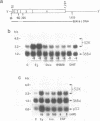
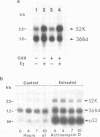
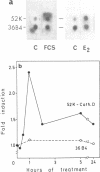
Abstract
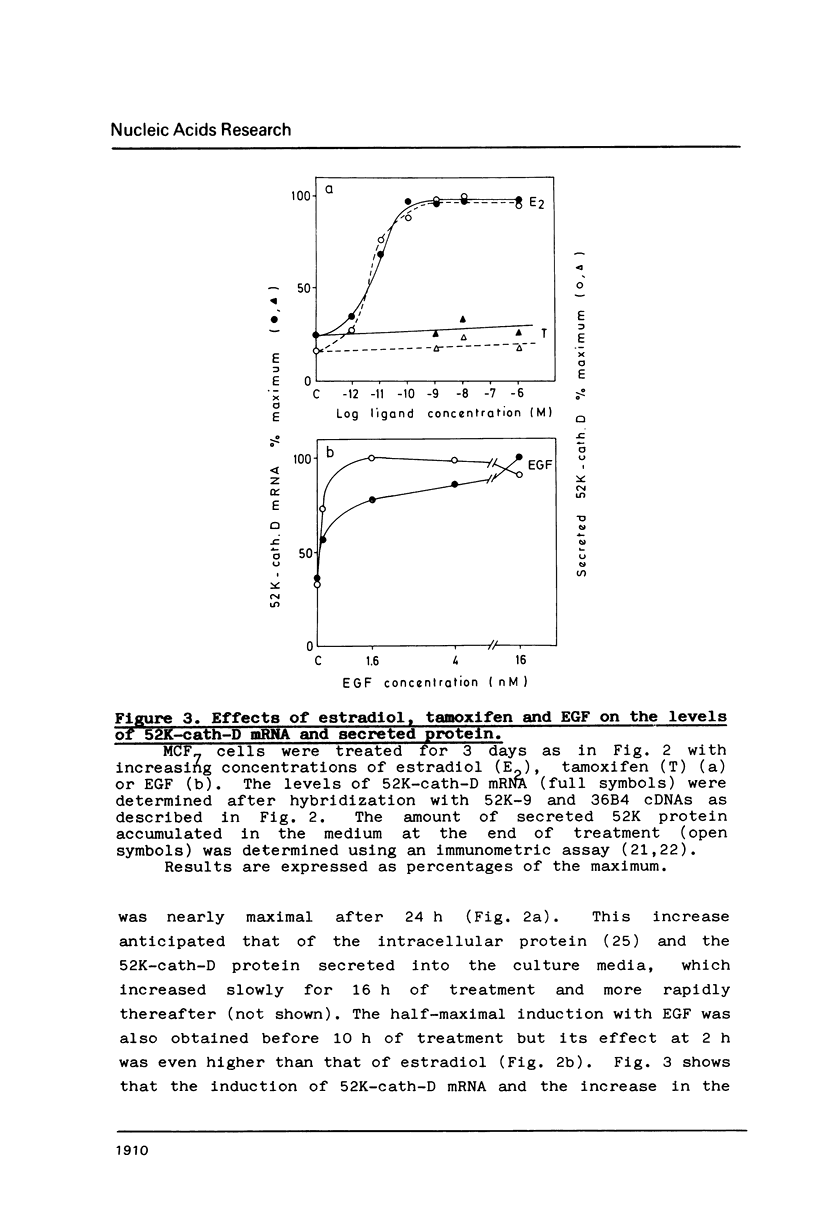
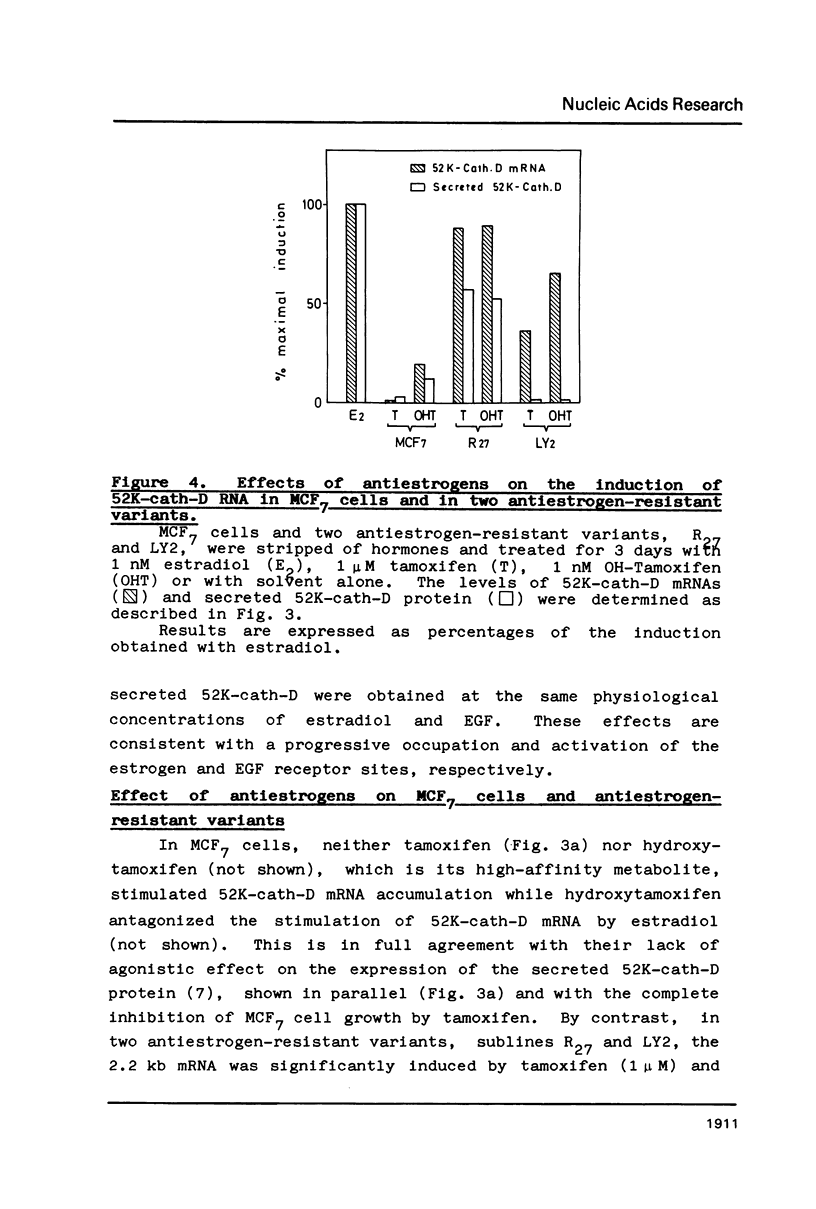
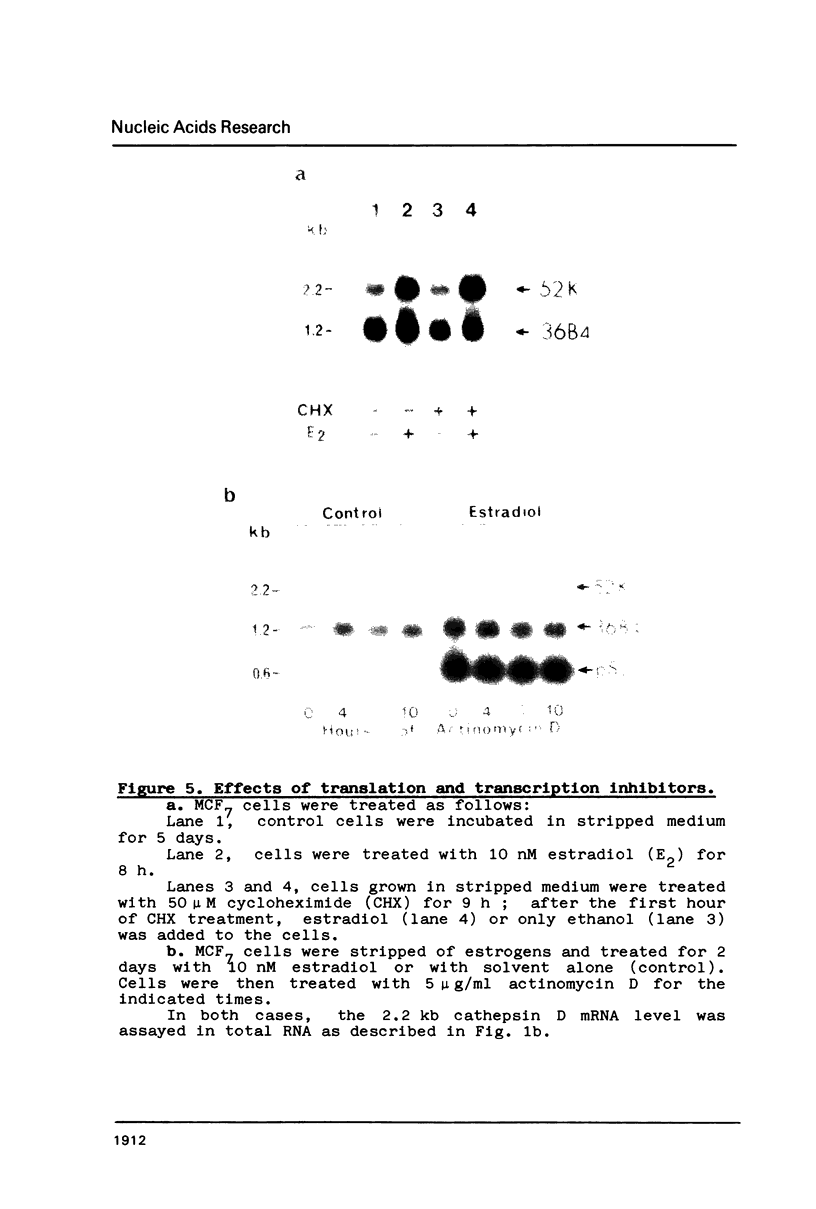
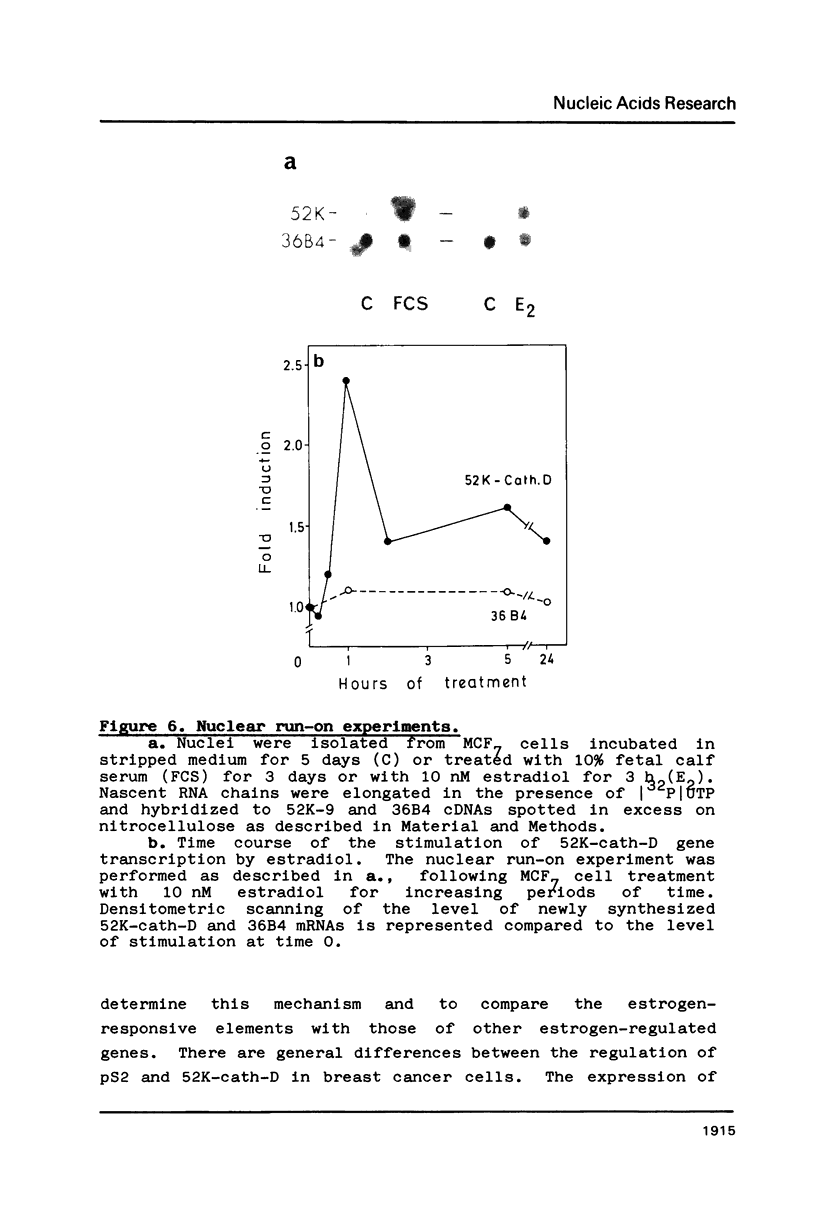
The estrogen-induced 52K protein secreted by human breast cancer cells is a lysosomal protease recently identified as a pro-cathepsin D by sequencing several cDNA clones isolated from MCF7 cells (Augereau et al., Mol. Endocr.). Using one of these clones, we detected, in MCF7 cells, a 2.2 kb mRNA whose level was rapidly increased 4- to 10-fold by estradiol, but not by other classes of steroids. Other mitogens, such as epidermal growth factor and insulin, also induced the 2.2 kb mRNA in a dose-dependent manner. Induction with epidermal growth factor was as rapid but was 2- to 3-fold lower than with estradiol. Antiestrogens had no effect on the 52K-cathepsin-D mRNA in MCF7 cells, but became estrogen agonists in two antiestrogen-resistant sublines R27 and LY2. The use of transcription and translation inhibitors and nuclear run-on experiments indicate that estradiol enhances transcription of the 52K-cathepsin-D gene in MCF7 cells.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Edwards D. P., McGuire W. L. Estrogen regulation of specific messinger RNA's in human breast cancer cells. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1354–1361. doi: 10.1016/s0006-291x(80)80016-7. [DOI] [PubMed] [Google Scholar]
- Aitken S. C., Lippman M. E., Kasid A., Schoenberg D. R. Relationship between the expression of estrogen-regulated genes and estrogen-stimulated proliferation of MCF-7 mammary tumor cells. Cancer Res. 1985 Jun;45(6):2608–2615. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bronzert D. A., Greene G. L., Lippman M. E. Selection and characterization of a breast cancer cell line resistant to the antiestrogen LY 117018. Endocrinology. 1985 Oct;117(4):1409–1417. doi: 10.1210/endo-117-4-1409. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capony F., Morisset M., Barrett A. J., Capony J. P., Broquet P., Vignon F., Chambon M., Louisot P., Rochefort H. Phosphorylation, glycosylation, and proteolytic activity of the 52-kD estrogen-induced protein secreted by MCF7 cells. J Cell Biol. 1987 Feb;104(2):253–262. doi: 10.1083/jcb.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalbos D., Westley B., May F., Alibert C., Rochefort H. Cloning of cDNA sequences of a progestin-regulated mRNA from MCF7 human breast cancer cells. Nucleic Acids Res. 1986 Jan 24;14(2):965–982. doi: 10.1093/nar/14.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N. E., Bronzert D. A., Chambon P., Gelmann E. P., Lippman M. E. Use of two MCF-7 cell variants to evaluate the growth regulatory potential of estrogen-induced products. Cancer Res. 1986 Apr;46(4 Pt 2):1904–1908. [PubMed] [Google Scholar]
- Faust P. L., Kornfeld S., Chirgwin J. M. Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4910–4914. doi: 10.1073/pnas.82.15.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Garcia M., Capony F., Derocq D., Simon D., Pau B., Rochefort H. Characterization of monoclonal antibodies to the estrogen-regulated Mr 52,000 glycoprotein and their use in MCF7 cells. Cancer Res. 1985 Feb;45(2):709–716. [PubMed] [Google Scholar]
- Garcia M., Lacombe M. J., Duplay H., Cavailles V., Derocq D., Delarue J. C., Krebs B., Contesso G., Sancho-Garnier H., Richer G. Immunohistochemical distribution of the 52-kDa protein in mammary tumors: a marker associated with cell proliferation rather than with hormone responsiveness. J Steroid Biochem. 1987;27(1-3):439–445. doi: 10.1016/0022-4731(87)90338-4. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Bates S., Knabbe C., Huff K., Swain S., McManaway M., Bronzert D., Kasid A., Gelmann E. P. 8th San Antonio Breast Cancer Symposium--Plenary lecture. Autocrine and paracrine growth regulation of human breast cancer. Breast Cancer Res Treat. 1986;7(2):59–70. doi: 10.1007/BF01806790. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset M., Capony F., Rochefort H. Processing and estrogen regulation of the 52-kilodalton protein inside MCF7 breast cancer cells. Endocrinology. 1986 Dec;119(6):2773–2782. doi: 10.1210/endo-119-6-2773. [DOI] [PubMed] [Google Scholar]
- Morisset M., Capony F., Rochefort H. The 52-kDa estrogen-induced protein secreted by MCF7 cells is a lysosomal acidic protease. Biochem Biophys Res Commun. 1986 Jul 16;138(1):102–109. doi: 10.1016/0006-291x(86)90252-4. [DOI] [PubMed] [Google Scholar]
- Moulton B. C., Koenig B. B. Progestin increases cathepsin D synthesis in uterine luminal epithelial cells. Am J Physiol. 1983 May;244(5):E442–E446. doi: 10.1152/ajpendo.1983.244.5.E442. [DOI] [PubMed] [Google Scholar]
- Nawata H., Bronzert D., Lippman M. E. Isolation and characterization of a tamoxifen-resistant cell line derived from MCF-7 human breast cancer cells. J Biol Chem. 1981 May 25;256(10):5016–5021. [PubMed] [Google Scholar]
- Osborne C. K., Bolan G., Monaco M. E., Lippman M. E. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4536–4540. doi: 10.1073/pnas.73.12.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin M. S., Doherty P. J., Gottesman M. M. The tumor promoter phorbol 12-myristate 13-acetate induces a program of altered gene expression similar to that induced by platelet-derived growth factor and transforming oncogenes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):357–360. doi: 10.1073/pnas.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort H., Capony F., Garcia M., Cavaillès V., Freiss G., Chambon M., Morisset M., Vignon F. Estrogen-induced lysosomal proteases secreted by breast cancer cells: a role in carcinogenesis? J Cell Biochem. 1987 Sep;35(1):17–29. doi: 10.1002/jcb.240350103. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Vignon F., Capony F., Chambon M., Freiss G., Garcia M., Rochefort H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinology. 1986 Apr;118(4):1537–1545. doi: 10.1210/endo-118-4-1537. [DOI] [PubMed] [Google Scholar]
- Vignon F., Lippman M. E., Nawata H., Derocq D., Rochefort H. Induction of two estrogen-responsive proteins by antiestrogens in R27, a tamoxifen-resistant clone of MCF7 cells. Cancer Res. 1984 May;44(5):2084–2088. [PubMed] [Google Scholar]
- Westley B. R., May F. E. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res. 1987 May 11;15(9):3773–3786. doi: 10.1093/nar/15.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley B., May F. E., Brown A. M., Krust A., Chambon P., Lippman M. E., Rochefort H. Effects of antiestrogens on the estrogen-regulated pS2 RNA and the 52- and 160-kilodalton proteins in MCF7 cells and two tamoxifen-resistant sublines. J Biol Chem. 1984 Aug 25;259(16):10030–10035. [PubMed] [Google Scholar]
- Westley B., Rochefort H. A secreted glycoprotein induced by estrogen in human breast cancer cell lines. Cell. 1980 Jun;20(2):353–362. doi: 10.1016/0092-8674(80)90621-2. [DOI] [PubMed] [Google Scholar]