Il1lr1−/− (herein referred to as ST2−/−) animals. The animals were treated in accordance with a research protocol approved by the Institutional Animal Care and Use Committee of the University of Maryland. The adenoviruses were instilled intratracheally as previously described in detail (21)(22)(23)(24)(25). Quantitative PCR of lung homogenates was used to confirm overexpression of mRNAs for the delivered cytokines. The ELISA of pulmonary homogenates from these mice were used to confirm the overexpression of the delivered cytokines on days 7, 14, and 21. Bronchoalveolar lavage (BAL), pulmonary homogenates, histological and immunohistological analyses, flow cytometry, and all other assays were performed as previously described (21)(22)(23)(24)(25). Multiplex analyses (Luminex, Austin, TX) of cytokine levels in BAL and lung homogenates were performed in triplicate in each sample from each animal, and the mean values and standard deviations were calculated for each triplicate. Cell culture, preparation of intracellular and cytoplasmic cell fractions, quantitative reverse transcription-real time polymerase chain reaction (RT-Q-PCR), ELISA, western blotting, and statistical analyses of data were performed as previously described (21) (22)(23)(24)(25)(27)(28).
RESULTS
Expression of IL-33 in human lungs
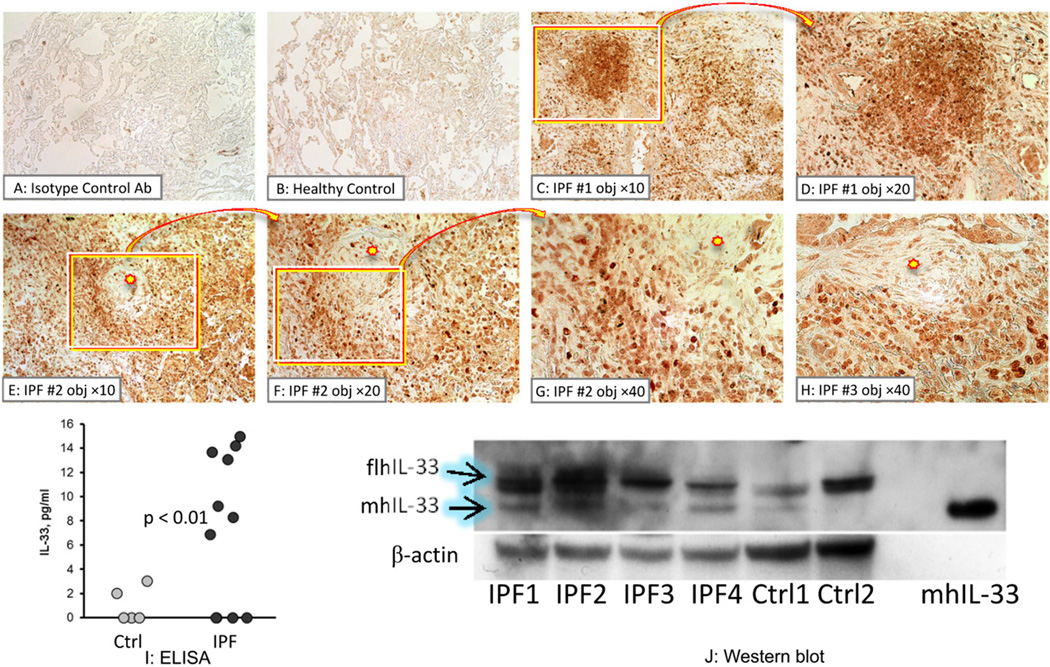
Initial experiments were aimed at determining whether IL-33 is present in human lungs. As a prototypic diffuse parenchymal lung disease, idiopathic pulmonary fibrosis (IPF), was selected, which is characterized by pulmonary inflammation and architectural remodeling of the lung. Immunohistochemical analyses used a commercial antibody developed against the mature (proteolytically processed) human (h)IL-33, and was thus reactive against both full-length and processed isoforms. Although some staining for hIL-33 was observed in healthy lungs (Figure 1B), significantly more intense staining was observed in the lungs of patients with IPF than in healthy controls (Figure 1C-H). Cells within inflammatory infiltrates (Figure 1C, D) and in the vicinity of fibrotic foci – a hallmark feature of IPF (Figure 1E-H) – stained positively for hIL-33 in these patients. These observations suggested that the overall level of hIL-33 is increased in the lungs of patients with IPF compared to healthy controls. ELISA assays of BAL samples confirmed that total hIL-33 was elevated in the lungs of these IPF patients (Figure 1I).
Figure 1.
Expression of IL-33 in human lung. A-H. Immunohistochemistry for IL-33 in lung tissue obtained from one healthy volunteer and three patients with idiopathic pulmonary fibrosis (IPF), as indicated. Similar observations were obtained in two additional healthy controls and three additional patients with IPF. This anti-IL-33 antibody (Ab) indiscriminately reacts with full-length and mature forms of hIL-33 (brown staining). Lung tissue stained with isotype control Ab (A), or with anti-IL-33 Ab from the healthy control (B) and 3 patients with IPF were imaged with a ×10 objective (C, E). Selected areas were imaged with a ×20 objective (D, F) or a ×40 objective (G, H). I. ELISA for total IL-33 in BAL from five healthy volunteers (light circles) and ten patients with IPF (dark circles). J. Western blotting of lung tissue homogenates from four patients with IPF (IPF) and two healthy controls (Ctrl) for IL-33 (upper gel) and β-actin (lower gel). Recombinant mature human (mh) IL-33 protein was used as a positive control in the right-most column. Loading of the samples was normalized to total protein.
Subsequent western blotting analyses of human lung homogenates were performed using an antibody reactive against both full-length and mature hIL-33. Because of the differential electrophoretic mobility of these IL-33 isoforms, these assays allowed for clear separation of full-length and mature IL-33 isoforms in human lungs (Figure 1J). The full-length IL-33 isoform was predominant in human lungs, although both full-length and mature human isoforms were present in the majority of samples.
Distinct and similar effects of full-length and mature IL-33 on pulmonary cellularity and cytokine milieu
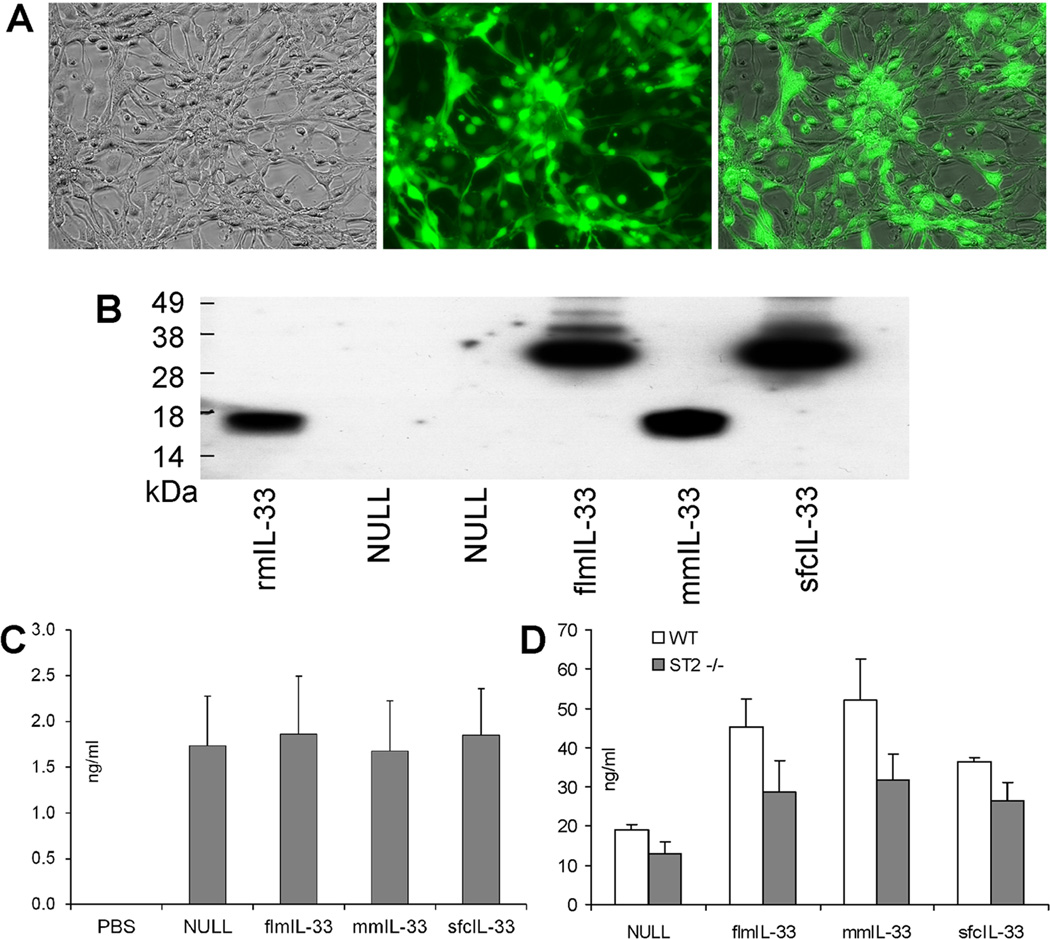
In light of the findings in human lungs (Figure 1), further experiments were aimed at assessing possible similar and different effects of flmIL-33 and mmIL-33 in vivo. Recombinant adenoviruses encoding IL-33 isoforms were prepared and validated as described in the Methods. Infectivity of the AdV preparations was confirmed by GFP fluorescence of cultured cells at 24–72 h after infection (Figure 2A), and expression of IL-33 in these cultures was confirmed by western blot (Figure 2B). Intratracheal instillations of the AdV in mice in vivo were also performed, leading to increased expression of GFP (Figure 2C) and IL-33 (Figure 2D) in the lungs.
Figure 2.
Validation of infectivity and IL-33 gene delivery by recombinant AdV constructs. A. Recombinant adenoviruses infect cells causing green fluorescence due to GFP expression encoded in the viral backbone. Bright field microscopy (left), GFP fluorescence microscopy (middle), and image overlay (right) of AdV-flmIL-33-infected mouse epithelial TC-1 cells 48 h after infection (×20 objective). Similar results were obtained with all other constructs in these cells and also in A549 primary small airway epithelial cells and primary pulmonary mouse fibroblasts. No fluorescence was observed in cells without AdV infection. B. Western blotting with anti-mIL-33 antibody of fibroblast culture lysates infected with AdV encoding the indicated proteins. rmIL-33 is a commercial preparation of mature IL-33 (R&D Systems). Samples were normalized to total protein for loading. C. ELISA of whole lung homogenates for GFP, ng/ml ± SD, on day 14 after instillation of adenoviruses encoding the indicated proteins. D. ELISA of whole lung homogenates for mIL-33, ng/ml ± SD. WT mice (open bars) and ST2−/− mice (closed bars) were analyzed 14 days after infection with AdV encoding the indicated proteins, with 3–5 animals per group.
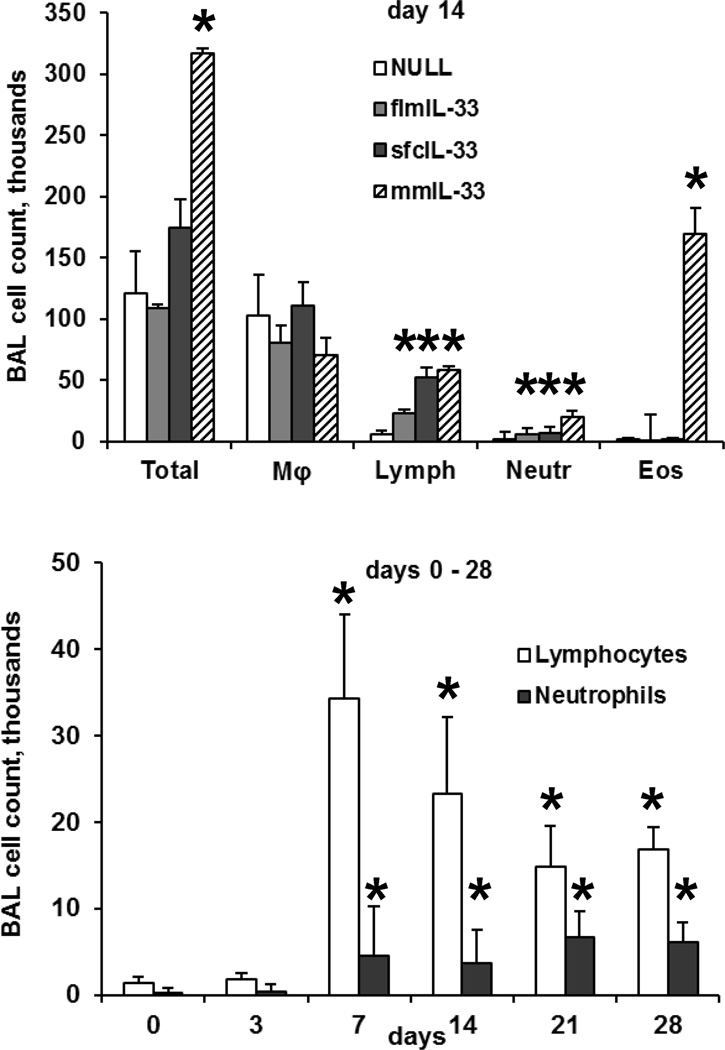
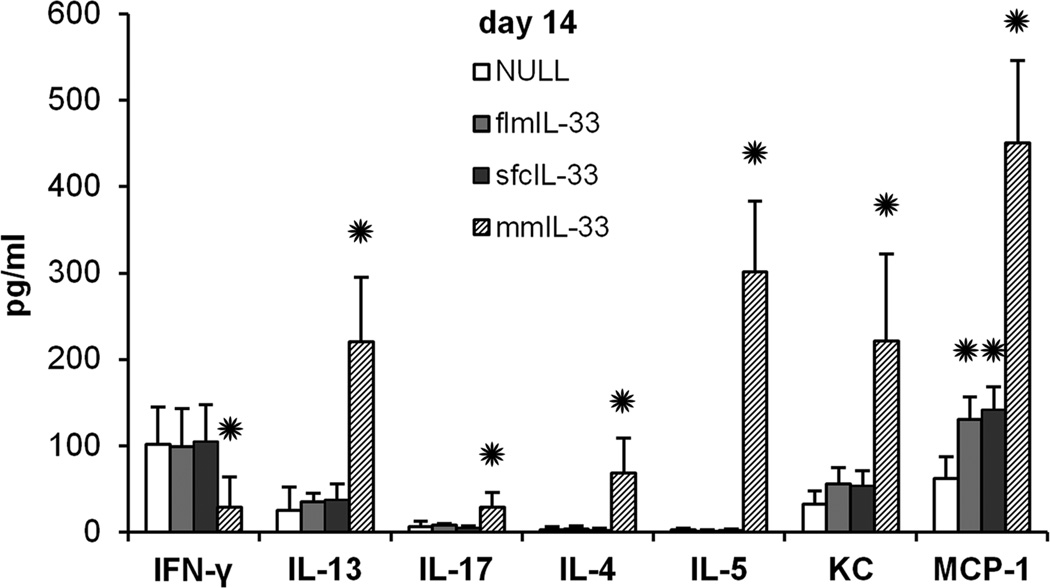
Mice instilled intratracheally with any of the AdV showed no signs of morbidity, such as body weight loss, ruffled fur, dehydration, diarrhea, hunched posture, or decreased motor activity at any time post-infection. There was minimal mortality (<5%) immediately following the instillation procedure, but it was not significantly different between the study groups. At days 7 and 14 after the instillations, BAL samples showed significant changes induced by flmIL-33 and mmIL-33. BAL lymphocytes and, to a lesser extent, neutrophils were elevated in the flmIL-33-expressing animals, whereas profound eosinophilia, along with increases in lymphocytes and neutrophils were observed in mmIL-33-expressing animals (Figure 3). A more detailed analysis of the kinetics of changes in the BAL cellularity following AdV-flmIL-33 administration confirmed increases in lymphocytes and neutrophils on days 7–28 (Figure 3), whereas no changes were observed in the numbers of macrophages or eosinophils (p > 0.05, one-way ANOVA). Analyses of lung homogenates for cytokines revealed that, as expected for mmIL33, a significant skewing toward the Th2 pattern occurs, but no such changes were observed in flmIL-33-expressing mice (Figure 4). In addition to the cytokines shown in Figure 4 there were no significant differences in pulmonary levels of IL-1β, IL-6, IL-12p40, IL-12p70, TNF-α, MIP-1α, RANTES, IFN-α, or IFN-β among the groups of animals tested.
Figure 3.
Changes in cellular composition of bronchoalveolar lavage induced by gene delivery of IL-33 to the lungs of C57Bl/6 mice in vivo (mean ± SD), with 3–7 animals per group and with significant differences from AdV-NULL-challenged mice indicated with asterisks (p < 0.05). The top panel shows BAL cell counts in mice overexpressing the indicated IL-33 isoforms on day 14. The bottom panel shows the dynamics of BAL lymphocytes and neutrophils in flmIL-33-overexpressing mice.
Figure 4.
Levels of pulmonary cytokines (pg/ml) in multiplex assays of lung homogenates (mean ± SD, with 5–10 animals per group) in mice expressing IL-33 isoforms as indicated.
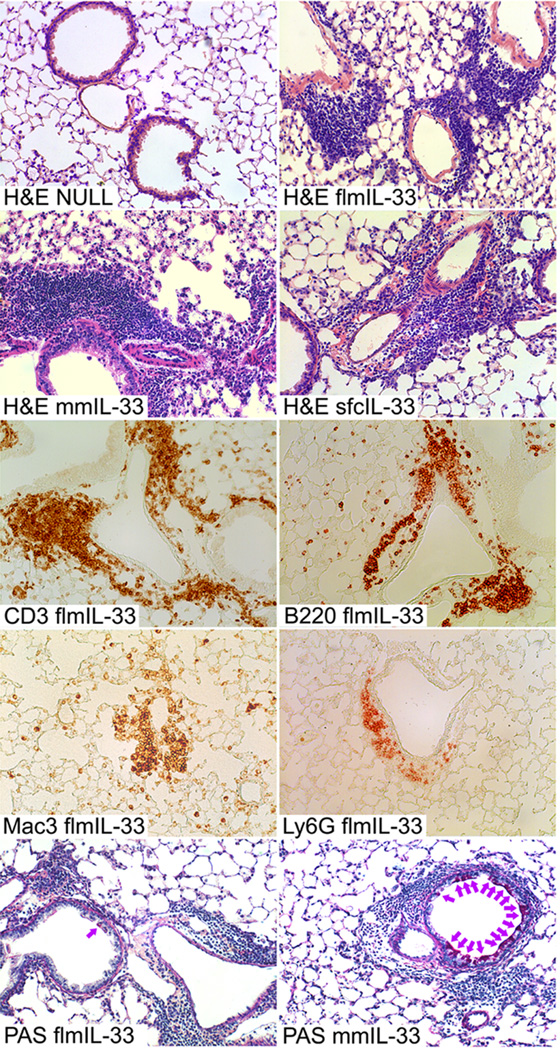
The changes observed in mmIL-33-expressing mice were consistent with the well-known pro-Th2 activity of mature IL-33. However, the observations in flmIL-33-expressing mice were somewhat unexpected, as full-length IL-33 is considered to be inactive as a cytokine. In light of the findings above, subsequent analyses focused on histological changes in the flmIL-33-expressing mice. On days 3, 7, and 14 after instillation, minimal accumulation of scattered neutrophils and mononuclear cells was observed in the peribronchial and perivascular areas and in the lung parenchyma of mice infected with AdV-NULL (Figure 5). Minimal histologic changes were noted 3 days after intratracheal instillation of AdV-flmIL33. However, in agreement with the observed changes in the BAL cellularity, there were perivascular and peribronchial infiltrates consisting of lymphocytes, but also neutrophils and macrophages (Figure 5). This was associated with severe endothelialitis, as evidenced by endothelial karyorrhexis, perivascular nuclear dust, pericyte vacuoles, perivascular edema, loosening of the sub-endothelial connective tissue, denuded endothelium, and by the presence of hemosiderin-laden macrophages. The delivery of flmIL-33 did not, whereas the delivery of mmIL-33 did, induce goblet cell hyperplasia (Figure 5). Combined, these observations suggest that flmIL-33 is independently active in mouse lungs in vivo in a fashion different from that of mmIL-33.
Figure 5.
Histological changes in mouse lungs following flmIL-33 gene delivery 14 days after AdV construct instillation. Shown from top to bottom are H&E staining of lung sections from mice infected with viral construct for delivery of indicated IL-33 isoforms; immunohistochemistry of flmIL-33-expressing mouse lungs for CD3, B220, Mac3, and Ly6G, as indicated; periodic acid-Schiff staining of flmIL-33- (lower left) and mmIL-33-expressing (lower right) mouse lungs.
Secretion and subcellular distribution of IL-33 isoforms
The observed different and similar effects of IL-33 isoforms in the lung in vivo require mechanistic explanations. Four mechanisms were considered to explain the differences and similarities: 1) secretion from the producing cells, 2) intracellular distribution, 3) utilization of cell surface receptor, and 4) regulation of gene expression.
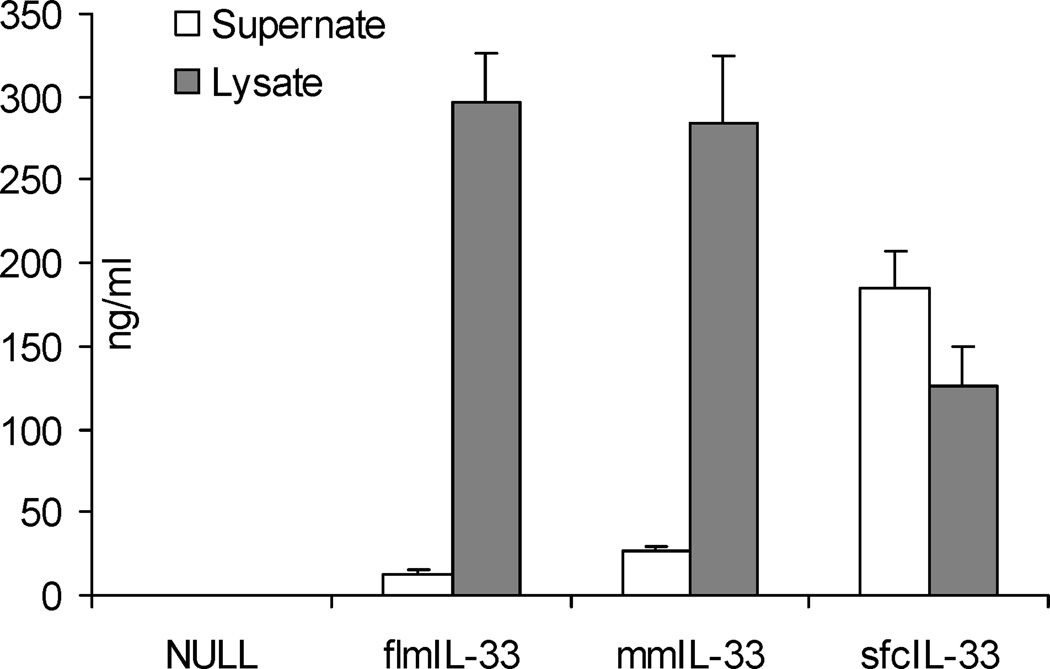
First, the possibility was considered that the difference in the observed phenotypes upon gene delivery of flmIL-33 and mmIL-33 may be due to differences in secretion of these isoforms from cells. Both flmIL-33 and mmIL-33 remained predominantly intracellular and were not released into the supernate of expressing cell cultures (Figure 6). Consistent with this observation, expression of flmIL-33 and mmIL-33 were observed in lung homogenates (Figure 2D), but not in BAL fluids, in which IL-33 was undetectable by ELISA. Thus, the major portion of expressed full-length or mature IL-33 remains predominantly intracellular, and only a small fraction of the protein is released from cells. Subsequent experiments tested similar in vivo delivery of sfcIL-33, which is readily secreted from expressing cells (Figure 6). The changes induced by sfcIL-33 in BAL cellularity, pulmonary cytokines, and lung histology were indistinguishable from those induced by flmIL-33 (Figures 3–5). Thus, differences in protein secretion are unlikely to drive the differential effects of flmIL-33 and mmIL-33 in vivo.
Figure 6.
ELISA for mIL-33 of TC-1 cell culture supernates (open bars) and lysates (shaded bars), in triplicate, 48 h after infection with AdV constructs encoding the indicated proteins. The same ELISAs were repeated in A549 and SAEC cells with similar results.
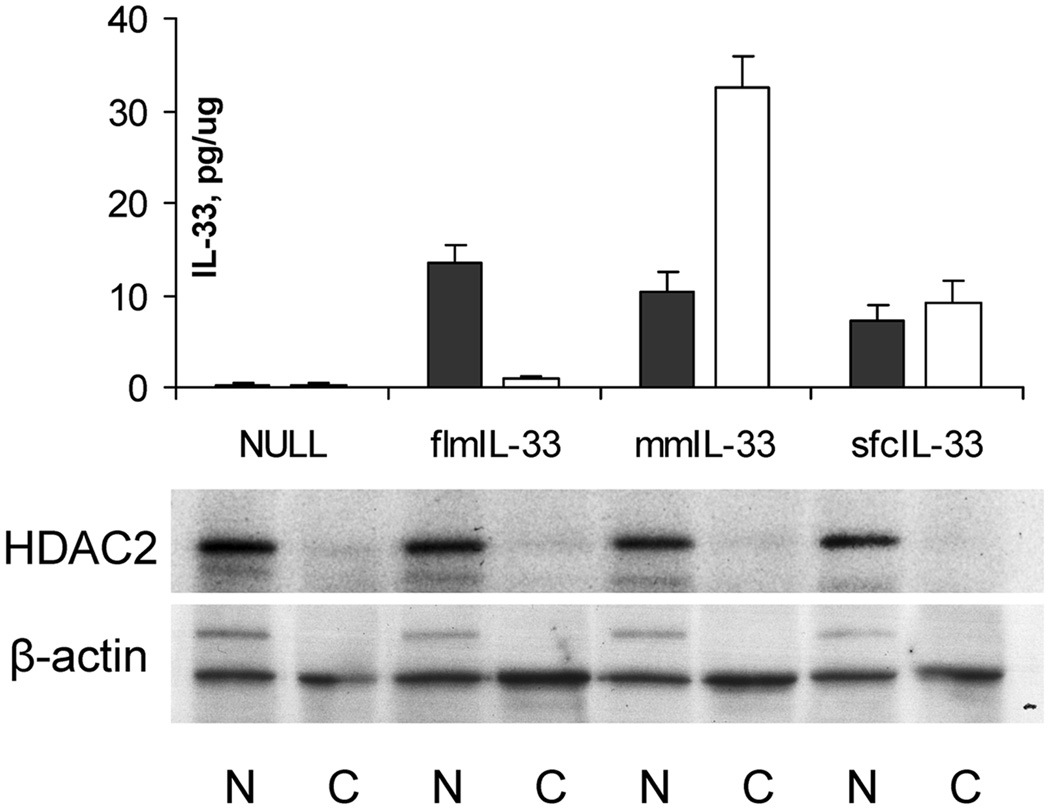
Then, to assess possible differences in the intracellular localizations of flmIL-33 and mmIL-33, nuclear and cytoplasmic fractions of the cells infected with AdV particles encoding the corresponding proteins were isolated and tested for IL-33 (Figure 7). In contrast to flmIL-33, which was localized predominantly in the cell nucleus, mmIL-33 was present in both nuclear and cytoplasmic compartments, with the cytoplasmic levels exceeding nuclear levels by approximately 3 fold. This observation suggests that flmIL-33 exerts its effects through intranuclear activities in the expressing cells, whereas mmIL-33 acts in both nuclei and cytoplasm. The latter notion is somewhat challenged by the observation that sfcIL-33 was distributed approximately equally between the nuclear and cytoplasmic fractions, yet the in vivo effects of sfcIL-33 were the same as those of flmIL-33. This observation suggests that perhaps not only cytoplasmic localization but also IL-33 maturity or even protein maturity alone may be important for the Th2 effects of mmIL-33. However, there appeared to be no clear association between subcellular localization of IL-33 isoforms and their in vivo effects. The subsequent experiments focused on the remaining two mechanistic considerations, utilization of cell surface receptor and effects on gene expression.
Figure 7.
Levels of IL-33 in nuclear (closed bars) and cytoplasmic (open bars) fractions of TC-1 cells infected with AdV encoding the indicated proteins (pg per μg total protein ± SD) measured by ELISA. Below, western blotting of the same nuclear (N) and cytoplasmic (C) samples for histone deacetylase 2 (HDAC2) and β-actin.
Independence of the effect of full-length IL-33 from ST2
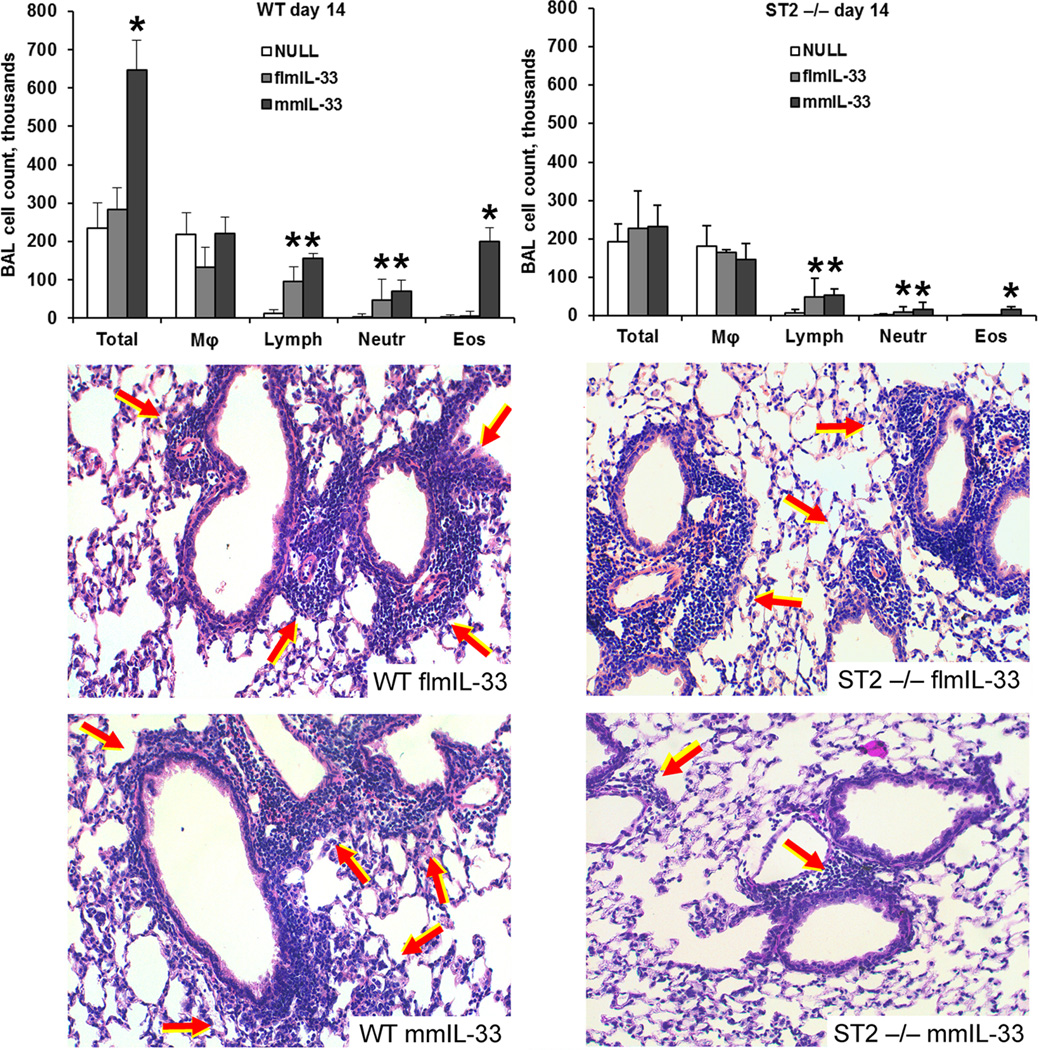
Since secretion and subcellular distribution of IL-33 isoforms did not appear to be associated with their functional effects, subsequent experiments were performed in ST2−/− mice, with the goal of determining whether this known mmIL-33 receptor also mediates the effects of flmIL-33 in vivo. Deficiency of ST2 significantly strongly attenuated the mmIL-33-driven pulmonary eosinophilia (Figure 8), and ELISA assays revealed that ST2-deficient animals had no increase in pulmonary IL-4 or IL-5 following mmIL-33 expression. However, the increases in lymphocytes persisted in flmIL-33- and mmIL-33-expressing ST2-deficient animals (Figure 8). Moreover, deficiency of ST2 failed to attenuate flmIL-33-induced pulmonary infiltration, and attenuated the size of, but did not completely abrogate, mmIL-33-induced infiltrates (Figure 8). In contrast to WT mice, ST2-deficient animals did not show hyperplasia of goblet cells in response to mmIL-33 delivery. Thus, it appears that the effects of flmIL-33 on the lung are ST2-independent, as is mmIL-33-induced lymphocytosis, whereas mmIL-33-driven Th2 deviation is ST2-dependent. This observation may explain the different effects of flmIL-33 and mmIL-33 in the lung in vivo.
Figure 8.
Responses to IL-33 gene delivery in ST2-deficient animals. The top two panels show BAL cell counts in flmIL-33- and mmIL-33-expressing mice on day 14 after construct instillation in wild-type (left) and ST2 −/− (right) mice. Significant differences from AdV-NULL-challenged mice are indicated with asterisks (p < 0.05). Below, histological changes in the lungs following flmIL-33 or mmIL-33 gene delivery (H&E staining).
Similar effects of IL-33 isoforms on gene expression
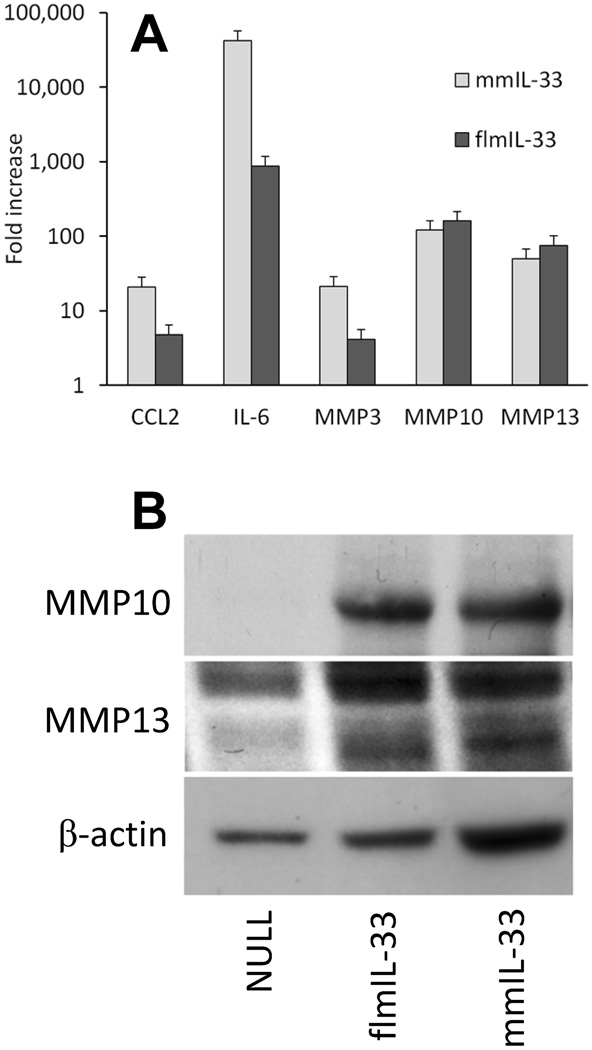
As stated above, a difference in engaging the ST2 receptor molecule appeared to drive differences in the effects of IL-33 isoforms on Th2 skewing of cytokines, goblet cell hyperplasia, and pulmonary eosinophilia. However, those findings did not explain observed similar effects of IL-33 isoforms, such as induction of pulmonary infiltration with inflammatory cells. To assess the possibility that flmIL-33 and mmIL-33 may exert their similar functional effects by similarly regulating gene expression, pilot experiments were performed in which TC-1 cells were infected with either AdV-flmIL-33 or AdV-mmIL-33, and gene expression profiling was performed using Affymetrix Mouse Genome 430 2.0 short oligomer arrays. The results suggested that each of the IL-33 isoforms similarly upregulated gene expression of CCL2 (MCP-1), IL-6, and matrix metalloproteinases (MMP)3, MMP10, and MMP13 in comparison with AdV-NULL-infected cells. To assess the magnitude of these changes more precisely, quantitative reverse transcription-polymerase chain reaction (RT-Q-PCR) experiments were carried out, and revealed that expression of these genes was similarly and significantly increased by gene delivery of either of flmIL-33 or mmIL-33 (Figure 9A). To demonstrate the relevance of these findings to the in vivo situation, pulmonary homogenates were prepared and western blotting performed for MMP10 and MMP13, revealing similar increases in the lungs of IL-33 isoform-overexpressing mice (Figure 9B). These observations suggest that the similar effects of IL-33 isoforms on the lungs in vivo may be associated with similar regulation of gene expression.
Figure 9.
Regulation of gene expression by flmIL-33 and mmIL-33 in cell culture (A) and in vivo (B). A. Mean fold increase ± SD in the levels of indicated mRNAs in TC-1 cells infected with indicated constructs versus cells infected with AdV-NULL, 48 h after infection, by RT-Q-PCR. Repeated on two occasions with similar results. Note the log scale on the vertical axis. B. Western blot of mouse lung homogenates for MMP10 and β-actin, 14 days after instillation of adenoviruses encoding IL-33 isoforms. Sample loading was normalized to the entire left lung: the whole lung was homogenized and an equal fraction of the homogenate loaded on the gel in each case.
DISCUSSION
The observed results indicate that IL-33 is elevated in the lungs of patients (Figure 1A, B), and that full-length IL-33 is the predominant naturally expressed form in health and disease (Figure 1C). Further studies in experimental animals indicated that full-length IL-33 promotes inflammation differently from the mature form of the cytokine in an ST2-independent fashion. Although flmIL-33 and mmIL-33 were similarly expressed in cell culture and in mouse lungs in vivo (Figures 2, 6), their functional effects were different. Expression of both isoforms caused accumulation of lymphocytes and neutrophils (Figures 3, 5), but in contrast to the mature isoform, the full-length isoform did not induce pulmonary eosinophilia, goblet cell hyperplasia, or Th2 skewing of the cytokine profile (Figure 3–5).
These observations may resolve the existing controversy about IL-33 functional effects. On the one hand, in numerous studies, activation of the IL-33R was associated with the Th2 cytokine pattern. IL-33 was found to be a chemoattractant for human Th2 cells (30). Intranasal administration of IL-33 triggered an allergic response in the airways (31), and the systemic in vivo treatment with mature IL-33 lead to eosinophilia and a significant increase in serum IgE along with Th2-driven pathological changes in the lungs and the digestive tract (6), perhaps due to the preferential expression of the IL-33R on Th2 cells under the control of a transcription factor GATA-3 (32)(33), independently of stimulation with Th2 cytokines IL-4, IL-5, or IL-10 (34)(35). Treatment with an anti-IL-33, but not a control antibody, significantly reduced serum IgE secretion, the numbers of eosinophils and lymphocytes, and the concentrations of IL-4, IL-5, and IL-13 in bronchoalveolar lavage fluid in a mouse model of asthma (36). Administration of either anti-T1/ST2 antibody or T1/ST2-immunoglobulin fusion protein abrogated Th2 cytokine production and pulmonary eosinophilia, but not Th1 cytokine production in an ovalbumin-induced animal model of the Th2-driven immune response (37). In respiratory syncytial virus infection in mice, inhibition of T1/ST2 with a monoclional antibody attenuated Th2-driven, but not Th1-driven, immunopathology (38). In T1/ST2-deficient mice, formation of pulmonary granuloma induced with Schistosoma mansoni eggs and characterized by eosinophil infiltration was abrogated compared with wild-type controls, and the levels of Th2 cytokines were significantly reduced in T1/ST2-deficient mice (29).
However, IL-33 may also promote responses other than Th2. IL-33 synergizes with T cell receptor (TCR) signaling and IL-12 in promoting IFN-γ production and the effector function of CD8+ T cells (39). Primary responses to IL-33 in bone marrow-derived human macrophages favored M1 chemokine generation, while the addition of IL-33 to polarized human macrophages amplified M2 chemokine expression (40). In human invariant NKT cells, IL-33 dose-dependently enhanced production of IL-4, IL-5, IL-13, but also IL-2, TNF-α, and IFN-γ (41). House dust mite preparation combined with APC, IL-2, and IL-33 induced production of both IL-4 and IFN-γ in Th2-polarized cells (41). IL-33 also mediated antigen-induced cutaneous and articular mechanical hypernociception through the induction of TNF-α, IL-1β, and IFN-γ (42). It is thus possible that IL-33 is naturally produced and acts in more than one form, each with different functions.
Detailed mechanistic explanations for the independent effects of flmIL-33 and for the observed differences and similarities between flmIL-33 and mmIL-33 will require further analysis, but some important conclusions can be made based on the data presented. Although flmIL-33, mmIL-33, and sfcIL-33 differed in secretion from cells (Figure 6) and in subcellular distribution (Figure 7), these differences did not correlate with corresponding functional effects in vivo. The flm-33-induced cellular infiltration of the lungs was independent of ST2, whereas mmIL-33 required ST2 to induce Th2-associated effects (Figure 8). These findings are in agreement with the notion that IL-33 may act without engaging a cell surface receptor, by acting as a transcription factor (5) or co-factor (43). Indeed, flmIL-33 remained predominantly intranuclear (Figure 7), and both flmIL-33 and mmIL-33 similarly regulated gene expression, most notably of matrix metalloproteinases MMP3, MMP10, and MMP13 (Figure 9). These enzymes are known for their contribution to pulmonary inflammation (44–47), and their similar regulation by flmIL-33 and mmIL-33 may explain the similar in vivo effects of these isoforms on lung inflammation (Figure 3, 5).
These observed effects were specifically induced by the delivered IL-33 isoforms and were not artifacts induced by the adenoviral vehicle. First, mice instilled with AdV-NULL showed minimal, if any, infiltration of cells in the lungs, suggesting that the response to the infection with the non-replicating adenovirus was minimal within the timeframe of the experiments. Second, all adenoviral constructs encode a foreign protein (GFP) in their backbone under a different (RSV) promoter, leading to strong GFP expression in cells infected with any of these viruses. AdV-NULL-infected mice over-expressing GFP in their lungs show minimal if any pulmonary response, suggesting that over-expression of a foreign protein has minimal effect on the lung within the experimental timeframe. A similar unpublished observation was made by us using recombinant adenovirus-mediated delivery of an unrelated protein, keratoepithelin, in which expression in mouse lungs did not cause pulmonary infiltration of inflammatory cells or changes in the cytokine profile. Third, delivery to mouse lung of an unrelated protein (chemokine CCL18) using the same adenoviral system resulted in a highly selective accumulation of T cells, but with no accumulation of B cells, macrophages, or eosinophils, and with minimal disturbance of the pulmonary cytokine milieu (21–23), all of which are in contrast to the observed effects of IL-33 isoforms. Fourth, in contrast to the observed effects of IL-33 isoforms, similar recombinant adenovirus-mediated delivery of IL-4 resulted in the accumulation of both T and B cells, but without macrophages in pulmonary infiltrates, and with different changes in the cytokine milieu (24, 25). Moreover, the observed effects, particularly on pulmonary eosinophilia and the cytokine profile, were distinctly specific to IL-4 splice variants delivered (24, 25). Finally, adenoviral vectors have undergone significant investigation in the clinic and showed immense promise for delivery of desired genes (48). Together, these observations suggest that the observed outcomes are specific to the proteins being delivered, and are different from an overall inflammatory response or broad immune activation.
It may appear that our findings on flmIL-33 by AdV-mediated delivery to the lungs contradict those previously reported in a transgenic model (14). Although there was mild pulmonary eosinophilia along with Th2 skewing of BAL cytokines in the flmIL-33 transgenic model, these effects may be explained by their observation of partial spontaneous activation of IL-33. Additionally, our model of acute IL-33 gene delivery in an organ-specific fashion arguably represents a more relevant realistic tissue response than the constitutive ubiquitous over-expression model. Therefore, these two models should not be compared directly, as transgenic animals with continued overexpression of a gene are well known to “re-wire” their regulatory pathways during pre- and post-natal development.
In summary, full-length IL-33 is independently active in vivo in an ST2-independent fashion, causing infiltration of the lung with inflammatory cells, and with particularly notable accumulation of lymphocytes in lung tissue and bronchoalveolar lavage. The Th2 effects of mature IL-33 are ST2-dependent, yet this isoform induces some lymphocytic infiltration in the lungs even in the absence of ST2. The similar non-Th2 effects of full-length and mature IL-33 isoforms are associated with similar regulation of gene expression, particularly matrix metalloproteinase, in the lungs.
Acknowledgments
Supported by VA Merit Awards 5I01CX000101 (IGL) and 5I01CX000107 (SPA), NIH-NIAMS 1R03AR054946 (IGL), and NIH-NHLBI 1R21HL106196 (SPA).
REFERENCES
- 1.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 2.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 3.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 4.Murphy GE, Xu D, Liew FY, McInnes IB. Role of interleukin 33 in human immunopathology. Ann. Rheum. Dis. 2010;69(Suppl 1):i43–i47. doi: 10.1136/ard.2009.120113. [DOI] [PubMed] [Google Scholar]
- 5.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. U. S. A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Lecart S, Lecointe N, Subramaniam A, Alkan S, Ni D, Chen R, Boulay V, Pene J, Kuroiwa K, Tominaga S, Yssel H. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur. J. Immunol. 2002;32:2979–2987. doi: 10.1002/1521-4141(2002010)32:10<2979::AID-IMMU2979>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa M, Hayakawa H, Matsuyama Y, Tamemoto H, Okazaki H, Tominaga S. Mature interleukin-33 is produced by calpain-mediated cleavage in vivo. Biochem. Biophys. Res. Commun. 2009;387:218–222. doi: 10.1016/j.bbrc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J. Immunol. 2009;183:7890–7897. doi: 10.4049/jimmunol.0802449. [DOI] [PubMed] [Google Scholar]
- 10.Ali S, Nguyen DQ, Falk W, Martin MU. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem. Biophys. Res. Commun. 2010;391:1512–1516. doi: 10.1016/j.bbrc.2009.12.107. [DOI] [PubMed] [Google Scholar]
- 11.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J. Biol. Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhiguang X, Wei C, Steven R, Wei D, Wei Z, Rong M, Zhanguo L, Lianfeng Z. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol. Lett. 2010;131:159–165. doi: 10.1016/j.imlet.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J. Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa M, Matsukura S, Kawaguchi M, Ieki K, Suzuki S, Odaka M, Watanabe S, Homma T, Sato M, Yamaguchi M, Takeuchi H, Adachi M. Expression and effects of IL-33 and ST2 in allergic bronchial asthma: IL-33 induces eotaxin production in lung fibroblasts. Int. Arch. Allergy Immunol. 2011;155(Suppl 1):12–20. doi: 10.1159/000327259. [DOI] [PubMed] [Google Scholar]
- 17.Le Goffic R, Arshad MI, Rauch M, L'helgoualc'h A, Delmas B, Piquet-Pellorce C, Samson M. Influenza Virus Infection Induces IL-33 in Mouse Lungs. Am. J. Respir. Cell Mol. Biol. 2011 doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 18.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum IL-33 levels are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Clin. Rheumatol. 2011;30:825–830. doi: 10.1007/s10067-011-1686-5. [DOI] [PubMed] [Google Scholar]
- 19.Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am. J. Respir. Crit. Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 20.Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124:1206–1214. doi: 10.1378/chest.124.4.1206. [DOI] [PubMed] [Google Scholar]
- 21.Luzina IG, Papadimitriou JC, Anderson R, Pochetuhen K, Atamas SP. Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18. Arthritis Rheum. 2006;54:2643–2655. doi: 10.1002/art.21950. [DOI] [PubMed] [Google Scholar]
- 22.Pochetuhen K, Luzina IG, Lockatell V, Choi J, Todd NW, Atamas SP. Complex regulation of pulmonary inflammation and fibrosis by CCL18. Am. J. Pathol. 2007;171:428–437. doi: 10.2353/ajpath.2007.061167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luzina IG, Todd NW, Nacu N, Lockatell V, Choi J, Hummers LK, Atamas SP. Regulation of pulmonary inflammation and fibrosis through expression of integrins alphaVbeta3 and alphaVbeta5 on pulmonary T lymphocytes. Arthritis Rheum. 2009;60:1530–1539. doi: 10.1002/art.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzina IG, Lockatell V, Todd NW, Keegan AD, Hasday JD, Atamas SP. Splice isoforms of human interleukin-4 are functionally active in mice in vivo. Immunology. 2011;132:385–393. doi: 10.1111/j.1365-2567.2010.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luzina IG, Lockatell V, Todd NW, Highsmith K, Keegan AD, Hasday JD, Atamas SP. Alternatively spliced variants of interleukin-4 promote inflammation differentially. J. Leukoc. Biol. 2011;89:763–770. doi: 10.1189/jlb.0510271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushime H, Roussel MF, Matsushima K, Hishinuma A, Sherr CJ. Cloning and expression of murine interleukin-1 receptor antagonist in macrophages stimulated by colony-stimulating factor 1. Blood. 1991;78:616–623. [PubMed] [Google Scholar]
- 27.Luzina IG, Highsmith K, Pochetuhen K, Nacu N, Rao JN, Atamas SP. PKCalpha mediates CCL18-stimulated collagen production in pulmonary fibroblasts. Am. J. Respir. Cell Mol. Biol. 2006;35:298–305. doi: 10.1165/rcmb.2006-0033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luzina IG, Tsymbalyuk N, Choi J, Hasday JD, Atamas SP. CCL18-stimulated upregulation of collagen production in lung fibroblasts requires Sp1 signaling and basal Smad3 activity. J. Cell. Physiol. 2006;206:221–228. doi: 10.1002/jcp.20452. [DOI] [PubMed] [Google Scholar]
- 29.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur. J. Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 31.Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, Bourne P, McClanahan TK, Pflanz S, de Waal Malefyt R. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int. Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 32.Nawijn MC, Dingjan GM, Ferreira R, Lambrecht BN, Karis A, Grosveld F, Savelkoul H, Hendriks RW. Enforced expression of GATA-3 in transgenic mice inhibits Th1 differentiation and induces the formation of a T1/ST2-expressing Th2-committed T cell compartment in vivo. J. Immunol. 2001;167:724–732. doi: 10.4049/jimmunol.167.2.724. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa M, Yanagisawa K, Aoki S, Hayakawa H, Takezako N, Tominaga S. T-helper type 2 cell-specific expression of the ST2 gene is regulated by transcription factor GATA-3. Biochim. Biophys. Acta. 2005;1728:53–64. doi: 10.1016/j.bbaexp.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisel C, Bonhagen K, Lohning M, Coyle AJ, Gutierrez-Ramos JC, Radbruch A, Kamradt T. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J. Immunol. 2001;166:3143–3150. doi: 10.4049/jimmunol.166.5.3143. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 2009;386:181–185. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walzl G, Matthews S, Kendall S, Gutierrez-Ramos JC, Coyle AJ, Openshaw PJ, Hussell T. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J. Exp. Med. 2001;193:785–792. doi: 10.1084/jem.193.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8(+) T cells. Eur. J. Immunol. 2011 doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, Das A, Hogaboam CM. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. doi: 10.1186/1471-2172-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 42.Verri WA, Jr, Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, Ferreira SH, Liew FY, Cunha FQ. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, Martin MU. The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB- stimulated gene transcription. J. Immunol. 2011;187:1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- 44.Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am. J. Respir. Cell Mol. Biol. 2001;24:537–544. doi: 10.1165/ajrcmb.24.5.4160. [DOI] [PubMed] [Google Scholar]
- 45.Nerusu KC, Warner RL, Bhagavathula N, McClintock SD, Johnson KJ, Varani J. Matrix metalloproteinase-3 (stromelysin-1) in acute inflammatory tissue injury. Exp. Mol. Pathol. 2007;83:169–176. doi: 10.1016/j.yexmp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, Wright C, Sin D, Pare PD, Pierce JA, Pierce RA, Patterson A, Cooper J, Hogg JC. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;181:1329–1335. doi: 10.1164/rccm.200812-1902OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flechsig P, Hartenstein B, Teurich S, Dadrich M, Hauser K, Abdollahi A, Grone HJ, Angel P, Huber PE. Loss of matrix metalloproteinase-13 attenuates murine radiation-induced pulmonary fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:582–590. doi: 10.1016/j.ijrobp.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 48.Parker AL, Nicklin SA, Baker AH. Interactions of adenovirus vectors with blood: implications for intravascular gene therapy applications. Curr. Opin. Mol. Ther. 2008;10:439–448. [PubMed] [Google Scholar]