Abstract
Background
Lumbar intrathecal injection of oxytocin produces antinociception in rats and analgesia in humans. Classically, oxytocin receptors couple to stimulatory G proteins, increase inositol-3-phosphate production, and result in neuronal excitation. Most work to date has focused on a spinal site of oxytocin to excite γ-aminobutyric acid interneurons to produce analgesia. Here we ask whether oxytocin might also affect primary sensory afferents by modulating high voltage-gated calcium channels, such as it does in the brain.
Methods
Dorsal root ganglion cells from adult rats were acutely dissociated and cultured, and changes in intracellular calcium determined by fluorescent microscopy using an indicator dye. The effects of oxytocin alone and in the presence of transient depolarization from increased extracellular KCl concentration were determined, then the pharmacology of these effects were studied. Cells from injured dorsal root ganglion cells after spinal nerve ligation were also studied.
Results
Oxytocin produced a concentration-dependent inhibition of the increase in intracellular calcium from membrane depolarization, an effect blocked more efficiently by oxytocin- than vasopressin-receptor selective antagonists. Oxytocin-induced inhibition was present in cells responding to capsaicin, and when internal stores of calcium were depleted with thapsigargin. Oxytocin produced similar inhibition in cells from animals with spinal nerve ligation.
Conclusions
These data suggest that oxytocin produces antinociception after intrathecal delivery in part by reducing excitatory neurotransmitter release from the central terminals of nociceptors.
Introduction
Oxytocin, a neuropeptide mainly synthesized in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus, exerts diverse effects across the life cycle from actions within and outside the central nervous system.1 A role for oxytocin in analgesia and antihypersensitivity has been demonstrated and is postulated to reflect actions primarily within the spinal cord. Oxytocin-containing PVN neurons project to the superficial and deep dorsal horn of the spinal cord,2–4 and are activated by stress and pain, including that of obstetric labor.5 PVN stimulation temporarily reverses second order spinal neuronal6,7 and behavioral8 hypersensitivity from nerve injury in a manner reversed by oxytocin receptor antagonists. These effects are mimicked by intrathecal injection of oxytocin itself8,9 and intrathecal oxytocin transiently reversed chronic low back pain in 970 men and women in a report from China.10 Thus, spinally released oxytocin would be expected to relieve acute and chronic pain.
Most previous work has focused on excitatory actions of oxytocin on γ-amino-butyric acid (GABA)-containing spinal neurons to produce analgesia. Oxytocin receptors classically couple to Gq and enhance inositol-3-phosphate (IP3) signaling, leading to increased intracellular Ca2+ and neuronal excitation.11 Electrophysiologic and behavioral studies of dorsal horn neurons suggest that oxytocin inhibits sensory neurotransmission between primary afferents and dorsal horn neurons by modulating glutamate release12 by direct postsynaptic inhibition of neurons receiving afferent input,13,14 and by enhancing GABA release from spinal interneurons.15–17
A less explored target for spinal oxytocin analgesia is an action on central terminals of primary afferents. Only one study has examined the effects of oxytocin on primary sensitive afferents, and showed that excitatory adenosine triphosphate-activated currents (present only on a subset of nociceptors) were acutely reduced by oxytocin.18 In the SON, oxytocin inhibits glutamate release by modulating high voltage-gated Ca2+ channels, especially N-type channels,19 and it is conceivable that oxytocin could, by a similar mechanism, reduce nociceptive afferent input into the spinal cord. We hypothesized that oxytocin would affect primary sensory afferent excitability, as reflected in changes in membrane depolarization-induced increases in intracellular Ca2+. We first used a population-based approach to determine what proportion of small diameter afferents were affected by oxytocin, then determined the pharmacology of its action. Additionally, since transient receptor potential vanilloid (TRPV)-1 expressing nociceptors are considered important in many pain states,20 we tested whether this subset of primary sensory afferents was differentially affected by oxytocin. Finally, because peripheral nerve injury, which can lead to neuropathic pain, affects intracellular Ca2+ regulation,21,22 we compared the action of oxytocin on primary sensory afferents from normal animals and injured afferents from those with spinal nerve ligation (SNL), a model of neuropathic pain.
Methods
Animals
Male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN, USA), weighing 200–250 g, were used in this study. All the experiments were approved by Animal Care and Use Committee at Wake Forest University (Winston Salem, NC, USA). Animals were housed under a 12-h light/dark cycle and food and water were available ad libitum.
Spinal nerve ligation (SNL)
L5–L6 SNL was performed as previously described.23 In brief, animals were anesthetized with 2–3% isoflurane in oxygen and the right L5 and L6 spinal nerves were isolated and tightly ligated with 5–0 silk sutures. After surgery, animals were allowed to recover for 2 weeks. Allodynia was confirmed 2 weeks after SNL surgery by measuring withdrawal threshold to the hindpaw in response to application of von Frey filaments. Only animals with a withdrawal threshold < 4 g were used in this study.
Cell dissociation
Animals were deeply anesthetized and killed by decapitation. Dorsal root ganglion (DRG) cells were acutely dissociated as previously described.24 In brief, bilateral L4, L5 and L6 DRGs in normal animals or L5 and L6 DRGs ipsilateral to SNL in nerve-injured animals were collected in cold Hanks' balanced salt solution (Lonza, Walkersville, MD, USA). After mincing into small pieces, tissues were incubated in 0.25% collagenase (Sigma-Aldrich, St. Louis, MO, USA) in Ham's F12 medium (Gibco, Carlsbad, CA, USA) at 37 °C for 60 min, with gentle agitation at 10-min intervals. Tissue were transferred to a Hanks' balanced salt solution, centrifuged at 500xG for 3min, and mechanically dissociated with flame-polished pipette in this solution. The tissues were then centrifuged at 500xG for 3min again and the resulting pellet was re-suspended with Dulbecco's Modified Eagle Medium (Gibco BRL, Invitrogen Corp., Carlsbad, CA, USA), containing 1% HEPES, 10% heat-inactivated fetal bovine serum (Fisher Scientific USA), and 100 U/ml penicillin with 100 mg/ml streptomycin (Gibco BRL, Invitrogen Corp., Carlsbad, CA, USA). Cells were plated onto 0.02% poly-l-lysine (Sigma-Aldrich) -coated glass coverslips and kept at 37 °C, 5% CO2 atmosphere. Satellite cells are not present in this culture. All experiments were performed within 12–24 h after preparation.
Ca2+ videomicroscopy
Ca2+ videomicroscopy was performed in small and medium size (<36 □m diameter) DRG neurons as previously described.24,25 Cells were incubated with 3 μM Fura-2 AM and 0.01% Pluronic F-127 (Molecular Probes, Eugene, OR, USA) for 60 min at room temperature. After washing with normal buffer solution (mM:140 NaCl, 4 KCl, 2 CaCl2, 1.2 MgSO4, 10 glucose, and 10 HEPES, pH 7.4), cells were placed in a dark environment at room temperature for 10–20 min. Coverslips were then mounted on a chamber equipped with a perfusion system (ALA-VM8, ALA Scientific Instruments, New York, NY, USA) which perfused locally and totally covered the recording area and was viewed through an inverted microscope. Fura-2 fluorescence was recorded at 510 nm during alternating excitation at 340 and 380 nm at 1 Hz using a monochromater (PTI Deltascan, Photon Technology International, South Brunswick, NJ, USA). Regions of interest were defined on a computer connected to a CCD camera and ratio of emission at 510 nm from excitation at 340 and 380 was analyzed. High KCL buffer solution (mM: 119 NaCl, 25 KCl, 2 CaCl2, 1.2 MgSO4, 10 glucose, and 10 HEPES) was applied for 30 sec three times, separated by a 10-min period of perfusion with normal buffer alone or containing test drugs. Only cells with a resting 340/380 fluorescence ratio of 0.55–0.90 and a signal to noise ratio from the second train of potassium stimulation of ≥3.0 were included.
Drugs and treatment groups
To determine the effect of oxytocin on intracellular Ca2+ response to KCl-induced depolarization, oxytocin (0, 10, 100, and 1000 nM; Sigma) was added to all buffers beginning 5 min before the third stimulus with 25 mM KCl. To determine the receptor acted upon by oxytocin, the oxytocin receptor antagonist, atosiban alone (1-deamino-2D-Tyr (O-ethyl)-4-Thr-8-Orn-oxytocin; 10–1000 nM, Tocris Bioscience, Ellisville, MO, USA) or combined with 100 μM oxytocin was studied. To determine the receptors upon which oxytocin acts, similar experiments were performed with the oxytocin receptor selective antagonist, desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT and the arginine vasopressin (AVP)-V1a receptor selective antagonist, d(CH2)5[Tyr(me) 2]AVP, 10 and 100 nM (kindly supplied by Dr. Maurice Manning, University of Toledo, Toledo, OH). To determine the role of internal Ca2+ stores, thapsigargin (5 μM; Sigma) was added to all buffers beginning 12–15 min before the second stimulus with 25 mM KCl. Finally, to determine the effect of oxytocin on capsaicin-sensitive DRG cells, at the end of the study, capsaicin (Sigma; 100 μM) was added in some experiments. Capsaicin-sensitive cells were defined as those responding to capsaicin with an increase in Fura-2 fluorescence ratio > 2 SD of that from the second KCl response.
Data analysis
Data are presented as the ratio of emission of Fura-2 loaded cells at 510 nm from excitation at 340 nm divided by excitation at 380 nm (I 340/380). We did not permeabilize cells to determine the maximum and minimum I 340/380 in these experiments, so could not calculate estimated intracellular Ca2+ concentrations. From previous validation work, typically baseline I 340/380 values in the current set of studies (0.75) represent an approximate intracellular Ca2+ concentration of 100 nM. I 340/380 values were normally distributed and are presented as mean ± SD. The proportion of responding cells was similar between dishes and cultures, and an average of 3.6 cells per field were included in analysis, with an average yield of 2.3 coverslips per culture (animal). All groups contained data from a minimum of 8 animals.
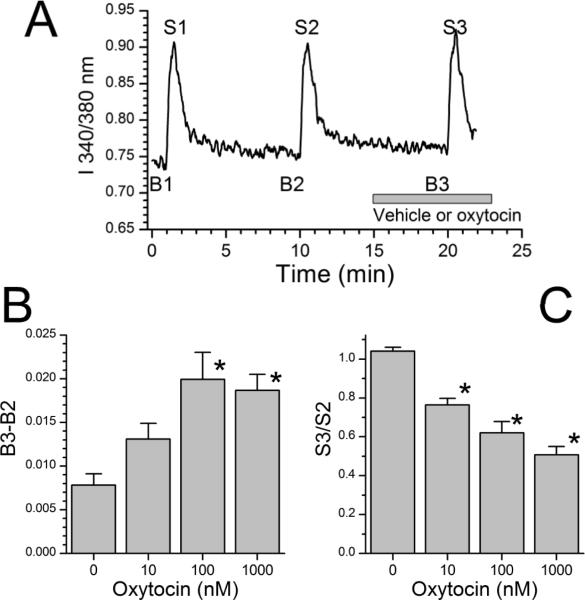
Data for each recording were reduced as previously described.24,25 In brief, 3 baselines and 3 responses to high KCl stimulation were calculated (Figure 1A). Each baseline consisted of the average of 30 sec of recordings ending 20 sec before high KCl stimulation, each stimulation consisted of the average of 60 sec of recording beginning at the time high KCl solution was started, and the net effect of KCl was calculated as their difference. We also recorded the maximum value during stimulation. To compare effects of drug infusion in the absence of high KCl stimulation, the third baseline (obtained in the presence of drug) was subtracted from the second baseline (obtained in the absence of drug), and treatment groups compared for this difference using an ANOVA on ranks followed by Dunn's test or, for two groups only, for Mann-Whitney rank sum test.
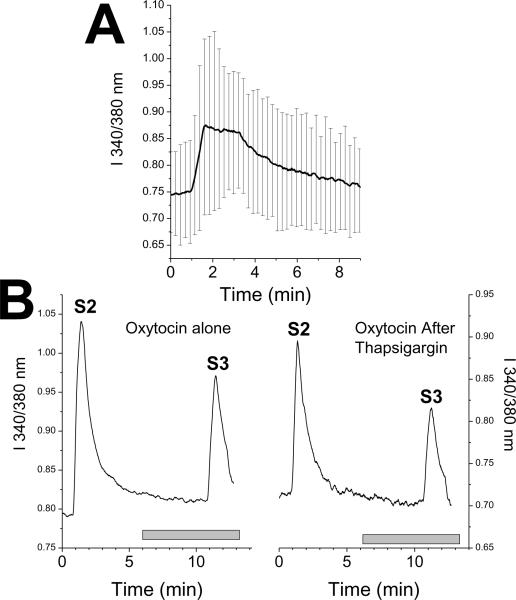
Fig.1.
(A) Representative intracellular Ca2+ imaging experiment from a dorsal root ganglion neuron and vehicle control. Ratio of fluorescence of Fura-2 at 550 nm after excitation at either 340 or 380nm, a measure of intracellular Ca2+ concentration. Dorsal root ganglion neurons were repeatedly stimulated with 25 mM KCl for 30 sec (S1, 2, and 3). Baseline values (B1, 2, and 3) were determined from the average ratio for 30 sec before each stimulus. Gray bar represents time of vehicle or oxytocin superfusion.
(B) Change in Fura-2 fluorescence ratio at third baseline (B3) after vehicle or oxytocin compared to preceding baseline (B2) before experimental treatment. Values represent mean of 57–218 cells. *P < 0.05 compared to vehicle control.
(C) Fura-2 fluorescence ratio at third KCl stimulus (S3) after vehicle or oxytocin divided by that of the preceding KCl stimulus (S2) before experimental treatment. Values represent mean of 57–218 cells. *P < 0.05 compared to vehicle control.
To compare the effect of drug infusion on response to high KCl stimulation, two analyses were performed. For the primary analysis, the net effect of the third KCl stimulation (in the presence of drug) was divided by the net effect of the second KCl stimulation (in the absence of drug). Treatment groups were compared for this ratio using an ANOVA on ranks followed by Dunn's test or, for two groups only, for Mann-Whitney rank sum test. For the secondary analysis, we calculated the 2.5th percentile of this ratio in vehicle-treated groups and considered any cell with a ratio below this value after treatment to have been inhibited. Groups were compared in this secondary analysis using χ2 analysis. P<0.05 was considered significant.
Results
Effect of oxytocin and atosiban on KCl-evoked Ca2+ response in dorsal root ganglion neurons
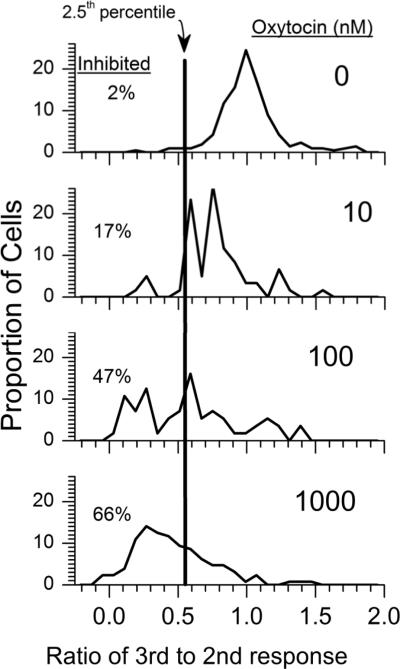
Cells perfused with buffer alone showed reproducible baselines in intracellular Ca2+ concentration, as reflected by I 340/380 in FURA2 loaded cells of approximately 0.75, and reproducible responses to high KCl stimulation (Figure 1A). Oxytocin produced two concentration-dependent effects, a very minor increase in intracellular Ca2+ concentration in normal KCl concentration buffer (Figure 1B; H = 47; P < 0.001), and a pronounced decrease in rise of Ca2+ concentration in response to high KCl (Figure 1C; H = 203; P < 0.001). There was no significant correlation between the degree of increase and the degree of inhibition of Ca2+ concentration. The increase in I 340/340 from oxytocin in normal KCl concentration buffer was significant only with the two highest concentrations of oxytocin, 100 nM and 1000 nM (Figure 1B; Q = 83 and 96, respectively; P < 0.001) and was small, likely reflecting an approximate change in intracellular Ca2+ of 5–6 nM. Oxytocin-induced inhibition of response to high KCl was, in contrast, large and significant at all concentrations (Figure 1C; Q = 6.4, 11, and 15 for 10, 100, and 1000 nM oxytocin, respectively, all P < 0.05). Similar results were obtained with the peak response becaise with this primary analysis of the average response over 60 sec from KCl (data not shown). There was no significant correlation between the small excitatory effect and the large inhibitory effect of oxytocin at any concentration (Pearson product moment, P > 0.05). In a secondary analysis we calculated the proportion of cells inhibited by oxytocin, as defined by a response to the third KCl stimulus compared to the second below the 2.5th percentile in buffer-treated controls. As shown in Figure 2, oxytocin produced a concentration-dependent increase in the proportion and extent of cells inhibited (χ2 = 14, 81, and 167 for 10, 100, and 1000 nM oxytocin, respectively, all P < 0.001).
Fig.2.
Proportion of cells inhibited by treatment, as defined as below the 2.5th percentile of the S3 to S2 response, after continued perfusion with buffer or with oxytocin. Groups differ as noted in text.
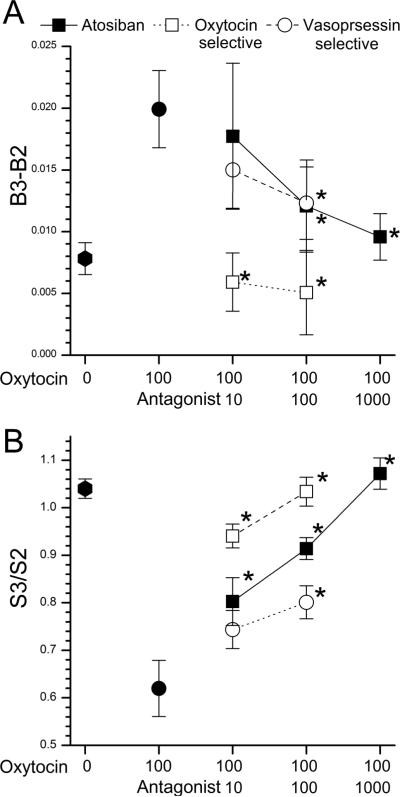
The oxytocin receptor-preferring antagonist atosiban produced a concentration-dependent blockade of the 100 nM oxytocin-induced small increase in intracellular Ca2+, first present at 100 nM atosiban (Figure 3 A; H=12.49; P = 0.006) and the oxytocin-induced reduction in response to KCl, first present at 10 nM atosiban (Figure 3B; H=53.26; P < 0.001). Similarly, the proportion of cells meeting the criterion for inhibition from 100 nM oxytocin (47%) was reduced to 10% when co-administered with 100 nM atosiban (χ2 = 16.9; P < 0.001). Both an oxytocin receptor selective antagonist and an AVP-V1a receptor selective antagonist reduced excitatory (H=20.42; P < 0.001 and H=7.2; P =0.027, respectively) and inhibitory (H=43.9; P < 0.001 and H=11.4; P = 0.003, respectively) actions of oxytocin. Only the oxytocin receptor selective antagonist produced a block at 10 nM and it produced a greater degree of block at 100 nM than the AVPV1a receptor selective antagonist (Figure 3A and 3B). None of the antagonists alone affected resting I 340/380 or on the response to KCl (n=5; data not shown).
Fig.3.
(A) Change in Fura-2 fluorescence ratio at third baseline (B3) after vehicle or oxytocin, 100 nM alone or in the presence of antagonists compared to preceding baseline (B2). Atosiban and oxytocin- and arginine vasopressin (AVP)-V1a receptor-selective antagonists produced concentration-dependent inhibitions of the effect of oxytocin. The oxytocin receptor-selective antagonist blocked the effect of oxytocin at a lower concentration and more effectively than the AVP-V1a receptor selective antagonist. Values represent mean of 50–62 cells. *P < 0.05 compared to oxytocin, 100 nM alone. (B) Fura-2 fluorescence ratio at third KCl stimulus (S3) after vehicle or oxytocin, 100 nM alone or in the presence of antagonists divided by that of the preceding KCl stimulus (S2) before experimental treatment. Atosiban and oxytocin- and AVP-V1a receptor-selective antagonists produced concentration-dependent inhibitions of the effect of oxytocin. The oxytocin receptor-selective antagonist blocked the effect of oxytocin at a lower concentration and more effectively than the AVP-V1a receptor-selective antagonist. Values represent mean of 50–62 cells. *P < 0.05 compared to oxytocin, 100 nM alone.
A majority of small diameter neurons in this preparation respond to the TRPV1 receptor agonist, capsaicin25 and are presumed nociceptors, so we tested the effect of oxytocin on 21 capsaicin-sensitive cells. Compared to the general population of cells in this preparation, a similar proportion of capsaicin-sensitive neurons met the criterion for inhibition by 100 nM oxytocin (47% overall, 43% in capsaicin-sensitive cells) and the reduction in response to KCl was a similar magnitude (38 ± 44% inhibition overall, 35 ± 23% inhibition in capsaicin-sensitive cells).
Role of internal Ca2+ stores in oxytocin actions in DRG neurons
Thapsigargin, which acutely depletes Ca2+ stores, induced a transient increase in I 340/380 which returned to the baseline level within 10 min (Figure 4A), but had no effect on subsequent KCl depolarization (Figure 4B). This increase in I 340/380 was over 5-fold more than that induced by oxytocin. Oxytocin's small excitatory effect was blocked in cells treated with thapsigargin (Figure 4B; T = 3.6; P < 0.001), whereas its inhibition of KCl-induced depolarization was unaffected (Figure 4B; T = 359; P = 0.29).
Fig.4.
(A) Response of Fura-2 fluorescence ratio to perfusion with thapsigargin, 5 □M, begun at time 1 min. Values are mean ± SD of 9 cells.
(B) Response of Fura-2 fluorescence ratio to repeated KCl exposure in cells perfused between S2 and S3 with oxytocin, 100 nM alone (left panel) or with ongoing perfusion with thapsigargin, 5 □M (right panel). Values are mean of 9 (thapsigargin) or 57 (control) cells. Groups differ in baseline just before S3 but not in S3 response.
Effect of nerve injury on oxytocin actions in DRG neurons
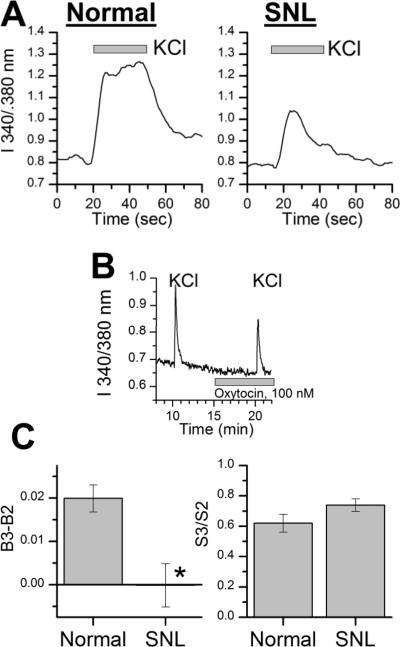
As previously observed,25 DRG neurons from L5 ipsilateral to L5 SNL had similar resting Fura-2 fluorescent ratios, but a smaller response to KCl stimulation (Figure 5A). The excitatory effect of oxytocin, 100 nM, was absent in cells ipsilateral to SNL ligation, and significantly different than cells from normal animals (Figure 5B and C; t=2266; P < 0.001). In contrast, the inhibitory effect of oxytocin, 100 nM on KCl-induced change in Fura-2 fluorescence was similar in cells ipsilateral to SNL ligation and cells from normal animals (Figure 5B and C; t=1.65; P = 0.10).
Fig. 5.
(A) Representative trace of Fura-2 fluorescence ratio in response to KCl exposure in cells from normal animals and those after spinal nerve ligation (SNL).
(B) Representative trace of Fura-2 fluorescence ratio in dorsal root ganglion neuron ipsilateral to spinal nerve ligation in response to repeated KCl exposures and oxytocin, 100 nM infusion.
(C) Left panel: Change in Fura-2 fluorescence ratio at third baseline (B3) after oxytocin, 100 nM to preceding baseline (B2). Right panel: Fura-2 fluorescence ratio at third KCl stimulus (S3) after oxytocin, 100 nM divided by that of the preceding KCl stimulus (S2) before experimental treatment. Values are mean of 52–57 cells from normal animals and those after spinal nerve ligation. *P < 0.05 compared to cells from normal animals.
Discussion
Oxytocin is released from the central nervous system to the systemic circulation where it induces uterine contractions, protects the fetal brain during delivery, and facilitates lactation1,26 and, within the central nervous system, regulates disparate behavior in both sexes across the life cycle,1 including analgesia.10 The current study adds to our understanding by demonstrating a mechanism for oxytocin to produce analgesia by actions on the peripheral nervous system via inhibition of responses in primary sensory afferents.
Oxytocin produces antinociception and reduces hypersensitivity in models of nerve injury primarily by actions in the spinal cord. Initial studies have focused on oxytocin-induced inhibition of sensory neurotransmission indirectly via excitation of inhibitory GABA interneurons in the spinal cord as a potential cellular site for oxytocin analgesia15–17 or by direct inhibition of spinal cord neurons which receive sensory information.12–14 Potential actions on primary sensory afferents have been largely ignored, because systemically administered oxytocin (which cannot re-enter the central nervous system27) fails to produce antinociception in rats28 or to produce analgesia in women in labor. Nonetheless, oxytocin could target central terminals of primary sensory afferents for analgesia, analogous to opioids, which produce minor analgesic effects at peripheral sites but profound analgesia when given intrathecally, primarily by actions at this target.29
Only one previous study examined the action of oxytocin on primary sensory afferents observed inhibition to response to adenosine triphosphate, a stimulator of a subset of nociceptors.18 The current study extends these observations by demonstrating oxytocin-dependent inhibition in a majority of small diameter neurons in vitro with an enriched population of nociceptors.25
Oxytocin produced two effects in these small diameter afferent cell bodies, a small increase in intracellular Ca2+ and a large inhibition of KCl depolarization-induced increase in intracellular Ca2+ concentration. The former is consistent with the known action of oxytocin to stimulate, via IP3 generation, release of Ca2+ from intracellular stores. Consistent with such an action, thapsigargin, which acutely depletes intracellular Ca2+ stores, prevented this minor increase in intracellular Ca2+ from oxytocin. In contrast, internal Ca2+ stores are not essential to the increase in intracellular Ca2+ observed with KCl-induced depolarization, because this increase was not blocked by thapsigargin and because thapsigargin inhibition of oxytocin-induced increase in intracellular Ca2+ did not prevent the inhibition by oxytocin of the intracellular Ca2+ response to KCl. It is unlikely that the small increase in intracellular Ca2+ after oxytocin was causally related to oxytocin-induced inhibition of intracellular increases in Ca2+ from depolarization, because the two were not correlated in individual cells and because two manipulations (thapsigargin and SNL surgery) completely blocked the small excitatory effect without change in the inhibitory effect.
Systemically administered oxytocin produces antinociception in mice by an action mediated by AVP-V1a receptors, although the very high doses required in that study could have led to acute hypertension, which produces analgesia.30 The receptors by which oxytocin slightly increased intracellular Ca2+ and more dramatically reduced depolarization-induced intracellular Ca2+ increases are not fully identified by this study, because oxytocin can stimulate both oxytocin and vasopressin receptors. Atosiban, an oxytocin-receptor preferring antagonist, produced a concentration-dependent blockade of both phenomena in a concentration range consistent with an action on oxytocin receptors. Similarly, the results of studies examining blockade of oxytocin's effects, although largely consistent with an action on oxytocin receptors, cannot exclude a small contribution by AVP-V1a receptors, because high concentrations of a selective antagonist for that receptor partially inhibited the effects of oxytocin. Taken together, however, the current data support a primary engagement of oxytocin receptors in the actions of oxytocin on primary sensory afferents. In addition, oxytocin modulates high voltage-gated Ca2+ channels, primarily of the N-type, to reduce presynaptic glutamate release in the SON19 and we speculate that a similar mechanism could be responsible for oxytocin's inhibitory effects in small diameter primary sensory afferents. Future electrophysiologic studies could directly test this hypothesis.
The large majority of DRG neurons in this preparation are presumed nociceptors, because they either express calcitonin gene-related peptide or isolectin B4 and/or respond to the TRPV1 agonist, capsaicin.25,31 A similar proportion of cells responding to TRPV1 were inhibited by oxytocin as in the general cell population in this preparation in the current study, consistent with an inhibitory action of oxytocin on nociceptors.
Whether oxytocin produces analgesia to acute painful stimuli or is selectively active in reducing spontaneous pain or hypersensitivity in chronic pain states is unclear. Some electrophysiologic studies suggest a selective dampening action of nociceptive sensory input in the spinal cord by oxytocin only after peripheral nerve injury6 yet other similar work by the same laboratory15 and others17 found antinociceptive effects of intrathecal oxytocin in normal rats. In humans, intrathecal oxytocin has been reported to relieve both acute and chronic low back pain.10
In the current study oxytocin produced similar inhibition in primary sensory afferent neurons ipsilateral to SNL injury as in normal rats, consistent with an antihypersensitivity effect of intrathecal oxytocin in animals and humans that reflects, in part, actions on primary sensory afferents. We confirmed in the current study that the KCl-induced increase in intracellular Ca2+ is less in primary sensory afferent neurons ipsilateral to SNL injury than in normal animals, most likely reflecting reduced Ca2+ stores and/or voltage-gated Ca2+ currents.21,22,25,32 Similar mechanisms could have resulted in the lack of the small excitatory effect of oxytocin in cells from SNL animals.
In conclusion, oxytocin produces a concentration-dependent inhibition of KCl depolarization-induced increase in intracellular Ca2+ in rat small diameter primary sensory afferent neurons, including TRPV1-expressing nociceptors. This action most likely reflects stimulation of oxytocin receptors because it is reversed by the selective oxytocin receptor antagonist, atosiban. A small increase in intracellular Ca2+ induced by oxytocin most likely represents release from intracellular Ca2+ stores but is not essential to this inhibition. Oxytocin-induced inhibition occurs in normal primary sensory afferents and in injured afferents after a surgical model of chronic neuropathic pain, consistent with analgesia observed in humans by intrathecal oxytocin to treat both acute and chronic pain. These results suggest that spinally released oxytocin or intrathecally administered oxytocin may produce analgesia in part by actions on primary sensory afferents, including nociceptors.
Acknowledgments
Funding: NIH: GM48085
Footnotes
The authors declare no conflicts of interest, but they do have a conflict with Tony Yaksh (he is co-investigator on this grant and also handled this manuscript).
DISCLOSURES: Name: Shotaro Hobo, MD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Shotaro Hobo has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Ken-ichiro Hayashida, DVM, PhD
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Attestation: Ken-ichiro Hayashida has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: James C. Eisenach, MD
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: James C. Eisenach has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee HJ, Macbeth AH, Pagani JH, Young WS., III Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–51. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condes-Lara M, Martinez-Lorenzana G, Rojas-Piloni G, Rodriguez-Jimenez J. Branched oxytocinergic innervations from the paraventricular hypothalamic nuclei to superficial layers in the spinal cord. Brain Res. 2007;1160:20–9. doi: 10.1016/j.brainres.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–12. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 4.Rousselot P, Papadopoulos G, Merighi A, Poulain DA, Theodosis DT. Oxytocinergic innervation of the rat spinal cord. An electron microscopic study. Brain Res. 1990;529:178–84. doi: 10.1016/0006-8993(90)90825-v. [DOI] [PubMed] [Google Scholar]
- 5.Takeda S, Kuwabara Y, Mizuno M. Effects of pregnancy and labor on oxytocin concentrations in human plasma and cerebrospinal fluid. Endocrinol Japon. 1985;32:875–80. doi: 10.1507/endocrj1954.32.875. [DOI] [PubMed] [Google Scholar]
- 6.Condes-Lara M, Maie IA, Dickenson AH. Oxytocin actions on afferent evoked spinal cord neuronal activities in neuropathic but not in normal rats. Brain Res. 2005;1045:124–33. doi: 10.1016/j.brainres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Lorenzana G, Espinosa-Lopez L, Carranza M, Aramburo C, Paz-Tres C, Rojas-Piloni G, Condes-Lara M. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain. 2008;140:265–73. doi: 10.1016/j.pain.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Miranda-Cardenas Y, Rojas-Piloni G, Martínez-Lorenzana G, Rodríguez-Jiménez J, López-Hidalgo M, Freund-Mercier MJ, Condés-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122:182–9. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 9.DeLaTorre S, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Villanueva L, Condes-Lara M. Paraventricular oxytocinergic hypothalamic prevention or interruption of long-term potentiation in dorsal horn nociceptive neurons: electrophysiological and behavioral evidence. Pain. 2009;144:320–8. doi: 10.1016/j.pain.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Yang J. Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine. 1994;19:867–71. doi: 10.1097/00007632-199404150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 12.Jo YH, Stoeckel ME, Freund-Mercier MJ, Schlichter R. Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J Neurosci. 1998;18:2377–86. doi: 10.1523/JNEUROSCI.18-07-02377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, Muglia LJ, Zhuo M. Oxytocin mediates stress-induced analgesia in adult mice. J Physiol. 2002;540:593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breton JD, Poisbeau P, Darbon P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol Pain. 2009;5:63. doi: 10.1186/1744-8069-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas-Piloni G, Lopez-Hidalgo M, Martinez-Lorenzana G, Rodriguez-Jimenez J, Condes-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137:69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Condes-Lara M, Rojas-Piloni G, Martinez-Lorenzana G, Lopez-Hidalgo M, Rodriguez-Jimenez J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 2009;1247:38–49. doi: 10.1016/j.brainres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Breton JD, Veinante P, Uhl-Bronner S, Vergnano AM, Freund-Mercier MJ, Schlichter R, Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in laminas I-II which amplify GABAergic inhibition. Mol Pain. 2008;4:19. doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Wu ZZ, Li X, Li ZW, Wei JB, Hu QS. Modulation by oxytocin of ATP-activated currents in rat dorsal root ganglion neurons. Neuropharmacology. 2002;43:910–6. doi: 10.1016/s0028-3908(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa M, Kombian SB, Pittman QJ. Oxytocin retrogradely inhibits evoked, but not miniature, EPSCs in the rat supraoptic nucleus: role of N- and P/Q-type calcium channels. J Physiol. 2001;532:595–607. doi: 10.1111/j.1469-7793.2001.0595e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagy I, Sántha P, Jancsó G, Urbán L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–69. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 21.Gemes G, Rigaud M, Weyker PD, Abram SE, Weihrauch D, Poroli M, Zoga V, Hogan QH. Depletion of calcium stores in injured sensory neurons: anatomic and functional correlates. Anesthesiology. 2009;111:393–405. doi: 10.1097/ALN.0b013e3181ae63b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigaud M, Gemes G, Weyker PD, Cruikshank JM, Kawano T, Wu HE, Hogan QH. Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology. 2009;111:381–92. doi: 10.1097/ALN.0b013e3181ae6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SH, Chung JM. Sympathectomy alleviates mechanical allodynia in an experimental animal model for neuropathy in the rat. Neurosci Lett. 1991;134:131–4. doi: 10.1016/0304-3940(91)90524-w. [DOI] [PubMed] [Google Scholar]
- 24.Duflo F, Zhang Y, Eisenach JC. Electrical field stimulation to study inhibitory mechanisms in individual sensory neurons in culture. Anesthesiology. 2004;100:740–3. doi: 10.1097/00000542-200403000-00041. [DOI] [PubMed] [Google Scholar]
- 25.Hayashida K, Bynum T, Vincler M, Eisenach JC. Inhibitory M2 muscarinic receptors are upregulated in both axotomized and intact small diameter dorsal root ganglion cells after peripheral nerve injury. Neuroscience. 2006;140:259–68. doi: 10.1016/j.neuroscience.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–92. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 27.Kang YS, Park JH. Brain uptake and the analgesic effect of oxytocin--its usefulness as an analgesic agent. Arch Pharm Res. 2000;23:391–5. doi: 10.1007/BF02975453. [DOI] [PubMed] [Google Scholar]
- 28.Xu XJ, Wiesenfeld-Hallin Z. Is systemically administered oxytocin an analgesic in rats? Pain. 1994;57:193–6. doi: 10.1016/0304-3959(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 29.Yaksh TL. Spinal opiate analgesia: Characteristics and principles of action. Pain. 1981;11:293–346. doi: 10.1016/0304-3959(81)90633-3. [DOI] [PubMed] [Google Scholar]
- 30.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–84. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenach JC, Zhang Y, Duflo F. alpha2-adrenoceptors inhibit the intracellular Ca(2+) response to electrical stimulation in normal and injured sensory neurons, with increased inhibition of calcitonin gene-related peptide expressing neurons after injury. Neuroscience. 2005;131:189–97. doi: 10.1016/j.neuroscience.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 32.McCallum JB, Kwok WM, Sapunar D, Fuchs A, Hogan QH. Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons. Anesthesiology. 2006;105:160–8. doi: 10.1097/00000542-200607000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]