Abstract
Antibody mediated allograft rejection is an increasingly recognized problem in clinical transplantation. However, the primary location of donor specific alloantibody (DSA) producing cells after transplantation have not been identified. The purpose of this study was to test the contribution of allospecific antibody secreting cells (ASCs) from different anatomical compartments in a mouse transplantation model. Fully MHC-mismatched heart allografts were transplanted into three groups of recipients: non-sensitized wild type, alloantigen-sensitized wild type and CCR5−/− mice that have exaggerated alloantibody responses. We found that previous sensitization to donor alloantigens resulted in the development of anti-donor alloantibody (alloAb) with accelerated kinetics. Nevertheless, the numbers of alloantibody secreting cells and the serum titers of anti-donor IgG alloantibody were equivalent in sensitized and non-sensitized recipients six weeks after transplantation. Regardless of recipient sensitization status, the spleen contained higher numbers of donor-reactive ASCs than bone marrow at days 7–21 after transplantation. Furthermore, individual spleen ASCs produced more anti-donor IgG alloantibody than bone marrow ASCs. Taken together, our results indicate that the spleen rather than bone marrow is the major source of donor-reactive alloAb early after transplantation in both sensitized and non-sensitized recipients.
Keywords: alloantibody, plasma cells, sensitization, spleen, bone marrow
Introduction
Despite advances in immunosuppression and routine panel reactive antibody screening prior to transplantation, donor specific alloantibodies (DSA) mediate an unacceptably high incidence of acute allograft rejection (1–3) and contribute to the development of chronic rejection (4, 5). In order to develop therapies that effectively target DSA secreting cells, it is crucial to understand the cellular mechanisms of DSA production.
Following migration from bone marrow (BM) to secondary lymphoid organs, naïve B cells can be activated after encountering antigen in the presence of primed helper T cells in the extrafollicular T cell rich environment (6). Activated B cells then can either develop into short-lived plasma cells (PCs) that secrete low affinity antibodies, or enter into the germinal center reaction driven by a specialized subset of follicular helper T cells (7). After proliferation, somatic hypermutation, affinity maturation and isotype switching, germinal center B cells can differentiate into long-lived PCs or memory B cells (8, 9). Long-lived PCs either migrate back to the BM or remain in the spleen or lymph nodes. These non-dividing cells continually secrete antibodies in the absence of antigen and increase antibody production in response to specific cytokines (10, 11).
The contribution of different antibody secreting cell (ASC) subsets to maintaining serum antibody levels can be determined by multiple factors such as the antigen type and dose, site of antigen administration, antigen persistence and the magnitude of initial inflammatory response (10, 12, 13). Current information on the role of ASCs from various anatomical compartments is predominantly derived from various models of infection and immunization (11). Isotype-switched ASCs can be detected in lymphoid organs draining infection sites as early as several days after infection. The early peak in spleen and lymph node ASCs is followed by a gradual decline, while antigen-specific ASCs in the BM increase during this time and typically reach a plateau 3–4 weeks after infection coinciding with the presence of high serum antibody titers (14–17). Therefore, it is generally believed that BM ASCs are responsible for maintaining anti-viral serum antibody levels and protection following re-infection. In addition, antigen-specific ASCs have been detected in non-lymphoid tissues following inflammation. Thus, during infections of the central nervous system (CNS), virus-specific ASCs accumulate within the CNS where they play a local protective role (18–21). In a murine model of influenza, viral antigen-specific ACSs within the lung have been observed to persist longer compared to those in the spleen (22). Chronic inflammation can also lead to the formation of ectopic lymphoid follicles containing long-lived ASCs such as those detected in the meninges of multiple sclerosis patients (23).
In contrast to the extensively characterized humoral responses after infection or vaccination, the sequence of events leading to the appearance and maintenance of donor-specific alloantibodies in the serum of transplant recipients is less well defined. The current concept of alloantibody production after transplantation has been recently summarized by Stegall et al (24). In particular, due to the low frequencies of alloreactive ASCs and a paucity of assays to identify these cells, considerable gaps remain in identifying the locations of alloantibody producing cells and their relative importance. It has been recently demonstrated that a PC-enriched population of cells isolated from the BM of sensitized renal allograft candidates was able to produce alloantibodies in vitro with specificities identical to the alloantibodies found in serum (25). However, it remains difficult to evaluate the relative importance of the BM alloantibody production as spleen and lymph node samples are generally not available from human subjects. Furthermore, the main source of alloreactive antibodies prior to versus following transplantation could be different in sensitized recipients.
In the current study, we used a mouse cardiac transplantation model to test whether the contribution of allospecific ASCs from different anatomical compartments depends on the sensitization status of the recipient and on the time elapsed after transplantation. We show that regardless of sensitization status, ASCs from the spleen and graft-draining lymph nodes, rather than from the BM, are the main sources of donor-specific alloantibodies at early stages after transplantation. By 6 weeks after transplantation, the difference in allospecific antibody production between these anatomical compartments becomes less significant, and the magnitude of the anti-donor humoral response is not affected by the initial sensitization status of the recipient. We also investigated the mechanisms of the previously reported exaggerated DSA production in CCR5−/− heart allografts recipients (26–28). We demonstrate that while CCR5−/− mice do not possess anti-donor T cell or humoral reactivity prior to transplantation, their response to allografts are similar to those in sensitized wild type (WT) recipients.
Materials and Methods
Animals and procedures
The following mice, aged 6–8 weeks, were purchased from the Jackson Laboratories (Bar Harbor, ME): female C57Bl/6 (B6, H-2b: Kb, Db, and I-Ab), male BALB/c (H-2d: Kd, Dd, Ld, I-Ad and I-Ed), male SJL (H-2s: Ks, Ds, Ls, I-As and I-Es) and male CCR5−/− on the C57Bl/6 background. All animals were maintained and bred in the pathogen-free facility at the Cleveland Clinic. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Vascularized heterotopic cardiac allografts were placed and monitored as previously described (29–31). Rejection was defined as a loss of palpable heartbeat and was confirmed by laparotomy. To generate allosensitized recipients, female B6 mice were subcutaneously injected with 30×106 BALB/c splenocytes in 100 μl of Complete Freund’s Adjuvant (CFA) that was prepared by mixing Incomplete Freund’s Adjuvant (Sigma-Aldrich, St. Louis, MO) and lyophilized M. Tuberculosis H37RA (2.5 mg/ml, Difco Laboratories, Detroit, MI). The efficiency of sensitization was monitored in all recipients by measuring serum titers of BALB/c reactive IgG alloantibodies on d. 14 after immunization. BALB/c heart allografts were transplanted into sensitized B6 recipients 4 weeks after immunization.
To induce anti-viral immune responses, male B6 mice were intraperitoneally injected with 5 × 106 plaque forming units (PFU) of the mouse hepatitis virus strain JHMV, designated 2.2v-1 (14, 20, 32).
Histologic examination of recipient spleen tissues
For immunohistochemistry, tissues were fixed with acid methanol (60% methanol, 10% acetic acid). Paraffin-embedded sections (5 μm) were steamed in two changes of Trilogy-EDTA, pH 8 (Cell Marque, Hot Springs, AR) for 1 hr. Endogenous peroxidase activity was blocked by incubation with 0.3% H2O2 in 80% methanol and nonspecific protein interactions were blocked by incubation with a serum-free protein block (DAKO Corp, Carpinteria, CA). Slides were incubated with a 1:3,000 dilution of biotin-SP-conjugated Affinity purified F(ab′)2 fragments of goat antibodies to the Fc-gamma of mouse IgG (Jackson ImmunoResearch, West Grove, PA) for 60 min at room temperature. Slides were subsequently incubated for 30 minutes with Avidin-Biotin-Enzyme Complex (ABC elite PK6100; Vector, Burlingame, CA), followed by diaminobenzidine and counterstained with Hematoxylin.
Flow cytometry
Phycoerythrin (PE)-conjugated anti-mouse B220, allophycocyanin (APC)-conjugated anti-mouse CD138 and fluorescein isothiocyanate (FITC)-conjugated Annexin V were purchased from BD Pharmingen (San Diego, CA). Cells freshly isolated from spleen, lymph nodes or bone of heart allograft recipients or cells obtained after 24 or 48 h. of in vitro culture were stained with indicated reagents as previously described (29–31) and analyzed on a BD Biosciences FACSCalibur using CellQuest software.
In vitro cell cultures
BM or spleen cells were isolated from individual recipients at the time of sacrifice and 5 × 106 cells were cultured in 1 ml of HL1 media (LONZA, Walkersville, MD). The supernatants were collected after 48 hours and the presence of donor-reactive IgG antibody was determined by a thymocyte binding assay (33). The apoptosis rates and viability of spleen and BM cells were evaluated prior to the culture and at 24 and 48 h after the culture by flow cytometry.
Measurement of alloantibody titers
Donor BALB/c and third party SJL thymocytes were isolated and 1×106 cell aliquots were incubated with 100μl of serially diluted recipient serum or with non-diluted cell culture supernatant. FITC-conjugated goat anti-mouse IgG, PE-conjugated anti-mouse IgG1, FITC-conjugated anti-mouse IgG2a, FITC-conjugated anti-mouse IgG3 and biotinylated anti-mouse IgG2b followed by PE-Streptavidin conjugate were used as detecting antibodies at a 1:50 – 1:100 dilution (all from BD Pharmingen). Cells were washed, fixed in 1% paraformaldehyde, and analyzed by flow cytometry. For cell culture supernatants, the results were reported as mean fluorescent intensity (MFI). The titer of BALB/c-reactive IgG alloantibody in serum was calculated as previously published (33, 34). For each IgG isotype, the MFI of each dilution was determined. The dilution that returned the MFI to the level observed when thymocytes were stained with a 1:90 dilution of naïve B6 serum was divided by two and reported as the titer.
IgG ELISPOT Assay
The frequencies of IgG secreting donor-reactive ASCs were determined using ELISpot PLUS for mouse IgG kit (MABTECH AB, Nacka Strand, Sweden). Briefly, serial dilutions of recipient cells isolated from the spleen, graft-draining mediastinal lymph nodes and BM were cultured for 20 h in RPMI media supplemented with 5% of Fetal Bovine Serum (FBS) in 96-well plates coated with anti-mouse IgG antibody (4 × 106 – 0.06 × 106 cells/well). Then, either biotinylated-anti-IgG or biotinylated Dd molecules (5μg/ml, kindly provided by Dr. Cornelia Bergmann at the Cleveland Clinic) were added as detection reagents for two hours at RT. After extensive washes, the plates were incubated with Streptavidin-Alkaline Phosphatase for one hour at RT, followed by BCIP/NBT substrate.
To detect JHMV-specific IgG producing ACSs, 96-well plates were coated with JHMV virus at 5×105 PFU/well. Serial dilutions of cells were plated and incubated for 20 h at 37°C. ASCs were detected by subsequent incubations with biotinylated rabbit anti-mouse IgG (Southern Biotech, Birmingham, AL), streptavidin-horseradish peroxidase (BD Pharmingen) and 3, 3′-diaminobenzidine substrate as previously described (14). The numbers of spots per well and the cumulative spot size distribution were analyzed using an ImmunoSpot Series 2 Analyzer (Cellular Technology Ltd., Shaker Heights, OH). The results were calculated as total numbers of ASCs per compartment, BM size was estimated by multiplying number of cells isolated from femurs by 8.3 as previously described (35).
ELISPOT assay
Assays were performed as previously outlined using capture and detecting anti-mouse IFNγ antibody from BD Pharmingen (36). Recipient spleen cells were stimulated with mitomycin C-treated BALB/c or third party SJL spleen cells. The resulting spots were analyzed using an ImmunoSpot Series 2 Analyzer.
Statistical Analysis
Heart allograft survival was compared between groups by Kaplan-Meier analysis. Unless noted otherwise, the data are represented as mean values ± SD. The antibody titers were analyzed using nonparametric Mann-Whitney test. All other results were analyzed by ANOVA followed by Bonferroni post-test. A p value < 0.05 was considered a significant difference.
Results
Generation of allosensitized transplant recipients
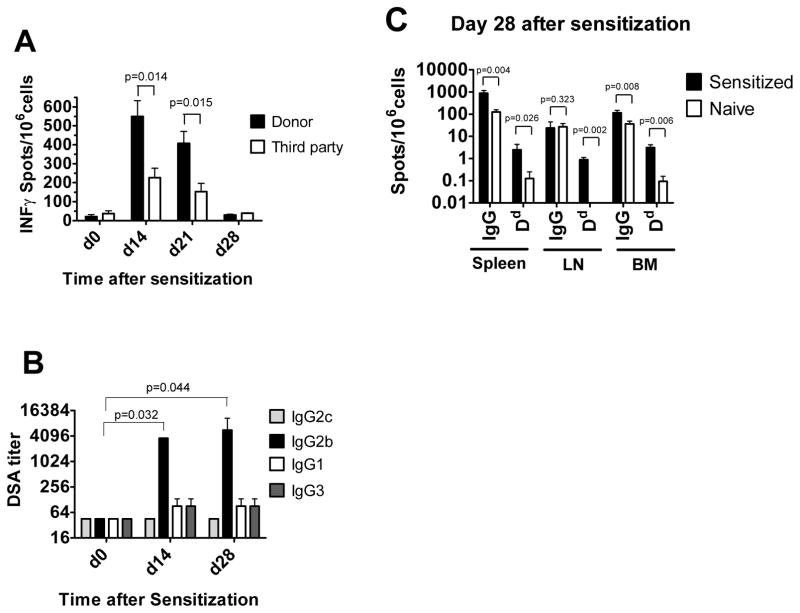
Female B6 mice were subcutaneously injected with BALB/c splenocytes in CFA. In order to test the efficiency and the specificity of this allo-immunization, we performed the following experiments. First, spleen cells from sensitized mice were analyzed by ELISPOT to enumerate IFNγ-secreting alloantigen-specific T cells. The responses to BALB/c stimulators were significantly higher than those to third party stimulators at days 14 and 21 and declined to baseline levels by day 28 after immunization (Figure 1A). Second, sera from immunized mice were tested for the presence of BALB/c-reactive alloantibody. High titers of BALB/c reactive IgG2b were detected in sensitized but not in naïve B6 mice at days 14 and 28, althogh immunized mice had low serum titers of IgG2c, IgG1 and IgG3 (Figure 1B). Third, the numbers of total IgG-producing ASCs as well as ASCs secreting Dd-specific IgG were evaluated on day 28 after immunization by ELISpot assay. While very few ACSs producing Dd binding IgG were detected in naïve B6 mice, immunization with BALB/c spleen cells led to significant increases in numbers of ASCs secreting total and Dd-binding IgG in the spleen, BM and mediastinal lymph nodes (Figure 1C). Consistent with the sensitized status of immunized B6 mice, they rejected BALB/c heart allografts with accelerated kinetics compared to non-immunized B6 recipients (MST 4.9 ± 0.8 days vs 7.4 ± 0.7 days, respectively; n=16 per group, p < 0.0001).
Figure 1. Generation of allosensitized transplant recipients.
B6 mice were subcutaneously injected with BALB/c splenocytes in CFA. A. IFN γ ELISPOT assays were performed on spleen cells isolated prior to sensitization and at days 14 (n=3), 21 (n=3) and 28 (n=4) after sensitization and re-stimulated with BALB/c or third party splenocytes. B. Sera were collected prior to sensitization and on days 14 (n=4) and 28 (n=4) after sensitization, BALB/c reactive antibody titers were measured as outlined in the Methods. The titers of BALB/c reactive antibody in the serum of naïve B6 mice was < 45 for all IgG isotypes. C. IgG ELISPOT assays performed on spleen, BM and inguinal lymph node cells to enumerate the frequencies of ASCs producing total and Dd-binding IgG (n=4).
Sensitized heart allograft recipients develop high titers of anti-donor alloantibody with accelerated kinetics
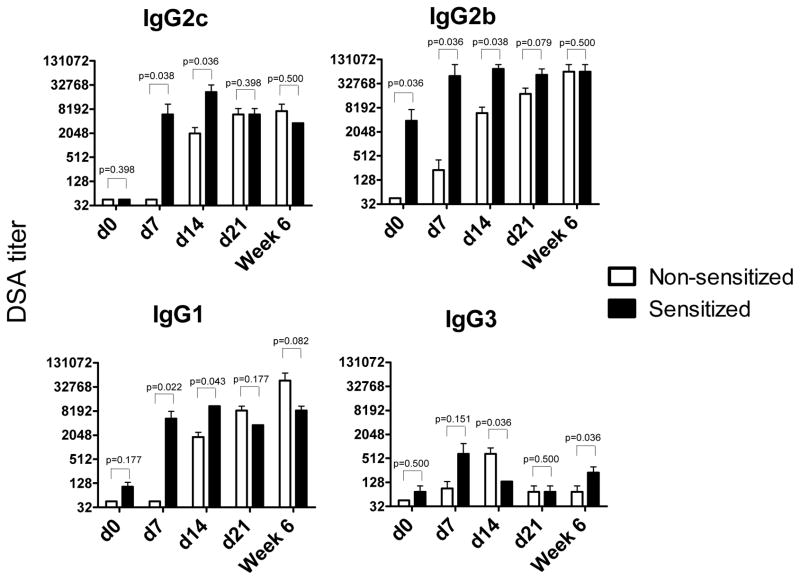
The sera from sensitized and non-sensitized heart allograft recipients were tested for the development of donor-reactive alloantibodies. Anti-donor IgG antibody titers were significantly higher in the sensitized than in non-sensitized recipients at day 7 after transplantation (7–200-fold increases for different IgG isotypes, Figure 2). While donor-reactive alloantibody titers remained stable in sensitized recipients between days 7 and 21 after transplantation, they gradually increased in non-sensitized recipients. Notably, by day 21 and up to 6 weeks after transplantation, the anti-donor IgG antibody titers were comparable between the groups (Figure 2).
Figure 2. The kinetics of DSA production in sensitized versus non-sensitized heart allograft recipients.
Heterotopic BALB/c heart transplants were placed into non-sensitized B6 mice or into sensitized B6 recipients four weeks after immunization with BALB/c alloantigens. Serum was collected on days 7, 14 and 21 and at six weeks post transplant, and the titers of BALB/c- and third party-reactive alloAb were determined by cell binding assay. The titers of third party-reactive alloAb in all serum samples were < 45 for IgG2c and IgG2b and < 135 for IgG1 and IgG3. The results were compared using non-parametric Mann-Whitney test. N = 4–5 mice per group at each time point.
The spleen is the main source of anti-donor ASCs in both sensitized and non-sensitized recipients
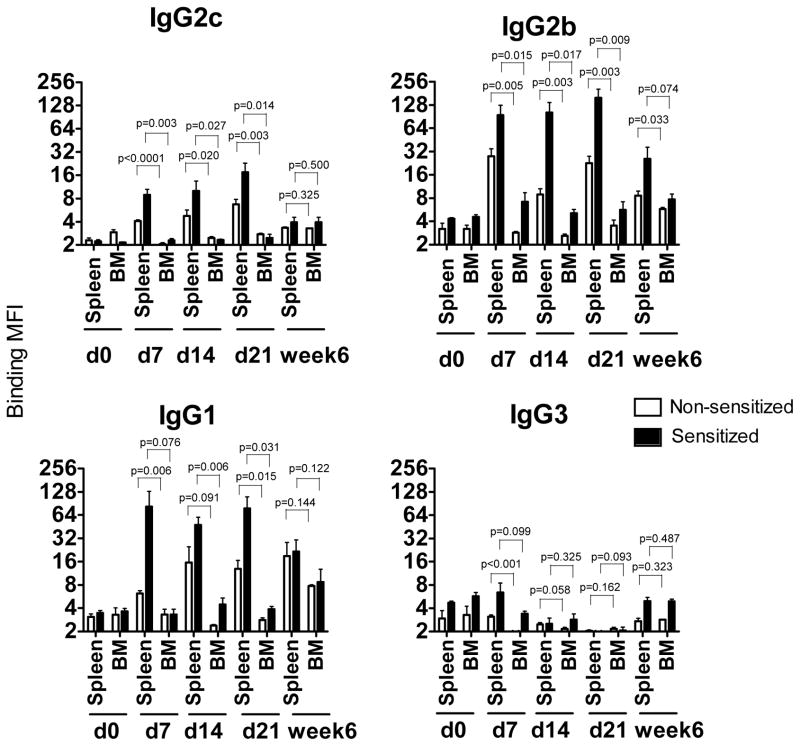
To investigate the relative contribution of the spleen versus BM as a source of cells producing alloantibody, spleen and BM cell suspensions were prepared from heart allograft recipients and cultured in vitro for 48 hours without further re-stimulation. The presence of donor-specific antibodies was evaluated by testing the ability of the culture supernatants to bind donor thymocytes. The binding MFI levels for spleen cell supernatants were higher in the sensitized than in non-sensitized group at days 7, 14 and 21 after transplantation. However, the binding of spleen cell supernatants from sensitized and non-sensitized recipients was comparable at 6 weeks post transplant (Figure 3). The binding MFIs were significantly higher for the spleen compared to the BM supernatants in both sensitized and non-sensitized recipients (Figure 3). These findings were not due to the inferior survival of BM plasma cells during in vitro culture as B220+CD138+ cells from spleen and BM demonstrated similar viability and apoptotic rates (Figure S1). The differences between spleen and BM cell supernatants became less prominent at 6 weeks after transplantation.
Figure 3. In vitro DSA secretion by spleen and bone marrow cells.
Spleen and BM cells were isolated from heart allograft recipients at 7, 14, 21 and at six weeks post transplant and cultured in vitro for 48 hours without re-stimulation. The presence of DSA was evaluated by testing the ability of the culture supernatants to bind donor thymocytes. Results are expressed as Mean Fluorescent Intensity (MFI). Spleen cells isolated from naïve B6 mice and cultured for 48 hours were used as a control. N = 4–5 mice per group at each time point.
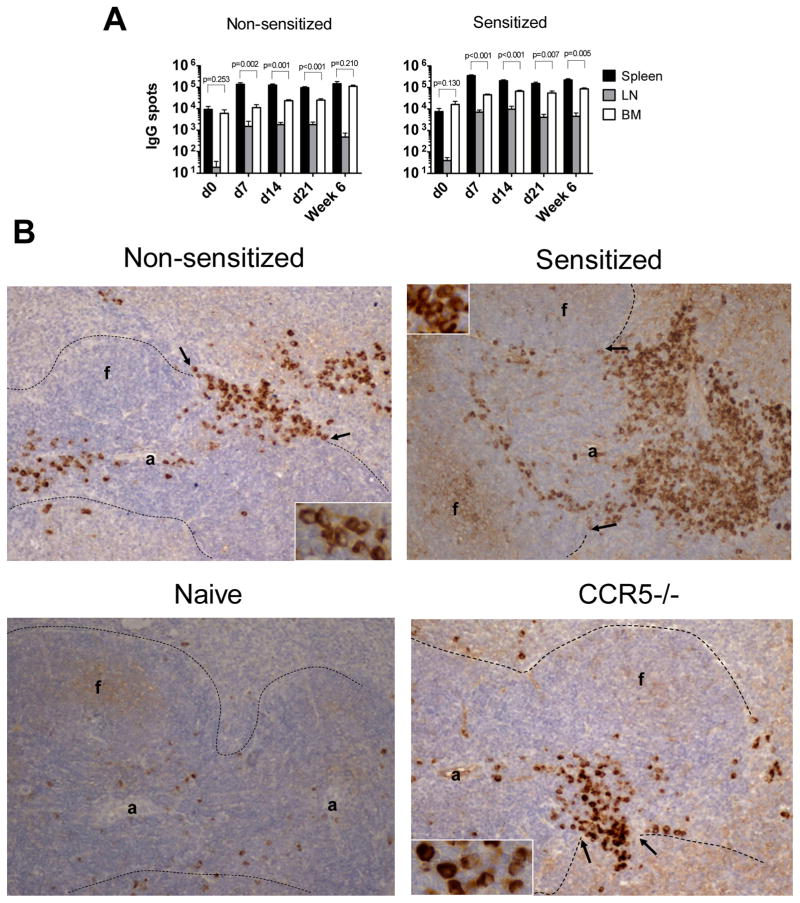
To investigate whether the differences in alloantibody production between anatomical compartments could be explained by the relative frequencies of ASCs, we quantified IgG secreting cells in the spleen, BM and graft-draining mediastinal lymph nodes. The numbers of total IgG spots were significantly higher in the spleen than in the BM at days 7, 14 and 21 after transplantation. While the numbers of spleen IgG-secreting ACS did not change between days 7 and 42 after transplantation, such cells gradually accumulated in the bone marrow, resulting in comparable numbers of IgG secreting cells between the two compartments at 6 weeks post transplant (Figure 4A).
Figure 4. Distribution of IgG producing ASCs within spleen, mediastinal lymph nodes and bone marrow of sensitized and non-sensitized recipients.
A. Spleen, BM and mediastinal lymph nodes cells were isolated at indicated time points after transplantation or from naïve B6 mice as a control. The total numbers of IgG-secreting cells per each compartment were determined by ELISPOT assay. N = 4–5 mice per group at each time point. B. Immunohistochemical staining of recipient spleen sections for IgG secreting cells at day 7 post transplant. Spleen tissues were excised, fixed and embedded in paraffin. 5μm sections were prepared and stained with anti-mouse IgG antibodies. Dotted lines indicate the border between white and red pulp. Arrows point to bridging channels with clusters of IgG+ ASCs; “a” = arteriole, “f” = follicle. Original magnification 20x, insets 60x.
Compared to non-sensitized mice, recipient sensitization resulted in increased numbers of IgG-producing ASCs within the spleen and draining lymph nodes early after transplantation (Figure 4A). This was confirmed by immunohistochemical staining of recipient spleen sections. In non-sensitized recipients, IgG producing ASCs appeared to be in transit through the bridging channels between the periarteriolar lymphatic sheets and the red pulp whereas large numbers of ASCs accumulated at the border of the marginal zone and red pulp in sensitized mice (Figure 4B). The majority of ASCs in non-sensitized recipients appeared to be plasmacytes with characteristic uneven and granular staining of cytoplasm. In contrast, a larger proportion of ASCs in sensitized recipients had uniformly and densely stained cytoplasm consistent with the morphology of mature plasma cells (Figure 4B insets).
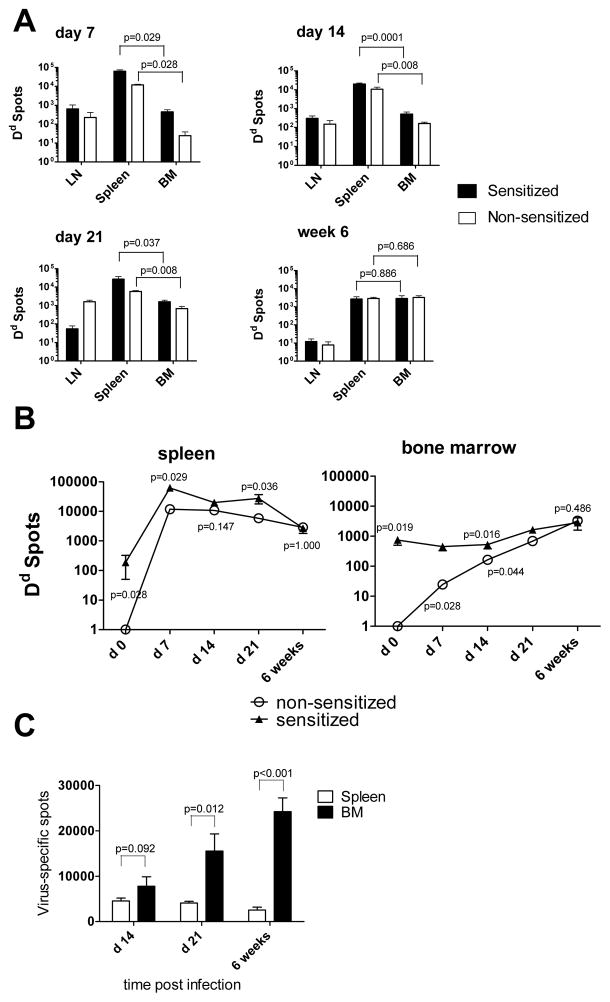
Next, we evaluated the frequencies and total numbers of donor MHC-reactive ASCs within distinct anatomical compartments of sensitized and non-sensitized recipients. Regardless of recipient sensitization status, we detected more cells secreting Dd-binding IgG in the spleen than in the BM at days 7, 14 and 21 after transplantation. However, the frequencies of donor-reactive ASCs among CD138+ cells and their total numbers gradually decreased in the spleen and increased in the BM of non-sensitized recipients and became comparable in the twp compartments by 6 weeks after transplantation (Figure 5A, B and Figure S2). The sensitized recipients demonstrated similar trends in the kinetics of ASC accumulation, but had significantly higher frequencies and numbers of allo-reactive ASCs in the spleen at days 7 and 21, and in the BM at days 7, 14 and 21.
Figure 5. Distribution of antigen-specific IgG producing ASCs in heart allograft recipients is distinct from that in virus-infected mice.
A–B. Heterotopic BALB/c heart transplants were placed into non-sensitized B6 mice or into sensitized B6 recipients four weeks after immunization with BALB/c alloantigens. Spleen, BM and mediastinal lymph nodes cells were isolated at indicated time points after transplantation. The total numbers per compartment of cells secreting Dd-binding IgG were determined by ELISPOT assay (A). The kinetics of Dd-reactive ASC frequencies in the spleen and in the BM of sensitized versus non-sensitized recipients (B). C. B6 mice were intraperitoneally injected with 5 × 106 PFU of the mouse hepatitis virus strain JHMV. The numbers of virus-specific ASCs were determined by ELISPOT assay at indicated time points after infection. N= 4–5 mice per group at each time point.
To exclude the possibility that our assay for BM ASCs lacks sensitivity and to compare the B cell responses to a heart allograft with responses to an infectious agent, we tested whether we can detect antigen-specific BM ASCs in a model of viral infection. B6 mice were intraperitoneally injected with a sublethal strain of mouse hepatitis virus JHMV that has been reported to elicit a potent humoral response (14, 20, 32). The frequencies and total numbers of ASCs producing virus-specific IgG were evaluated by ELISPOT assay on days 7, 14, 21 and at 6 weeks after infection. Notably, the frequencies of ASCs on day 7 post infection were below the detection limit. In contrast to our findings in allograft recipients, BM of the infected mice contained higher numbers of virus-specific ASCs at all time points.
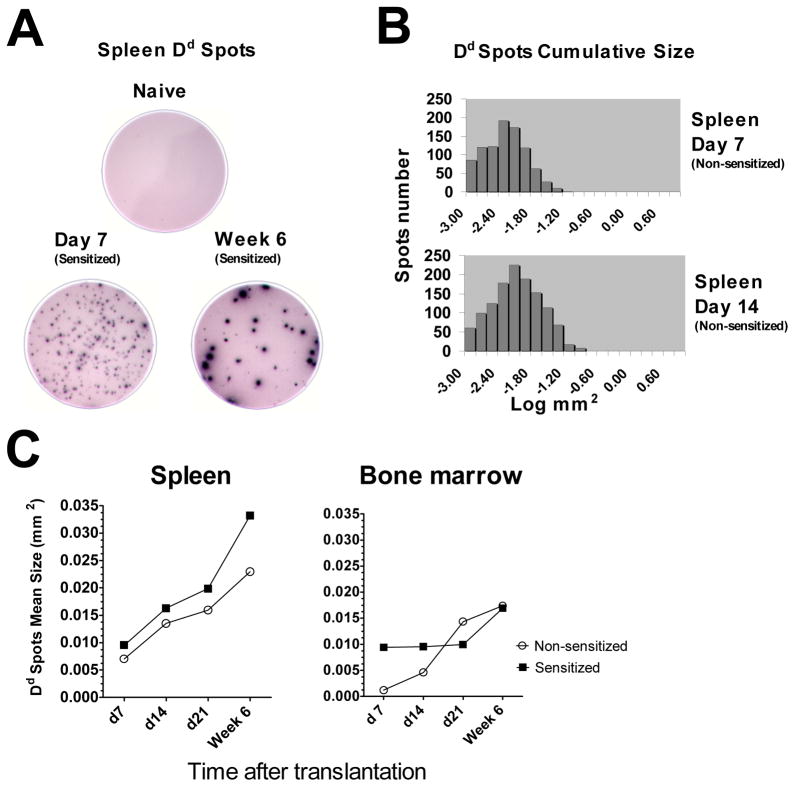
Despite the increase in donor-reactive alloantibody titers in the serum of non-sensitized recipients between days 7 and 14 after transplantation, we observed similar numbers of donor-reactive ASCs at these time points. Therefore, we compared the ability of individual ASCs to secrete Dd-binding IgG by analyzing spot size distrubution in ELISPOT assay. The Dd-binding spot sizes increased over time after transplantation indicating an increasing production of allo-reactive antibody by individual cells (Figure 6). In general, spleen ASCs from sensitized recipients formed larger spots than cells from non-sensitized mice at all time points examined. For BM ASCs, sensitization resulted in enhanced secretion of donor-reactive alloAb only at days 7 and 14 whereas comparable amounts of antibody were produced by cells from sensitized and non-sensitized recipients at 6 weeks post transplant. Notably, individual spleen ASCs produced more Dd-binding antibody compared to BM ASCs regardless of recipient sensitization status or time elapsed after transplantation (Figure 6C).
Figure 6. Secretion of donor MHC binding IgG by individual ASCs.
A–B. ELISPOT well images and cumulative spot size histograms illustrate increasing production of allo-reactive antibody by individual cells. C. The kinetics of average Dd-binding spot size in sensitized versus non-sensitized recipients. All ELISPOT assays were performed in duplicates, average spot sizes were determined for 4–5 recipients per group at each time point.
The accelerated kinetics of humoral alloresponses in CCR5−/− heart allograft recipients
It has been previously shown that mice deficient in the chemokine receptor CCR5 have enhanced humoral immune responses against donor alloantigens compared to WT animals and develop acute humoral rejection of heart and kidney allografts (26–28). In order to investigate the underlying mechanisms of exaggerated alloAb production under these conditions, we evaluated the parameters of anti-donor humoral immune responses in CCR5−/− heart allograft recipients. As in previous studies, WT and CCR5−/− recipients rejected BALB/c heart allografts with similar kinetics (MST of 7.4 ± 0.7 days vs 7.8 ± 0.7 days, respectively). Increased numbers of donor-specific IFNγ secreting T cells were present in CCR5−/− compared to WT recipients, consistent with previously reported exaggerated T helper activity in the absence of CCR5 (8).
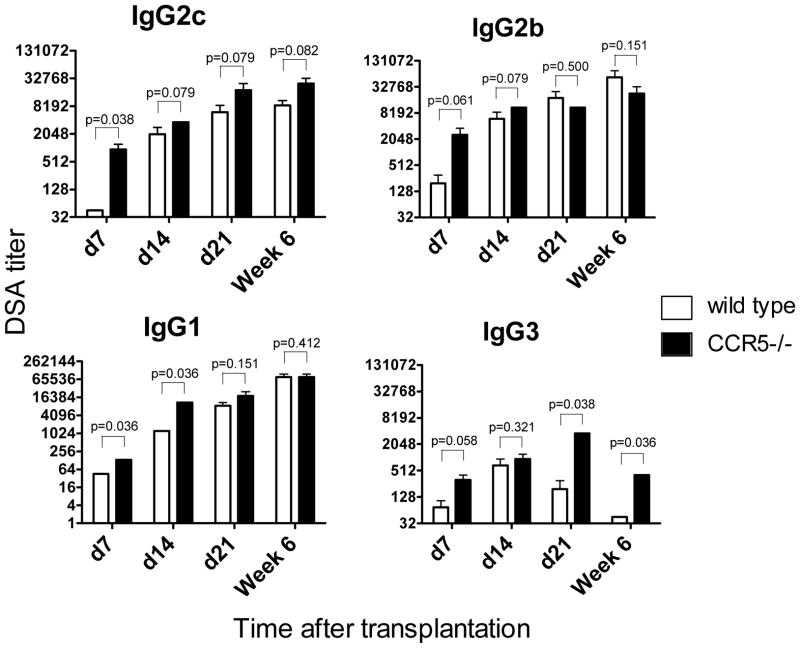
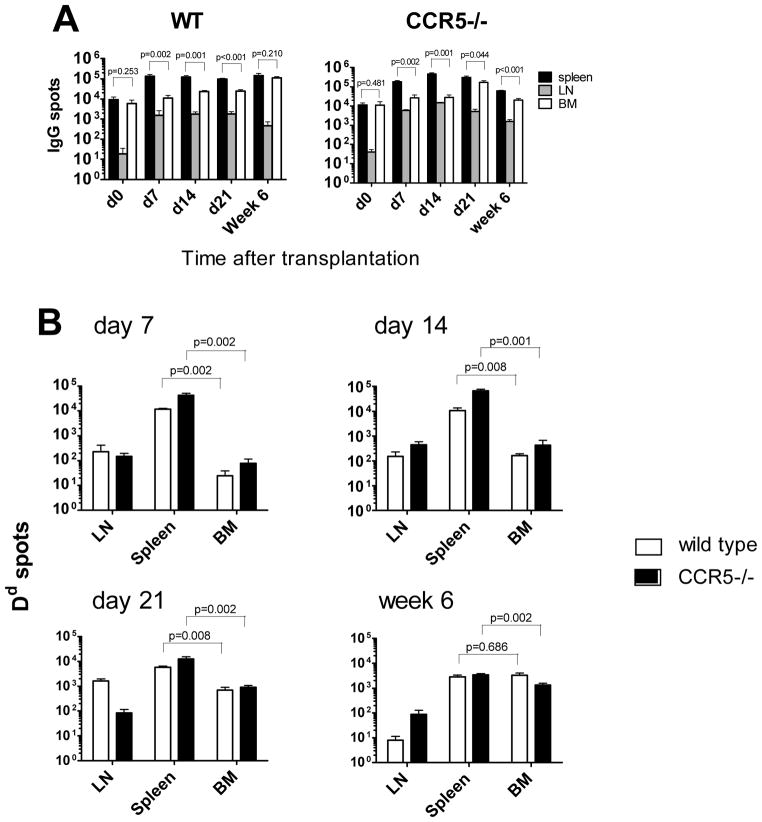
CCR5−/− recipients developed high titers of anti-donor IgG antibodies with accelerated kinetics compared to WT mice. However, the titers of donor-reactive IgG2c, IgG2b and IgG1 alloAb became comparable between groups by day 21 after transplantation (Figure 7). The numbers of total IgG secreting cells did not change over time after transplantation and were slightly, but not significantly, elevated in CCR5−/− compared to WT recipients (Figure 8A). In contrast, CCR5−/− recipients contained significantly higher numbers of Dd-binding IgG secreting cells in the spleen at days 7, 14 and 21 (Figure 8B). Similar to our findings in sensitized wild type recipients, the average Dd-bindingspot size in the spleen was larger at day 7 in CCR5−/− compared to WT recipients. At later time points, the ability of individual cells to secrete donor-reactive IgG antibody became comparable between both groups (Figure S3). Taken together, these results indicate that despite the absence of pre-transplant sensitization, the parameters of humoral alloimmune responses in CCR5−/− allograft recipients are similar to those observed in sensitized WT mice.
Figure 7. The kinetics of DSA production in wild type and CCR5−/− heart allograft recipients.
WT and congenic CCR5−/− B6 recipients received heterotopic heart transplants from BALB/c donors. Serum was collected on days 7, 14 and 21 and at six weeks post transplant, and the titers of BALB/c and third party reactive alloAb were determined by thymocyte binding assay. The titers of third party-reactive alloAb were < 45 for all IgG isotypes. N = 4–5 mice per group at each time point.
Figure 8. Frequencies of ASCs producing total and donor-reactive IgG in WT versus CCR5−/− recipients.
Spleen, BM and mediastinal lymph nodes cells were isolated from WT or CCR5−/− heart allograft recipients at indicated time points after transplantation and the total numbers of ASCs per compartment were determined by ELISPOT assay. A. Total IgG ASCs. B. Dd-binding IgG ASCs. N = 4–5 mice per group at each time point.
Discussion
In the past two decades, the frequencies, kinetics, and functional parameters of T cell immune responses following transplantation have been well characterized. However, analogous studies on humoral alloimmunity have been hindered by the comparatively low frequencies of alloreactive ASCs, by the paucity of assays to identify these cells and by the challenges of evaluating different anatomical compartments in human transplant recipients. A recent study by Perry et al. successfully detected alloreactive ASCs in the BM of sensitized patients using purified HLA molecules as targets (25). However, the role of allospecific ASCs from secondary lymphoid organs of allograft recipients has not been previously addressed.
In the current study, we used three different models of murine cardiac allotransplantation to compare the contribution of cells in the BM, graft draining lymph nodes and spleen to donor-reactive alloantibody production. Parameters of humoral alloimmune responses were evaluated at various time points after transplantation by measuring serum titers of donor-reactive alloantibody, by comparing the ability of spleen and BM cells to secrete anti-donor alloantibody during culture, and by enumerating total and donor-antigen reactive ASCs from various compartments by a modified ELISPOT assay.
We show for the first time that the spleen rather than BM is the major source of alloAb at early stages after heart transplantation. First, spleen cells produced higher levels of donor-reactive alloAb than BM cells during in vitro culture. Second, higher numbers of ASCs producing donor-reactive IgG were detected within the spleen compared to the BM. Third, individual spleen ASCs secreted more antibody than BM ASCs at all examined time points.
Previous studies of viral infections and immunizations had demonstrated that the initial antigen-specific antibody production occurs in the spleen but BM antigen-specific ASCs rapidly take over maintaining serum antibody levels as antibody production by spleen cells wanes (12, 15–17). We observed similar kinetics and distribution of ASCs in a model of viral infection (Figure 5C). In contrast, the numbers of donor antigen-specific ASCs in bone marrow are negligibly low for the first three weeks after transplantation and the numbers of spleen and bone marrow ASCs become comparable only long after high serum levels of alloantibody have been achieved (d. 14 – d. 21 for different IgG isotypes).
The dynamics of ASCs distribution observed in our study raise questions about the longevity of ASCs in the spleen versus BM. In contrast to acute viral infections, the alloantigens are not rapidly cleared after heart transplantation and may continue to recruit new B cells into the response and to stimulate induction of short-lived PCs in the spleen. Thus, it is possible that spleen ACSs are mostly comprised of short-lived PCs while ASCs that migrate into the BM persist for a long time, analogous to the responses induced against pathogens or model antigens (10, 11). Studies evaluating the life span, antigen dependence and phenotypical characteristics of spleen versus BM ASCs following transplantation are currently ongoing in our laboratory.
In clinical practice, splenectomy has been used as a preemptive treatment of ABO incompatible recipients or patients with high DSA levels and increased risk of antibody mediated rejection (37–40). Performed prior to transplantation, splenectomy may reduce T and B cell alloresponses against the graft as well as decrease the numbers of plasmablasts and mature PCs. Interestingly, two groups have recently reported the successful use of splenectomy for the treatment of refractory antibody mediated renal allograft rejection in small groups of renal transplant patients (41, 42). In both studies, the DSA or ABO antibody titers were decreased in all splenectomized recipients and resolution of antibody mediated rejection (AMR) has been achieved. These outcomes are consistent with our findings on the role of the spleen in the maintenance of DSA serum titers. However, it is possible that in some cases lymph node and/or BM can harbor sufficient numbers of ASCs to overcome the beneficial effects of splenectomy at later time points after transplantation.
Mediastinal lymph nodes have been established as important sites of antigen drainage for the peritoneum. Previous studies on heterotopic cardiac allografts have demonstrated that donor dendritic cells migrate to the mediastinal lymph nodes and PCs expand at these sites within 1 week after transplantation (43, 44). The current data indicate that mediastinal lymph nodes are a site of rapid and prolonged production of donor specific antibodies. Nevertheless, the relative size of mediastinal lymph nodes was modest compared to the spleen (5–10 × 106 versus 80–100 × 106 cells) and the numbers of lymph node ASCs were consistently lower than those observed in the spleen (Figures 5 and 8), suggesting the more prominent contribution of the spleen in the generation and maintenance of cells producing serum alloantibodies. While the frequencies of alloantigen-specific ASCs were below the detection level in non-draining axillary, brachial and popliteal lymph nodes (data not shown), it can’t be entirely ruled out that ASCs from other locations such as GALT may also contribute to alloantibody production after transplantation.
A previous study of sensitized patients by Perry et al. estimated frequencies of BM cells secreting antibody against specific HLA antigens to be as low as 0.5/1 × 106 (45). Analogously, we found that BM cells secreting IgG antibodies binding a representative donor MHC class I molecule, Dd, were extremely rare in sensitized mice and at earlier stages after transplantation (0.5–2/1 × 106, Figure 5). The frequencies of ASCs in BM significantly increased by six weeks post transplant and reached 16/1 × 106. The dynamics of spleen ASC frequencies was exactly the opposite with the numbers peaking at day 7 post transplant and then steadily declining by week six (from 100/1 × 106 to 30/1 × 106 and from 400/1 × 106 to 30/1 × 106 in non-sensitized and sensitized recipients, respectively).
Our data indicate that the sensitization status of the recipient does not alter the anatomical distribution of the allo-reactive ASCs in that the spleen cells appear to be the major contributors to allo-reactive antibody production in sensitized recipients as well. However, the results do not exclude the possibility that BM ASCs contribute to the increased levels of early antibody production in sensitized recipients. While BM cells did not appear to secrete donor-reactive IgG2c and IgG1 at day 7 (Figure 3), they contained higher frequencies of total and donor MHC-reactive IgG producing cells (Figures 4A and 5A) and produced anti-donor IgG 2b (Figure 3). In addition, while sensitized recipients in our study were uniformly exposed to donor alloantigens four weeks prior to transplantation, the contribution of various anatomical compartments may be influenced by the time elapsed after initial sensitization and/or by the strength of the priming stimulus.
As anticipated, previous sensitization with donor alloantigens resulted in an accelerated allo-reactive antibody response following heart allograft placement. As immunization with donor cells induced high titers of IgG2b but not of other IgG isotypes, the accelerated response cannot be entirely attributed to the presence of allospecific antibodies prior to transplantation. The numbers of total IgG and donor-reactive IgG secreting cells in sensitized recipients increased significantly by day 7 after transplantation compared to pre-transplant levels, suggesting the rapid differentiation of memory B cells to PCs as well as robust secondary T helper responses.
Unexpectedly, in the absence of immunosuppression the donor-reactive antibody responses in sensitized and non-sensitized recipients became comparable at later stages after transplantation. Furthermore, the findings in CCR5−/− mice indicate that even in non-sensitized recipients, anti-donor humoral responses can be accelerated in the presence of higher frequiencies of donor-reactive helper T cells. The clinical implication of these results is that the magnitude of de novo DSA production in transplant recipients that have negative PRA and/or cross-match but contain increased frequencies of donor-reactive helper T cells may reach that observed in sensitized recipients.
In summary, our study identifies the spleen as the major source of donor-reactive alloAb in both sensitized and non-sensitized recipients. These findings contribute to the understanding of humoral responses after transplantation and will facilitate future therapies targeting the development and persistence of ASCs producing donor-reactive alloantibody.
Supplementary Material
Acknowledgments
This work was supported by a 1P01 AI087586 grant from the NIH (AV, WMB, RLF) and by a research grant from the Roche Organ Transplantation Research Foundation (AV).
We thank Nina Volokh, Earla Biekert and Katayoun Ayasoufi for expert technical assistance.
Abbreviations
- AMR
antibody mediated rejection
- ASC
antibody secreting cell
- BM
bone marrow
- CFA
complete Freund’s adjuvant
- DSA
donor specific alloantibody
- MFI
mean fluorescence intensity
- MST
mean survival time
- PBST
PBS with 0.025% Tween
- PBS-1%BSA
PBS with 1% bovine serum albumin
- PC
plasma cell
- PFU
plaque forming units
- WT
wild type
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article:
References
- 1.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5(10):807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 2.Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, et al. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant. 2007;7(4):832–841. doi: 10.1111/j.1600-6143.2006.01686.x. [DOI] [PubMed] [Google Scholar]
- 3.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4(7):1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74(8):1192–1194. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 5.Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12(3):574–582. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 6.Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18(3):278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 8.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarlinton DM, Batista F, Smith KG. The B-cell response to protein antigens in immunity and transplantation. Transplantation. 2008;85(12):1698–1704. doi: 10.1097/TP.0b013e3181777a39. [DOI] [PubMed] [Google Scholar]
- 10.Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol Rev. 2010;237(1):140–159. doi: 10.1111/j.1600-065X.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 11.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10(3):252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 12.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 13.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 14.Marques CP, Kapil P, Hinton DR, Hindinger C, Nutt SL, Ransohoff RM, et al. CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J Virol. 2011;85(13):6136–6147. doi: 10.1128/JVI.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangster M, Hyland L, Sealy R, Coleclough C. Distinctive kinetics of the antibody-forming cell response to Sendai virus infection of mice in different anatomical compartments. Virology. 1995;207(1):287–291. doi: 10.1006/viro.1995.1079. [DOI] [PubMed] [Google Scholar]
- 16.Sangster MY, Topham DJ, D’Costa S, Cardin RD, Marion TN, Myers LK, et al. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol. 2000;164(4):1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 17.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69(3):1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin D, Levine B, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–161. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 19.Hooper DC, Phares TW, Fabis MJ, Roy A. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl Trop Dis. 2009;3(10):e535. doi: 10.1371/journal.pntd.0000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschen SI, Stohlman SA, Ramakrishna C, Hinton DR, Atkinson RD, Bergmann CC. CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur J Immunol. 2006;36(3):603–612. doi: 10.1002/eji.200535123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyor WR, Griffin DE. Virus specificity and isotype expression of intraparenchymal antibody-secreting cells during Sindbis virus encephalitis in mice. J Neuroimmunol. 1993;48(1):37–44. doi: 10.1016/0165-5728(93)90056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones PD, Ada GL. Influenza virus-specific antibody-secreting cells in the murine lung during primary influenza virus infection. J Virol. 1986;60(2):614–619. doi: 10.1128/jvi.60.2.614-619.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens GP, Bennett JL, Gilden DH, Burgoon MP. The B cell response in multiple sclerosis. Neurol Res. 2006;28(3):236–244. doi: 10.1179/016164106X98099. [DOI] [PubMed] [Google Scholar]
- 24.Stegall MD, Dean PG, Gloor J. Mechanisms of alloantibody production in sensitized renal allograft recipients. Am J Transplant. 2009;9(5):998–1005. doi: 10.1111/j.1600-6143.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 25.Perry DK, Pollinger HS, Burns JM, Rea D, Ramos E, Platt JL, et al. Two novel assays of alloantibody-secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant. 2008;8(1):133–143. doi: 10.1111/j.1600-6143.2007.02039.x. [DOI] [PubMed] [Google Scholar]
- 26.Amano H, Bickerstaff A, Orosz CG, Novick AC, Toma H, Fairchild RL. Absence of recipient CCR5 promotes early and increased allospecific antibody responses to cardiac allografts. J Immunol. 2005;174(10):6499–6508. doi: 10.4049/jimmunol.174.10.6499. [DOI] [PubMed] [Google Scholar]
- 27.Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, et al. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am J Transplant. 2008;8(3):557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 28.Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, et al. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J Immunol. 2007;179(8):5238–5245. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172(9):5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 30.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol. 2006;176(2):770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4(2):121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant. 2008;8(3):497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 34.Amano H, Bickerstaff A, Orosz CG, Novick AC, Toma H, Fairchild RL. Absence of recipient CCR5 promotes early and increased allospecific antibody responses to cardiac allografts. J Immunol. 2005;174(10):6499–6508. doi: 10.4049/jimmunol.174.10.6499. [DOI] [PubMed] [Google Scholar]
- 35.Benner R, van Oudenaren A, Koch G. Induction of antibody formation in mouse bone marrow. Immunological Methods. 1981;II:247–261. [Google Scholar]
- 36.Valujskikh A, Heeger P. Enzyme linked immunosorbent spot (ELISPOT) assay for detection of alloreactive cytokine-secreting cells - detailed methods. Graft. 2000;3(5):250–258. [Google Scholar]
- 37.Alexandre GP, Squifflet JP, De Bruyere M, Latinne D, Reding R, Gianello P, et al. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19(6):4538–4542. [PubMed] [Google Scholar]
- 38.Lucas JG, Co JP, Nwaogwugwu UT, Dosani I, Sureshkumar KK. Antibody-mediated rejection in kidney transplantation: an update. Expert Opin Pharmacother. 2011;12(4):579–592. doi: 10.1517/14656566.2011.525219. [DOI] [PubMed] [Google Scholar]
- 39.Sawada T, Fuchinoue S, Kawase T, Kubota K, Teraoka S. Preconditioning regimen consisting of anti-CD20 monoclonal antibody infusions, splenectomy and DFPP-enabled non-responders to undergo ABO-incompatible kidney transplantation. Clin Transplant. 2004;18(3):254–260. doi: 10.1111/j.1399-0012.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- 40.Sawada T, Fuchinoue S, Teraoka S. Successful A1-to-O ABO-incompatible kidney transplantation after a preconditioning regimen consisting of anti-CD20 monoclonal antibody infusions, splenectomy, and double-filtration plasmapheresis. Transplantation. 2002;74(9):1207–1210. doi: 10.1097/00007890-200211150-00001. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan B, Gangemi A, Thielke J, Oberholzer J, Sankary H, Benedetti E. Successful rescue of refractory, severe antibody mediated rejection with splenectomy. Transplantation. 2007;83(1):99–100. doi: 10.1097/01.tp.0000243739.31440.2b. [DOI] [PubMed] [Google Scholar]
- 42.Locke JE, Zachary AA, Haas M, Melancon JK, Warren DS, Simpkins CE, et al. The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. Am J Transplant. 2007;7(4):842–846. doi: 10.1111/j.1600-6143.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 43.Baldwin WM, 3rd, Hendry W, Birinyi LK, Jr, Tilney NL. Immune responses to organ allografts. I. Intense B-cell response to heart allografts in lymphoid tissues of unmodified rats. Lab Invest. 1979;40(6):695–702. [PubMed] [Google Scholar]
- 44.Brown K, Badar A, Sunassee K, Fernandes MA, Shariff H, Jurcevic S, et al. SPECT/CT lymphoscintigraphy of heterotopic cardiac grafts reveals novel sites of lymphatic drainage and T cell priming. Am J Transplant. 2011;11(2):225–234. doi: 10.1111/j.1600-6143.2010.03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry DK, Pollinger HS, Burns JM, Rea D, Ramos E, Platt JL, et al. Two novel assays of alloantibody-secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant. 2008;8(1):133–143. doi: 10.1111/j.1600-6143.2007.02039.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.