Abstract
Accumulation of amyloid-β peptide (Aβ), the neurotoxic peptide implicated in the pathogenesis of Alzheimer's disease (AD), has been shown in brain mitochondria of AD patients and of AD transgenic mouse models. The presence of Aβ in mitochondria leads to free radical generation and neuronal stress. Recently, we identified the presequence protease, PreP, localized in the mitochondrial matrix in mammalian mitochondria as the novel mitochondrial Aβ-degrading enzyme. In the present study, we examined PreP activity in the mitochondrial matrix of the human brain's temporal lobe, an area of the brain highly susceptible to Aβ accumulation and reactive oxygen species (ROS) production. We found significantly lower hPreP activity in AD brains compared with non-AD age-matched controls. By contrast, in the cerebellum, a brain region typically spared from Aβ accumulation, there was no significant difference in hPreP activity when comparing AD samples to non-AD controls. We also found significantly reduced PreP activity in the mitochondrial matrix of AD transgenic mouse brains (Tg mAβPP and Tg mAβPP/ABAD) when compared to non-transgenic aged-matched mice. Furthermore, mitochondrial fractions isolated from AD brains and Tg mAβPP mice had higher levels of 4-hydroxynonenal, an oxidative product, as compared with those from non-AD and nonTg mice. Accordingly, activity of cytochrome c oxidase was significantly reduced in the AD mitochondria. These findings suggest that decreased PreP proteolytic activity, possibly due to enhanced ROS production, contributes to Aβ accumulation in mitochondria leading to the mitochondrial toxicity and neuronal death that is exacerbated in AD. Clearance of mitochondrial Aβ by PreP may thus be of importance in the pathology of AD.
Keywords: Mitochondrial amyloid-β, mitochondrial function, oxidative stress, presequence protease (PreP), proteolysis
INTRODUCTION
Accumulation of amyloid-β peptide (Aβ) is a hallmark of Alzheimer's disease (AD) according to the amyloid cascade hypothesis, and is produced by regulated intramembrane proteolysis of the amyloid-β protein precursor (AβPP) via sequential cleavage by β- and γ-secretases [1, 2]. Extracellular accumulation of Aβ in the form of senile plaques has been detected in individuals with AD [3, 4]. It is also known that there is a reduction of mitochondrial volume during early stage AD, and this fact has led to an increased focus on intracellular events, especially the role of mitochondria, in AD [5–7]. Aβ peptides have been found in mitochondria of the AD brains as well as in AD transgenic mice overexpressing Aβ [8–14]. Accumulation of mitochondrial Aβ has been shown at a young age in AD transgenic mice, before extracellular plaques are formed [8, 9]. Notably, mitochondrial Aβ accumulation significantly correlates to the abnormal mitochondrial function as shown by impaired mitochondrial respiratory function, inactivated mitochondrial respiratory key enzyme, and reduced ATP levels [8–13, 15–17].
AβPP binds to mitochondria and is incompletely translocated leaving the Aβ region outside the mitochondrial membrane [18–20], suggesting that Aβ cannot be produced inside the mitochondria. We have recently shown in vitro that Aβ is transported into mitochondria via the protein Translocase of the Outer Membrane (TOM) machinery [14]. The cell surface RAGE receptor (receptor for advanced glycation end product) also contributes to transport of Aβ from the cell surface to the intracellular space including mitochondrial localization. RAGE-deficient neurons displayed a decrease in uptake of Aβ and protection from Aβ-induced mitochondrial dysfunction [21]. Further, Aβ-targeted mitochondrial proteins, ABAD (Aβ-binding alcohol dehydrogenase) and cyclophilin D, are upregulated in neurons of AD patients and AD transgenic mice [10, 12,13, 15, 22, 23]. Interaction of Aβ with ABAD in AD mitochondria leads to elevated reactive oxygen species (ROS) in neurons and neuronal death [10, 24–26]. The abrogation of cyclophilin D in Aβ-rich mitochondria attenuates mitochondrial ROS accumulation/production and protects against aberrant mitochondrial and neuronal function.
A few years ago, we identified a novel mitochondrial peptidasome, the presequence protease (PreP), that is responsible for Aβ degradation in mitochondria [27, 28]. PreP is a metallopeptidase, localized in the mitochondrial matrix in mammals, containing an inverted zinc-binding motif. It belongs to the pitrilysin oligopeptidase family (M16 C), which also includes insulin degrading enzyme (IDE), implicated in AD [29–31]. PreP was originally identified as a protease degrading mitochondrial presequences [32], but has also been shown to degrade other unstruc tured peptides up to 70 amino acid residues in length [33, 34], including Aβ. We have characterized the human PreP homologue (hPreP) in brain mitochondria and shown its capacity to degrade the Aβ peptides including Aβ1-40, Aβ1-42, and Arctic Aβ. Immunoinactivation studies in human brain mitochondria using hPreP antibodies showed complete inhibition of proteolytic activity against Aβ, demonstrating that hPreP is the protease responsible for degradation of this toxic peptide [27]. However, it has not yet been proven whether PreP activity is relevant to amyloid pathology and mitochondrial Aβ accumulation and whether its activity is altered in an Aβ-rich environment, such as in the AD brain and AD mouse models.
Molecular homology modeling of hPreP based on the crystal structure of Arabidopsis thaliana PreP, AtPreP, refined at 2.1Å [35], revealed four topologically similar domains creating two halves, connected by a hinge region. These two halves enclose a large chamber wherein the proteolytic activity occurs. This molecular modeling was followed by the identification of Cys90 in the first domain and Cys527 at the hinge region that together form a disulfide bridge (under oxidizing conditions) resulting in a complete inhibition of the enzyme.
In the present study, we investigated the alterations of hPreP activity in AD individuals and transgenic AD mouse models. We studied hPreP activity in human brain (temporal lobe) mitochondrial matrix from AD individuals and in non-AD age-matched controls. We also investigated hPreP activity in the AD spared region, the cerebellum, which is usually free from Aβ accumulation. In an AD transgenic mouse model, we compared PreP activity overexpressing mAβPP/ Aβ and mAβPP/ABAD at different ages to aged-matched non-transgenic littermates. The selected AD mouse models were well-suited to our strategy for determining whether enhancing/accelerating Aβ accumulation and oxidative stress in mitochondria alter PreP expression/activity since these animals have been characterized with respect to mitochondrial Aβ accumulation, mitochondrial and neuronal function, and neuropathological and behavioral endpoints [8–10, 12, 13, 26, 51].
MATERIALS AND METHODS
Human brain samples and transgenic (Tg) mice
Human brain tissues of temporal lobe and cerebellum from individuals with AD and age-matched non-AD controls were obtained from the New York Brain Bank at Columbia University (Table 1). The following Tg mice were used in these studies: Tg mAβPP overexpressing human mutant form of amyloid-β protein precursor (mAβPP, J-20 line) and Aβ, Tg ABAD mice overexpressing neuronal ABAD driven by PDGF-B chain promoter, Tg mAβPP/ABAD mice overexpressing both mutant human form of AβPP and ABAD, and non-transgenic littermate controls (nonTg) [8–10, 12, 13, 37, 51]. Animal studies were approved by the Animal Care and Use Committee of Columbia University in accordance with the National Institute of Health guidelines for animal care.
Table 1.
The information on the human brain tissues used in the experiments
| Case | Gender | Age (yr) | PMI (h) | Braak stage | Neuritic plaques – Temporal pole | Neuritic plaques – Hippocampus |
|---|---|---|---|---|---|---|
| AD1 | M | 86 | 4.4 | VI/VI | 25–30 per 100 × microscope field | Rare |
| AD2 | F | 89 | 3.3 | VI/VI | 10–15 per 100× microscope field | Rare |
| AD3 | F | 89 | 13.6 | VI/VI | 10–20 per 100× microscope field | Occasional |
| AD4 | F | 81 | 2.5 | VI/VI | Up to 30 per 100 × microscope field | Many |
| AD5 | F | 89 | 5.5 | VI/VI | Up to 30 per 100× microscope field | Many |
| AD6 | F | 89 | 13.8 | VI/VI | Up to 10 per 100× microscope field | Up to 6 per 100× microscope field |
| AD7 | F | 83 | NA | V/V | Severe | Up to 20 per 100× microscope field |
| AD8 | M | 75 | 8.3 | III/III | Severe | Up to 20 per 100× microscope field |
| AD9 | M | 72 | 2.3 | VI/VI | Severe | Up to 20 per 100× microscope field |
| AD10 | F | 89 | 3.5 | VI/VI | Many | Up to 20 per 100× microscope field |
| AD11 | F | 89 | 6.7 | VI/VI | Up to 25 per 100× microscope field | Present |
| AD12 | F | 89 | 4.2 | VI/VI | Up to 30 per 100× microscope field | Up to 15 per 100× microscope field |
| Mean ± SE | 9F/3M | 85 ± 1.75 | 6.19 ± 1.24 | |||
| ND1 | M | 89 | 4.9 | II/II | Very rare | Very rare |
| ND2 | M | 74 | 4.3 | II/0 | Absent | Absent |
| ND3 | F | 67 | 5 | 0 | Absent | Absent |
| ND4 | M | 62 | 4.3 | 0 | Absent | Absent |
| ND5 | M | 87 | 5.2 | III/I | Absent | Absent |
| ND6 | F | 76 | 3.6 | 0/I | Absent | Absent |
| ND7 | M | 89 | 3 | I/I | Absent | Absent |
| NAD8 | F | 74 | 18.7 | II/0 | Absent | Absent |
| Mean ± SE | 3F/5M | 77.25 ± 3.61 | 6.13 ± 1.81 |
AD, Alzheimer's disease; ND, non-Alzheimer disease; F, female; M, male; PMI, postmortem interval; SE, standard error.
Isolation of mitochondria
Human and mice brain mitochondria were isolated using Percoll gradient as described previously [8, 12]. Briefly, human temporal lobe, human cerebellum and cerebral lobe from mice brain were minced with scissors and washed two times with isolation buffer (225 mM mannitol, 75 mM sucrose, 1 mM EGTA, 5 mM HEPES, and 1 mg/ml bovine serum albumin [BSA], pH 7.2) to remove contaminating blood, followed by homogenization in a glass Teflon homogenizer in 14 ml isolation buffer. Homogenates were then centrifuged at 1500 g for 5 min. Supernatant was applied to 14% Percoll and centrifuged at 12,000 g for 10 min. The mitochondrial pellets were re-suspended and centrifuged at 8000 g for 10 min followed by a washing and re-suspension in 100 μl sonication buffer (20 mM HEPES-KOH, 10 mM MgCl2, pH 7.5). Protein concentration was determined by Bio-Rad protein concentration kit with BSA as standard.
Preparation of brain mitochondria matrix
Isolated brain mitochondria were diluted with a sonication buffer to a final concentration of 8 μg/μl and incubated 30 min at 4°C followed by sonication 4×30 s. To obtain the matrix fraction, samples were then centrifuged at 70,000 rpm for 1 h and the super-natant containing the matrix proteins was used in the PreP degradation assay studies.
PreP expression levels
20 μg of the isolated human and mice brain mitochondrial matrix were subjected to a 12% Bis-Tris gel (Invitrogen, CA), run in 1 × MES buffer, and transferred to nitrocellulose membrane Hybond™ (Amersham Biosceinece) for 1 h at 100 V. The membrane was blocked overnight in 5% milk-PBS. For detection of PreP, antibodies against hPreP were applied at a dilution of [1:1500–2000] for 1 h followed by detection with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:2500) and ECL (GE Healthcare). Hsp60 expression levels were detected by antibody raised against Hsp60, 1:2000 for 1 h followed by detection with horseradish peroxidase-conjugated anti-mouse secondary antibody (1:2500) and ECL (GE Healthcare).
Degradation assays
The degradation assay for studying proteolytic activity of hPreP in human brain mitochondrial matrix fractions was conducted by using 20 μg isolated matrix protein and the following substrates: 0.25 μg pF1β, a mitochondrial presequence derived from the F1β-subunit of the Nicotiana plumbaginifolia ATP synthase N5.7 pF1β(2–54) [36], 0.25 μg Biotin-labeled Aβ1-40 and 0.25 μg Biotin-labeled Aβ1-42 in a degradation buffer containing 20 mM HEPES-KOH pH 8.0, and 10 mM MgCl2. For inhibitory studies, 20 mM orthophenathroline (oPh) was pre-incubated with 20 μg of brain mitochondrial matrix protein on ice for 10 min before the addition of substrate. For studies of PreP activity under oxidized conditions, 20 μg matrix protein was pre-incubated with 0.1 mM and 1 mM K3Fe(CN)6 for 10 min on ice before addition of 1 μg Aβ1-40. The immuno-inactivation was performed by pre-incubating mitochondrial fractions with 6 μg of antibodies raised against hPreP or 18 μg of antibodies raised against the presequence of F1β subunit of the ATP synthase from N. plumbaginifolia, pF1β at 4°C for 30 min before the addition of Biotin-labeled Aβ1-40. Samples were incubated for 2.5 h at 37°C. Reactions were stopped by the addition of 2 × sample buffer, analyzed on NuPAGE 12% Bis-Tris gel (Invitrogen, CA), and run in 1×MES buffer. Proteins were electrophoretically transferred to nitrocellulose membrane Hybond™ (Amersham Bioscience) for 1 h at 100 V. For pF1β identification, the nitrocellulose membrane was blocked overnight in 5% milk-PBS followed by incubation with pF1β antibody (1:2000) and detection with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:2500) and ECL (GE Healthcare). For analyzing the degradation of Biotin labeled Aβ1-40 and Biotin labeled Aβ1-42 (Biotin-LC- Aβ1-40, Biotin-LC- Aβ1-42), the nitrocellulose membrane was dried overnight at 25°C followed by blocking with 2% milk-PBS for 1 h. Immunoblotting was performed with ExtraAvidin Peroxidase Conjugate 1:3000 (Sigma) and detection with ECL.
Measurement of HNE in brain mitochondrial fractions
Brain mitochondria isolated from human subjects and Tg mice were sonicated in the cold isolation buffer, then centrifuged at 10,000 g for 10 minutes at 4°C. Levels of 4-hydroxynonenal (HNE) were measured using commercial ELISA Kit (Cell Biolabs, Inc.).
Measurement of cytochrome c oxidase activity
Cytochrome c oxidase activity in the cortical mitochondria from AD and non-AD temporal pole and cerebellum was measured as previously described [12, 13].
Statistical analysis
Quantification analysis was performed by using NIH Image J software. Statistical analyses were performed using STATVIEW software. One-way ANOVA was used for repeated measures followed by Fisher's Protected Least Significant Difference for post-hoc comparisons. Results are expressed as mean ± Standard Error Mean (SEM). p < 0.05 was considered significant.
RESULTS
Decreased PreP proteolytic activity in AD brain mitochondria
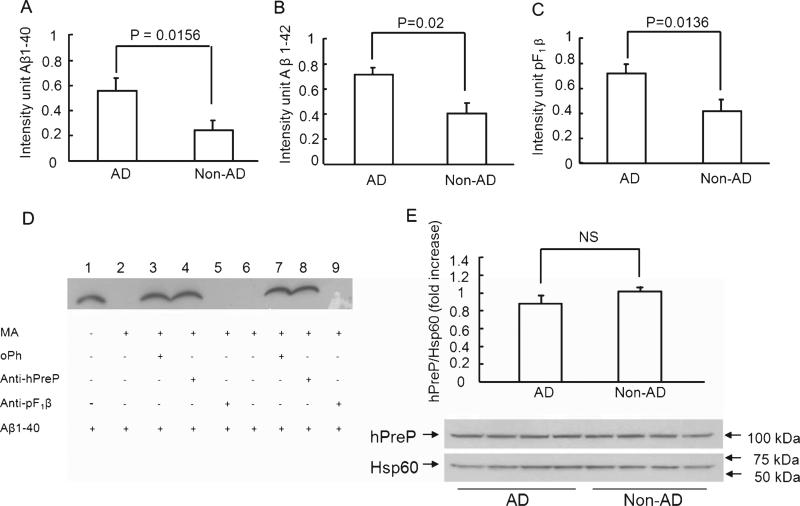
It is well established that the temporal lobe is one of the most highly susceptible regions to the Aβ accumulation in the AD brain, whereas Aβ accumulation and aggregation are usually not found in the cerebellum. Therefore, we investigated hPreP activity in AD brain mitochondrial matrix fractions from these two areas of the brain and compared such activity to age-matched non-AD individuals. Measurement of PreP proteolytic activity in AD and mAβPP brain mitochondria was based on the capacity of PreP in the mitochondrial matrix for degrading exogenous biotin-labeled Aβ (Aβ40 or Aβ42) or non-Aβ peptide (F1β presequence, pF1β). The amount of Aβ or pF1β peptides degraded by PreP from AD brains or AβPP mice compared to those from non-AD or nonTg mice was representative of changes in PreP activity. We isolated brain mitochondrial matrix proteins from the temporal lobes and cerebella of 12 AD patients and 8 control patients (Table 1). The isolated human brain mitochondrial fraction showed proteolytic activity against biotin- labeled Aβ1-40, Aβ1-42, and pF1β (Fig. 1A-C). There was a significant reduction in proteolytic activity of hPreP in the mitochondrial matrix extracted from temporal lobes of AD patients compared with age-matched non-AD individuals for all three substrates [biotin-labeled Aβ1-40 (p = 0.0156), biotin-labeled Aβ1-42 (p = 0.02), and pF1β (p = 0.0136)] (Fig. 1A-C).
Fig. 1.
Changes in hPreP activity/expression in brain mitochondria of AD temporal lobe. Degradation of biotin-Aβ (A, B) and F1β presequence peptide (C). hPreP proteins extracted from the cortical mitochondria of the indicated AD and non-AD brains (A, B) were incubated with biotin-labeled Aβ (0.25 μg), and then subjected to immunoblotting with Extravidin peroxidase conjugated IgG and detection with ECL to reveal immunoreactive biotin Aβ. C) For degradation of F1β substrate, mitochondrial hPreP proteins were incubated with pF1β followed by the immunoblot with antibody to pF1β. Densitometry of the combined immunoreactive Aβ (A, B) or F1β bands (C) using NIH Image program is shown. D) Determination of hPreP proteolytic activity and hPreP-dependent degradation of Aβ in brain mitochondrial fraction. Biotin-labeled Aβ was completely degraded by the isolated human mitochondrial matrix hPreP protein (MA-hPreP) (lanes 2 and 6). The oPh (ortho-phenantroline, an inhibitor of PreP proteolytic activity) blocked the degradation of biotin Aβ (lanes 3 and 7). The immuno-inactivation assay confirmed hPreP-induced degradation of Aβ. When isolated human mitochondrial matrix hPreP protein (MA-hPreP) was pre-incubated with antibody against hPreP, degradation of Aβ1-40 (lane 4 & 8) was almost completely inhibited, whereas incubation of pF1β presequence antibodies did not affect Aβ degradation (lanes 5 and 9). E) Immunoblotting of brain mitochondria from AD and non-AD brains for hPreP. The upper panel denotes representative immunoblots for human PreP and Hsp60. Hsp60 was used as a mitochondrial marker and protein loading controls. The lower panel shows densitometry of hPreP immunoreactive bands from the indicated brain mitochondria. NS: no statistical significance between these groups (p > 0.05). Mitochondria were isolated from 12 AD brains and 8 non-AD brains. All experiments were performed in triplicates.
To determine the effect of PreP proteolytic activity, ortho-phenantroline (oPh), a metalloprotease inhibitor, was used for the inhibition study in the degradation assay. The degradation activity of PreP was completely abolished in the presence of oPh (Fig. 1D). The strong immunoreactive biotin Aβ or pF1β bands were noticeable due to the loss of PreP proteolytic activity in the presence of oPh inhibitor (Fig. 1D, lanes 3 and 7 for degrading Aβ and Fig. 2D, lane 3 for degrading pF1β), as compared to those without oPh treatment (Figs. 1D and 2D, lane 2).
Fig. 2.
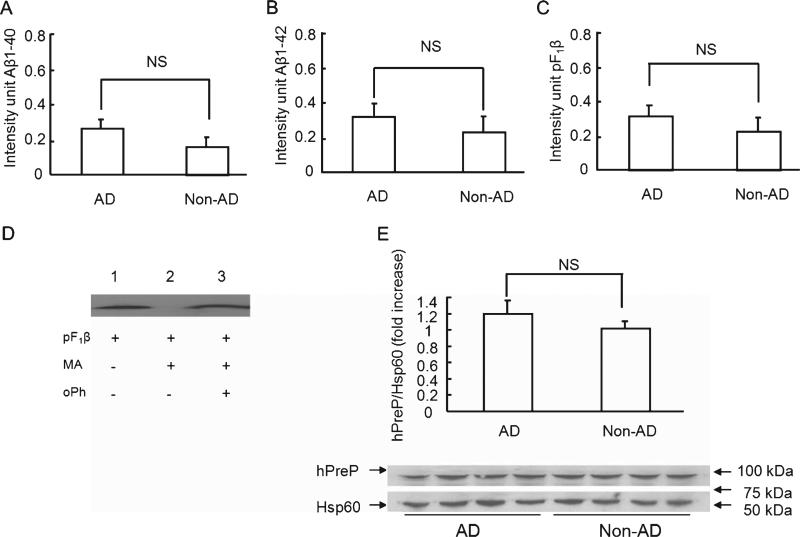
hPreP activity/expression in the cerebellum of AD and non-AD brains. Mitochondrial hPreP extracted from cerebellum for degrading biotin Aβ40/42 (A,B) and pF1β (C). Densitometry of Aβ or pF1β immunoreactive bands are shown by diagram bars. D) Effect of hPreP activity on degrading of F1β presequence (pF1β). pF1β was completely degraded by mitochondrial matrix hPreP protein (MA-hPreP, no F1β immunreactive band in lane 2 versus F1β immunoreactive band in lane 1 without MA-hPreP). In the presence of oPh, MA-PreP was not able to degrade pF1β (lane 3 versus lane 2 without oPh). E) Immunoblotting of cortical mitochondria from AD and non-AD cerebellum for human PreP. NS: no significant difference. Mitochondria were isolated from 12 AD brains and 8 non-AD brains. All experiments were performed in triplicates.
To determine the PreP-dependent degradation of Aβ, we performed the following two experiments as shown in Fig. 1D: 1) immuno-inactivation assay with a specific antibody against PreP; and, 2) immunoinactivation assay with unrelated control antibody, such as a specific antibody to the F1β presequence, which is a non-Aβ substrate for PreP. In the absence of PreP antibody, mitochondrial matrix PreP completely degraded biotin-labeled Aβ (Fig. 1D, lanes 2 and 6). By contrast, neutralization of mitochondrial matrix PreP protein with PreP antibody revealed an almost complete inhibition of the degradation of biotin-labeled Aβ1-40 (Fig. 1D, lanes 4 and 8) as demonstrated by Aβ immunoreactive bands, whereas no effect on PreP proteolytic activity for degrading Aβ was seen in the presence of pF1β antibodies, in which no Aβ immunoreactive bands were observed (Fig. 1D, lanes 5 and 9). These results verified that hPreP is the protease responsible for degrading these two different peptides (Aβ and pF1β) in isolated human brain mitochondrial matrix and that these in vitro degradation assays can be used to study proteolytic activity in human and mouse brain mitochondrial matrix.
To determine whether reduced activity of hPreP in the mitochondrial matrix fraction extracted from temporal lobes of AD patients was due to lower protein expression of hPreP, we performed Western blot analysis of mitochondrial fractions using specific antibody to hPreP. Statistical analysis of intensity of immunoreactive hPreP bands normalized to Hsp60 (mitochondrial protein marker) revealed a slight (11%) but not significant decrease in relative expression levels of hPreP in the temporal lobe of AD individuals compared to age-matched non-AD subjects (Fig. 1E).
When comparing degradation activity of hPreP in the cerebella of AD and non-demented individuals, there was a trend toward lower (but not significantly lower) proteolytic activity of hPreP in this part of AD human brains against biotin-labeled Aβ1-40 (p = 0.185), biotin-labeled Aβ1-42 (p = 0.230), and pF1β (p = 0.312) (Fig. 2A–C). One likely explanation for these findings might be the fact that there is a lack of, or at least lower, levels of Aβ accumulation in the cerebellum, less toxicity in the form of elevated ROS production (Fig. 6B), and therefore less inhibition of hPreP activity. Proteolytic activity of hPreP was completely abolished in the presence of oPh (Fig. 1D lanes 3 and 7, Figs. 2D and 3D, lanes 3). Comparison of hPreP expression levels in the cerebellum mitochondrial matrix of AD and non-AD individuals revealed no significant change in hPreP protein levels (Fig. 2E). We also examined whether PreP is able to degrade the reversed Aβ peptide (Aβ40-1). Incubation of Aβ40-1 with the recombinant hPreP protein revealed that the enzyme is able to degrade Aβ40-1 and that the proteolytic activity is totally inhibited by 20 mM orthophenanthroline (oPh), a metalloprotease inhibitor (data not shown).
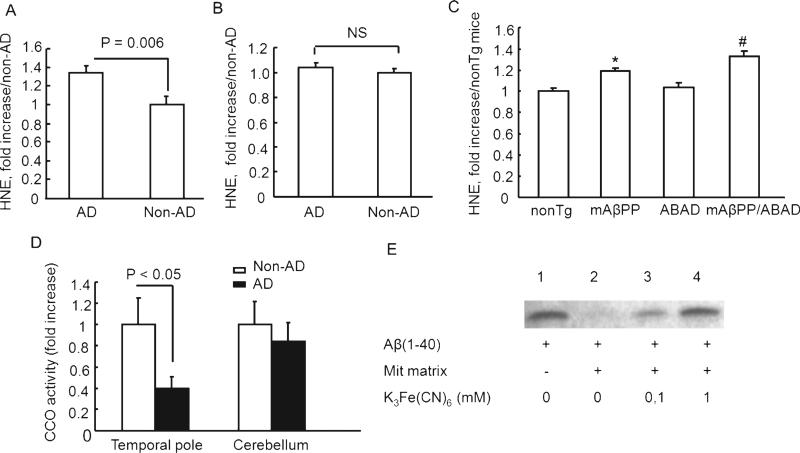
Fig. 6.
A. HNE levels and cytochrome c oxidase activity in brain mitochondria. HNE levels in cortical mitochondria isolated from temporal lobe (A) and cerebellum (B) of AD and non-AD brains were measured by ELISA. n = 10–11 mice per group. C) HNE levels in cortical mitochondria from the indicated Tg mice were determined by ELISA. *p < 0.05 vs. nonTg mice, #p < 0.05 vs. nonTg and mAβPP mice. n = 8–9 mice per group. D) Cytochrome c oxidase activity in cortical mitochondria from temporal lobe and cerebellum of AD and non-AD subjects. E) Decreased hPreP activity in mitochondrial matrix in the presence of 0.1 mM and 1 mM K3Fe(CN)6 (lanes 3 and 4).
Fig. 3.
Alterations of PreP activity/expression in transgenic AD mice. Cortical mitochondrial PreP extracted from 5-month-old indicated Tg mice degraded the biotin Aβ40 (A), Aβ42 (B), and pF1β (C), respectively. Densitometry of Aβ40/42 or pF1β immunoreactive bands was performed using NIH image program. The upper panels indicate the representative immunoblots for Aβ (A, B) and pF1β (D). In the presence of oPh, mitochondrial PreP was not able to degrade pF1β peptide (lane 3 vs. lane 2). E) Immunoblotting of cortical mitochondrial protein from the indicated Tg mice for PreP. Representative immunoblots for PreP and Hsp60 are shown in the upper panel. Hsp60 (mitochondrial marker) was used as protein loading control and mitochondrial rich fractions. n = 4–7 mice per group. All experiments were performed in triplicates.
Decreased PreP proteolytic activity in transgenic AD mice
Since cellular and mitochondrial integrity might be deteriorated significantly soon after death and the accompanying tissue disruption might allow potentially non-physiological effects to occur, we studied the mitochondria isolated from cerebral cortices of transgenic AD mice where sample quality was carefully controlled. Tg mAβPP mice overexpressing AβPP/Aβ have been well characterized in terms of AD-type functional, neuropathological, behavioral and electro-physiological changes [8–10, 12, 26, 37, 38]. Notably, accumulation of mitochondrial Aβ occurs as early as 4 months of age [8] before the onset of Aβ pathology and is associated with mitochondrial abnormalities [8–10, 26]. Furthermore, previous studies have shown that transgenic mice overexpressing mAβPP and ABAD (Tg mAβPP/ABAD) manifested excessive production of reactive free radicals, as well as aberrant mitochondrial and neuronal function [8, 10, 26]. Therefore, we investigated proteolytic activity of PreP in Tg mAβPP/ABAD mice (accelerated AD model) as well as in Tg mAβPP mice and compared the results to Tg ABAD mice and nonTg littermate controls.
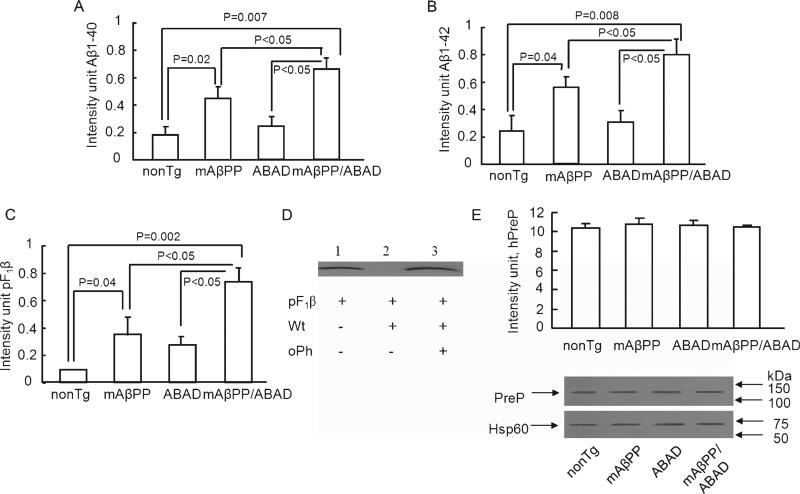
First, proteolytic activity of PreP against all the three substrates was significantly reduced in the cortical mitochondrial matrix isolated from 5-months-old Tg mAβPP mice compared to nonTg littermate cortical mitochondria: Aβ1-40 (p = 0.02), Aβ1-42 (p = 0.04), and pF1β (p = 0.04) (Fig. 3A-C). Second, the activity in Tg mAβPP/ABAD mice was significantly lower than that in both nonTg mice (Aβ1-40, p = 0.007; Aβ1-42, p = 0.008; and pF1β, p = 0.002) and Tg mAβPP mice (p < 0.05). Notably, compared to nonTg cortical mitochondria, PreP activity for degrading Aβ (Fig. 3A–B) was decreased by 2.3- and 3.8-fold in mAβPP and mAβPP/ABAD mitochondria, respectively; these data suggest that increased expression of ABAD in an Aβ-rich environment significantly diminishes PreP proteolytic activity. Similarly, PreP activity for degrading pF1β was also diminished by 3.8- and 7.5-fold in mAβPP and mAβPP/ABAD mitochondria in comparison to the results from nonTg mitochondria (Fig. 3 C). The PreP activity against pF1β was inhibited in the presence of oPh (Fig. 3D). Third, PreP in the brain cortex mitochondrial matrix isolated from twelve-month-old Tg mAβPP/ABAD mice was less active in its ability to degrade Aβ1-40 (p = 0.004), Aβ1-42 (p = 0.002), and pF1β (p = 0.003) compared to age-matched nonTg mice (Fig 4A–C). The ability of PreP to degrade these three peptides was also significantly reduced in Tg mAβPP mouse brain cortex mitochondrial matrix compared to nonTg mice of the same age (Aβ1-40 (p = 0.004), Aβ1-42 (p = 0.002), and pF1β (p = 0.007)) (Fig. 4A–C). We did not find a significant difference of PreP activity between mAβPP and mAβPP/ABAD at 12 month old mice as compared with those of 5 month old mice. ABAD is a co-factor for exacerbating mitochondrial and neuronal dysfunction, in particular early stage of Alzheimer's disease. Previously, we have demonstrated that neuronal over-expression of ABAD in mAβPP mice had early deficits in mitochondrial and cognitive dysfunction, which occurred at 4–5 months old mice [10]. These results are consistent with our observation that mAβPP/ABAD mice at 5 (not 12) months of age had a significant decreased PreP activity in brain mitochondria compared to mAβPP mitochondria. The results generated from 12 months old mice could be due to mouse model used in our study. Because 12 months old mAβPP mice are the end stage of amyloid pathology together with abnormalities in mitochondrial and neuronal function, overexpression of ABAD in mAβPP mice might not further exaggerate Aβ-mediated deficits in mitochondrial and neuronal properties. To determine whether decreased PreP activity was due to alteration in PreP expression, mitochondrial proteins extracted from Tg mice were subjected to the immunoblotting with the specific antibody to PreP. Similar levels of PreP protein were expressed in all groups of Tg mice (Fig. 3E and 4D, p > 0.05). To determine whether the alterations in PreP activity occurred before accumulation of mitochondrial Aβ, we measured PreP activity in 2 months old cortical mitochondria. There were no significant differences of PreP activity between mAβPP cortical mitochondria and nonTg cortical mitochondria at the age of 2 month prior to detectable mitochondrial Aβ accumulation (data not shown).
Fig. 4.
PreP activity/expression in Tg AD mice at age of twelve months. The cortical mitochondrial PreP from twelve-months-old Tg mice degraded biotin Aβ40/42 (A,B) and pF1β (C) Densitometry of combined immunoreactive bands (Aβ or pF1β) from the indicated Tg mice. D) Immunoblotting of mitochondrial fractions from the indicated Tg mice for PreP or Hsp60. n = 4–5 mice per group. All experiments were performed in triplicates.
When comparing the activity of PreP in five-months and twelve-months-old nonTg, and Tg mAβPP mice, we found that proteolytic activity decreased in an age-dependent manner (Fig. 5A–C). There was a significant difference in the capacity of PreP to degrade pF1β in five-months-old nonTg and Tg mAβPP mice compared to twelve-months old mice (Fig. 5 C). A probable explanation for these observations may be the effect of aging. Interestingly, when comparing the proteolytic activity of PreP in five-months and twelve-months-old Tg mAβPP mice, a significant difference was noted in degradation against all three peptides, possibly due to overproduction of mAβPP and consequently elevated Aβ generation and accumulation that enhances the effect of aging thereby affecting PreP activity.
Fig. 5.
Age-dependent decreased PreP activity in Tg mAβPP mice. Cortical mitochondrial PreP was extracted from Tg mAβPP and nonTg mice at ages of five and twelve months, and then incubated with biotin Aβ40/42 (A, B) and pF1β (C), respectively. Densitomery of all immunoreactive bands for Aβ and pF1β is shown. n = 4–6 mice per group. All experiments were performed in triplicates.
Mitochondrial oxidative stress and mitochondrial dysfunction in Aβ-rich mitochondria
In light of elevated levels of oxidative stress in the AD brain and Aβ-rich mitochondria, and the potential effects of oxidative stress on PreP activity, we measured HNE levels, an oxidative stress marker, in the mitochondrial fractions used in the present studies. As shown in Fig. 6, HNE levels were significantly elevated in the AD temporal lobe mitochondria compared with non-AD mitochondria (Fig. 6A), while levels of HNE in cerebellum mitochondria were comparable between AD and non-AD (Fig. 6B). Similarly, HNE levels were significantly higher in mAβPP mitochondria than in nonTg mitochondria. The mAβPP/ABAD mitochondria exhibited increased HNE levels compared to mAβPP mitochondria (Fig. 6C) as early as five months old. These results are consistent with our previous observation that overexpressed ABAD in Tg mAβPP mice enhanced generation of reactive free radicals [10].
Next, we measured the activity of cytochrome c oxidase, a key respiratory enzyme, in human brain mitochondria to determine the correlation of mitochondrial function to the alterations in PreP. Cytochrome c oxidase activity was significantly decreased in cortical mitochondria from AD temporal lobe ( 50–60% lower than non-AD mitochondria); but only a small change in cytochrome c oxidase activity (10% change) was noted in cerebellum mitochondria when comparing AD and non-AD brains (Fig. 6D). These studies suggest that aberrant mitochondrial function from Aβ-rich mitochondria of AD-affected brain correlates to alterations in PreP proteolytic activity.
We have also investigated PreP activity against Aβ1-40 in the mitochondrial matrix under oxidized conditions, in the presence of increasing concentrations of K3Fe(CN)6. PreP activity was completely inhibited at 1 mM K3Fe(CN)6 (Fig. 6E). This is in agreement with our previous results [27], in which we have shown that degradation of Aβ1-40 with recombinant hPreP was totally reduced under oxidized conditions.
DISCUSSION
Several lines of evidence suggest that steady-state levels of Aβ, a fundamental feature of AD, are achieved by a balance between Aβ generation and clearance and that disruption of the clearance system gives rise to increased Aβ accumulation. Several proteases have recently been shown to be involved in regulation of the steady-state level of Aβ including IDE, a cytoplasmic functional analogue of hPreP, and neprilysine (NEP), a plasma membrane-anchored zinc metallprotease. Both have been extensively studied and are implicated in AD [39–41]. Several reports emphasize the potentially important role of mitochondrial dysfunction and elevated ROS production in AD development [10, 12, 26, 42–44]. Aβ accumulation in this vital organelle has been reported to occur in brain mitochondria of AD transgenic mice models prior to the extracellular Aβ deposits and to cause increases in ROS production [10, 26]. Elevated levels of oxidative stress have also been observed in Aβ-affected areas in postmortem AD brains [8]. We have identified PreP as the novel Aβ-degrading protease in human brain mitochondria [27].
In the present studies, we show that hPreP proteolytic activity is reduced in the AD-affected region, temporal lobe, while similar results were not observed in the AD-spared cerebellum, a region often free of Aβ accumulation. Proteolytic activity of hPreP in mitochondrial matrix isolated from temporal lobe of AD patients and non-AD age-matched controls was measured with three different peptides (biotin labeled Aβ1-40, biotin labeled Aβ1-42, and pF1β) using an in vitro degradation assay; further, hPreP activity in isolated cerebellum mitochondrial matrix of AD individuals was compared to age-matched controls. A significant reduction in PreP function was also confirmed in AD transgenic mice models (Tg mAβPP and Tg mAβPP/ABAD) compared to nonTg mice using the above listed peptides as substrates. Biotin labeled Aβ in the degradation assays was used to distinguish it from the Aβ accumulated in AD brain mitochondria and transgenic mice models. pF1β, a 53 amino acid long mitochondrial presequence peptide, was used as a non-Aβ substrate to further determine PreP proteolytic activity. Although these peptides have slightly different properties (as Aβ peptides are negatively charged, whereas pF1β is positively charged), hPreP degraded these substrates with similar efficiency. Importantly, Aβ-containing mitochondria from AD, mAβPP, or mAβPP/ABAD brains showed significantly decreased PreP proteolytic activity for degrading both Aβ and non-Aβ substrates. Accordingly, mitochondrial respiratory function was also reduced in these AD mitochondria as well as in mAβPP or mAβPP/ABAD mitochondria [8, 9, 12, 13, 26]. The strong negative correlation between PreP activity and mitochondrial Aβ accumulation suggests that functional PreP is important for maintenance of mitochondrial function through its capabilities to detoxify and to clear mitochondrial Aβ.
hPreP is a metalloprotease that is able to degrade mitochondrial presequences after they have been cleaved off from precursor proteins as well as other unstructured peptides in the range of 10 to 70 amino acid residues. We have previously reported that recombinant hPreP degraded both the mitochondrial presequence pF1β as well as Aβ1-40, Aβ1-42, and Aβ Arctic (E22G) peptides [26]. Here, we demonstrate that hPreP is also able to degrade Aβ40-1. This finding is not surprising as PreP is a general peptidasome with a preference for positively charged amino acids in the P1′ position and small-uncharged residues or serine residues in the P1 position and it does not exhibit any unique cleavage specificity [33]. Most importantly, under physiological and pathological conditions such as AD, reversed Aβ40-1 peptide is not present in both human and mouse brains, which is not relevant to human disease.
Since no change was observed in the protein expression level of hPreP in AD cases versus non-AD individuals or in the AD transgenic mice models versus nonTg mice, the reduced proteolytic ability of PreP is most probably due to AD-related PreP functional alteration. When comparing the gene expression levels in the brain of AβPP overexpressing mice of different ages to age-matched nonTg mice, Reddy et al. [45] observed a consistent increase of mRNA corresponding to proteins involved in mitochondrial energy metabolism and apoptosis, but they did not report any change in the mRNA levels of PreP, which is in agreement with our protein expression data. hPreP contains two cysteines situated close to each other. Under oxidized conditions these cysteines form a disulphide bridge locking the two protein halves of the enzyme in a closed conformation generating an inactive PreP [27]. These cysteines are conserved in all mammalian homologues of PreP. Measurement of the proteolytic activity of hPreP in the presence of an oxidizing K3Fe (CN)6 shows complete inhibition. Reduction of hPreP proteolytic activity observed in AD-affected areas of brain may thus be due to formation of the disulphide bridge that occurs because of the elevated ROS formation (that occurs in the vulnerable regions of AD brain). However, attempts to reactivate the enzyme by pre-incubating isolated matrix fraction with DTT, a reducing agent, revealed inconsistent results indicating that other factors, such as amino acid modification rather than disulphide bond formation may be responsible for inactivation of the enzyme.
Notably, PreP activity was significantly diminished in the Aβ-enriched mitochondria as compared with the nonTg mitochondria, suggesting the association of mitochondrial Aβ with alterations in PreP activity. Indeed, our recent study indicates that increased PreP activity in Tg mAβPP mice significantly reduced mitochondrial Aβ accumulation [51] Furthermore, our data also revealed an age-related reduction of PreP function in transgenic mice models, which suggests that the PreP activity may decrease during aging.
In summary, our data clearly demonstrate that PreP proteolytic activity was significantly reduced in Aβ-enriched mitochondria such as in AD-affected brains and AD mouse models. Levels of PreP activity negatively correlate to mitochondrial oxidative stress and positively associate with mitochondrial respiratory function. Deficits in PreP proteolytic activity observed in AD brain may be a significant contributing factor leading to cerebral Aβ accumulation, which in turn results in mitochondrial dysfunction. For future areas of investigation, it will be important to elucidate the mechanisms underlying reduced PreP function in AD-affected regions of the brain. The current data provides important evidence for AD- and age-related alterations of this mitochondrial Aβ-degrading pro-tease, and may open avenues to new pharmacological approaches or gene therapy to upregulate PreP activity or increase PreP levels that will enhance Aβ clearance in mitochondria and thereby halt disease progression.
ACKNOWLEDGMENTS
This work was supported by grants from the USPHS (P01AG17490, AG037319, and AG040011), Alzheimer's Association, The Swedish Research Council (M2005-4831, NT-2006-4393), Lennanders Foundation and Fundacao para a Ciência e a Tecnologia, Portugal, through PhD fellowship SFRH/BD/ 60783/2009.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=890).
REFERENCES
- 1.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 3.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soderberg L, Bogdanovic N, Axelsson B, Winblad B, Naslund J, Tjernberg LO. Analysis of single Alzheimer solid plaque cores by laser capture microscopy and nano-electrospray/tandem mass spectrometry. Biochemistry. 2006;45:9849–9856. doi: 10.1021/bi060331+. [DOI] [PubMed] [Google Scholar]
- 5.Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer's disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Su B, Perry G, Smith MA, Zhu X. Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free Radic Biol Med. 2007;43:1569–1573. doi: 10.1016/j.freeradbiomed.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Wirths O, Multhaup G, Bayer TA. A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide–the first step of a fatal cascade. J Neurochem. 2004;91:513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- 8.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 9.Du H, Yan S, Sosunov AA, Mckhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 11.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 12.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert A, Hauptmann S, Scherping I, Meinhardt J, Rhein V, Drose S, Brandt U, Fandrich M, Muller WE, Gotz J. Oligomeric and fibrillar species of beta-amyloid (A beta 42) both impair mitochondrial function in P301L tau transgenic mice. J Mol Med. 2008;86:1255–1267. doi: 10.1007/s00109-008-0391-6. [DOI] [PubMed] [Google Scholar]
- 17.Eckert A, Hauptmann S, Scherping I, Rhein V, Muller-Spahn F, Gotz J, Muller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- 18.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlov PF, Wiehager B, Sakai J, Frykman S, Behbahani H, Winblad B, Ankarcrona M. Mitochondrial gamma-secretase participates in the metabolism of mitochondria-associated amyloid precursor protein. FASEB J. 2011;25:78–88. doi: 10.1096/fj.10-157230. [DOI] [PubMed] [Google Scholar]
- 21.Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009;106:20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He XY, Wen GY, Merz G, Lin D, Yang YZ, Mehta P, Schulz H, Yang SY. Abundant type 10 17 beta-hydroxysteroid dehydrogenase in the hippocampus of mouse Alzheimer's disease model. Brain Res Mol Brain Res. 2002;99:46–53. doi: 10.1016/s0169-328x(02)00102-x. [DOI] [PubMed] [Google Scholar]
- 23.Chen JX, Yan SD. Amyloid-beta-induced mitochondrial dysfunction. J Alzheimers Dis. 2007;12:177–184. doi: 10.3233/jad-2007-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, Fang F, Chen JX, Yan SD, Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Ren Y, Xu HW, Davey F, Taylor M, Aiton J, Coote P, Fang F, Yao J, Chen D, Chen JX, Yan SD, Gunn-Moore FJ. Endophilin I expression is increased in the brains of Alzheimer disease patients. J Biol Chem. 2008;283:5685–5691. doi: 10.1074/jbc.M707932200. [DOI] [PubMed] [Google Scholar]
- 26.Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 27.Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, Eneqvist T, Tjernberg L, Ankarcrona M, Glaser E. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem. 2006;281:29096–29104. doi: 10.1074/jbc.M602532200. [DOI] [PubMed] [Google Scholar]
- 28.Alikhani N, Ankarcrona M, Glaser E. Mitochondria and Alzheimer's disease: amyloid-beta peptide uptake and degradation by the presequence protease, hPreP. J Bioenerg Biomembr. 2009;41:447–451. doi: 10.1007/s10863-009-9244-4. [DOI] [PubMed] [Google Scholar]
- 29.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller BC, Eckman EA, Sambamurti K, Dobbs N, Chow KM, Eckman CB, Hersh LB, Thiele DL. Amyloid-beta peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc Natl Acad Sci U S A. 2003;100:6221–6226. doi: 10.1073/pnas.1031520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Stahl A, Moberg P, Ytterberg J, Panfilov O, Brockenhuus Von Lowenhielm H, Nilsson F, Glaser E. Isolation and identification of a novel mitochondrial metalloprotease (PreP) that degrades targeting presequences in plants. J Biol Chem. 2002;277:41931–41939. doi: 10.1074/jbc.M205500200. [DOI] [PubMed] [Google Scholar]
- 33.Stahl A, Nilsson S, Lundberg P, Bhushan S, Biverstahl H, Moberg P, Morisset M, Vener A, Maler L, Langel U, Glaser E. Two novel targeting peptide degrading proteases, PrePs, in mitochondria and chloroplasts, so similar and still different. J Mol Biol. 2005;349:847–860. doi: 10.1016/j.jmb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Glaser E, Alikhani N. The organellar peptidasome, PreP: a journey from Arabidopsis to Alzheimer's disease. Biochim Biophys Acta. 2010;1797:1076–1080. doi: 10.1016/j.bbabio.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Johnson KA, Bhushan S, Stahl A, Hallberg BM, Frohn A, Glaser E, Eneqvist T. The closed structure of presequence protease PreP forms a unique 10,000 Angstroms3 chamber for proteolysis. EMBO J. 2006;25:1977–1986. doi: 10.1038/sj.emboj.7601080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moberg P, Nilsson S, Stahl A, Eriksson AC, Glaser E, Maler L. NMR solution structure of the mitochondrial F1beta presequence from Nicotiana plumbaginifolia. J Mol Biol. 2004;336:1129–1140. doi: 10.1016/j.jmb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 40.Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 41.Yasojima K, Akiyama H, McGeer EG, McGeer PL. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci Lett. 2001;297:97–100. doi: 10.1016/s0304-3940(00)01675-x. [DOI] [PubMed] [Google Scholar]
- 42.Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer's disease?. Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 46.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 47.Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA, et al. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1-42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 49.Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM. Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging. 2005;26:645–654. doi: 10.1016/j.neurobiolaging.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Wang DS, Iwata N, Hama E, Saido TC, Dickson DW. Oxidized neprilysin in aging and Alzheimer's disease brains. Biochem Biophys Res Commun. 2003;310:236–241. doi: 10.1016/j.bbrc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Yao J, Du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SS. Inhibition of amyloid-β (Aβ) peptide-binding alcohol dehydrogenase-Aβ interaction reduces Aβ accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease. J Neurosci. 2011;31:2313–2320. doi: 10.1523/JNEUROSCI.4717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]