Abstract
Purpose
To study the effects of radiation dose, chemotherapy and their interaction in patients with unresectable or medically inoperable stage III non-small cell lung cancer (NSCLC).
Methods and Materials
A total of 237 consecutive stage III NSCLC patients were evaluated. Median follow-up was 69.0 months. Patients were treated with radiation therapy (RT) alone (n = 106), sequential chemoradiation (n = 69) or concurrent chemoradiation (n = 62). The primary endpoint was overall survival (OS). Radiation dose ranged from 30 to 102.9 Gy (median 60 Gy), corresponding to a bioequivalent dose (BED) of 39 to 124.5Gy (median 72 Gy).
Results
The median OS of the entire cohort was 12.6 months, and 2- and 5-year survival rates were 22.4% and 10.0%, respectively. Multivariable Cox regression model demonstrated that Karnofsky performance status (P = 0.020), weight loss < 5% (P = 0.017), chemotherapy (yes vs. no), sequence of chemoradiation (sequential vs. concurrent) (P < 0.001) and BED (P < 0.001) were significant predictors of OS. For patients treated with RT alone, sequential chemoradiation and concurrent chemoradiation, median survival was 7.4, 14.9 and 15.8 months, and five-year OS was 3.3%, 7.5% and 19.4% respectively (P < 0.001). The effect of higher radiation doses on survival was independent of whether chemotherapy was given.
Conclusion
Radiation dose and use of chemotherapy are independent predictors of OS in stage III NSCLC, and concurrent chemoradiation is associated with the best survival. There is no interaction between RT dose and chemotherapy.
Keywords: NSCLC, Stage III, Dose, Chemotherapy, Radiation
Introduction
In 2007, there were an estimated 213,380 new cases and 160,390 deaths of lung cancer in the United States (1). The majority of them are locally advanced non-small cell lung cancer (NSCLC). The standard care for locally advanced unresectable stage III NSCLC is combined modality therapy with chemotherapy and radiation therapy (RT). Despite improved survival with combined modality therapy (2, 3), local-regional recurrences and the development of distant metastases are still problematic and the prognosis of the majority of patients remains poor. Recent trials suggest that local-regional control can be improved with RT dose escalation and that this improvement in local-regional control could be translated into an overall survival (OS) benefit (4-8). Several studies have suggested that radiation dose is a significant prognostic factor for tumor control and OS in patients with locally advanced or medically inoperable early-stage NSCLC. A prospective dose-escalation trial at our institution showed a positive relationship between dose and local-regional tumor control, as well as OS, with RT doses in the range of 63 to 103 Gy in patients with stage I-III NSCLC largely treated with RT alone (9). Radiation dose was the only significant factor affecting both local tumor control and OS for patients with stage I-III disease in our trial (9, 10). In a series of 114 patients with stage I-II NSCLC, we have recently confirmed the positive dose effect in early stage NSCLC and demonstrated that high-dose radiation (higher than 66 Gy in 2 Gy fractions) is more important for patients with larger tumors (> 4cm) (11). Using 3-D conformal techniques, recent studies have demonstrated the safety of high dose radiation in patients with unresectable or medically inoperable stage III NSCLC treated with radiation alone or neoadjuvant chemotherapy plus radiation, provided that normal structure dose–volume limits are not excessive (4-10, 12). Researchers from the University of North Carolina reported that doses of 74 Gy (13, 14) and 90 Gy (15) can be delivered safely with induction chemotherapy followed by concurrent chemoradiation.
In this study, we aimed to investigate the effects of radiation dose and chemotherapy on OS and determine if the radiation dose effect is independent from that of chemotherapy in patients with unresectable or medically inoperable stage III NSCLC
Materials and Methods
Patient Selection
This is an Institutional Review Board approved retrospective study. Eligible subjects included all patients with stage III NSCLC registered in the radiation oncology database and treated with radiation-based therapy at University of Michigan Hospital (UM) and the Veterans Affairs Ann Arbor Healthcare System (VA) between January 1992 and July 2004. Patients treated with preoperative and postoperative radiation or for recurrent disease were excluded. All 237 patients included in this study were restaged according to American Joint Cancer Committee (AJCC) 2002 criteria: stage IIIA (T1-3N2M0, T3N1M0) and IIIB (T1-3N3M0, T4N0-3M0). The Charlson scoring system was used to assess the degree of co-morbidity (16). Consistent 3-D conformal techniques were used throughout the study period. Positron emission tomography (PET) staging was based on physician's preference at University of Michigan Hospital; and it was routinely not performed at the Veterans' hospital during the study period. A total of 75 patients (55 at UM and 20 at VA) underwent PET scan.
General Treatment Decision
Treatment decisions were generally made by an institutional tumor board consisting of thoracic surgeons, medical oncologists, and radiation oncologists. Treatment strategies were determined based on tumor status and a patient's performance status and co-morbidities. Most of the patients with stage III NSCLC were treated with RT with definitive or palliative intent either with or without chemotherapy based on the time of treatment (more concurrent chemoradiation in recent years) and the decision of the treating physicians based on the patient's candidacy for chemotherapy.
Radiation Therapy Technique
CT simulation guided 3D plans were used throughout the study period. Intensity modulated radiation therapy was not utilized for the treatment of lung cancer, the planning techniques and dose calculation algorithm were unchanged during the study period. Patients who enrolled into the dose escalation protocol received RT based on the following protocol specifications, which has been described previously (9, 11). The fraction size was 2.1 Gy for protocol patients. For patients who received radiation in the off-protocol setting, the radiation technique was similar to protocol patients, except that fraction size ranged from 1.8 Gy to 3.0 Gy.
Data Analysis and Statistical Considerations
OS was the primary endpoint of this study. Survival was defined as the duration between the dates of pathological diagnosis and death. Age at diagnosis, gender, Karnofsky Performance Score (KPS), percent of total body weight loss (< 5 vs. ≥ 5%), smoking history (yes vs. no), pre-RT oxygen use (yes vs. no), non-malignant pleural effusion (yes vs. no), disease stage (IIIA vs. IIIB), Charlson's comorbidity score, RT dose, gross tumor volume (GTV), on-protocol vs. off-protocol, chemotherapy use (yes vs. no) and timing of chemoradiation (sequential vs. concurrent) were collected from original medical records and assessed for their impact on survival. To further study the radiation dose effect, biological equivalent dose (BED) was estimated for every patient using an α/β ratio of 10.
Data were considered right-censored for OS outcomes if no events occurred by the end of follow-up. Cox's proportional hazards model was used for multivariate analysis to estimate the simultaneous impact of clinical factors on OS, and to test the significance of the interaction between BED and chemotherapy in terms of OS. The Kaplan-Meier method was used to estimate survival curves for different groups, and the Log-Rank test was used to determine whether there were significant differences between groups in terms of the survival curves. All P values were two-sided, with P ≤ 0.05 considered significant.
Results
Patient and Treatment Characteristics
Characteristics of all 237 patients are provided in Table 1. A total of 106 patients were treated with RT alone, 69 with sequential chemoradiation and 62 with concurrent chemoradiation. The agents commonly used for chemotherapy were carboplatin, etoposide, paclitaxel, cisplatin and vinorelbine. For sequential chemoradiation, 24 patients were treated with carboplatin and paclitaxel, 19 with cisplatin and vinorelbine, 6 with cisplatin and etoposide and 20 with other regimens. For concurrent chemoradiation, 21 patients were treated with carboplatin and etoposide, 18 with cisplatin and etoposide, 17 with carboplatin and paclitaxel and 6 with other regimens. Thirty-one patients were enrolled in the radiation dose escalation protocol and 206 patients were treated off-protocol. GTV data were retrievable for only 101 patients.
Table 1. Patient, tumor and treatment characteristics and their effects on overall survival (univariate analysis).
| Characteristic | No. of Patients | Median OS (months) | 5 year OS | P value | |
|---|---|---|---|---|---|
| Age | Median | 65 years | |||
| Range | 34 – 89 years | 0.377‡ | |||
| 34-65 years | 121 (51.1%) | 13.6 | 11.9% | ||
| >65 years | 116 (48.9%) | 11.0 | 5.3% | 0.157† | |
| Gender | Male | 170 (71.7%) | 11.3 | 8.2% | |
| Female | 67 (28.3%) | 14.2 | 9.8% | 0.667† | |
| KPS | Median | 80 | |||
| Range | 60 – 100 | 0.029‡ | |||
| < 80 | 11 (4.6%) | 7.2 | 0.0% | ||
| ≥ 80 | 226 (95.4%) | 12.8 | 10.4% | 0.003† | |
| Weight loss ≥ 5% | Yes | 85 (35.9%) | 9.3 | 4.2% | |
| No | 152 (64.1%) | 15.0 | 11.3 | 0.0001† | |
| Smoking history | Yes | 211 (89.0%) | 12.2 | 8.9% | |
| No | 26 (11.0%) | 12.8 | 11.5% | 0.509† | |
| Oxygen use pre-RT | Yes | 19 (8.0%) | 9.6 | 0.0% | |
| No | 218 (92.0%) | 12.8 | 10.1% | 0.093† | |
| Comorbidity score | Median | 2.0 | |||
| Range | 0-10 | 0.245‡ | |||
| 0 - 2.0 | 152 (64.1%) | 14.0 | 11.2% | ||
| > 2 | 85 (35.9) | 10.8 | 5.7% | 0.154† | |
| Pleural effusion | Yes | 52 (21.9%) | 11.1 | 5.1% | |
| No | 185 (78.1%) | 12.8 | 10.6% | 0.181† | |
| Stage | IIIA | 102 (43.0%) | 14.1 | 9.7% | 0.089† |
| IIIB | 135 (57.0%) | 11.1 | 8.8% | ||
| Radiation dose escalation protocol | Yes | 31 (13.1%) | 15.6 | 6.5% | 0.825† |
| No | 206 (86.9%) | 12.1 | 9.7% | ||
| Chemoradiation | Yes | 131 (55.3%) | 15.4 | 13.2% | <0.001† |
| No | 106 (44.7%) | 7.4 | 3.3% | ||
| BED | Median | 72.2 Gy | |||
| Range | 39 – 125 Gy | <0.001‡ | |||
| ≤ 72 | 118 (49.8%) | 10.2 | 7.2% | ||
| > 72 | 119 (50.2%) | 15.6 | 10.2% | 0.018 | |
| GTV* | Median | 187 cm3 | |||
| Range | 1.6 – 1425 cm3 | 0.447‡ | |||
| ≤ 187 cm3 | 51 (50.5%) | 16.4 | 7.4 % | ||
| > 187 cm3 | 50 (49.5%) | 14.7 | 7.0% | 0.619† | |
Abbreviations: KPS = Karnofsky performance score, RT = radiation therapy, BED = bioequivalent dose, GTV = gross tumor volume, OS = overall survival.
GTV data only available in 101 patients.
Log-rank test, categorical covariates or continuous covariate transform to categorical covariates by the median.
Cox-regression analysis, continuous covariate.
The radiation dose for the whole group ranged from 30 Gy in 3 Gy fractions to 102.9 Gy in 2 or 2.1 Gy fractions, with a median dose of 60 Gy in 2 Gy fractions. The median BED was 72.2 Gy (range: 39-124.5 Gy). There were 169 patients treated with definitive radiotherapy and 68 with palliative dose of radiotherapy. The median BED was 78 Gy (95%CI of 77-79 Gy) for patients treated with definitive intent and 50 Gy (95% CI of 47-53Gy) for patients treated with palliative intent. These dose distributions were too narrow to allow further meaningful analysis for these groups individually.
Overall Survival
Median follow-up was 69.0 months (range, 0.2-146). The median OS for the whole group was 12.6 months (95% CI: 10.5-14.7 months, range: 0.8–153.2 months). The two and five-year OS were 22.4% and 10% respectively. Figure 1A shows the OS of the entire group. The median OS for patients treated off-protocol (n = 206) and those within the dose escalation protocol (n = 31) were 12.1 months (95% CI: 9.7-14.5 months, range: 0.8-153.2 months) and 15.6 months (95% CI: 9.6-21.5 months, range: 4.8-107.6 months) respectively; two and five-year OS were 22.3%, 9.7% and 16.1%, 6.5% respectively. There was no significant difference for OS between the protocol and off-protocol groups (P = 0.825).
Figure 1.
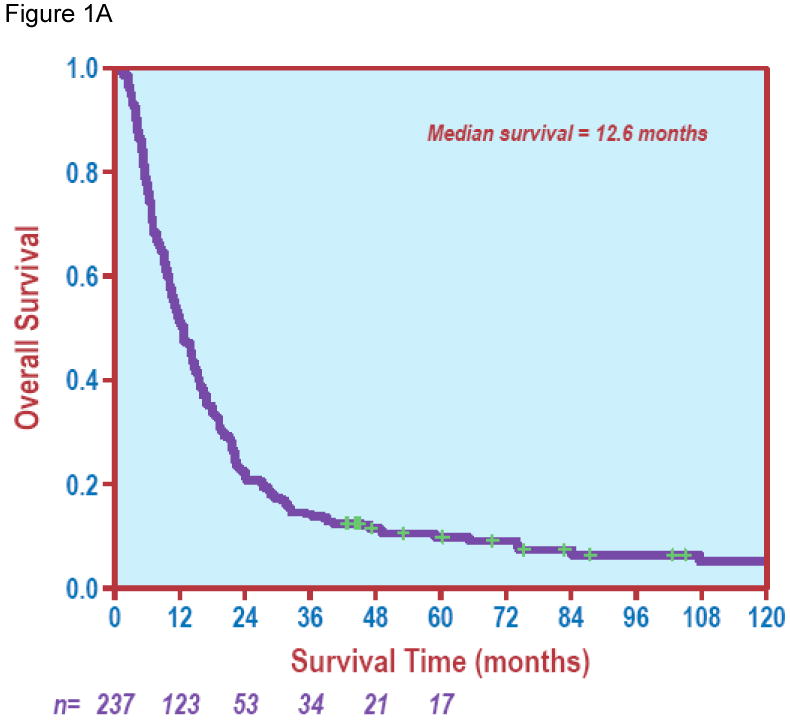
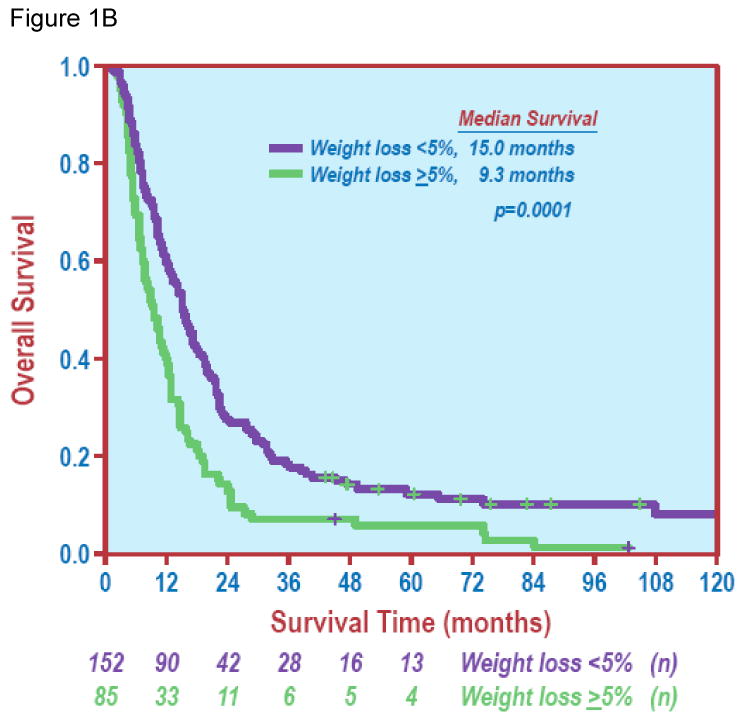
Overall survival. Figure 1A shows overall survival of the entire study group. Figure 1B depicts overall survival curves by weight loss (< 5% vs. ≥ 5%) with a significant difference in survival between the two groups (Log Rank test, P = 0.0001).
Factors Associated with OS - Univariate analysis
The effects of patient characteristics, tumor factors and treatment parameters on OS are shown in Table 1. Univariate analysis demonstrated that higher KPS (HR 0.965, 95% CI: 0.935-0.996, P = 0.029), < 5% of total body weight loss (Figure 1B, P < 0.001), PET scan before treatment (P = 0.044) and use of chemotherapy (Figure 2A, P < 0.001) were significantly associated with improved OS. There were no significant differences in OS based on gender, smoking history, or pre-RT oxygen use (P ≥ 0.093 for all factors). Neither advanced age nor higher co-morbidity score was associated with worse OS (P all ≥ 0.245).
Figure 2.
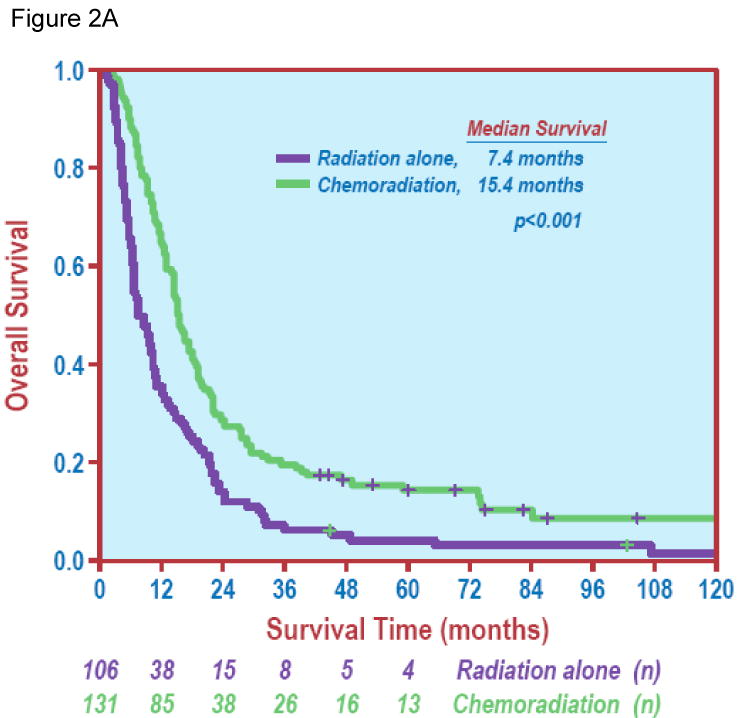
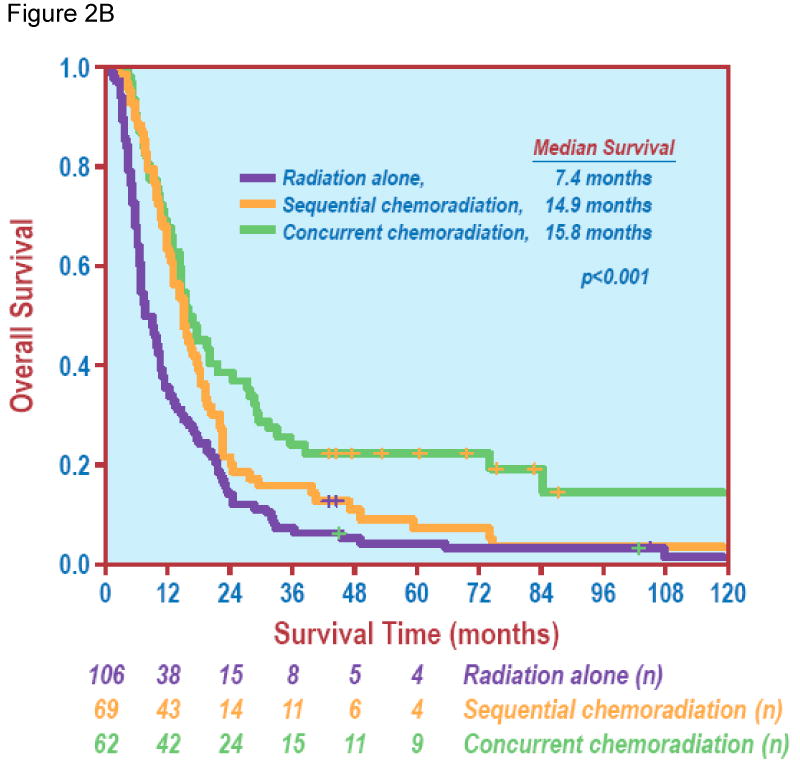
Chemotherapy and overall survival. Figure 2A demonstrates a significant difference in overall survival between radiation alone vs. chemoradiation (Log Rank test, P < 0.001). Figure 2B shows overall survival of patients treated with sequential chemoradiation or concurrent chemoradiation vs. radiation alone (Log Rank test, P < 0.001).
The number of patients who received definitive RT for stage IIIA and IIIB disease were 86 (84.3%) and 83 (61.5%) respectively (P < 0.001). There was a trend for difference in OS between patients with stage IIIA and IIIB disease. The median, two-year, and five-year survivals were 14.1 months (95% CI: 11.7-16.6 months, range: 1.7-129 months), 26.5%, and 9.7% for patients with stage IIIA disease, and 11.1 months (95% CI: 9.2-13.0 months, range: 0.8-153 months), 17.8% and 8.8% for patients with stage IIIB disease (P = 0.089). The presence of a non-malignant pleural effusion was not a significant factor for OS (P = 0.181). GTV was not significantly associated with OS when analyzed as a continuous variable (P = 0.447) or as a categorical variable based on the median value (P = 0.619).
In terms of treatment variables, increasing BED was associated with improved OS (HR 0.972, 95% CI: 0.962-0.981, P < 0.001) in the entire group. The median survival for patients treated with RT alone, sequential chemoradiation and concurrent chemoradiation were 7.4 months (95% CI: 5.3-9.5 months, range: 0.8-152 months), 14.9 months (95% CI: 13.2-16.7 months, range: 2.2-132.2 months) and 15.8 months (95% CI: 11.2-20.4 months, range: 4.2-153.2 months); and five-year OS were 3.3%, 7.5% and 19.4%, respectively (P < 0.001). Figure 2B shows OS in different treatment groups.
Factors Associated with OS – Multivariate analysis
To further evaluate the independent effect of patient characteristics, tumor factors and treatment parameters on OS, a multivariate Cox proportional hazards regression analysis was performed (Table 2). This analysis included those factors with P≤ 0.1 from univariate analysis. As a consequence, KPS, weight loss < 5%, pre-RT oxygen use (yes vs. no), stage IIIA vs. IIIB, PET scan before treatment, addition of chemotherapy, sequence of chemoradiation, and BED were included as covariates in the Cox regression model.
Table 2. Sequential and concurrent chemoradiation compared to radiation alone (multivariate analysis).
| Factors | Estimated HR of death (95% CI) | P value* |
|---|---|---|
| KPS | 0.964 (0.934 – 0.994) | 0.020 |
| Weight loss < 5% | 0.702 (0.524 – 0.939) | 0.017 |
| Pre-RT oxygen use (yes vs. no) | 1.393 (0.824 – 2.354) | 0.215 |
| Stage (IIIA vs. IIIB) | 1.062 (0.799 –1.413) | 0.677 |
| Concurrent CT-RT vs. RT alone | 0.461 (0.322 – 0.662) | < 0.001 |
| Sequential CT-RT vs. RT alone | 0.692 (0.502 – 0.955) | 0.025 |
| BED (Gy) | 0.976 (0.966 – 0.985) | < 0.001 |
Abbreviations: KPS = Karnofsky performance score, RT = radiation therapy, CT = chemotherapy, BED = bioequivalent dose, Gy = Gray, CI = confidence interval, HR = hazard ratio (risk of death).
Multivariate Cox's proportional hazards model.
Higher KPS (P = 0.020) and weight loss < 5% of total body weight (P = 0.017) remained significantly correlated with improved OS on multivariate analysis. However, neither pre-RT oxygen use (P = 0.215), lower disease stage (IIIA vs. IIIB) (P = 0.677) nor PET scan before treatment (P = 0.327) was associated with OS.
Radiation dose remained a significant factor on multivariate analysis. The hazard ratio of BED for death was 0.976 per Gy (95% CI: 0.966-0.985, P < 0.001). The relationship between BED and OS is shown in Figure 3. Figure 3A depicts the BED distribution of different treatment regimens, while Figure 3B shows OS over radiation dose for each patient. Although the narrow BED distribution did not allow stratified analysis within each treatment group, a significant dose-response relationship was seen between BED as a continuous variable and survival (P<0.001).
Figure 3.
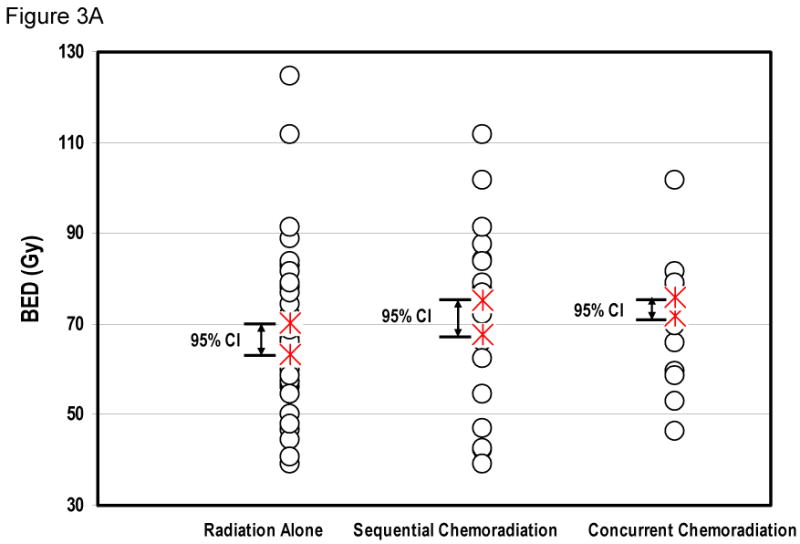
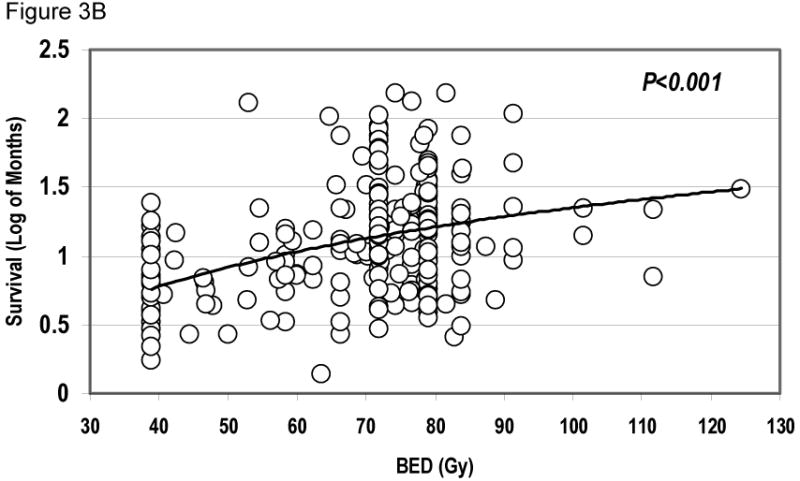
Radiation dose and overall survival. Figure 3A, showing the distribution of BED in patients treated with radiation alone, sequential chemoradiation, or concurrent chemoradiation. Figure 3B plots the relationship of survival over BED for all individual patients, showing that BED was a significant factor for overall survival (Cox-regression analysis, continuous covariate, P < 0.001).
The sequence of chemoradiation remained a significant prognostic factor. Concurrent chemoradiation (P < 0.001) and sequential chemoradiation (P = 0.025) were both associated with significantly better OS than RT alone (Table 2). Further comparing the effects of concurrent and sequential chemoradiation revealed that concurrent chemoradiation was associated with a significantly better prognosis than sequential chemoradiation (P = 0.034).
Interaction between Radiation Dose and Chemotherapy
To investigate whether there was a significant interaction between radiation dose and chemotherapy, the product of BED and chemotherapy (BED*CHEMO) was included as a covariate in the Cox model. No significant interaction was confirmed between BED and chemotherapy (HR = 1.013, 95% CI: 0.993-1.034, P = 0.200) (Table 3). This suggests that radiation dose and chemotherapy were independent factors for OS, and the impact of BED on survival outcomes did not vary by the administration of chemotherapy.
Table 3. Interaction between radiation dose and chemotherapy on overall survival, sequential and concurrent chemoradiation compared to radiation alone.
| Factors | Estimated HR of death (95% CI) | P value* |
|---|---|---|
| KPS | 0.962 (0.933 – 0.993) | 0.016 |
| Weight loss < 5% | 0.717 (0.535 – 0.963) | 0.027 |
| Pre-RT oxygen use (yes vs. no) | 1.466 (0.861 – 2.497) | 0.159 |
| Stage (IIIA vs. IIIB) | 1.079 (0.810 –1.438) | 0.603 |
| Concurrent CT-RT vs. RT alone | 0.180 (0.041 – 0.794) | 0.024 |
| Sequential CT-RT vs. RT alone | 0.277 (0.065 – 1.175) | 0.082 |
| BED (Gy) | 0.970 (0.957 – 0.983) | < 0.001 |
| BED* CHEMO | 1.013 (.993 – 1.034) | 0.200 |
Abbreviations: KPS = Karnofsky performance score, RT = radiation therapy, CT = chemotherapy, BED = bioequivalent dose, Gy = Gray, CI = confidence interval, HR = hazard ratio (hazard of death).
Multivariate Cox's proportional hazards model.
The hazard ratio of BED for death was 0.973 per Gy (95% CI: 0.962-0.985, P < 0.001) in patients treated with RT alone and 0.979 per Gy (95% CI: 0.962-0.996, P = 0.015) in patients treated with chemoradiation.
Discussion
The radiation dose effect in NSCLC has been reported previously, mostly in the context of stage I/II NSCLC (7, 9-11, 17-21). Several retrospective analyses have suggested that doses higher than 60 Gy may improve local disease control and OS (5, 7, 19, 20). Schwegler et al. (21) carried out bronchoscopiec biopsies after radiation and demonstrated the elimination of macro and microscopic tumor in 20.5% of patients after a dose of 60 Gy and 64% of patients after 80 Gy, with improved local relapse–free and OS with the higher dose. The radiation dose effect in stage III NSCLC is relatively limited (7, 22). In 72 patients with GTVs greater than 100 cc treated with radiation alone, Rengan et al. showed that radiation doses of 64 Gy and higher were associated with better survival than doses less than 64 Gy (median survival 20 months vs. 15 months, P = 0.068) (7). Radiation dose was also an independent predictor of local failure-free survival with each 1 Gy increase in dose resulting in a 3.6% decreased risk of local failure. Machtay et al. performed a secondary analysis of 1,290 patients from six RTOG trials utilizing chemoradiation and reported a 1.8% decrease in the risk of death (P < 0.0001) for every 1 Gy increase in BED. The odds ratio for local-regional failure was 0.77 for a10 Gy increase in BED (P < 0.0001) (22). The current analysis demonstrates that higher radiation doses were significantly associated with significantly improved OS in patients with stage III NSCLC treated with RT alone or with chemoradiation. For patients who received RT alone vs. chemoradiation, the hazard ratio was 0.973 vs. 0.979, respectively, per 1 Gy increase in BED (P < 0.001). Thus, higher radiation doses may improve OS not only for patients with stage III NSCLC treated with RT alone, but also for those treated with chemoradiation.
Although it has been established that the standard of care for locally advanced NSCLC is a combination of chemotherapy and radiation, the optimal strategy in terms of timing of chemotherapy and radiation continues to be investigated. There have been a few randomized studies which demonstrated that concurrent, as opposed to sequential, administration of chemotherapy and RT leads to a survival advantage (2, 3, 23, 24-26). The most common practice in the United States is concurrent chemoradiation (27). The supportive evidence of this practice, however, is not extensive. The phase II randomized LAMP trial showed that concurrent chemoradiation followed by adjuvant chemotherapy resulted in a median survival of 16.3 months compared to 13.0 months for sequential chemoradiation and 12.7 months for, induction chemotherapy followed by concurrent chemoradiation (23). The only phase III trial from the United States evaluating the timing of chemoradiotherapy is RTOG 9410, which has only been published in abstract form (3). This trial reported a median OS of 17 months in patients treated with concurrent chemotherapy with once daily radiation compared to 14.6 months in those treated with sequential chemoradiotherapy (P = 0.046). In a large number of patients treated outside of a clinical trial, the current analysis confirms the superiority of concurrent chemoradiation over sequential chemoradiation.
The role of RT dose escalation in locally advanced NSCLC continues to be researched. Important questions remain to be answered about the potential benefits and risks of the use of higher RT doses in combination with concurrent chemotherapy. Similarly, the potential benefits of chemotherapy remain unclear in patients treated with higher doses of RT. In an attempt to address these issues, this analysis evaluated the product of BED and chemotherapy (BED*CHEMO) as a covariate in the Cox model to determine whether or not there was a significant interaction between RT dose and chemotherapy. The analysis showed no significant interaction between RT dose and chemotherapy suggesting that the impact of BED on survival did not vary with the use of chemotherapy. Thus, patients receiving chemotherapy may benefit from high dose RT and among those treated with high dose RT, chemotherapy may still be beneficial.
Many previous studies demonstrated that KPS and weight loss are significant factors affecting OS in patients with NSCLC (28, 29). The current study confirmed these findings with higher KPS (P = 0.020) and weight loss < 5% of total body weight (P = 0.017) were being significantly correlated with improved OS. The current study, however, failed to show a significant prognostic effect for age, gender, smoking history, pre-RT oxygen use, or co-morbidity score.
This analysis has limitations. It is a retrospective study which carries all of the weakness inherent in such an analysis. Considering the presence of many confounding factors, the number of cases was relatively small, so the study may be underpowered to detect relatively small differences in outcome. Because the range of radiation doses was rather narrow for all three groups of patients, the radiation dose effect should be interpreted with caution. The dose effect for patients treated with definitive intent of concurrent chemotherapy is not answered due to the relatively narrow dose-range given to these patients, Due to missing data points, no strong conclusions can be drawn regarding the overall effect of GTV. Non-uniform clinical conditions and the possible presence of other confounding factors, such as the inclusion of patients treated with palliative intent, may have obscured the effects of chemotherapy and RT on survival, though performance status and comorbidity scores were also considered during the analysis.
In summary, this analysis suggests that a higher radiation dose, the addition of chemotherapy and the use of concurrent chemoradiation are independent factors associated with improved OS in patients with stage III NSCLC. There was no interaction between the use of chemotherapy and the dose of radiation, suggesting the potential importance of both high-dose radiation and the use of chemotherapy. Future prospective studies are needed to establish optimal radiation doses both alone and in the setting of chemoradiation, RTOG 617 was recently activated to study the dose effect in patients with stage III NSCLC treated with concurrent chemoradiation.
Acknowledgments
This work was partially supported by the Pardee Foundation and Career Developmental Award from American Society of Clinical Oncology. We express our gratitude to all our patients. We also thank Brady T. West, the statistician lead of Center for Statistical Consultation and Research (CSCAR) for his assistance on statistical analysis.
Footnotes
The study has been presented in 49th ASTRO, October 30, 2007, Los Angeles as an oral presentation. It has been regarded as *Best of ASTRO 2007* and was asked to be quoted in 3rd Annual Mid-Atlantic ASTRO Review Course on January 10, 2008, the University of Maryland*s.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Furuse K, Fukuoka M, Kawakara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ, Scott CB, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential vs. concurrent chemo-radiation for patients with unresectable stage III NSCLC: RTOG 9410. Proc ASCO. 2003;22:621A. abstr 2449. [Google Scholar]
- 4.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various radiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–2753. doi: 10.1002/1097-0142(19800601)45:11<2744::aid-cncr2820451108>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Kupelian PA, Komaki R, Allen P. Prognostic factors in the treatment of node-negative nonsmall cell lung carcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1996;36:607–613. doi: 10.1016/s0360-3016(96)00364-1. [DOI] [PubMed] [Google Scholar]
- 6.Sibley GS, Jamieson TA, Marks LB, et al. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys. 1998;40:149–154. doi: 10.1016/s0360-3016(97)00589-0. [DOI] [PubMed] [Google Scholar]
- 7.Rengan R, Rosenzweig KE, Venkatraman E, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:741–747. doi: 10.1016/j.ijrobp.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118–2127. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 9.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Hayman JA, Ten Haken RK, et al. Long-term results of high-dose conformal radiotherapy for patients with medically inoperable T1–3N0 non-small-cell lung cancer: is low incidence of regional failure due to incidental nodal Radiation? Int J Radiat Oncol Biol Phys. 2006;64:120–126. doi: 10.1016/j.ijrobp.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, West BT, Hayman JA, et al. High radiation dose may reduce the negative effect of large gross tumor volume in patients with medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:103–10. doi: 10.1016/j.ijrobp.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 12.Belderbos JS, De Jaeger K, Heemsbergen WD, et al. First results of a phase I/II dose escalation trial in non-small cell lung cancer using three-dimensional conformal radiotherapy. Radiother Oncol. 2003;66:119–26. doi: 10.1016/s0167-8140(02)00377-8. [DOI] [PubMed] [Google Scholar]
- 13.Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma. Cancer. 2001;92:1213–1223. doi: 10.1002/1097-0142(20010901)92:5<1213::aid-cncr1440>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Rosenman JG, Halle JS, Socinski MA, et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2002;54:348–356. doi: 10.1016/s0360-3016(02)02958-9. [DOI] [PubMed] [Google Scholar]
- 15.Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non–small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004;22:4341–4350. doi: 10.1200/JCO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Perez CA, Bauer M, Edelstein S, et al. Impact of tumor control on survival in carcinoma of the lung treated with irradiation. Int J Radiat Oncol Biol Phys. 1986;12:539–547. doi: 10.1016/0360-3016(86)90061-1. [DOI] [PubMed] [Google Scholar]
- 18.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer. 1987;59:1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Willner J, Baier K, Caragiani E, et al. Dose, volume, and tumor control prediction in primary radiotherapy of nonsmall-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:382–389. doi: 10.1016/s0360-3016(01)01823-5. [DOI] [PubMed] [Google Scholar]
- 20.Bradley JD, Ieumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for nonsmall-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:49–57. doi: 10.1016/s0360-3016(01)01772-2. [DOI] [PubMed] [Google Scholar]
- 21.Schwegler N, Gordic J, Ragaz A, et al. Inoperable non-small cell bronchial carcinoma: macroscopic and microscopic tumor behavior during and after radiotherapy with curative intent. Schweiz Rundsch Med Prax. 1999;88:1825–1836. [PubMed] [Google Scholar]
- 22.Machtay M, Swann S, Komaki R, et al. Higher BED is associated with improved local-regional control and survival for NSCLC treated with chemoradiotherapy: An RTOG Analysis. Int J Radiat Oncol Biol Phys. 2005;63(1):S40. abstr 66. [Google Scholar]
- 23.Belani PC, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 24.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemo-radiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 25.Zatloukal P, Peruzelka L, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized phase II study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Huber RM, Flentje M, Schmidt M, et al. Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non small-cell lung cancer: study CTRT 99/97 by the Bronchial Carcinoma Therapy Group. J Clin Oncol. 2006;24:4397–4404. doi: 10.1200/JCO.2005.05.4163. [DOI] [PubMed] [Google Scholar]
- 27.Vokes EE. Optimal therapy for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2005;23:5853–5. doi: 10.1200/JCO.2005.95.028. [DOI] [PubMed] [Google Scholar]
- 28.Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 29.Firat S, Byhardt RW, Gore E. Comorbidity and Karnofsky performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated under RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54:357–364. doi: 10.1016/s0360-3016(02)02939-5. [DOI] [PubMed] [Google Scholar]


