Abstract
Purpose
This study aimed to (1) examine changes in dyspnea, global pulmonary function test (PFT) results, and functional activity on ventilation (V)/perfusion (Q) single-photon emission computerized tomography (SPECT) scans during the course of radiation (RT), and (2) factors associated with the changes in patients with non-small-cell lung cancer (NSCLC).
Methods and Materials
Fifty-six stage I to III NSCLC patients treated with definitive RT with or without chemotherapy were enrolled prospectively. Dyspnea was graded according to Common Terminology Criteria for Adverse Events version 3.0 prior to and weekly during RT. V/Q SPECT-computed tomography (CT) and PFTs were performed prior to and during RT at approximately 45 Gy. Functions of V and Q activities were assessed using a semiquantitative scoring of SPECT images.
Results
Breathing improved significantly at the third week (mean dyspnea grade, 0.8 vs. 0.6; paired t-test p = 0.011) and worsened during the later course of RT (p > 0.05). Global PFT results did not change significantly, while regional lung function on V/Q SPECT improved significantly after ~45 Gy. The V defect score (DS) was 4.9 pre-RT versus 4.3 during RT (p = 0.01); Q DS was 4.3 pre-RT versus 4.0 during RT (p < 0.01). Improvements in V and Q functions were seen primarily in the ipsilateral lung (V DS, 1.9 pre-RT versus 1.4 during RT, p < 0.01; Q DS, 1.7 pre-RT versus 1.5 during RT, p < 0.01). Baseline primary tumor volume was significantly correlated with pre-RT V/Q DS (p < 0.01). Patients with central lung tumors had greater interval changes in V and Q than those with more peripheral tumors (p < 0.05 for both V and Q DS).
Conclusions
Regional ventilation and perfusion improved during RT at 45 Gy. This suggests that adaptive planning based on V/Q SPECT during RT may allow sparing of functionally recoverable lung tissue.
Keywords: Non-small-cell lung cancer, Perfusion, Radiotherapy, Single-photon emission computerized tomography, Ventilation
Introduction
Lung cancer is the leading cause of cancer death (1). Approximately, 80% to 85% of lung cancer cases are non-small-cell lung cancer (NSCLC). Of these cases, over 60% of patients require radiation therapy (RT) during the course of the disease (2). Thoracic RT can damage lung tissues and result in reduction of pulmonary function (3, 4). Global pulmonary function tests (PFTs) are commonly used at baseline to assess RT tolerance. Significant comorbidities, especially smoking-related conditions such as chronic obstructive pulmonary disease (COPD), may increase overall treatment risk (5). Definitive RT or RT dose escalation is often considered unsafe for patients with poor lung function, and many patients are excluded from receiving definitive RT or dose escalation. However, lung cancer often causes bronchial obstruction leading to atelectasis and or large vessel compression, which effects regional lung ventilation (V) and/or perfusion (Q). When tumor-induced bronchial or pulmonary artery compression results in functional defect, tumor regression from RT may lead to regional lung reperfusion and/or recovery of ventilation because of partial or complete relief of obstructions. How the dynamics of radiation-induced lung damage and tumor regression from radiation treatment on V/Q single-photon emission computed tomography (SPECT) is unknown.
Lung Q SPECT scans obtained at baseline have been used to guide radiation planning for functional region avoidance (5–12). Recently, ventilation scans generated from four-dimensional computed tomography (CT) were applied for functional avoidance in RT planning (13), although this has yet to be validated. We have recently studied V and Q defects at baseline using SPECT, analyzed for potential causes and classified as to potential for recovery and applications in RT planning. We demonstrated that V SPECT provided additional value in some patients over that of Q SPECT alone in the assessment of local pulmonary function and application in guiding RT (16). However, it is unknown whether local lung function actually changes during RT; SPECT-guided RT plans based on pre-RT SPECT may actually target functional regions as conditions change during the course of treatment (14). Considering that the tumor shrinks during the course of RT (15), in this study, we hypothesized that global and regional pulmonary functions change during RT, as measured by dyspnea grade, PFT results, and local-regional functions on V/Q SPECT. Additionally, we investigated factors associated with the change during the course of therapy in patients with NSCLC.
Methods and Materials
Patient population
The study population included patients enrolled in institutional review board-approved prospective studies (UMCC 2003-0376 and UMCC 2006-040) between 2006 and 2009. Written informed consent was obtained from all subjects before enrollment. Patients were treated with conventionally fractionated three-dimension conformal RT doses of ≥60 Gy, with or without chemotherapy based on stage of disease and medical condition. PFTs, V/Q SPECT-CT scans, and fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT) studies were performed within 2 weeks before the initiation of RT and during RT at approximately 45 Gy (during RT).
Dyspnea and pulmonary function tests
Dyspnea was diagnosed and graded according to National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events version 3.0 immediately prior to and weekly during the course of fractionated RT. PFTs were administered using Jaeger Masterlab equipment (Würzburg, Germany). In this study, we evaluated the forced expiratory volume at 1 sec (FEV1), forced vital capacity (FVC), and diffusing capacity of the lung for carbon monoxide (DLCO). Outcomes of these measurements were expressed as a percentage of the normal value (determined by weight, height, and gender). Changes in PFTs during RT were assessed as the ratio of results during RT minus those pre-RT divided by pre-RT. V/Q SPECT-CT, FDG-PET/CT, and global PFTs were performed during the week before RT (pre-RT) and around the time tumor dose delivery (during RT) was 45 Gy.
SPECT-CT imaging and analysis
SPECT images were acquired as previously described (16). Fifteen patients from study UMCC2003-0376 underwent SPECT alone; all remaining patients underwent SPECT-CT. For patients with CT scans, SPECT images coregistered with low-dose-X-ray CT scans were obtained with the patient in the same position. Pulmonary V/Q SPECT functions were measured by a semiquantitative score. The largest localized V/Q defect scores (DS) in each lung on SPECT were assessed as 0 = no defect or 1, 2, 3, or 4, which represented <25%, 25% to 49%, 50% to 74%, or 75% to 100%, respectively, of one lung. The remaining lung DSs as seen on SPECT images were assessed as 1 = homogeneous, or 2, 3, or 4, which represented mild, moderate, or marked heterogeneity, respectively. Total lung function score = ipsilateral lung DS + contralateral lung DS + remaining lung DS ranging from 1 to 12 (11).
Central tumors were defined as ≤2 cm from the proximal bronchial tree, carina, main bronchus, and lobe bronchus. Peripheral tumors were defined as >2 cm from this location. In cases where tumors were detected in both central and peripheral locations, the tumor with the largest lesion was used to define localization.
To assess the impact of V/Q SPECT on adaptive radiation planning during RT, lung regions were further classified as the following (16): region A, tumor; region B1, complete functional loss induced by COPD or other unrecoverable diseases; region B2, reduced lung function induced by unrecoverable diseases; region B3, temporarily dysfunctional lung caused by tumor-induced constriction and other potentially recoverable diseases; and region C, normally functioning lung. Coregistered V/Q-SPECT scans were interpreted by experienced physicians, together with comprehensive analysis of CT anatomy findings, PET/CT findings, and other clinical data. For example, a defect shaped as segments or a lobe located distal to a centrally located tumor was classified as B3, while a region with reduced function in the contralateral lung was most likely from an unrecoverable B2 region.
Statistical analysis
Values at different time points were compared using a nonparametric test or paired t-test. Pearson correlations were performed for correlation analysis. p values of <0.05 were considered significant. Analyses were performed using Statistical Package for Social Sciences version 13.0 software.
Results
Study population
Fifty-six stage I to III NSCLC patients (45 men, 11 women; 51–87 years old) considered definitive RT candidates were eligible for enrollment (characteristics are given in Table 1). Twenty-one patients had medically inoperable stage I to II disease, and the other 35 patients had locally advanced stage IIIA to -B disease. Twenty-six patients had central primary tumors, and 30 patients had peripheral primary tumors. Twenty-six patients had COPD.
Table 1.
Patient characteristics
| Variable | No. of patients |
|---|---|
| Gender | |
| Male | 45 |
| Female | 11 |
| Age | |
| ≤70 | 26 |
| >70 | 30 |
| History of COPD | |
| Yes | 26 |
| No | 30 |
| History of smoking | |
| Yes | 44 |
| No | 12 |
| T stage | |
| T1 | 13 |
| T2 | 11 |
| T3 | 18 |
| T4 | 14 |
| Clinical stage | |
| I | 11 |
| II | 10 |
| III | 35 |
| Tumor location | |
| Central type | 26 |
| Peripheral type | 30 |
| “During-RT” time point dose (Gy) | |
| Mean tumor dose (95% CI) | 45 (42.8–46.5) |
| Mean lung dose (95% CI) | 9.1 (8.2–9.9) |
| Concurrent chemotherapy | |
| Yes | 39 |
| No | 17 |
Abbreviations: CI = confident interval; COPD = chronic obstructive pulmonary disease; RT = radiation therapy.
Changes in dyspnea and global PFT results during RT
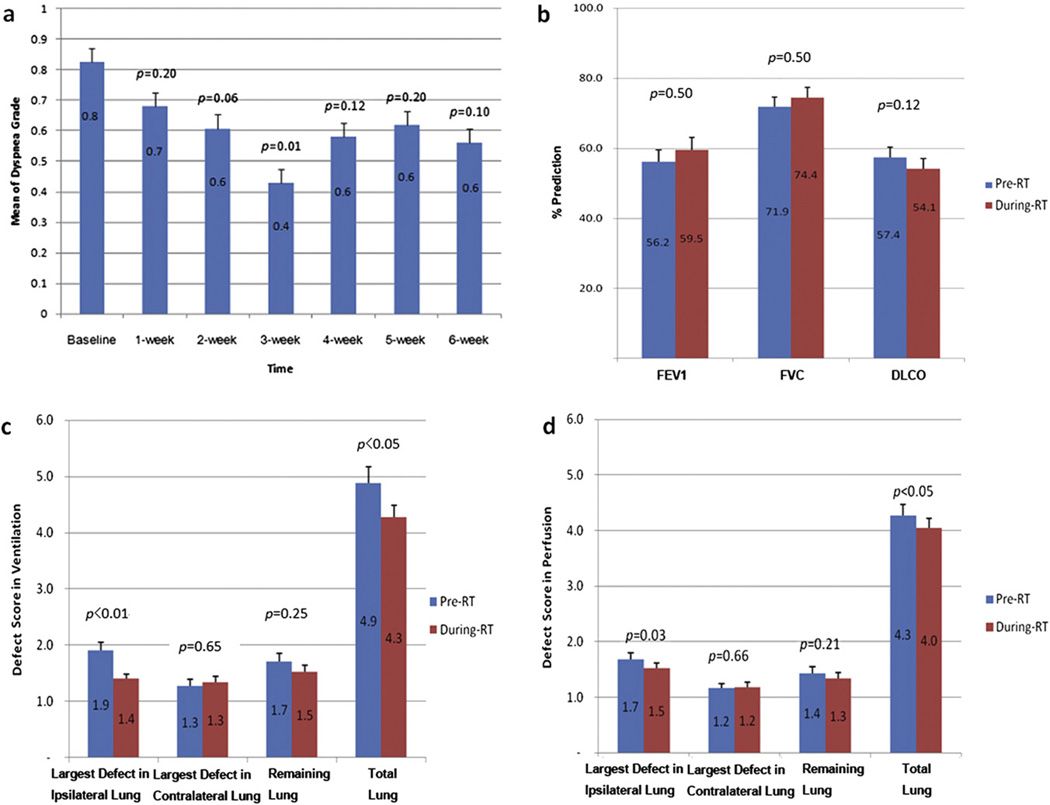
Changes in dyspnea and PFT results during RT are presented in Figure 1a and b. Compared to baseline values, the grade of dyspnea decreased (breathing improved) significantly by the third week during RT (pre-RT, 0.8 ± 0.1, vs. 0.6 ± 0.1 during RT, p = 0.01) but did not change significantly during the remaining course of RT (p > 0.05). No significant changes were observed during RT in individual PFT parameters (FEV1 pre-RT, 56.2% ± 3.3%, vs. 59.5% ± 3.5% during RT, p = 0.50; FVC, 71.9% ± 2.5%, versus 74.4% during RT, p = 0.50; and DLCO, 57.4% vs. 54.1% ± 2.8% during RT, p = 0.12). Changes in PFT results during RT were remarkably heterogeneous. For example, DLCO values remained stable for 47% of patients (±10% of baseline level), increased for 20% of patients (more than 10% elevation), and decreased for 30% of patients (more than 10% reduction).
Fig. 1.
Changes in lung functions during RT are compared to baseline values. Mean dyspnea grade decreased (breathing improved) gradually and became significant by 3 weeks during RT (a). Dyspnea was graded according to Common Terminology Criteria for Adverse Events version 3.0. Compared to baseline values, mean levels of FEV1, FVC, and DLCO during RT were not statistically different (b). For regional lung functions, DSs in ventilation (c) and perfusion (d) seen on SPECT decreased (lung function improved) significantly. Improvements are seen mostly in the ipsilateral lung; there were no significant changes in the contralateral lung.
Changes in V/Q DS on SPECT during RT
V and Q DSs of total lung decreased (lung function improved) significantly after 45-Gy RT (V DS pre-RT, 4.9 ± 0.3, vs. 4.3 ± 0.2 during RT, p = 0.03; Q DS pre-RT, 4.3 ± 0.2 vs. 4.0 ± 0.2 during RT, p < 0.05). Improvements were seen mostly in the ipsilateral lung (V DS pre-RT, 1.9 ± 0.1 vs. 1.4 ± 0.1 during RT, p < 0.01; Q DS pre-RT, 1.7 ± 0.1 vs. 1.5 ± 0.1 during RT, p = 0.03). There were no significant changes in V or Q DSs in the contralateral lung (p > 0.05) (Fig. 1c and d).
Factors associated with V/Q DSs during RT
Tumor volume was significantly associated with changes in lung function on SPECT. Mean primary tumor volume decreased significantly by the time 45-Gy RT was reached (72.3 cm3 pre-RT vs. 22.3 cm3 during RT, p < 0.01). Nodal volume also decreased significantly (pre-RT, 27.2 cm3, vs. 9.8 cm3 during RT, p = 0.02). Primary tumor volume at baseline was significantly associated with both V (r = 0.4, p = 0.02) and Q (r = 0.5, p < 0.01) DSs. Primary tumor volume during RT was not associated with V (r = −0.1, p = 0.58) or Q (r = 0.1, p = 0.76) DS during RT. Changes in primary tumor volume were not associated with changes in V (r = −0.1, p = 0.49) or Q (r = −0.1, p = 0.72) DS from baseline to during RT.
Baseline nodal volume was not associated with baseline V (r = −0.3, p = 0.21) or Q (r = 0.1, p = 0.68) DS. Nodal volume during RT was not a significant factor of V (r = 0.4, p = 0.16) or Q (r = 0.1, p = 0.62) DS. Change in nodal volume during RT was associated with changes in Q DS during RT (r = 0.7, p < 0.01), although it was not associated with a change in V DS (r = −0.0, p = 0.92).
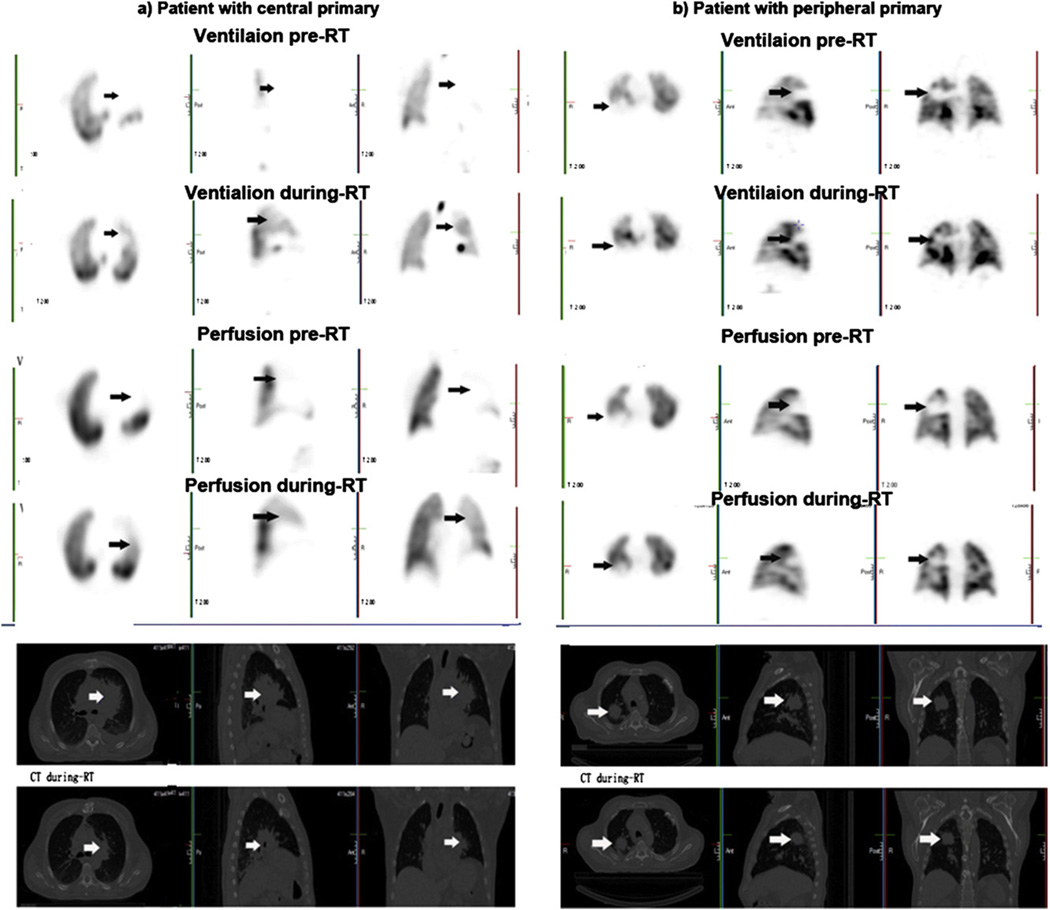
Tumor location was associated with PFT result levels and their changes during RT. Pulmonary functions of patients with central tumors appeared to be worse than those of patients with peripheral tumors, in all parameters, particularly for DLCO pre-RT (49.8% ± 3.16% vs. 66.5% ± 4.9%, respectively, p = 0.01), ipsilateral lung V DS (2.2 ± 0.2 vs. 1.7 ± 0.1, respectively, p = 0.02), and Q DS (2.1 ± 0.2 vs. 1.4 ± 0.1, respectively, p < 0.01). Ipsilateral lung functions of patients with central type tumors improved in V function (DS, 2.3 ± 0.2 versus 1.5 ± 0.1, p = 0.01) and Q function (DS 2.1 ± 0.1 vs. 1.8 ± 0.1, p = 0.01) during RT. Peripheral type tumors did not change significantly during RT in either V DS (1.6 ± 0.1 vs.1.3 ± 0.1, p = 0.06) or Q DS (1.3 ± 0.1 vs. 1.3 ± 0.1, p = 0.67). Neither V DS nor Q DS changed significantly during RT in contralateral lungs in any type of tumors. Overall, patients with central tumors had significantly greater interval changes in the whole lung than those with peripheral tumors for both V DS (−1.0 ± 0.3 vs. −0.4 ± 0.1, respectively, p = 0.03) and Q DS (−0.4 ± 0.1 vs. 0 ± 0.1, respectively, p < 0.01) (Fig. 2.).
Fig. 2.
Tumor location and V/Q SPECT-CT scans are shown during RT. Above pictures show V and Q SPECT scans from patients with central and peripheral tumors. Centrally located tumors had greater changes at 45 Gy during RT.
COPD was associated with local-regional lung function. Pre-RT pulmonary functions of COPD patients were worse than those of non-COPD patients. Mean pre-RT FEV1 was significantly different between COPD and non-COPD patients (49.4 % ± 3.6% vs.64.0 % ± 5.4%, respectively, p = 0.03). The difference in FEV1 between COPD and non-COPD patients was marginally significant during RT (53.2% ± 0.4.1% vs. 66.3% ± 0.5.5%, respectively, p = 0.06). There were no significant differences between the FVC and DLCO parameters of COPD patients and those of non-COPD patients (p > 0.05). There were no significant differences between the mean changes in PFT results and Vand Q DSs of patients without COPD and those of patients with COPD during RT (p > 0.05). The changes in PFT results were not significantly associated with the stage of COPD. There were no significant differences in interval changes when patients were analyzed for gender, age, and clinical stage.
Pulmonary functional mapping on V/Q SPECT during RT and its underlying mechanism
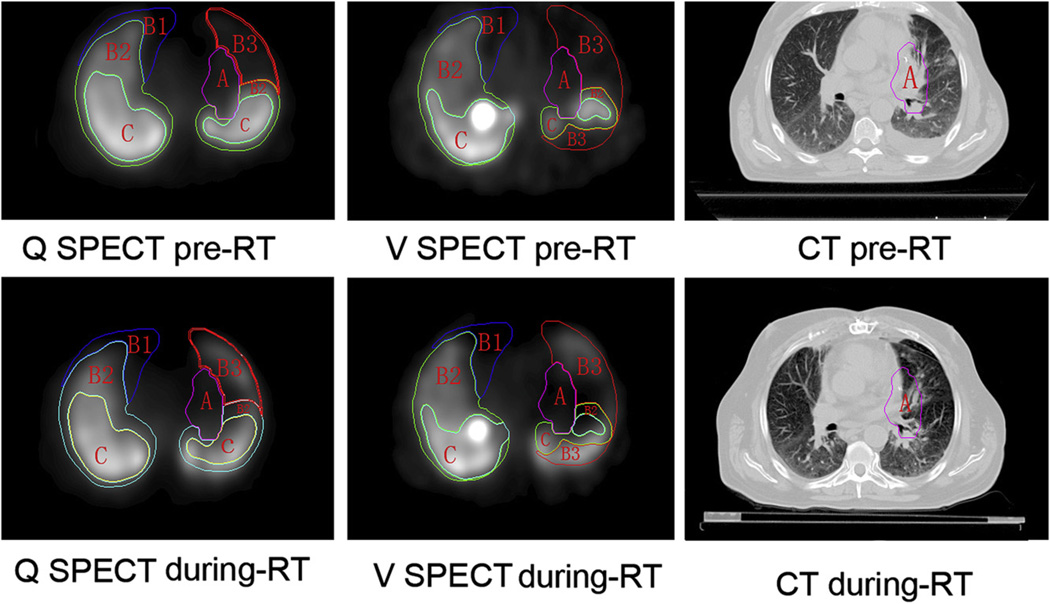
Regional V/Q functional mapping changed in most cases by the time a mean dose of 45 Gy had been delivered (Table 2 and Fig. 3). Such changes varied due to underlying mechanisms.
Table 2.
Changes in pulmonary function in specific regions during RT
| Changes in specific regions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ventilation | Perfusion | |||||||
| Region type |
Total no. of patients |
Improved no. of patients/total no. (%) |
Stable no. of patients/total no. (%) |
Worsened no. of patients/total no. (%) |
Total no. of patients |
Improved no. of patients/total no.(%) |
Stable no. of patients/total no. (%) |
Worsened no. of patients/total no. (%) |
| A | 56 | 38/56 (67.9) | 15/56 (26.8) | 3/56 (5.3) | 56 | 38/56 (67.9) | 15/56 (26.8) | 3/56 (5.3) |
| B1 | 34 | 0 | 34/34 (100) | 0 | 29 | 0 | 29/29 (100) | 0 |
| B2 | 56 | 0 | 54/56 (96.4) | 2/56 (3.6) | 56 | 0 | 54 (96.4) | 2 (3.6) |
| B3 | 43 | 22/43 (51.2) | 17/43 (39.5) | 4/43 (9.3) | 40 | 22/40 (55) | 19/40 (47.5) | 2/40 (5.0) |
| C | 56 | 0 | 44/56 (78.6) | 12/56 (21.4) | 56 | 0 | 43/56 (76.8) | 13/56 (23.2) |
Abbreviations: Type A region = functional defects corresponding to the location of tumor. Type B1 region = complete function defect induced by COPD or other unrecoverable diseases. Type B2 region = reduced lung function induced by unrecoverable diseases (e.g., COPD) or other pathologic conditions. Type B3 region = temporarily dysfunctional lung induced by tumor and other potentially recoverable diseases. Type C region = normal functioning “good” lung.
Compared to the baseline volume, improved = defect volume decrease of >25%; stable = defect volume change of ≤25%; and worsened = defect volume increase of >25%.
Fig. 3.
Lung functional map is shown on SPECT-CT during RT. Scans show changes in functional mapping on SPECT according to underlying causes and their potential impact for radiation planning. Type B3 defects partially recovered, while B1 and B2 defects did not change significantly during RT. The defect classification has been previously described (16). Briefly the A region consists of functional defects corresponding to the location of tumor. The B1 region represents complete function defect induced by COPD or other unrecoverable diseases. The B2 region is reduced lung function induced by unrecoverable diseases. B3 region consists of temporarily dysfunctional lung induced by tumor and other potentially recoverable diseases. The C region is normally functioning lung.
Based on the functional classifications for the purpose of RT that we previously described (16), all patients had type A defects, defined by the location of tumors on the pre-RT scan. Most patients, 67.9% (38/56), improved (defect volume decreased), 26.8% of patients (15/56) remained stable, and 5.3% of patients (3/56) became worse on both V and Q SPECT during RT.
B1 regions, observed in 60.7% of patients (34/56) on V SPECT and 48.2% (29/56) patients on Q SPECT did not change remarkably during RT. B2 regions, observed in all patients in both Vand Q SPECT, remained stable in 96.4% of patients (54/56) and worsened in 3.6% of patients (2/56). B3 regions on V SPECT, observed in 76.8% of patients (43/56) partially or completely recovered to normal levels of function in 22/43 patients (51.2%), remained stable in 17/43 patients (39.5%), and worsenedin 4/43 patients (9.3%). B3 regions on Q SPECT were observed in 71.4% of patients (40/56); 55% of patients (22/40) partially or completely recovered to normal function levels, while 47.5% of patients (19/40) remained stable, and 5% of patients (2/40) got worse. In C regions, 78.6% of patients remained stable, and 21.4% of those patients worsenedon V SPECT; and 76.8% of them remained stable, and 23.2% of them worsened on Q SPECT.
Discussion
This study demonstrated significant improvement in pulmonary function as assessed by dyspnea grade during RT and remarkable regional V/Q SPECT by the time a mean dose of 45 Gy was delivered. Regional V and Q improvements were mostly in the ipsilateral lung in patients with central tumors, which may be due to tumor volume reduction. Additionally, regional V or Q functional mapping changed remarkably during RT and remarkable changes were seen in B3 regions. An adaptive plan based on V/Q SPECT during RT may better spare the functioning lung.
To our knowledge, this is the first study to examine a full spectrum of pulmonary function from laboratory measurement of global PFTs to local regional functional mapping on V/Q SPECT to clinical symptoms of dyspnea during the course of RT in patients with NSCLC. Improvement as seen on V/Q SPECT were statistically significant but not for dyspnea grades, suggesting that V/Q SPECT DSs may be more sensitive than dyspnea grade in detecting pulmonary function changes during RT. Conventionally, investigators focused on post-RT pulmonary function changes, and dose-dependent pulmonary function reductions were observed in most studies. Borst et al. (3) reported post-RT global pulmonary function loss in 34 patients with inoperable NSCLC and no recovery until 18 months. Zhang et al. (17) reported overall dose-dependent reductions in Q valuesat post-RT up to 12 years on SPECT in 123 patients. However, regional differences in dose response curves were observed between ipsilateral and contralateral lungs. Tumor shrinkage appears to confound the observed dose-response relationships. For patients with central mass/positive nodes, increased lung perfusion was noted at 3 and 6 months after RT in low-dose regions. These reports of lung function changes post-RT were consistent with our findings of functional improvement during the course of RT.
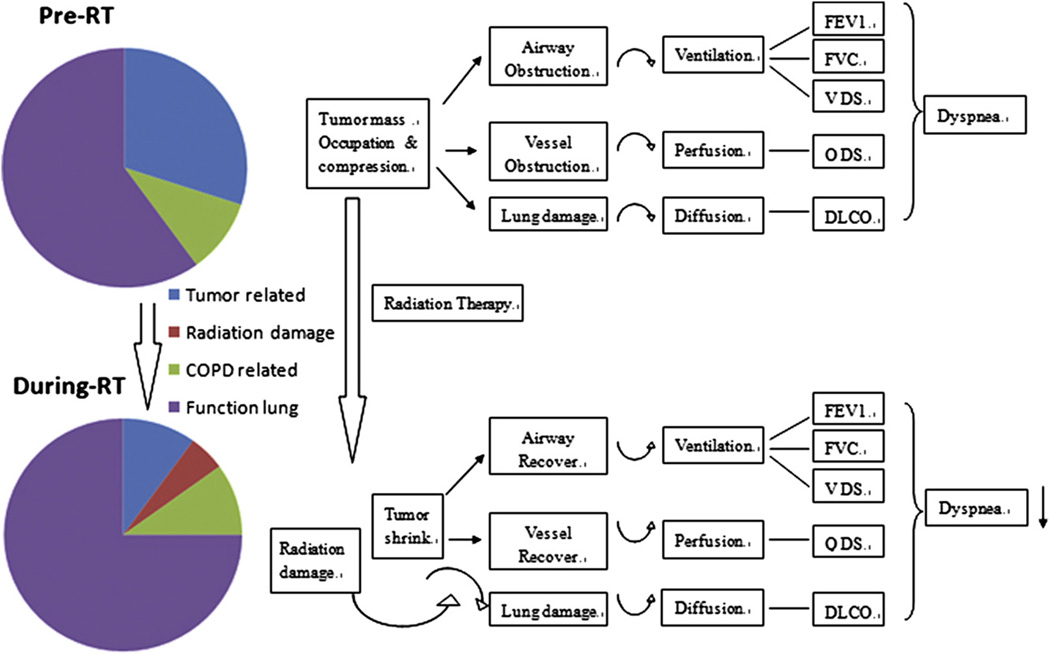
Tumor response during RT may directly contribute to pulmonary function improvement. We reported that tumor FDG-PET/CT activity decreased at 40 to 50 Gy during RT (18) and demonstrated remarkable tumor volume reduction during RT (19). RT-induced tumor volume reduction may cause V /Q recovery because tumor shrinkage reduces pressure on the central airway and blood vessels. In this series of patients, significant tumor volume reductions during RT were observed. There was significant correlation between baseline tumor volume and pre-RT ipsilateral V and Q functions. However, primary tumor volume was not associated withV and Q functions during RT; and tumor volume reduction was not significantly correlated with Q and V changes during RT. This is most likely due to the confounding effect of tumor location (central tumors changed more than peripheral tumors), which is a more significant factor for regional function recovery. This suggests that V/Q recovery may result mainly from decompression of vessels and airways, and further tumor volume reduction cannot induce V/Q function improvements when vessels and airways are already functioning under normal conditions. Recovery of V and Q functions during RT result from decompression due to shrinkage of centrally located tumors. In summary, changes in global and regional pulmonary function are complicated; Figure 4 provides a simplified illustration of the involved dynamics. Tumor-related functional defects were less on SPECT during RT than pre-RT. We were not able to visually detect radiation-induced function reduction or complete defect during RT.
Fig. 4.
Schematic illustrates pulmonary function changes during RT.
Interval changes in V and Q during RT have clinical significance. V/Q SPECT images, particularly pre-RT Q SPECT scans have been applied to guide treatment planning of RT for lung cancer to minimize dose to functional lung (5–11). Significant V/Q SPECT changes during RT suggest the value of obtaining V/Q SPECT scans to reoptimize treatment plans, especially in patients with central tumors. V/Q functional mapping based on our recently proposed classification on pre-RT V/Q SPECT may guide the potential applications of SPECT on RT planning, based on regional function level, cause, and potentially recoverability (16). SPECT may provide an opportunity for RT plan optimization by the use of the following principle: the type A tumor occupies a lung region given as high an RT dose as possible; type B1 regions, with unrecoverable nonfunctioning “bad” lung, can be given high-dose RT without causing changes in global lung function; B2 regions, with unrecoverable low-functioning lung, may be given high doses (if no worse lung is available) without causing remarkable changes in global lung function; type B3 should be spared whenever possible and may be given high-dose RT if it remains nonfunctioning during RT SPECT; the RT dose to type C regions should be minimized to decrease functionally or clinically significant complications.
By further studying functional mapping on V/Q scans during RT, the current study validated, to some degree, our previous functional classification. Type B3 regions, potentially recoverable, were observed in 71.4% of patients (43/56) on pre-RT SPECT. The V and Q functions recovered in 51.2% of patients (22/43) in these regions, accounting for 39.3% of patients (22/56). This group of patients may benefit from V/Q SPECT scans acquired during RT for RT plan reoptimization for sparing of functional lung.
There are some limitations to this study. First, the semi-quantitative method may not be able to identify some small defects. Second, a sample size of 56 patients may not be powerful enough to detect small differences in many functional endpoints. Third, all patients underwent V/Q SPECT rescanning at about 45 Gy, but it is unclear whether 45 Gy is the right dose or the best time (generally this dose was given at week 4 of treatment) for V/Q rescans and replanning or if this dose made the damage irreversible. Improvement in dyspneaby the third week in this study suggests that V/Q functions actually changed earlier than the 45-Gy dose point. Studies focusing on quantitative analyses in more patients at different time points are being considered in our institution.
Conclusions
This study demonstrated that regional ventilation and perfusion functions improved remarkably at the 45-Gy dose point during RT. These changes may be associated with tumor shrinkage. Adaptive planning based on V/Q SPECT during RT is ongoing in our institute, which may allow sparing of functioning lung and improve therapeutic ratio.
Summary.
This study demonstrated that lung function improved during the course of fractionated radiation therapy. The improvement was seen in the significant reduction of dyspnea score and defect score of ventilation and perfusion on SPECT scan.
Acknowledgments
This study was supported in part by ASCO-CDA Lance Armstrong Foundation grants R21CA127057 and P01 CA059827, the Shandong Provincial Health Bureau (2007QW036), and the Foundation for Excellent Young Scientists of Shandong Province, China (BS2009YY012), and grant NSFC30700196 and NSFC81172133.
Footnotes
This work was presented in poster form at the 51th annual meeting of American Society of Therapeutic Radiology and Oncology in Chicago, Illinois, on November 1–5, 2009.
Conflict of interest: none.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Tyldesley S, Boyd C, Schulze K, et al. Estimating the need for radiotherapy for lung cancer: An evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973–985. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 3.Borst GR, De Jaeger K, Belderbos JS, et al. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys. 2005;62:639–644. doi: 10.1016/j.ijrobp.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Boersma LJ, Damen EM, de Boer RW, et al. Dose-effect relations for local functional and structural changes of the lung after irradiation for malignant lymphoma. Radiother Oncol. 1994;32:201–209. doi: 10.1016/0167-8140(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 5.Gayed IW, Chang J, Kim EE, et al. Lung perfusion imaging can risk stratify lung cancer patients for the development of pulmonary complications after chemoradiation. J Thorac Oncol. 2008;3:858–864. doi: 10.1097/JTO.0b013e31818020d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shioyama Y, Jang SY, Liu HH, et al. Preserving functional lung using perfusion imaging and intensity-modulated radiation therapy for advanced-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:1349–1358. doi: 10.1016/j.ijrobp.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Lavrenkov K, Christian JA, Partridge M, et al. A potential to reduce pulmonary toxicity: The use of perfusion SPECT with IMRT for functional lung avoidance in radiotherapy of non-small cell lung cancer. Radiother Oncol. 2007;83:156–162. doi: 10.1016/j.radonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Seppenwoolde Y, Engelsman M, De Jaeger K, et al. Optimizing radiation treatment plans for lung cancer using lung perfusion information. Radiother Oncol. 2002;63:165–177. doi: 10.1016/s0167-8140(02)00075-0. [DOI] [PubMed] [Google Scholar]
- 9.McGuire SM, Zhou S, Marks LB, et al. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol Biol Phys. 2006;66:1543–1552. doi: 10.1016/j.ijrobp.2006.07.1377. [DOI] [PubMed] [Google Scholar]
- 10.Christian JA, Partridge M, Nioutsikou E, et al. The incorporation of SPECT functional lung imaging into inverse radiotherapy planning for non-small cell lung cancer. Radiother Oncol. 2005;77:271–277. doi: 10.1016/j.radonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Lavrenkov K, Singh S, Christian JA, et al. Effective avoidance of a functional spect-perfused lung using intensity modulated radiotherapy (IMRT) for non-small cell lung cancer (NSCLC): An update of a planning study. Radiother Oncol. 2009;91:349–352. doi: 10.1016/j.radonc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Das SK, Miften MM, Zhou S, et al. Feasibility of optimizing the dose distribution in lung tumors using fluorine-18-fluorodeoxyglucose positron emission tomography and single photon emission computed tomography guided dose prescriptions. Med Phys. 2004;31:1452–1461. doi: 10.1118/1.1750991. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Kabus S, von Berg J, et al. Impact of four-dimensional computed tomography pulmonary ventilation imaging-based functional avoidance for lung cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:279–288. doi: 10.1016/j.ijrobp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Seppenwoolde Y, Muller SH, Theuws JC, et al. Radiation dose-effect relations and local recovery in perfusion for patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2000;47:681–690. doi: 10.1016/s0360-3016(00)00454-5. [DOI] [PubMed] [Google Scholar]
- 15.Kong FM, Frey KA, Quint LE, et al. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25:3116–3123. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- 16.Yuan S, Kirk FA, Gross MD, et al. Semi-quantification and classification of local pulmonary function by V/Q SPECT in patients with non-small cell lung cancer. J Thorac Oncol. 2011;6:71–78. doi: 10.1097/JTO.0b013e3181f77b40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Ma J, Zhou S, et al. Radiation-induced reductions in regional lung perfusion: 0.1–12 year data from a prospective clinical study. Int J Radiat Oncol Biol Phys. 2010;76:425–432. doi: 10.1016/j.ijrobp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Feng M, Kong FM, Gross M, et al. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys. 2009;73:1228–1234. doi: 10.1016/j.ijrobp.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong FM, Mahasittiwat P, Yuan S, et al. Define tumor volume during radiaotherapy to individualize adaptive radiation dose escalation in n non-small cell lung cancer. Imaging for treatment assessment in radiation therapy (ITART) 2010 Abstract 14327. [Google Scholar]