Perhaps the clearest organizing principle in sensory systems is the existence of maps, in which there is an orderly and systematic layout of the stimulus space on a two-dimensional array of neurons. Usually, the layout of the stimulus on the receptor sheet is reproduced so that neurons that innervate adjacent sites on the receptor sheet project to adjacent sites in the central map. Thus, in the visual system there are retinotopic maps in which layout of the visual field on the retina is reproduced across a sheet of neurons in the thalamus or cortex. Somatotopic maps of the body surface in the touch system and frequency maps in the auditory system are similar examples. In the olfactory system, the form of the representation is different in that projections from the olfactory epithelium to the first central olfactory nucleus, the olfactory bulb, are sorted according to olfactory receptor molecules (1) and not position on the olfactory epithelium, but the basic principle of sensory mapping seems to be the same.
In most systems, there are multiple repetitions of the map at both subcortical and cortical levels. Because all or most of the receptor sheet is contained in each map repetition, each separate representation is a unit in a system of serial/parallel channels making up the overall system. This organization suggests the hypothesis that each map is performing a different type of analysis on the sensory information from the receptors; perception ultimately involves integration of the information from these separate representations. Much recent effort in sensory neurophysiology has gone into trying to define the types of processing done in each map unit and the interactions among units. For example, the visual cortex consists of a complex series of interconnected units of this type (2) that differ in their responsiveness to color, motion, and other aspects of the visual stimulus. It has been suggested that this array of separate representations is organized into two pathways, the first important for motion and spatial relationships between objects and the second important for color, form, and object identification (3). However, the complexity of this system, and of other cortical systems, has made it difficult to assign particular perceptual or behavioral functions to individual maps. The paper by Metzner and Juranek (4) considers sensory maps in the brainstem electrosensory system of weakly electric fish and shows a clear example of a direct relationship between sensory maps and particular behaviors.
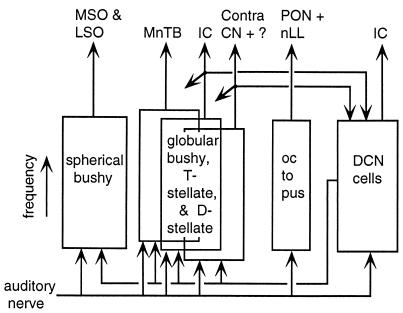
Parallel sensory maps have been defined in the brainstem in most sensory systems. For example, the mammalian cochlear nucleus contains at least six different principal-cell systems that project in parallel onto higher-order auditory nuclei (5) (Fig. 1). These systems differ dramatically in their morphology, their synaptic relationships to incoming auditory nerve fibers, their postsynaptic integration properties, the degree of interneuronal processing involved, and the targets of their axons. The cochlear nucleus serves below as a comparison with the primary electrosensory nucleus analyzed by Metzner and Juranek.
Figure 1.
Schematic of the mammalian cochlear nucleus, the equivalent of the ELL in the auditory system. Each box represents one principal cell type; the vertical extent of each box represents the cochlear frequency axis, which is fully mapped onto each cell type. The boxes for the globular bushy, T-stellate, and D-stellate cells are shown overlapped because these cell types occupy the same map in one region of the nucleus. Horizontal arrows show major interneuronal interconnections and collateral projections. The arrows at the top identify the projection sites of each cell type. MSO and LSO, medial and lateral superior olivary nuclei; MnTB, medial nucleus of the trapezoid body; IC, inferior colliculus; CN, cochlear nucleus; PON, periolivary nuclei; nLL, nuclei of the lateral lemniscus.
Weakly electric fish have an electric organ that produces an electric field in their vicinity. They are provided with an extensive electrosensory system containing two kinds of receptor cells, tuberous and ampullary, scattered along their outer surface from head to tail. By sensing changes in their own electric fields, the fish can gain information about nearby objects in the water (6). The axons innervating electroreceptors end in the electrosensory lateral line lobe (ELL) in the brainstem (7). The ELL actually contains four complete somatotopic maps of the electroreceptors on the body surface. Axons from the ampullary electroreceptors form one of the maps, and axons from the tuberous receptors form the other three (8); neurons in the three maps related to the tuberous receptors are sensitive to the electric organ discharge and are the subject of this paper.
Understanding the functional roles of the separate maps depends on three kinds of evidence. First, there is anatomical evidence. Once the connections, especially the output connections, of a map are known, it may be possible to infer the map’s function from known functions of the neurons that receive their inputs from the map. In the cochlear nucleus, for example, the bushy-cell subsystems (spherical and globular bushy cells in Fig. 1) project exclusively to the principal nuclei of the superior olivary complex (Fig. 1, MSO, LSO, and MnTB). The superior olive is the initial site of comparison of sound in the two ears; this comparison underlies the computation of sound source location based on interaural differences in the arrival time and loudness of a sound (9). These differences are produced by the acoustics of the head and vary in amplitude with the (mainly azimuthal) position of a sound source with respect to the head; thus, they convey information about the location of the source. Because cochlear-nucleus bushy cells project exclusively to the superior olive and are the only inputs to the cells analyzing interaural differences, the bushy-cell maps must be part of the sound localization system. In the case of the ELL, the parallel maps are essentially identical anatomically, do not share interconnections, and project axons to the same structures (10). Although there are some differences in the details of these projection patterns between maps, they do not appear to provide information about function.
A second source of evidence comes from the physiological properties of the neurons themselves. By analyzing the response properties of the neurons that make up a map, it may be possible to demonstrate that the neurons are encoding certain aspects of the sensory signal and not others, which may suggest a role for those neurons in the overall sensory analysis. In the example of the bushy cells, their synaptic and membrane physiology is specialized to preserve the timing of actions potentials from their auditory nerve inputs with a precision that is sufficient to support interaural time sensitivity at frequencies up to several kilohertz (5); they are the only neurons in the cochlear nucleus that do so. In the case of the ELL, there are differences in stimulus selectivity between the maps (11) that are consistent with the conclusions of this paper.
A third source of evidence is behavioral analysis of the consequences of activating or deactivating maps. If a particular behavior disappears or is modified when a particular map is lesioned, then it is likely that the map is involved in generating or controlling that behavior. This sort of experiment is often difficult to interpret because it is difficult to make a clean lesion that affects only one subsystem. In the case of the mammalian cochlear nucleus, for example, it is very difficult to lesion any one component of the system because the systems are anatomically intertwined. However, in birds the bushy and stellate cells are located in separate nuclei, and it has been possible to show that barn owls use a bushy-cell system for sound localization in the horizontal plane and a stellate-cell system for vertical localization (12).
The paper by Metzner and Juranek (4) offers evidence from the behavioral perspective about the roles of parallel maps in ELL of two species of weakly electric fish, Eigenmannia virescens and Apteronotus leptorhynchus. These species show two interesting electrosensory behaviors that are analyzed. The signals produced by the fishs’ electric organs are sinusoidal at frequencies of a few hundred hertz. Much of the information gathered by a fish depends on modulation in the amplitude of the sinusoidal electric field at the fish’s body surface. When two fish approach one another, their electric fields interact, and if their frequencies are similar, produce beats, i.e., regular oscillations in amplitude of the summed electrical signal at a frequency equal to the difference in the frequencies of the two fishs’ individual signals. To minimize the confusion inherent in this situation, the fish change their discharge frequencies to move them apart and increase the frequencies of the beats into a range where they do not interfere. This jamming avoidance response (JAR) appears to depend on one of the tuberous electroreceptor maps in the ELL, in that lesions of that component, and only that component, interfere with the JAR.
Similarly, the fish produce a chirp, meaning a rapid modulation in their electric organ discharge frequency, as a communication signal. The chirp can be evoked by the presence of another fish’s electrical signal. The chirp response in this situation was abolished by lesioning a second tuberous ELL map. The specificity of the maps for these two responses is complete in that each behavioral response seems to be affected by lesioning only one map region.
The association of the parallel maps in the electrosensory system with particular behavioral responses is an important and intriguing result that raises several questions. Why should there be essentially identical systems for these different behaviors? One possibility suggested by Metzner and Juranek’s discussion is that the ELL represents an intermediate stage in evolution. The sensory maps may have been duplicated to support the expanded behavioral repertoire of the fish but have not undergone the degree of specialization required in the auditory or visual systems by more sophisticated processing demands. A related question is why the streams that are separated at the level of the ELL are not kept in anatomically separate compartments at the next level. The four maps of the ELL project to the torus semicircularis of the midbrain in a convergent fashion, although the axons from different maps may terminate on different cells (10, 13). Why have separate maps at the level of the ELL and but not in the torus, especially because the systems diverge anatomically again at the level of the premotor pathways related to the two behaviors? A similar convergence occurs in the auditory system in the inferior colliculus, the mammalian equivalent of the torus semicircularis, and the nature of the convergence is similar in that the ascending subsystems terminate in a banded pattern that suggests some segregation of inputs from different sources (14). One possibility is that descending feedback and neuromodulatory projections, which exist in both the electrosensory and auditory systems, require separate maps at the level of the primary nucleus to function properly. Questions such as those raised by the results reported in this paper suggest that the organization of the brainstem electrosensory system may contain important clues about the parallel-hierarchical organization of the maps that make up sensory systems.
Footnotes
The companion to this commentary is published on page 14798 of volume 94.
References
- 1.Buck L B. Annu Rev Neurosci. 1996;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- 2.Felleman D J, Van Essen D C. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 3.Merigan W H, Maunsell J H R. Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- 4.Metzner W, Juranek J. Proc Natl Acad Sci. 1997;94:14798–14803. doi: 10.1073/pnas.94.26.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young E D. In: Synaptic Organization of the Brain. Shepherd G M, editor. New York: Oxford Univ. Press; 1998. pp. 121–158. [Google Scholar]
- 6.Bastian, J. (1994) Physics Today, February, 30–37.
- 7.Carr C, Maler L. In: Electroreception. Bullock T H, Heiligenberg W, editors. New York: Wiley; 1986. pp. 319–373. [Google Scholar]
- 8.Heiligenberg W, Dye J. J Comp Physiol. 1982;148:287–296. [Google Scholar]
- 9.Irvine D R F. The Auditory Brainstem. Berlin: Springer; 1986. [Google Scholar]
- 10.Shumway C A. J Neurosci. 1989b;9:4400–4415. doi: 10.1523/JNEUROSCI.09-12-04400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shumway C A. J Neurosci. 1989a;9:4388–4399. doi: 10.1523/JNEUROSCI.09-12-04388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi T, Moiseff A, Konishi M. J Neurosci. 1984;4:1781–1786. doi: 10.1523/JNEUROSCI.04-07-01781.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maler L, Sas E, Carr C, Matsubara L. J Comp Neurol. 1982;211:154–164. doi: 10.1002/cne.902110205. [DOI] [PubMed] [Google Scholar]
- 14.Oliver D L, Huerta M F. In: The Mammalian Auditory Pathway: Neuroanatomy. Webster D B, Popper A N, Fay R R, editors. New York: Springer; 1992. pp. 168–221. [Google Scholar]