Abstract
The adipokine leptin is primarily produced by white adipose tissue (AT) and is a potent monocyte/macrophage chemoattractant in vitro. The long form of the leptin receptor (LepR) is required for monocyte/macrophage chemotaxis towards leptin. In this study, we examined the effects of haematopoietic LepR as well as LepR with C-C Chemokine Receptor 2 (CCR2) deficiency (double knockout; DKO) on macrophage recruitment to AT after two different periods of high fat diet (HFD) feeding. Briefly, 8 week old C57BL/6 mice were transplanted with bone marrow from LepR+/+, LepR-/- or DKO donors (groups named BM-LepR+/+, BMLepR-/- and BM-DKO, respectively), and were placed on a HFD for 6 or 12 weeks. At the end of the study, macrophage infiltration and the inflammatory state of AT were evaluated by real-time RT-PCR, histology, and flow cytometry. In addition, glucose and insulin tolerance were assessed at both time points. Our results showed no differences in macrophage accumulation or AT inflammatory state between the BM-LepR+/+ and BM-LepR-/- mice after 6 or 12 weeks of HFD feeding; any effects observed in the BM-DKO were attributed to the haematopoietic deficiency of CCR2. In addition, no changes in glucose or insulin tolerance were observed between groups after either period of HFD feeding. Our findings suggest that although leptin is a potent chemoattractant in vitro, haematopoietic LepR deficiency does not affect macrophage accumulation in AT in early to moderate stages of diet induced obesity.
Keywords: Leptin, leptin receptor, macrophage, adipose tissue, obesity
INTRODUCTION
Accumulation of inflammatory macrophages in adipose tissue (AT) during obesity has been shown to correlate with AT inflammation and subsequent insulin resistance (IR) (Weisberg, McCann et al. 2003; Xu, Barnes et al. 2003). In addition, the polarization of the infiltrating macrophages is known to be an important factor in the inflammatory state of AT (Lumeng, Bodzin et al. 2007; Lumeng, DelProposto et al. 2008). Thus, understanding the mechanism(s) of macrophage recruitment to AT could lead to the development of therapies against IR and type 2 diabetes. Adipokines, which are hormones secreted from AT, could act as chemoattractants and contribute to macrophage recruitment into AT. The adipokine leptin has emerged as a strong candidate given its known role in immune regulation (La Cava and Matarese 2004). Leptin is a 16 kDa soluble protein encoded by the Ob gene and is expressed primarily by white adipose tissue, such that circulating levels of leptin are positively correlated with levels of adiposity and AT macrophage accumulation (Halaas, Gajiwala et al. 1995; Coenen, Gruen et al. 2007).
Leptin acts primarily through the long form of the leptin receptor (LepR) and plays an important role in weight regulation through its action in the hypothalamus (Leshan, Bjornholm et al. 2006). Mice that lack either leptin or the LepR are characterized by severe hyperphagia and morbid obesity, as well as other hormonal abnormalities (Robertson, Leinninger et al. 2008). Previous studies have shown that leptin promotes neutrophil and monocyte chemotaxis as well as the activation of monocytes/macrophages leading to secretion of pro-inflammatory cytokines and the upregulation of inducible nitric oxide synthase (iNOS) (Zarkesh-Esfahani, Pockley et al. 2001; Dixit, Mielenz et al. 2003; Gruen, Hao et al. 2007). We have shown that leptin is a potent monocyte chemoattractant in vitro and that this chemotaxis is LepR-dependent (Gruen, Hao et al. 2007). In addition, we have shown that expression of Emr1 (the gene for F4/80) in AT, an indicator of macrophage infiltration, is positively correlated with circulating levels of leptin during obesity (Supplementary Figure 1; r2 = 0.6973; P<0.0001) (Coenen, Gruen et al. 2007). Given that leptin is primarily secreted from AT and that its circulating levels are positively correlated with increases in macrophage infiltration, we hypothesize that leptin could act as a monocyte chemoattractant that regulates macrophage recruitment to AT during obesity. We designed a bone marrow transplant study to determine if haematopoietic deficiency of the functional long-form of the LepR, which we have shown to be required for monocyte chemotaxis towards leptin in vitro, would lead to decreases in macrophage recruitment to AT during HFD feeding. We transplanted bone marrow from LepR-/- and DKO mice into wild type (WT) recipients and placed these mice on a HFD for 6 or 12 weeks. These time points were chosen because of previously shown kinetics of macrophage recruitment to AT (Nishimura, Manabe et al. 2009). We examined macrophage infiltration to AT by flow cytometry, histology and real-time RT-PCR. Our data provides evidence that haematopoietic deficiency of the LepR does not affect macrophage recruitment to AT or insulin sensitivity during HFD-induced obesity.
MATERIALS AND METHODS
Mice and Diets
All animal care and experimental procedures were performed with approval from the Institutional Animal Care and Use Committee of Vanderbilt University. The LepR+/+, LepR-/- and CCR2-/- mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and were on a C57BL/6 background. DKO donor mice were generated by crossing CCR2-/- with LepR+/- mice. All studies were performed with 8 week old male donor and recipient mice. Mice were fed ad libitum and given free access to water. Mice were kept on antibiotic water for 1 week prior to bone marrow transplant (BMT) until 4 weeks post-BMT. Mice were then placed on a 60% fat diet from Research Diets, Inc. (D12492) with a caloric density of 5.24 kcal/gm; starting at 4 weeks post-BMT for a total of 6 or 12 weeks (Figure 1). Because we are only interested in changes between the two genotypes and not the diet effects we did not use chow fed mice in the original study; however, we performed an additional study in which we showed that HFD fed LepR+/+ mice have increased macrophage infiltration and become insulin resistant in comparison to LepR+/+ mice fed a chow diet (Supplementary Figure 2). Thus, we have the ability to identify any changes in these parameters in mice fed HFD for only 6 weeks.
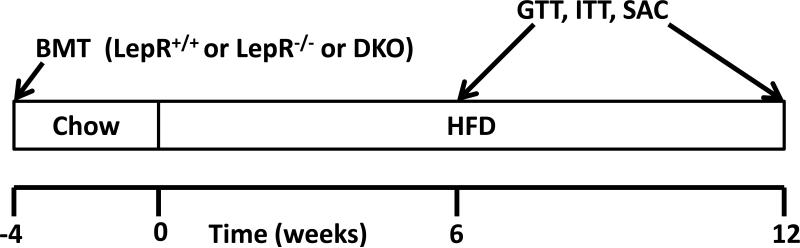
Figure 1. Study design.
C57BL/6 mice were transplanted with either LepR+/+, LepR-/- or DKO (LepR-/-; CCR2-/-) bone marrow at 8 weeks of age. After 4 weeks mice were placed on a 60% diet (HFD) for 6 or 12 weeks (n = 10 per group). GTTs and ITTs were performed after 6 and 12 weeks. Mice were sacrificed after the 6 or 12 week period for analysis of AT macrophage infiltration and inflammation.
Bone Marrow Transplantation
Bone marrow cells were collected from LepR+/+, LepR-/- and DKO donor mice and were injected into the retro-orbital venous plexus of lethally irradiated WT recipient mice. Complete reconstitution of the desired bone marrow was confirmed by PCR of splenic cells using primers specific for the db allele and the Neo cassette insertion in the CCR2-/- deficient mice (data not shown). The BM-LepR+/+ controls in this study were also used as negative controls for a different study performed simultaneously (Gutierrez, Kennedy et al. 2011). In addition, recipients of CCR2-/- marrow (BM-CCR2-/-) were included in the previous paper. There were no significant differences in any parameter studied between the CCR2-/- and DKO recipients. Thus, only the DKO mice are shown in the current report.
Body weight, Body Composition and Food Intake
Body weight and food intake were measured weekly for the duration of the study. Total lean, fat, and free fluid mass were measured by nuclear magnetic resonance (NMR) using a Bruker Minispec (Woodlands Texas) in the Vanderbilt Mouse Metabolic Phenotyping Centre (MMPC) at baseline, 6 and 12 weeks after placement on a HFD.
Insulin and Glucose Tolerance Tests
Mice were fasted for 5 h and baseline glucose levels were measured using a LifeScan One Touch Ultra glucometer (Johnson & Johnson, Northridge, CA) via the tail vein. Mice were then injected intraperitoneally with either 0.4 U (6 weeks) and 0.5 U (12 weeks) insulin per Kg of lean mass; or 2 g (6 weeks) and 1 g (12 weeks) dextrose per kg of lean mass. Glucose levels were then assessed at 15, 30, 45, 60, 90 and 150 min post-injection.
Plasma Collection and Measurements
Mice were fasted for 5 h and bled from the retro-orbital venous plexus using heparinized collection tubes. Plasma was separated by centrifugation and stored at -80 °C. Plasma insulin levels were measured using an ELISA kit from Millipore, Inc (Billerica, MA). Leptin levels were assessed at the Vanderbilt University Hormone Core.
Real-time RT-PCR
RNA extraction was performed with an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The iScript cDNA synthesis kit (BioRad, Hercules, CA) was used for reverse transcriptase reactions. Real-time RT-PCR experiments were performed using an iQ5 thermal cycler. The reactions were carried out using IQ Supermix (BioRad) and FAM conjugated Assay-on-Demand (Applied Biosystems, Foster City, California) primer/probe sets normalized to 18S. The following genes were assessed: 18S (4352930E), Emr1 (Mm00802530_m1), Arg1 (Mm01190441_g1), Cd68 (Mm00839636_g1), Tnf (Mm00443258_m1), Nos2 (Mm00440502_m1), Cd163 (Mm0118511), Mgl1 (Mm00546125_g1), Mgl2 (Mm00460844_m1), Il6 (Mm00446190_m1), and Il1b (Mm9999061_mH). The data was analyzed using the Pfaffl method (Pfaffl 2001) and presented as expression relative to the BM-LepR+/+ controls at each respective time point.
Histology
Epididymal AT was harvested from mice, weighed, and a portion was fixed overnight in 10% (v/v) formalin, transferred to 70% (v/v) ethanol, and paraffin embedded. Tissue was cut into 7μm sections and stained with 0.1% (w/v) toluidine blue O solution (TBO) (Newcomer Supply, Middleton, Wisconsin) as previously described (Coenen, Gruen et al. 2007).
Stromal Vascular Fraction (SVF) Separation
Epididymal AT pads were excised and minced in 3 ml of 0.5 % (w/v) BSA in PBS and placed on ice. Subsequently, 3 ml of 2 mg/ml collagenase was added to the minced fat and incubated at 37°C for 20 min with shaking. The cell suspension was filtered through a 100-μm filter and then spun at 300 g for 10 min to separate floating adipocytes from the SVF pellet. The SVF pellet was then resuspended in 3 ml of ACK lysis buffer and incubated at RT for 5 min for red blood cell lysis, followed by dilution in 10 ml of PBS. The cells were then washed twice with PBS.
Flow Cytometry
Stromal vascular and white blood cells were first incubated with Fc block for 5 min at RT, followed by incubation for 30 min at 4 °C with fluorophore-conjugated antibodies: F4/80-APC (eBioscience), Ly6C-FITC (BD Biosciences), Ly6G-PE (BD Biosciences), CD11b-APC-Cy7 (BD Bioscience). DAPI (0.2 μg/ml) was added to each sample as a viability stain 10 min before flow cytometry analysis. SVCs were processed on a MACS Quant analyzer (Miltenyi Biotech). Blood cells were processed using a 5 Laser LSRII machine in the Vanderbilt Flow Cytometry Core. All data was analyzed using FlowJo software version 7.6.1.
Statistics
GraphPad Prism 4.0 software was used for all statistical analyses. Data was analyzed using one way ANOVA to test differences between the 3 experimental groups and two-way ANOVA to compare measurements with two different variables (i.e. GTTs and ITTs). Outliers were excluded from the data for each individual parameter if outside the range of the mean ± 2SD and P≤0.05 was considered significant.
RESULTS
Metabolic Profile
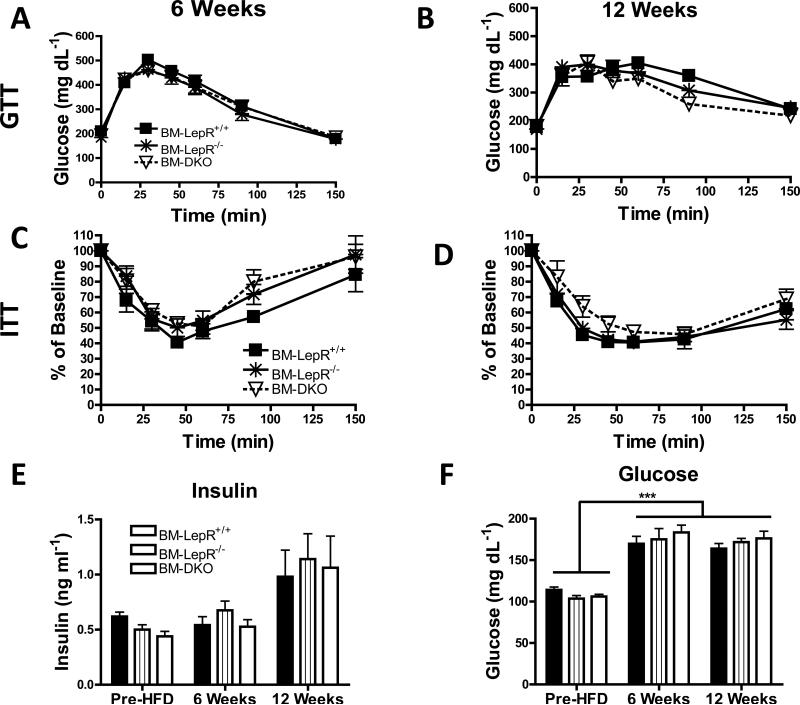
Male C57Bl/6 mice were transplanted with Lepr+/+, LepR-/- or DKO marrow followed by HFD feeding for 6 or 12 weeks as described in the Methods section (Figure 1). There were no differences in body weight (Figure 2A) or food intake (data not shown) between the groups at any point during the study. Likewise, there were no differences in adiposity among genotypes at any time although all groups increased in adiposity after 6 and 12 weeks of HFD feeding (Figure 2B). Epididymal fat pad weight (Figure 2C) and circulating leptin levels (Figure 2D) were not different among genotypes at any time point. In accordance with the NMR adiposity data, epididymal AT weight and leptin levels were generally elevated between the 6 and 12 week time points for each group (Figure 2C-D). Glucose and insulin tolerance tests were performed after 6 (Figure 3A & C) and 12 (Figure 3B & D) weeks of HFD feeding. All groups responded similarly to the intraperitoneal glucose and insulin challenges after both periods of HFD feeding. In general, fasting insulin levels were increased for all groups between the baseline measurements and 12 weeks of HFD feeding (Figure 3E). Fasting glucose levels were significantly increased (P<0.001) in all groups between the pre-HFD and both the 6 and 12 week time points (Figure 3F).
Figure 2. Weight gain was similar among groups upon HFD feeding.
A) Average weekly body weight of BM-LepR+/+, BM-LepR-/- and BM-DKO mice pre- and post-HFD. B) Percent adiposity (total fat tissue divided by total body weight) determined by nuclear magnetic resonance. C) Epididymal AT weight. D) Circulating plasma leptin levels assessed by ELISA. Data are presented as mean ± SEM. For pre-HFD up to 6 weeks post-HFD, n=20 mice per group. For 12 weeks post-HFD, n=9-10 mice per group.
Figure 3. All groups have similar glucose or insulin tolerance.
Glucose tolerance tests were performed by injecting mice intraperitoneally with dextrose at a concentration of 2 g (6 weeks) or 1 g (12 weeks) per kg lean mass after a 5 h morning fast. Glucose tolerance curves for all groups after A) 6 weeks and B) 12 weeks of HFD feeding. Insulin tolerance tests were performed by injecting mice intraperitoneally with 0.4 U (6 weeks) or 0.5 U (12 weeks) human insulin per kilogram lean mass after a 5 h morning fast. Insulin tolerance curves for all groups after C) 6 weeks and D) 12 weeks of HFD feeding. E) Fasting insulin levels were measured by ELISA at the pre-HFD, 6 and 12 weeks time points. F) Fasting glucose levels were assessed at the pre-HFD, 6 and 12 weeks time points. Data are presented as mean ± SEM. For GTT and ITT data, n=9-10 mice per group for 6 week data and n=5 mice per group for 12 week data. For fasting insulin and glucose data, n= 8-10 per group.
*** P<0.001 for all groups between Pre-HFD and 6 or 12 weeks
Blood Monocytes
Two phenotypic and functional subsets of mature blood monocytes have been described: Ly6Clo and Ly6Chi (Geissmann, Manz et al. 2010). The Ly6Chi circulating monocyte subset is thought to be the source of recruited inflammatory macrophages to inflamed tissues. The circulating levels of blood monocytes and neutrophils were assessed by flow cytometry after 12 weeks of HFD feeding by first gating on all live CD11b+ cells. The BM-LepR-/- mice had a ~40% (P<0.05) reduction in circulating Ly6Chi monocytes, and BM-DKO mice a ~90% (P<0.001) reduction, when compared to the BM-LepR+/+ controls (Figure 4A-D). No differences were observed in the Ly6Clo monocyte subset or the percentage of circulating neutrophils between groups (Figure 4E-F).
Figure 4. BM-LepR-/- mice have decreased circulating “classical” monocytes.
Blood cells were isolated from 12 week HFD fed recipient mice and analyzed by flow cytometry. Representative plots showing expression of Ly6C after gating on all CD11b+ live cells. A) BMLepR+/+, B) BM-LepR-/-, and C) BM-DKO. Quantification of the percentage of D) CD11b+Ly6Chi, E) CD11b+Ly6Clo and F) CD11b+Ly6CmidLy6Ghi cells in all groups (mean ± SEM; n = 4-5 mice per group).
* P<0.05 between BM-LepR+/+ and BM-LepR-/- mice
*** P<0.001 between BM-LepR+/+ and BM-DKO mice
^ P<0.05 between BM-LepR-/- and BM-DKO mice
Macrophage Accumulation in AT
The accumulation of macrophages in AT after 6 and 12 weeks of HFD feeding was evaluated by histology, real-time RT-PCR and flow cytometry. TBO staining showed that there were no overt differences in overall immune infiltration between groups after either period of HFD feeding (Figure 5A-F). Real-time RT-PCR gene expression analysis in total AT for the macrophage markers Emr1 and Cd68 showed no differences between groups after 6 (Figure 5G) or 12 weeks (Figure 5H) of HFD feeding.
Figure 5. Histology and real-time RT-PCR analysis of F4/80+ cells in AT.
Representative AT TBO images of A) BM-LepR+/+, B) BM-LepR-/- and C) BM-DKO mice after 6 weeks of HFD feeding. Representative AT TBO images of D) BM-LepR+/+, E) BM-LepR-/- and F) BM-DKO mice after 12 weeks of HFD feeding. Real-time RT-PCR gene expression analysis of Emr1 (F4/80) and Cd68 in total AT after G) 6 and H) 12 weeks of HFD feeding (mean ± SEM; n = 10 mice per group).
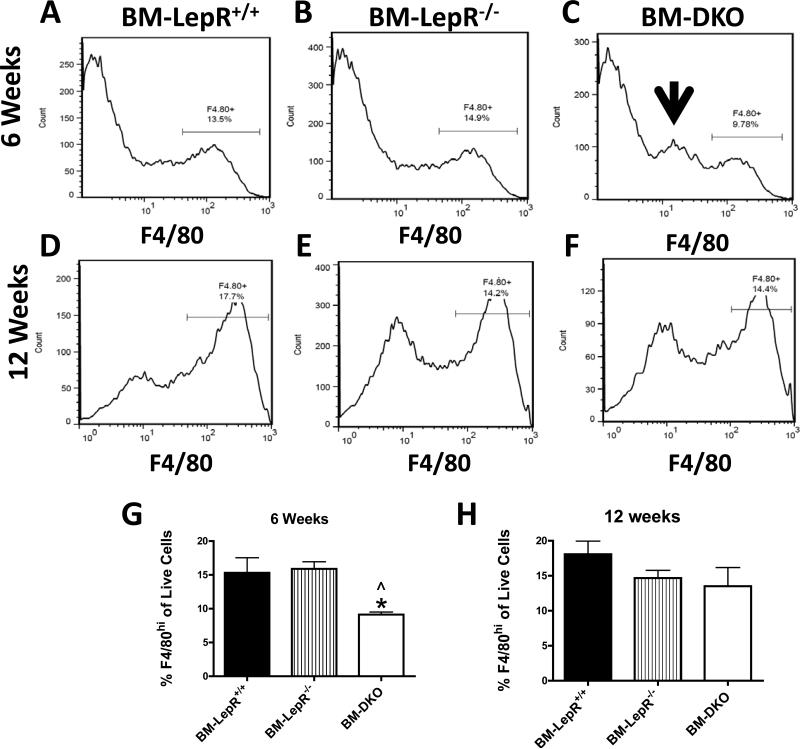
The stromal vascular fraction (SVF) was separated from the adipocytes of the epididymal AT by collagenase digestion. Macrophages from the SVF were quantified by flow cytometry using an antibody against F4/80. The representative histograms illustrate the percentage of live F4/80hi cells in the SVF after 6 (Figure 6A-C) and 12 weeks (Figure 6D-F) of HFD feeding. Quantification of the percentage of F4/80hi cells in the SVF showed a significant decrease (P<0.05) in the BM-DKO group when compared to the BM-LepR+/+ and BM-LepR-/- groups after 6 weeks of HFD feeding (Figure 6G). There were no differences in the percentage of F4/80hi cells in the AT of any group after 12 weeks of HFD feeding (Figure 6H). The BM-DKO mice had an increase in a discrete F4/80lo myeloid population (Figure 6C, arrow) which is due to the deficiency in haematopoietic CCR2, as we have previously reported (Gutierrez, Kennedy et al. 2011).
Figure 6. Flow cytometry analysis of stromal vascular cells.
Stromal vascular cells were isolated and the percentage of live F4/80+ cells was quantified. Representative histograms of F4/80hi cells for A) BM-LepR+/+, B) BM-LepR-/-, and C) BM-DKO mice after 6 weeks of HFD feeding; D) BM-LepR+/+ , E) BM-LepR-/- and F) BM-DKO mice after 12 weeks of HFD feeding. G-H) Quantification of the percentage of F4/80hi live cells in the SVF of all groups after 6 or 12 weeks of HFD feeding. Data represent mean ± SEM of 4-5 mice per group. Arrow indicates F4/80lo cells found in BM-DKO mice.
* P<0.05 between BM-LepR+/+ and BM-DKO mice
^ P<0.05 between BM-LepR-/- and BM-DKO mice
AT inflammatory State
The inflammatory state of total AT was determined by assessing the gene expression of several pro- and anti-inflammatory markers by real-time RT-PCR. Analysis of the expression of the inflammatory markers Tnf and Nos2 showed no differences in these genes among groups after 6 weeks on the HFD (Figure 7A). There was a significant decrease (P<0.001) in Nos2 expression in the BM-DKO group when compared to the BM-LepR+/+ and BM-LepR-/- groups at the 12 week time point (Figure 7B). Additionally, assessment of the anti-inflammatory “M2” markers Arg1, Mgl1 and Mgl2 showed that there was a significant increase (P<0.05) in Arg 1 expression in the BM-DKO mice in comparison to the BM-LepR+/+ mice after 6 weeks of HFD feeding (Figure 7A). Arg1, Mgl1 and Mgl2 were all significantly increased (P<0.05) in the BM-DKO group in comparison to the BM-LepR+/+ and/or BM-LepR-/- groups after 12 weeks of HFD (Figure 7B). Bone marrow LepR deficiency alone did not impact anti-inflammatory gene expression in AT.
Figure 7. Pro- and anti-inflammatory gene expression.
Real-time RT-PCR gene expression analysis of pro- and anti-inflammatory markers was performed on RNA isolated from total AT. Expression of Tnf, Nos2, Arg1, Mgl1 and Mgl2 in all groups after A) 6 weeks and B) 12 weeks of HFD feeding (mean ± SEM; n = 10 mice per group).
* P<0.05 between BM-LepR+/+ and BM-DKO mice
** P<0.01 between BM-LepR+/+ and BM-DKO mice
*** P<0.01 between BM-LepR+/+ and BM-DKO mice
^ P<0.05 between BM-LepR-/- and BM-DKO mice
DISCUSSION
Inflammatory macrophage infiltration into AT during obesity has been shown to be temporally correlated with local and systemic inflammation and IR (Weisberg, McCann et al. 2003; Xu, Barnes et al. 2003; Hotamisligil 2006; Lumeng, Bodzin et al. 2007). Several genetic models that either disrupt macrophage recruitment to AT (Kanda, Tateya et al. 2006; Weisberg, Hunter et al. 2006; Odegaard, Ricardo-Gonzalez et al. 2007; Surmi, Webb et al. 2010) or promote an adipose tissue macrophage switch towards an “M2” or anti-inflammatory phenotype (Bouhlel, Derudas et al. 2007; Odegaard, Ricardo-Gonzalez et al. 2007; Westcott, Delproposto et al. 2009) have been explored. Despite the great progress made in this field in the past decade, methods to actively decrease macrophage recruitment to AT or diminish their inflammatory impact on this tissue are poorly understood.
We and others have shown that the adipokine leptin plays an important role in macrophage chemotaxis in vitro directly via its ability to act as a macrophage chemoattractant (Gruen, Hao et al. 2007) or indirectly through the upregulation of adhesion molecules on endothelial cells (Curat, Miranville et al. 2004). Additionally, leptin has been shown to play a role in eosinophil (Kato, Ueki et al. 2011) and neutrophil (Caldefie-Chezet, Poulin et al. 2001; Caldefie-Chezet, Poulin et al. 2003) chemotaxis. These findings have led to the speculation that leptin, which has potent monocyte chemotactic activity, could be an important mediator of monocyte/macrophage chemotaxis to AT, where this adipokine is mainly produced (Surmi and Hasty 2008; Bourlier and Bouloumie 2009; Gutierrez, Puglisi et al. 2009; Maeda, Kiguchi et al. 2009; Vona-Davis and Rose 2009; Wood, de Heredia et al. 2009; Conde, Scotece et al. 2010; Fernandez-Riejos, Najib et al. 2010). This is of particular interest during the initial stages of inflammatory macrophage accumulation in AT when recruitment is positively correlated with adiposity and circulating leptin levels (Supplementary Figure 1) (Coenen, Gruen et al. 2007). However, this hypothesis had not been tested in vivo. In this study we assessed whether haematopoietic LepR deficiency led to a disruption in macrophage recruitment to AT during obesity with the hypothesis that BM-LepR-/- chimeras would have reduced macrophage accumulation and improvements in glucose and insulin tolerance. Given that compensation by other cytokines was possible, we generated BM-DKO chimeras that have a haematopoietic deficiency in both LepR and CCR2.
Our results showed that haematopoietic LepR deficiency led to a significant decrease in the percentage circulating Ly6Chi monocytes (Figure 4B & D). A similar but more dramatic phenotype has been observed in mice with CCR2 deficiency (Tsou, Peters et al. 2007; Gutierrez, Kennedy et al. 2011), in which Ly6Chi monocytes fail to egress from the bone marrow. In fact, the BM-DKO mice showed an identical phenotype to that of the BM-CCR2-/- mice (Figure 4C-D), indicating that having both CCR2 and LepR deficiency does not confer an additive or synergistic effect on the decreases in circulating inflammatory monocyte levels. The mechanism responsible for the reduction of Ly6Chi monocytes in BM-LepR-/- mice is unknown; however, because the levels of circulating monocyte in the BM-DKO mice are identical to those of the BM-CCR2-/- mice, it is likely that the BM-LepR-/- mutation is not affecting inflammatory monocyte egress but instead their proliferation and/or differentiation in the bone marrow. This hypothesis remains to be tested.
The main end point of this study was to determine if the deficiency of LepR led to a reduction in macrophage recruitment to AT. Previous studies have shown that the initial process of inflammatory macrophage accumulation in AT occurs between 6 and 12 weeks of HFD feeding (Nishimura, Manabe et al. 2009; Shaul, Bennett et al. 2010); thus we used these time points to assess macrophage accumulation using multiple approaches. Our histology (Figure 5AF), gene expression (Figure 5G-H) and flow cytometry (Figure 6) data showed no protective effect of LepR deficiency on macrophage accumulation in AT after 6 or 12 weeks of HFD feeding. There was a significant decrease (P<0.05) in the percentage of F4/80hi macrophages in the BM-DKO group after 6 weeks of HFD feeding (Figure 6G); however, this can be accounted for by the haematopoietic CCR2 deficiency, which leads to a decrease in F4/80hi cells and the accumulation of F4/80lo cells (Gutierrez, Kennedy et al. 2011). In addition, no changes in glucose or insulin tolerance were observed between the three groups (Figure 3A-D) despite the fact that all mice became hyperglycemic and hyperinsulinemic in response to the HFD feeding (Figure 3E-F). Thus, we can conclude that haematopoietic LepR deficiency alone or in combination with haematopoietic CCR2 deficiency does not affect inflammatory macrophage recruitment to AT or insulin sensitivity after 6 or 12 weeks of HFD feeding.
The negative results are not a consequence of the inability of these mice to have increased macrophage infiltration, become insulin resistant or glucose intolerant during HFD feeding. We performed an additional study in which we show that LepR+/+ mice fed a HFD have increased macrophage infiltration, glucose intolerance and insulin resistance when compared to LepR+/+ mice fed a chow diet (Supplementary Figure 2). Thus, within the limits of the sensitivity of our assays, haematopoietic LepR deficiency does not affect macrophage recruitment or insulin sensitivity during obesity.
Similar negative results have been obtained by our group and others when assessing inflammatory macrophage recruitment to AT after deletion of a single chemokine or chemokine receptor (Chen, Mumick et al. 2005; Inouye, Shi et al. 2007; Kirk, Sagawa et al. 2008; Surmi, Webb et al. 2010). Accordingly, the current study provides evidence for the redundancy of chemokines and chemokine receptors in the innate immune system. Despite the elimination of two receptors that mediate the function of four different chemokines (leptin, CCL2, CCL7 and CCL8) that are upregulated in the AT during obesity, macrophage infiltration to AT is not disrupted. This redundancy in the chemokine system contributes to the requirement for leukocyte chemotaxis in normal immune system function, making it “robust” to naturally occurring mutations (Mantovani 1999).
Previously published studies suggest that in vitro leptin treatment leads to the activation of monocyte/macrophages and have shown a dose-dependent induction of inflammatory mediators such as IL-6 and TNF-α (Zarkesh-Esfahani, Pockley et al. 2001). Macrophages are primarily responsible for AT inflammation during obesity (Hotamisligil, Shargill et al. 1993; Hotamisligil, Arner et al. 1995; Xu, Barnes et al. 2003); thus, we tested whether haematopoietic LepR deficiency led to changes in the inflammatory state of total AT by real-time RT-PCR. Our results showed no differences in pro- or anti-inflammatory marker gene expression between BMLepR+/+ and BM-LepR-/- mice after any period of HFD feeding (Figure 7A-B). Significant differences were observed in the inflammatory gene expression of BM-DKO mice; however, these are similar to the changes in gene expression found in the BM-CCR2-/- mice (Gutierrez, Kennedy et al. 2011). As with macrophage chemotaxis, the effects of leptin on inflammatory gene expression that have been found in vitro are likely compensated in vivo by other cytokines found in the AT environment during obesity.
In conclusion, our study provides evidence that despite the potency of leptin as a monocyte/macrophage chemoattractant (Gruen, Hao et al. 2007), haematopoietic deficiency of its functional receptor does not affect macrophage accumulation in AT. Our current finding that haematopoietic LepR deficiency decreases the number of circulating Ly6Chi monocyte levels should be further studied as it could provide a novel role for leptin in hematopoiesis and/or myeloid cell migration. Our results do not rule out the possibility that leptin can affect macrophage infiltration into AT through the upregulation of adhesion molecules in the endothelial cells of the SVF, as has been previously suggested by Curat et al. (Curat, Miranville et al. 2004).
Supplementary Material
ACKNOWLEDGEMENTS
Flow Cytometry experiments were performed in the VUMC Flow Cytometry Shared Resource supported by the Vanderbilt Digestive Disease Research Centre (DK058404). Leptin ELISAs were performed at the Vanderbilt Hormone Assay Core which is part of the Vanderbilt Mouse Metabolic Phenotyping Centre supported by grant U24 DK59637. We would like to thank the Major laboratory at Vanderbilt University for their assistance with flow cytometry.
FUNDING
This work was supported by NIH grant HL089466, a supplement to this grant to D.A.G, and by grant F31DK091128 to D.A.G. A.H.H is also supported by an American Diabetes Association Career Development Award (1-07-CD-10); and D.A.G was also supported by the Molecular Endocrinology Training Grant (NIH DK07563-23).
Footnotes
AUTHOR CONTRIBUTIONS
D.A.G. designed, performed and analyzed all experiments and wrote the manuscript. A.H.H aided in designing the experiments and edited the manuscript.
DECLARATION OF INTEREST
The authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Bouhlel MA, Derudas B, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Bourlier V, Bouloumie A. Role of macrophage tissue infiltration in obesity and insulin resistance. Diabetes Metab. 2009;35(4):251–60. doi: 10.1016/j.diabet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Caldefie-Chezet F, Poulin A, et al. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69(3):414–8. [PubMed] [Google Scholar]
- Caldefie-Chezet F, Poulin A, et al. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res. 2003;37(8):809–14. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- Chen A, Mumick S, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13(8):1311–20. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- Coenen KR, Gruen ML, et al. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56(3):564–73. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- Conde J, Scotece M, et al. At the crossroad between immunity and metabolism: focus on leptin. Expert Rev Clin Immunol. 2010;6(5):801–8. doi: 10.1586/eci.10.48. [DOI] [PubMed] [Google Scholar]
- Curat CA, Miranville A, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53(5):1285–92. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Mielenz M, et al. Leptin induces growth hormone secretion from peripheral blood mononuclear cells via a protein kinase C- and nitric oxide-dependent mechanism. Endocrinology. 2003;144(12):5595–603. doi: 10.1210/en.2003-0600. [DOI] [PubMed] [Google Scholar]
- Fernandez-Riejos P, Najib S, et al. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen ML, Hao M, et al. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol. 2007;293(5):C1481–8. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- Gutierrez DA, Kennedy A, et al. Aberrant Accumulation of Undifferentiated Myeloid Cells in the Adipose Tissue of CCR2-Deficient Mice Delays Improvements in Insulin Sensitivity. Diabetes. 2011 doi: 10.2337/db11-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez DA, Puglisi MJ, et al. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep. 2009;9(1):26–32. doi: 10.1007/s11892-009-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, et al. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Inouye KE, Shi H, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56(9):2242–50. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- Kanda H, Tateya S, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Ueki S, et al. Leptin Has a Priming Effect on Eotaxin-Induced Human Eosinophil Chemotaxis. Int Arch Allergy Immunol. 2011;155(4):335–344. doi: 10.1159/000321195. [DOI] [PubMed] [Google Scholar]
- Kirk EA, Sagawa ZK, et al. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes. 2008;57(5):1254–61. doi: 10.2337/db07-1061. [DOI] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4(5):371–9. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Bjornholm M, et al. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, et al. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, DelProposto JB, et al. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, et al. Leptin derived from adipocytes in injured peripheral nerves facilitates development of neuropathic pain via macrophage stimulation. Proc Natl Acad Sci U S A. 2009;106(31):13076–81. doi: 10.1073/pnas.0903524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20(6):254–7. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Leinninger GM, et al. Molecular and neural mediators of leptin action. Physiol Behav. 2008;94(5):637–42. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, et al. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59(5):1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3(5):545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmi BK, Webb CD, et al. Absence of macrophage inflammatory protein-1{alpha} does not impact macrophage accumulation in adipose tissue of diet-induced obese mice. Am J Physiol Endocrinol Metab. 2010;299(3):E437–45. doi: 10.1152/ajpendo.00050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou CL, Peters W, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117(4):902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009;20(3):193–201. doi: 10.1016/j.cytogfr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott DJ, Delproposto JB, et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206(13):3143–56. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood IS, de Heredia FP, et al. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68(4):370–7. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkesh-Esfahani H, Pockley G, et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167(8):4593–9. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.