Abstract
Introduction
SCLC patients unresponsive or relapsing within 90 days following frontline chemotherapy have poor prognosis and are treated with regimens different than the first-line regimen. Potential differences in the efficacy of second line therapy for refractory and sensitive SCLC have not been well studied.
Methods
Studies that enrolled sensitive and refractory (relapse more than or less than 90 days) SCLC patients for second-line therapy were identified using electronic databases (MEDLINE, EMBASE, and Cochrane library) and meeting abstracts databases. A systematic analysis was conducted using Comprehensive Meta Analysis (Version 2.2.048) software to calculate the Odds ratio of response and 95% confidence limits. Median overall survival time for sensitive and resistant SCLC patients was compared by 2-sided Student’s T-Test. We tested for significant heterogeneity by Cochran’s chi-square test and I square index.
Results
Twenty one studies published between 1984 and 2011 were eligible for this analysis with a total of 1692 patients enrolled; 912 with sensitive and 780 with refractory SCLC. The overall RR was 17.9% with a higher RR of 27.7% (range: 0 – 77%) for sensitive SCLC versus 14.8% (range: 0 – 70%) for refractory patients; p=0.0001. Pooled overall Odds ratio of response was 2.235 (95% CI: 1.518 – 3.291; p=0.001) favoring patients with sensitive disease. Median overall survival time was 6.7 months with a weighted survival of 7.7 and 5.4 months for sensitive and refractory SCLC respectively (p=0.0035).
Conclusions
Refractory SCLC patients derive modest clinical benefit from second line chemotherapy. However, response and survival outcomes are superior with chemosensitive disease.
Keywords: small cell lung cancer, chemotherapy, sensitive, resistant, refractory
Introduction
Approximately 30,000 new patients are diagnosed with small cell lung cancer (SCLC) in the US on an annual basis.1,2 Majority of these patients have extensive stage of the disease, which is incurable with currently available treatment options. The efficacy of platinum-based chemotherapy for frontline therapy has been established in randomized clinical trials3–7 with objective responses in approximately 70% of patients with limited stage SCLC and in 50% of patients with extensive stage disease.5,8–10 Despite this high initial response, majority of SCLC patients require salvage therapy for disease progression within several months following frontline therapy. Although various chemotherapeutic agents have been evaluated either singly or in combination for progressive SCLC following disease progression, topotecan is the only approved second line therapy for SCLC in the US population.11,12
The quality and duration of response to frontline therapy strongly predict the survival outcome in SCLC. Patients with durable response lasting more than 3 months are considered sensitive to the platinum-based frontline therapy. Refractory patients do not achieve any objective response while resistant disease is characterized by initial response followed by very early disease recurrence usually within 90 days of completing frontline therapy.12–14 Patients with chemosensitive disease and durable response lasting more than 6 months are treated with the original frontline regimen at the time of progression whereas patients with resistant or refractory disease are considered for treatment options different from the frontline regimen. Whether this treatment paradigm results in better outcome for patients with resistant/refractory SCLC is an area that has not been well studied.14,15 This systematic analysis is the first major attempt to bridge this knowledge gap by using data pooled from published results of clinical studies that enrolled sensitive and resistant/refractory SCLC patients to assess the clinical efficacy of systemic chemotherapy in the second line setting.
Materials and Methods
Study Eligibility
Prospective clinical trials that enrolled patients with both sensitive and resistant/refractory SCLC for the evaluation of second-line chemotherapy regimens were included in this analysis. In addition, qualifying studies must have enrolled minimum of 10 patients and reported on the clinical outcome (overall survival or response rate) for both subgroups of patients. Studies published in a language other than English were excluded.
Literature Search Strategy
We identified eligible clinical trials using the main computerized databases of published biomedical literature (MEDLINE, EMBASE, and Cochrane Library). We used the search terms “small cell lung cancer AND clinical trial” along with the following limit terms: humans, clinical trial, English, cancer, all adult: 19+ years for the MEDLINE search. Conference proceedings of the annual meetings of the American Society of Clinical Oncology (ASCO) and the International Association for the Study of Lung Cancer (IASLC) were also searched for relevant abstracts. The retrieved studies were reviewed independently by two of the authors (TKO, MB). Final determination of study eligibility was made by the concurrence of both investigators at a follow-up consensus meeting.
Data Extraction and Synthesis
Pertinent extracted data included patient demographics, number and distribution of enrolled patients, specific therapy and clinical outcome of response rate (RR) and overall survival (OS). The response rate data was pooled for the two patient subgroups to generate a weighted overall RR for sensitive and resistant/refractory SCLC. We also calculated a weighted mean survival time for the sensitive and resistant/refractory patient groups as the product of the median survival time and number of patients.
Statistical Approach
Analysis of the extracted data was conducted using Comprehensive Meta Analysis (Version 2.2.048) software. Using a random-effect model, the likelihood of response to second line treatment based on patients’ response to frontline chemotherapy (sensitive versus resistant/refractory) was calculated as Odds ratio along with 95% confidence interval. Statistical difference in the weighted mean RR and OS for sensitive and resistant/refractory SCLC patients was assessed by a 2-sided T-test.
Sensitivity testing
We conducted an initial analysis for the Odds ratio of response between sensitive and resistant/refractory population using the random effect model. A repeat analysis using a fixed effect model was performed to validate the initial results. Significant heterogeneity among the studies employed for this analysis was formally assessed by Cochran’s chi-square test and the I2 index where a p-value < 0.1 by chi-square test and I2 value < 0.25 indicate a low degree of heterogeneity.16 Finally, sensitivity analyses were conducted to exclude potential confounding of the results by an individual study or a group of studies by repeating the analysis after excluding each study in turn (leave-one-out model); by excluding studies of single agent and combination regimen; after excluding studies that evaluated topotecan and after excluding large studies that enrolled more than 50 patients.
Results
Study and patient demographics
Starting with 1141 studies, 53 studies published between 1984 and 2011 were identified as potentially eligible for this analysis. Based on the predefined eligibility criteria, we selected 21 studies that met the qualitative and quantitative requirements of the systematic analysis. A consort diagram of the stepwise identification of eligible studies is detailed in Figure 1. A total of 1692 patients were enrolled across the selected trials. Response data was available for 1055 patients across 20 studies; 570 (54.0%) with chemosensitive disease and 485 (45.9%) with resistant/refractory SCLC. Survival data from 1219 patients from 11 different studies was also analyzed; 678 (56%) and 541 (44%) with sensitive and resistant/refractory SCLC respectively. Details of patient demographics and study designs are included in Table 1.
Figure 1.
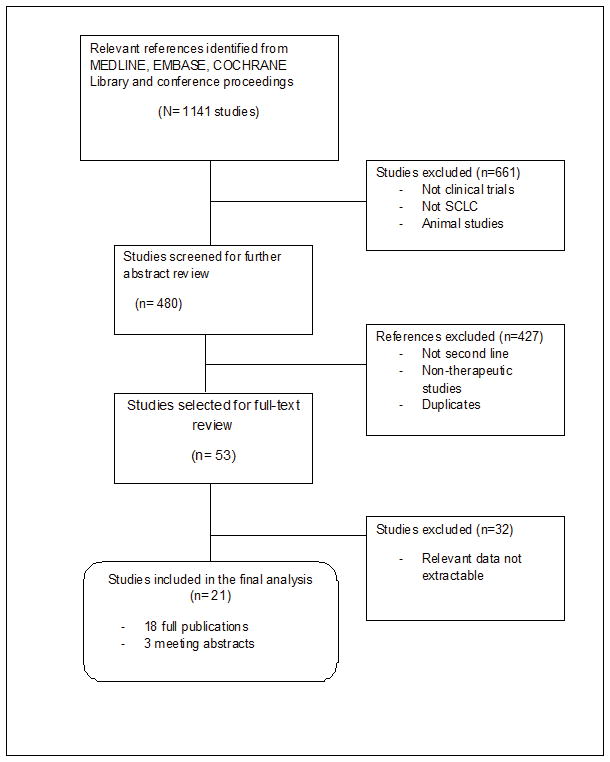
Consort diagram detailing search strategy and study selection for this systematic analysis
Table 1.
Study characteristics and clinical outcome of patients with sensitive or refractory SCLC following treatment with a second line chemotherapy. NR: Not reported. NA: Not adequate
| Study | Total (Sensitive/Refractory) | PS (0/1/2) | Study Design | Therapy | RR (%) | OS (months) | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Sensitive | Refractory | Sensitive | Refractory | |||||
|
| ||||||||
| Eckardt et al. 200917 | 77 (6/71) | 9/51/17 | Phase II single arm | Picoplatin | 17 | 8 | NR | NR |
|
| ||||||||
| Jalal et al. 200918 | 43 (20/23) | 14/18/11 | Single arm phase II | Pemetrexed | 5 | 4 | 4.4 | 2.7 |
|
| ||||||||
| Gronberg et al 200919 | 34 (25/9) | 5/18/11 | Multicenter phase II | Pemetrexed | 0 | 11 | 5.7 | 3.8 |
|
| ||||||||
| Rocha-Lima et al. 200720 | 71 (35/36) | 19/41/11 | Phase II single arm | Irinotecan Gemcitabine |
31 | 11 | 7.1 | 3.5 |
|
| ||||||||
| Ardizzoni et al. 200321 | 110 (68/42) | 17/75/18 | Multicenter single arm phase II | Topotecan + Cisplatin | 29 | 24 | NR | NR |
|
| ||||||||
| Naka et al. 200222 | 29 (16/13) | 0/16/13 | Single arm phase II | Irinotecan + Carboplatin | 38 | 23 | 6.1 | 5.7 |
|
| ||||||||
| Sculier et al. 200223 | 45 (29/16) | NR | Randomized phase II | Cisplatin-etoposide/carboplatin | 49 | 19 | 7.7 | 7.4 |
|
| ||||||||
| Kosmas et al. 200124 | 33 (13/20) | NR | Single arm phase II | Paclitaxel, Ifosfamide and Cisplatin | 77 | 70 | NR | NR |
|
| ||||||||
| Sonpavde et al. 200025 | 46 (32/14) | NR | Single arm phase II | Doxorubicin + Paclitaxel | 53 | 14 | NR | NR |
|
| ||||||||
| Ardizzoni et al. 199726 | 92 (45/47) | NR | Single arm phase II | Topotecan | 37.5 | 6.4 | 6.9 | 4.7 |
|
| ||||||||
| Schuette et al. 200527 | 35 (20/15) | 9/21/5 | Single arm phase II | Gemcitabine + Irinotecan | 10 | 26 | 4.5 | 4.5 |
|
| ||||||||
| Ichiki et al. 200328 | 34 (24/10) | 7/16/11 | Single arm phase II | Irinotecan + Ifosfamide | 62.5 | 30 | NR | NR |
|
| ||||||||
| Hensing et al. 200629 | 37 (20/17) | NR | Single arm phase II | BBR 3464 (Triplatin) | NR | NR | 6.8 | 2.5 |
|
| ||||||||
| Domine et al. 200130 | 20 (10/10) | NR | Multicenter phase II | Gemcitabine + Paclitaxel | 60 | 50 | NR | NR |
|
| ||||||||
| Dongiovanni et al. 200631 | 31 (21/10) | 3/20/8 | Single institution phase | Gemcitabine + Paclitaxel | 28.6 | 20 | NR | NR |
|
| ||||||||
| Hoang et al. 200332 | 27 (15/12) | 25/0/2 | Single arm phase II | Gemcitabine | NR | NR | 8.8 | 4.2 |
|
| ||||||||
| Hainsworth et al. 200333 | 29 (12/17) | NR | Single arm phase II | Gemcitabine and Vinorelbine | 25 | 0 | NR | NR |
|
| ||||||||
| Sessa et al. 200034 | 66 (37/29) | 15/39/12 | Single arm phase II | GI147211 (Camptothecin) | 21.6 | 10.3 | NR | NR |
|
| ||||||||
| Huber et al. 200635 | 169 (111/58) | 34/92/37 | Multicenter single arm phase II | Topotecan | 17.2 | 8.6 | 5.04 | 5.33 |
|
| ||||||||
| Von Pawel et al. 201136 | Amrubicin (225/199) | NR | Multicenter phase III trial | Amrubicin | NR | NR | 9.2 | 6.2 |
|
| ||||||||
| Topotecan (117/96) | Topotecan | NR | NR | 9.9 | 5.7 | |||
|
| ||||||||
| Vigano et al. 201137 | 27 | NA | Single arm phase II | NGR-hTNF + Doxorubicin | 27 | 19 | NR | NR |
|
| ||||||||
| Overall | 1692 (901/764) | 157/407/156 | 27.7 | 14.8 | 7.73 | 5.45 | ||
PS: Performance status using ECOG scale; NR: Not reported; NA: Not adequate
Tumor Response
The overall response rate of relapsed SCLC patients to second line treatment was 17.9% with 27.7% in patients with sensitive disease (range: 0 – 77%) and 14.8% (range: 0 – 70%) for resistant/refractory patients; p<0.0001. The overall Odds ratio of response was 2.235 (95% CI: 1.518 – 3.291; p<0.0001) in favor of patients with sensitive disease (Figure 2).
Figure 2.
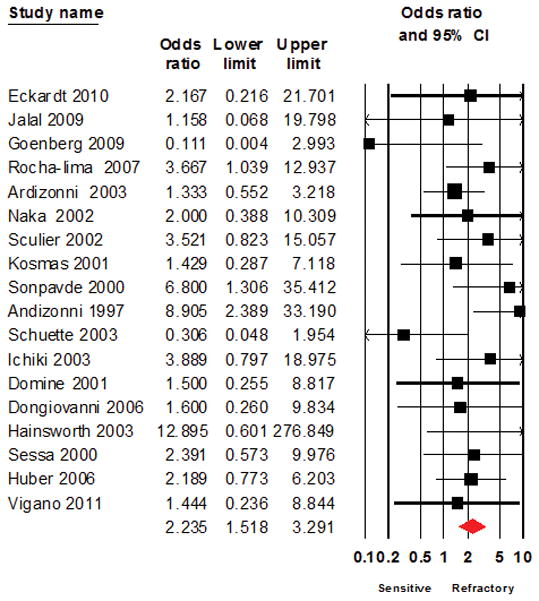
Forest plot showing the primary analysis using a random effect model for Odds ratio of response to salvage chemotherapy between patients with sensitive disease or refractory SCLC as defined based on response to frontline chemotherapy
Survival
The weighted average of the overall median survival time following second line therapy was 6.7 months with a weighted average of 7.73 months (range: 2.7 – 8.7) for sensitive SCLC and 5.45 months (range: 4.4 – 9.9) for resistant/refractory disease (p<0.0035).
Sensitivity testing
The test of heterogeneity using I2 test was 9.029% (p= 0.347) indicating a low degree of heterogeneity. The overall trends from the initial results remained unchanged after repeat analyses using the fixed effect model (Odds ratio of 2.227; 1.550 – 3.198; Figure 3) and after excluding studies with large sample size ( Odds ratio of 1.926; 1.093 – 3.393; Figure 4), studies of topotecan (2.170; 1.378 – 3.418; Figure 5), studies with combination regimens (2.480; 1.060 – 5.802, Figure 6) or studies of single agent treatment (2.040; 1.318 – 3.158, Figure 7) and following the leave-one-out analyses (Table 3).
Figure 3.
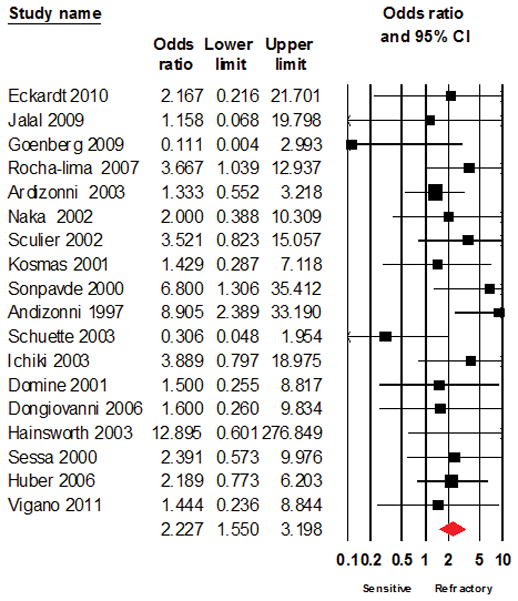
Forest plot of analysis using a fixed effect model for Odds ratio of response to salvage chemotherapy between patients with sensitive disease or refractory SCLC as defined based on response to frontline chemotherapy.
Figure 4.
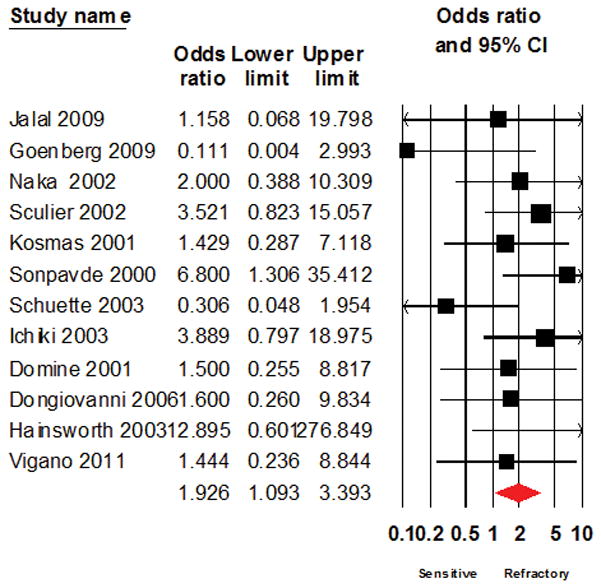
Forest plot showing the result of Odds ratio analysis after excluding large studies that enrolled 50 or more patients
Figure 5.
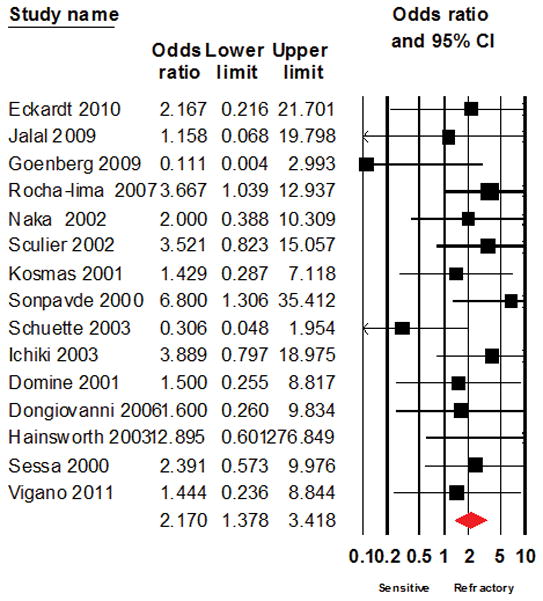
Forest plot of the result of systematic analysis for Odds ratio of response to salvage chemotherapy after excluding studies that evaluated topotecan
Figure 6.
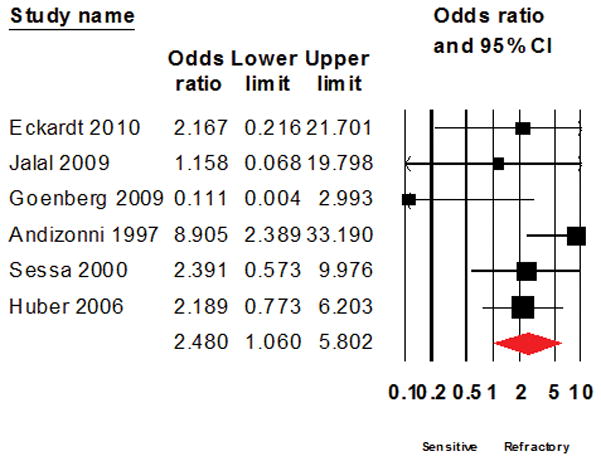
Forest plot of the result of systematic analysis for Odds ratio of response to salvage chemotherapy after excluding studies that evaluated combination multi-agent therapy
Figure 7.
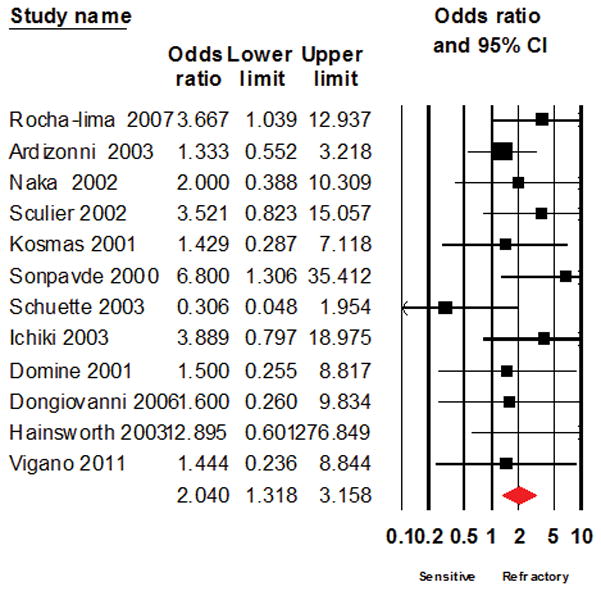
Forest plot of the result of systematic analysis for Odds ratio of response to salvage chemotherapy after excluding studies that evaluated single agent treatment regimens
Discussion
The result of this systematic analysis highlights the poor overall survival outcome for SCLC patients following progression on frontline therapy. More than 80% of patients enrolled in clinical trials employed for this systematic analysis did not achieve an objective response and a significant proportion of the patients died within 6 months. As previously observed with topotecan,11–13,15 we observed in this pooled analysis that patients with disease refractory or resistant to frontline therapy were also less likely to respond to second line chemotherapy in general and consequently had a worse survival outcome. Nonetheless, our data indicates that patients with resistant/refractory SCLC derive clinical benefit with the receipt of second line therapy in contrast to historical experience with untreated refractory SCLC where the survival is measured in weeks.6,38–40
The studies included in our analysis evaluated different types of investigational agents. It is therefore not unexpected that heterogeneity in study design, patient population and therapeutic agents may confound the result of this analysis. We carefully excluded any such possibility with the extensive sensitivity testing looking at all potential confounders including sample size, the use of topotecan, which is the only approved agent in this setting, as well as a leave-one-out analysis to examine if any of the included studies had a disproportionate influence on the overall result. Irrespective of the sensitivity test employed, we observed a consistent result of a worse survival and lower likelihood of response in refractory SCLC. Our result is also consistent with the report by Treat et al. who employed individual patient data from five large randomized studies of topotecan as salvage treatment for SCLC. They reported an overall response rate ranging between 14 and 17% with higher responses in chemosensitive disease (range of 18 – 24%) than in chemorefractory patients (range of 3 – 4%).15 Moreover, the reported outcome in this analysis is comparable to subset analysis of prospective studies of topotecan in the salvage setting.12,15 Since the use of intensive multi-agent chemotherapy has been shown to achieve higher response rates in SCLC albeit with heightened toxicities,41–43 we assessed whether this approach could result in higher likelihood of response in patients with resistant/refractory disease in the second line. However, we did not observe any reversal in the trend of higher odds of response in favor of patients with sensitive SCLC in studies evaluating both single agent and multi-agent chemotherapy regimens.
To our knowledge, this analysis using pooled data across 21 different prospective studies of second line chemotherapy is the largest such analysis in this patient population. Our data has now extended the results of previous small retrospective studies using local registry databases that reported superior survival with sensitive SCLC in the second line setting.39,44,45 Overgeneralization of the result of this analysis requires some caution since the patient population enrolled in clinical trials represents a select subset with good performance status and preserved organ function that may not accurately represent the general patient population.44 Indeed, majority of all patients in this analysis had an ECOG performance status of 0 or 1 but we were unable to ascertain whether this is balanced between the two subgroups of patients.
Based on our result and the observation in the frontline setting, we posit that the resistant/refractory SCLC patient population represents a biologically distinct subgroup of SCLC that requires a uniquely tailored therapeutic approach similar to the different approaches adopted for limited and extensive stage SCLC. In the absence of a highly effective salvage therapy regimen, we agree that patients with sensitive relapse should be retreated with a platinum/etoposide regimen in line with current management recommendations. In contrast, patient with resistant/refractory disease should be considered for innovative clinical trials, especially studies that are designed to exploit our evolving understanding of tumor biology and drug resistance. One such study is the ESCAPE study, a phase II study evaluating the efficacy of the combination of standard platinum/etoposide along with amuvatinib in patients with resistant/refractory SCLC [NCT01357395]. Amuvatinib is an oral multi-targeted tyrosine kinase inhibitor against mutant forms of c-Kit and PDGFR alpha and also suppresses DNA repair capacity by disrupting Rad51 protein activity and consequently homologous recombination, which is central to DNA damage repair capacity.
Pertinent limitations of our study include the retrospective nature of this analysis and the potential imbalances in important clinical characteristics that may also affect clinical outcome of SCLC patients such as gender, presence of brain metastasis, overall disease burden and dose intensity.46–48 However, the large number of patients included in the analysis and the use of tumor biology as defined by response to initial therapy for patient categorization makes our result a very important benchmark that could inform prospective clinical and translational research for this greatly understudied patient population.
Table 2.
Resultant odds ratio and P-value by t-test when the indicated study was excluded in the leave-one-out analysis for response and survival. No individual study had a dominant effect on the overall result of this analysis. NR: data required for analysis not reported
| Leave-One-Out Sensitivity testing for Odds Ratio and Survival Comparison | |||
|---|---|---|---|
| Excluded Study | Resultant Odds Ratio | 95% CI for OR | P-value for median survival estimate comparison |
| Eckardt et al. 200917 | 2.24 | 1.49–3.36 | NR |
|
| |||
| Jalal et al. et al. 200918 | 2.26 | 1.51–3.38 | P=<0.02 |
|
| |||
| Gronberg et al. 200919 | 2.31 | 1.60–3.32 | P=<0.004 |
|
| |||
| Rocha-lima et al. 200720 | 2.14 | 1.41–3.22 | P=<0.008 |
|
| |||
| Ardizzoni et al. 200321 | 2.45 | 1.61–3.70 | NR |
|
| |||
| Naka et al. 200222 | 2.25 | 1.48–3.4 | P=<0.005 |
|
| |||
| Sculier et al. 200223 | 2.16 | 1.43–3.26 | P=<0.005 |
|
| |||
| Kosmas et al. 200124 | 2.29 | 1.52–3.45 | NR |
|
| |||
| Sonpavde et al. 200025 | 2.10 | 1.43–3.09 | NR |
|
| |||
| Ardizzoni et al. 199726 | 1.98 | 1.36–2.89 | P=<0.0017 |
|
| |||
| Schuette et al. 200527 | 2.40 | 1.66–3.48 | P=<0.002 |
|
| |||
| Ichiki et al. 200328 | 2.16 | 1.44–3.25 | NR |
|
| |||
| Domine et al. 200130 | 2.27 | 1.51–3.42 | NR |
|
| |||
| Dongiovanni et al. 200631 | 2.27 | 1.50–3.41 | NR |
|
| |||
| Hainsworth et al. 200333 | 2.17 | 1.47–3.2 | NR |
|
| |||
| Sessa et al. 200034 | 2.22 | 1.46–3.37 | NR |
|
| |||
| Huber et al. 200635 | 2.24 | 1.46–3.44 | 0.0005 |
| Vigano et al. 201137 | 2.28 | 1.51–3.42 | NR |
| Hensing et al. 200629 | NR | NR | 0.006 |
| Hoang et al. 200332 | NR | NR | 0.007 |
| Von Pawel et al. 201136 | |||
| Amrubicin | NR | NR | P=<0.019 |
| Topotecan | NR | NR | P=<0.017 |
Acknowledgments
Supported by NIH P01 CA166999 grant awarded to FRK and P01 grant supplement award to TKO as well as unrestricted research funding to GS, WJC, FRK and SSR by the Georgia Cancer Coalition.
Footnotes
This work was presented as a poster at the 2010 Chicago Multidisciplinary Symposium in Thoracic Oncology. Chicago December 9–11, 2010.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–7. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 3.Owonikoko TK, Ramalingam S. Small cell lung cancer in elderly patients: a review. J Natl Compr Canc Netw. 2008;6:333–44. doi: 10.6004/jnccn.2008.0028. [DOI] [PubMed] [Google Scholar]
- 4.Lara PN, Jr, Natale R, Crowley J, et al. Phase III Trial of Irinotecan/Cisplatin Compared With Etoposide/Cisplatin in Extensive-Stage Small-Cell Lung Cancer: Clinical and Pharmacogenomic Results From SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark R, Ihde DC. Small-cell lung cancer: treatment progress and prospects. Oncology (Williston Park) 1998;12:647–58. discussion 661–3. [PubMed] [Google Scholar]
- 6.Pelayo Alvarez M, Gallego Rubio O, Bonfill Cosp X, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2009:CD001990. doi: 10.1002/14651858.CD001990.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin-Versus Carboplatin-Based Chemotherapy in First-Line Treatment of Advanced Non-Small-Cell Lung Cancer: An Individual Patient Data Meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 8.Owonikoko T, Ramalingam S. Minimal progress, potential promise in small-cell lung cancer. Oncology (Williston Park) 2008;22:1495–6. [PubMed] [Google Scholar]
- 9.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 10.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 11.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–7. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 13.Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593--a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:2114–22. doi: 10.1200/JCO.2001.19.8.2114. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S, Evans WK, Stys-Norman D, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2007;2:348–54. doi: 10.1097/01.JTO.0000263720.15062.51. [DOI] [PubMed] [Google Scholar]
- 15.Treat J, Huang CH, Lane SR, et al. Topotecan in the treatment of relapsed small cell lung cancer patients with poor performance status. Oncologist. 2004;9:173–81. doi: 10.1634/theoncologist.9-2-173. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckardt JR, Bentsion DL, Lipatov ON, et al. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J Clin Oncol. 2009;27:2046–51. doi: 10.1200/JCO.2008.19.3235. [DOI] [PubMed] [Google Scholar]
- 18.Jalal S, Ansari R, Govindan R, et al. Pemetrexed in second line and beyond small cell lung cancer: a Hoosier Oncology Group phase II study. J Thorac Oncol. 2009;4:93–6. doi: 10.1097/JTO.0b013e31818de1e6. [DOI] [PubMed] [Google Scholar]
- 19.Gronberg BH, Bremnes RM, Aasebo U, et al. A prospective phase II study: high-dose pemetrexed as second-line chemotherapy in small-cell lung cancer. Lung Cancer. 2009;63:88–93. doi: 10.1016/j.lungcan.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Rocha-Lima CM, Herndon JE, 2nd, Lee ME, et al. Phase II trial of irinotecan/gemcitabine as second-line therapy for relapsed and refractory small-cell lung cancer: Cancer and Leukemia Group B Study 39902. Ann Oncol. 2007;18:331–7. doi: 10.1093/annonc/mdl375. [DOI] [PubMed] [Google Scholar]
- 21.Ardizzoni A, Manegold C, Debruyne C, et al. European Organization for Research and Treatment of Cancer (EORTC) 08957 Phase II Study of Topotecan in Combination with Cisplatin as Second-Line Treatment of Refractory and Sensitive Small Cell Lung Cancer. Clin Cancer Res. 2003;9:143–150. [PubMed] [Google Scholar]
- 22.Naka N, Kawahara M, Okishio K, et al. Phase II study of weekly irinotecan and carboplatin for refractory or relapsed small-cell lung cancer. Lung Cancer. 2002;37:319–23. doi: 10.1016/s0169-5002(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 23.Sculier JP, Lafitte JJ, Lecomte J, et al. A phase II randomised trial comparing the cisplatin-etoposide combination chemotherapy with or without carboplatin as second-line therapy for small-cell lung cancer. Ann Oncol. 2002;13:1454–9. doi: 10.1093/annonc/mdf244. [DOI] [PubMed] [Google Scholar]
- 24.Kosmas C, Tsavaris NB, Malamos NA, et al. Phase II study of paclitaxel, ifosfamide, and cisplatin as second-line treatment in relapsed small-cell lung cancer. J Clin Oncol. 2001;19:119–26. doi: 10.1200/JCO.2001.19.1.119. [DOI] [PubMed] [Google Scholar]
- 25.Sonpavde G, Ansari R, Walker P, et al. Phase II study of doxorubicin and paclitaxel as second-line chemotherapy of small-cell lung cancer: a Hoosier Oncology Group Trial. Am J Clin Oncol. 2000;23:68–70. doi: 10.1097/00000421-200002000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol. 1997;15:2090–6. doi: 10.1200/JCO.1997.15.5.2090. [DOI] [PubMed] [Google Scholar]
- 27.Schuette W, Nagel S, Juergens S, et al. Phase II trial of gemcitabine/irinotecan in refractory or relapsed small-cell lung cancer. Clin Lung Cancer. 2005;7:133–7. doi: 10.3816/CLC.2005.n.029. [DOI] [PubMed] [Google Scholar]
- 28.Ichiki M, Gohara R, Rikimaru T, et al. Combination chemotherapy with irinotecan and ifosfamide as second-line treatment of refractory or sensitive relapsed small cell lung cancer: a phase II study. Chemotherapy. 2003;49:200–5. doi: 10.1159/000071145. [DOI] [PubMed] [Google Scholar]
- 29.Hensing TA, Hanna NH, Gillenwater HH, et al. Phase II study of BBR 3464 as treatment in patients with sensitive or refractory small cell lung cancer. Anticancer Drugs. 2006;17:697–704. doi: 10.1097/01.cad.0000215054.62942.7f. [DOI] [PubMed] [Google Scholar]
- 30.Dómine M, Larriba JLG, Morales S, et al. Gemcitabine and Paclitaxel as Second Line Treatment in Small Cell Lung Cancer (SCLC). A Multicentric Phase II Study. Proc Am Soc Clin Oncol. 2001;20:abstr 1263. [Google Scholar]
- 31.Dongiovanni V, Buffoni L, Berruti A, et al. Second-line chemotherapy with weekly paclitaxel and gemcitabine in patients with small-cell lung cancer pretreated with platinum and etoposide: a single institution phase II trial. Cancer Chemother Pharmacol. 2006;58:203–9. doi: 10.1007/s00280-005-0157-6. [DOI] [PubMed] [Google Scholar]
- 32.Hoang T, Kim K, Jaslowski A, et al. Phase II study of second-line gemcitabine in sensitive or refractory small cell lung cancer. Lung Cancer. 2003;42:97–102. doi: 10.1016/s0169-5002(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 33.Hainsworth JD, Burris HA, 3rd, Erland JB, et al. Combination chemotherapy with gemcitabine and vinorelbine in the treatment of patients with relapsed or refractory small cell lung cancer: a phase II trial of the Minnie Pearl Cancer Research Network. Cancer Invest. 2003;21:193–9. doi: 10.1081/cnv-120016415. [DOI] [PubMed] [Google Scholar]
- 34.Sessa C, Wanders J, Roelvink M, et al. Second-line treatment of small-cell lung cancer with the camptothecin-derivative GI147211: a study of the EORTC Early Clinical Studies Group (ECSG) Ann Oncol. 2000;11:207–10. doi: 10.1023/a:1008372404504. [DOI] [PubMed] [Google Scholar]
- 35.Huber RM, Reck M, Gosse H, et al. Efficacy of a toxicity-adjusted topotecan therapy in recurrent small cell lung cancer. Eur Respir J. 2006;27:1183–9. doi: 10.1183/09031936.06.00015605. [DOI] [PubMed] [Google Scholar]
- 36.von Pawel J, Jotte R, Spigel DR, et al. Randomized phase 3 trial of amrubicin versus topotecan as second-line treatment for small cell lung cancer (SCLC) Journal of Thoracic Oncology. 2011;6:S274, Abstract O01.02. doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]
- 37.Vigano MG, Cavina R, Novello S, et al. Phase II trial of NGR-hTNF and doxorubicin in relapsed small cell lung cancer (SCLC) J Clin Oncol. 2011;29 (suppl; abstr 7077) 29:suppl; abstr 7077, 2011. [Google Scholar]
- 38.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA: a cancer journal for clinicians. 2011;61:91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 39.Chute JP, Kelley MJ, Venzon D, et al. Retreatment of patients surviving cancer-free 2 or more years after initial treatment of small cell lung cancer. Chest. 1996;110:165–71. doi: 10.1378/chest.110.1.165. [DOI] [PubMed] [Google Scholar]
- 40.Agra Y, Pelayo M, Sacristan M, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2003:CD001990. doi: 10.1002/14651858.CD001990. [DOI] [PubMed] [Google Scholar]
- 41.Ettinger DS, Berkey BA, Abrams RA, et al. Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: a Radiation Therapy Oncology Group 9609 phase II study. J Clin Oncol. 2005;23:4991–8. doi: 10.1200/JCO.2005.00.414. [DOI] [PubMed] [Google Scholar]
- 42.Greco FA, Thompson DS, Morrissey LH, et al. Paclitaxel/carboplatin/etoposide versus paclitaxel/topotecan for extensive-stage small cell lung cancer: a Minnie Pearl Cancer Research Network randomized, prospective phase II trial. Oncologist. 2005;10:728–33. doi: 10.1634/theoncologist.10-9-728. [DOI] [PubMed] [Google Scholar]
- 43.Niell HB, Herndon JE, 2nd, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol. 2005;23:3752–9. doi: 10.1200/JCO.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 44.Froeschl S, Nicholas G, Gallant V, et al. Outcomes of second-line chemotherapy in patients with relapsed extensive small cell lung cancer. J Thorac Oncol. 2008;3:163–9. doi: 10.1097/JTO.0b013e318160c0cb. [DOI] [PubMed] [Google Scholar]
- 45.Kim YH, Goto K, Yoh K, et al. Performance status and sensitivity to first-line chemotherapy are significant prognostic factors in patients with recurrent small cell lung cancer receiving second-line chemotherapy. Cancer. 2008;113:2518–23. doi: 10.1002/cncr.23871. [DOI] [PubMed] [Google Scholar]
- 46.Lilenbaum RC, Huber RM, Treat J, et al. Topotecan therapy in patients with relapsed small-cell lung cancer and poor performance status. Clinical lung cancer. 2006;8:130–4. doi: 10.3816/CLC.2006.n.041. [DOI] [PubMed] [Google Scholar]
- 47.Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:1794–801. doi: 10.1200/JCO.1999.17.6.1794. [DOI] [PubMed] [Google Scholar]
- 48.Chute JP, Venzon DJ, Hankins L, et al. Mayo Clinic proceedings. Vol. 72. Mayo Clinic; 1997. Outcome of patients with small-cell lung cancer during 20 years of clinical research at the US National Cancer Institute; pp. 901–12. [DOI] [PubMed] [Google Scholar]


