Abstract
Objective
To determine whether expression of thrombospondin-1 (TSP1), an endogenous inhibitor of angiogenesis, is down-regulated during progression of uveal melanoma and if administration of TSP1 and/or its antiangiogenic peptides attenuate tumor growth.
Methods
Tyr-tag transgenic mice were used for evaluation of TSP1 expression during tumor progression using immunohistological methods. The therapeutic potential of TSP1 on tumor progression was evaluated by either crossing Tyr-tag mice to a line of transgenic mice over expressing TSP1 in the eye (Tyr-tag;TSP1), or by administration of TSP1 mimetic peptide with known antiangiogenic, antitumor activity. Tumor areas were measured in histological sections using Optima software.
Results
Tyr-tag tumors from 3-week-old mice showed significant TSP1 expression which was dramatically down-regulated in tumors from 12-week-old mice. Furthermore, the development and progression of tumor was significantly delayed in Tyr-tag;TSP1 transgenic mice or Tyr-tag mice receiving TSP1 mimetic peptides (100 mg/Kg/day).
Conclusions and Clinical relevance
TSP1 expression was decreased with the angiogenic switch during progression of uveal melanoma. TSP1 and/or its antiangiogenic peptides were effective in attenuation of tumor growth. Therefore, modulation of TSP1 expression and/or activity may be beneficial in treatment of uveal melanoma.
Introduction
Uveal melanoma is the most common primary intraocular malignant tumor in humans, and it occurs predominantly in a nonhereditary, sporadic manner (1, 2). The current treatments for uveal melanoma are enucleation, radiotherapy, transpupillary thermotherapy, laser photocoagulation, intravenous chemotherapy, immunotherapy, local tumor resection, or a combination of these treatments. Although some patients get successful treatment, approximately half of all patients ultimately develop metastases and die within a year. Angiogenesis, the formation of new blood vessels from pre-existing capillaries, is associated with progression of many solid tumors. Although the important role of angiogenesis in progression and metastasis of uveal melanoma has been recently recognized, the molecular and cellular mechanisms involved require investigation (3–6).
Angiogenesis is a very tightly regulated process and normally does not occur except during embryonic development and repair processes. This tight regulation is achieved by a balanced production of a variety of promoters and inhibitors of angiogenesis (7). The abrogation of this balance, under various pathological conditions such as cancer, promotes the growth of new blood vessels. Although many investigations have historically focused on identification of factors that promote angiogenesis, now more attention is also given to factors that inhibit angiogenesis. Thrombospondin-1 (TSP1) is one of the first potent endogenous inhibitor of angiogenesis, whose decreased expression with the angiogenic switch, contributes to progression of many solid tumors (8). This is accomplished, at least in part, through mutations that inactivate P53 (9).
The list of the inhibitors of angiogenesis has been growing in the past decades, and more studies have been focusing on potential expression and activity of these factors. Re-expression of TSP1 attenuates the growth and metastasis of a variety of solid tumors (10). TSP1 inhibits angiogenesis in vitro and in vivo by down regulation of bcl-2 expression and activation of caspases driving apoptosis of endothelial cells, the major cells that line inside of the blood vessels (11). We have previously shown that TSP1 and its antiangiogenic fragment are present in vitreous and aqueous humor samples prepared from normal human, rat, mouse and bovine eyes (12). Furthermore, TSP1 levels are dramatically decreased in ocular samples prepared from diabetic rats. Thus, TSP1 expression play a significant role in ocular vascular homeostasis and its altered production may contribute to the pathogenesis of eyes diseases with a neovascular component.
We have shown that expression of TSP1 plays a significant role during retinal vascular development such that in its absence developing retinal vasculature fails to undergo proper pruning and remodeling resulting in increased retinal vascular density (13). We also showed over-expression of TSP1 in the mouse eye, prior to postnatal retinal vascularization, results in attenuation of retinal neovascularization during oxygen-induced ischemic retinopathy (14). Thus, manipulation of TSP1 expression may provide a novel target for inhibition of ocular neovascularization. However, the expression of TSP1 and its altered production during progression of uveal melanoma has not been previously evaluated.
Uveal melanoma most often arises in the choroid and becomes vascularized, presumably via angiogenic mechanisms whose identity remains elusive. An important role for increased VEGF expression (6, 15, 16) and down-regulation of pigment epithelium derived factor (PEDF) (17) have been proposed in the progression and metastasis of uveal melanoma. In addition, inhibition of VEGF activity (16) and/or over-expression PEDF (18) have shown therapeutic benefit in preclinical models. However, the underlying mechanisms which drive tumor progression remain poorly defined. We hypothesized that down-regulation of TSP1 expression occurs during progression of uveal melanoma contributing to its pathogenesis. Here we demonstrate that the down-regulation of TSP1 expression occurs during progression of uveal melanoma using a murine transgenic pigmented ocular tumor model (19). Although in these mice the tumors develop from the retinal pigment epithelium, their histology, growth and response to treatment, closely resembles that of human choroidal melanoma. This model has proved a useful tool in the study of endogenous primary pigmented tumors limited to the eye, and we believe it to be the most useful murine model available for the study of human choroidal melanoma. We also show that increased expression of TSP1 in the eye, or administration of TSP1 mimetic peptide, attenuated tumor progression and growth in this model. Thus, TSP1 may be an important target for treatment of uveal melanoma.
Materials and Methods
Animals
All research using mouse models of uveal melanoma were carried out in accordance to the Association for Research in Vision and Ophthalmology Statement for the Use of animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care Committee of the University of Wisconsin School of Medicine and Public Health.
The generation of Tyr-Tag and TSP1 transgenic mice
The Tyr-Tag or TSP1-overexpressing transgeneic mice were generated and maintained as previously described (14, 19). The Tyr-Tag mice which over-express TSP1 in their eye (Tyr-Tag; TSP1) were generated by crossing the Tyr-Tag mice with TSP1 transgenic mice (all on C57BL/6J background) which express TSP1 driven by αA-crystalline promoter. In Tyr-Tag mice tumor development can be histologically detected by 3-weeks of age and is physically visible by 8–12 weeks of age. The tumor generally is quite large by 12-weeks such that it destroys the eye structure. Animals were sacrificed as soon as any sign of ocular discomfort was noted.
Treatment of Tyr-Tag mice with TSP1 mimetic peptide
The antiangiogenic activity of TSP1 is mapped to peptides from type 1 repeats and procollagen homology domain (20). An overlapping peptide that expands these regions has shown good efficacy for inhibition of angiogenesis in various tumor models (21) and was the basis for development ABT510, and most recently a newer generation (22), ABT898. The amino acid sequence of ABT-898 is N-acetyl-glycine-valine-D-alloisoleucine-serine-glutamine-isoleucine-argenine-prolin-ethylamid and was synthesized at UW-Biotechnology peptide synthesis core facility (Madison, WI). The purity and sequence of the peptide were confirmed using standard methods. The peptide was dissolved in 5%-dextran solution and used for intraperitoneal injections. Three-week-old Tyr-Tag transgenic mice received TSP1-antiangiogenic peptide or vehicle for five weeks at 100 mg/Kg/day, five days a week (at least 10 mice per group). The histopathological evaluations were performed with eyes obtained from 3- and 8-week-old Tyr-Tag transgenic mice receiving TSP1-antiangiogenic peptide or vehicle.
Tumor size determination
The mice were euthanized on the last day of experiment. Their eyes were then enucleated and placed in a 10% neutral-buffered formalin solution. Four serially sectioned 5-μm-thick sections were cut from each of the superior, middle, and inferior areas of the globe in the manner previously described (23–25) and stained with hematoxylin-eosin. All 4 of the sections from each globe area were examined under a microscope and the section with the largest area of tumor was used for measurement. The outline of the tumor was traced on a microscopically digitized image and the tumor area measured using Optimus software version 6.5 (Media Cybernetics, Silver Spring, MD). Three measurements from each tumor representation were averaged to obtain the mean tumor measurement. These methods have been described elsewhere (23–25). The mitotic count and capillary density evaluations were recorded on H&E and PECAM-1 stained sections using light and fluorescence microscope (x400), respectively. The mitotic figures and capillary densities were determined at the base of the tumors in the most active area in a minimum of 3 consecutive high powered fields and the mean number of mitosis and capillaries per high powered field was determined in eyes from 5 mice.
Immunohistochemical staining of the frozen eye sections
Mouse eyes were enucleated and embedded in optimal cutting temperature (OCT) compound at −80°C. Sections (9 μm) were cut on a cryostat, placed on glass slides, and allowed to dry for 2 h. For fluorescence microscopy, sections were fixed in cold acetone (4°C) on ice for 10 min, followed by three washes with PBS, 5 min each. Sections were incubated in blocker (1% BSA, 0.2% skim milk, and 0.3% Triton X-100 in PBS) for 15 min at room temperature. Sections were then incubated with rabbit polyclonal antibodies to human TSP1 (Neo Markers, Fremont, CA) or murine PECAM-1 (26) (prepared in our laboratory and diluted 1:250 in blocking solution) overnight at 4°C in humid environment. After three washes in PBS, 5 min each, sections were incubated with secondary antibody Alexa 594 goat-anti-rabbit (Invitrogen, Carlsbad, CA; 1:500 dilution prepared in blocking solution). Sections were washed three times in PBS, covered with PBS/glycerol (2 vol/1 vol), and mounted with a coverslip. Retina sections were viewed by fluorescence microscopy and images were captured in digital format using a Zeiss microscope (Carl Zeiss, Chester, VA).
Statistical analysis
All data were summarized as mean ± standard error. The effect of TSP1 mimetic peptide administration and ocular TSP1 over expression on tumor areas in Tyr-Tag and Tyr-Tag; TSP1 transgenic mice, respectively, were assessed using 1-way analysis of variance. The tumor area was transformed to the log scale before calculating the mean area. Differences were considered statistically significant at P< 0.05.
Results
Down-regulation of TSP1 expression and increased angiogenesis during tumor Progression
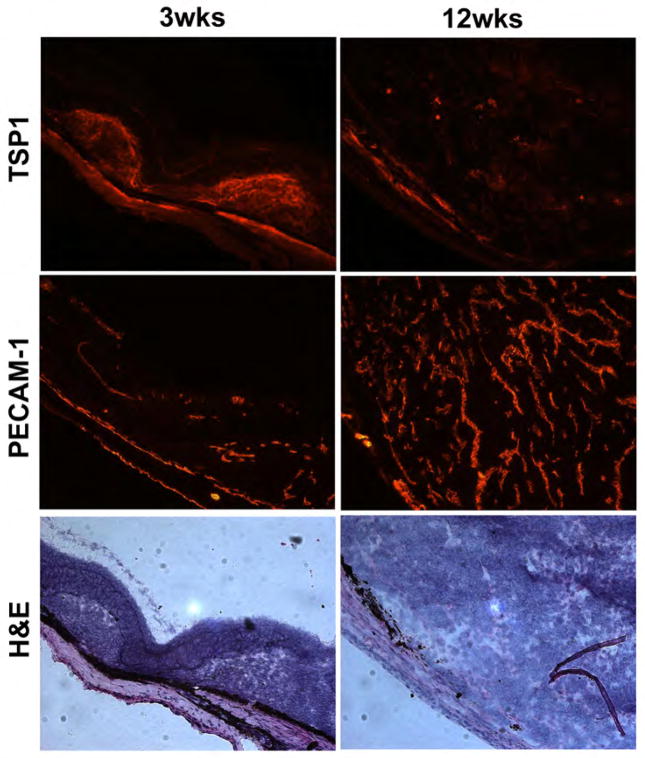
TSP1 expression during tumor progression was monitored by immunohistological staining. At 3 weeks, the tumor was small and TSP1 staining was strong suggesting increased expression at early stages of tumor development (Figure 1). At 12 weeks, the tumor grew significantly bigger and TSP1 expression was almost undetectable. This is consistent with previously reported negative staining of TSP1 in uveal melanoma samples (3). However, PECAM-1 staining in the tumors showed few blood vessels at 3 weeks of age, although the number of blood vessels significantly increased by 12 weeks of age in Tyr-Tag mice (Figure 1).
Figure 1.
TSP1 and PECAM-1 staining of frozen eye sections and histological examination of tumors in Tyr-Tag transgenic mice. TSP1 expression in Tyr-Tag tumors from 3-week and 12-week-old mice was examined by immunohistochemistry of frozen eye sections. Strong TSP1 staining was observed in eyes from 3 week-old mice, especially near the site of tumors. However, a significant decrease in TSP1 staining was observed in eyes from 12 week-old mice, where tumor size was increased significantly. The vascularity of tumors was similarly evaluated using anti-PECAM-1, a vascular marker. Although a few blood vessels were observed in eye sections from 3 week-old Tyr-Tag mice, a significant number of blood vessels were visible in section from 12-week-old mice. The H&E staining shows the tumor histology at 3- and 12 weeks. These images (x200) are reprehensive of images evaluated in eyes from at least 10 mice. Arrow heads indicate mitotic figures.
Attenuation of tumor growth in Tyr-Tag mice over-expressing TSP1 in their eyes
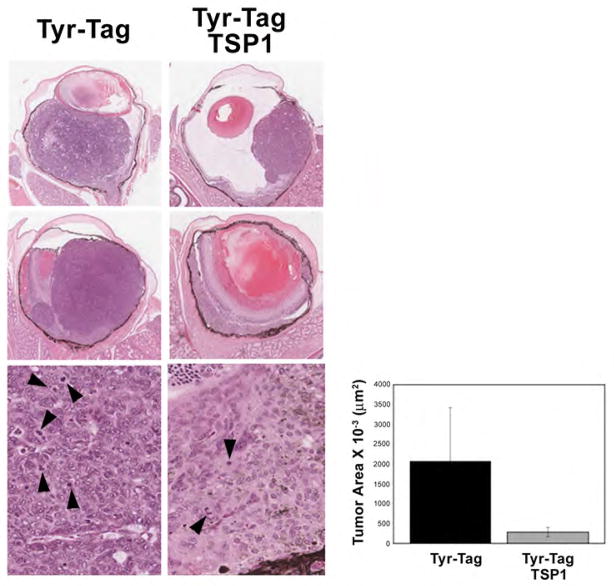
To provide additional evidence for the important role of TSP1 in modulation of tumor progression, and its potential use as an antitumor agent we determined the impact of TSP1 over-expression. We generated the Tyr-Tag; TSP1 transgenic mice. Tumor progression was assessed by histological examination of eyes at different postnatal days. Tumor development and progression was significantly delayed in Tyr-Tag; TSP1 transgenic mice compared to Tyr-Tag mice. The sizes of mouse ocular tumors were measured at 8 weeks for comparison. Average areas of ocular tumor in the Tyr-Tag mice expressing TSP1 were approximately 10-fold smaller than the areas of tumors in the parental Tyr-Tag mice (2.85×105 μm2 vs. 20.8×105 μm2). Although the tumor cells appeared similar in the two groups, Tyr-Tag; TSP1 mice showed less mitotic figures (8.80±0.86 vs. 22.83±2.56; P< 0.05, n=5), as well as capillary densities (27±4.0 vs. 55±6.95; P< 0.05, n=5) compared to parental Tyr-Tag mice.
Attenuation of tumor growth in Try-Tag mice receiving TSP1 antiangiogenic mimetic peptides
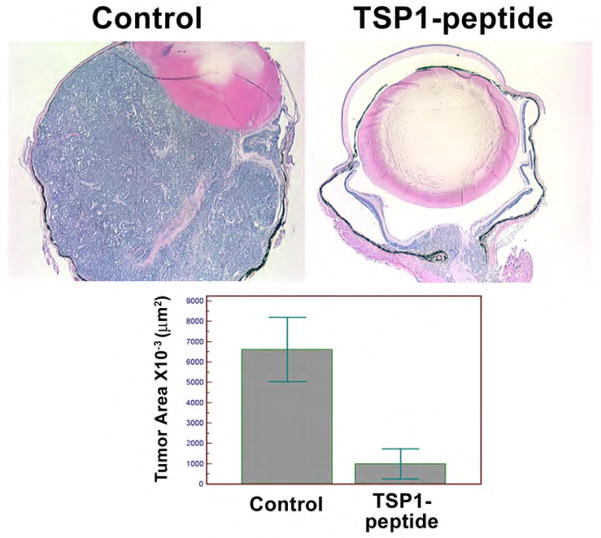
In order to demonstrate TSP1’s therapeutic potential, we synthesized the TSP1 antiangiogenic mimetic peptide as described in Methods. Tyr-Tag mice were injected intraperitoneally after initiation of tumors (3 week old) for 5 weeks. The tumor size in treated mice was significantly smaller than in control mice (Figure 3). The results were quite similar to that observed in transgenic Tyr-Tag; TSP1 mice. The average tumor areas in TSP1-treated mice were decreased by approximately 10-fold when compared to tumors in mice receiving vehicle alone (7×105 μm2 vs. 67×105 μm2). Thus, TSP1 mimetic peptide was efficacious in blocking the progression of tumor in this model.
Figure 3.
Inhibition of tumor growth in Tyr-tag mice receiving TSP1-anti-angiogenic mimetic peptide. The 3 week-old Tyr-Tag mice received TSP1 antiangiogenic mimetic peptide or vehicle for 5 weeks (100 mg/Kg/day, 5 days a week) and tumor volumes were evaluated as described in Methods. Histological sections showed a significant decrease in size of tumor in mice receiving the peptide compared to vehicle. The original magnification was x40. The quantification of tumor areas in mice receiving TSP1 peptide indicated that the tumor areas were approximately 10-fold smaller than the tumor areas of mice receiving vehicle (P< 0.05; n=10).
Discussion
The present study demonstrates, for the first time, that there is a decrease in expression of TSP1 during uveal melanoma progression in Tyr-tag mice, and shows a significant correlation between reduced TSP1 expression and increased tumor vascularity and size. Moreover, over-expression of TSP1 in the eye or administration of TSP1 mimetic peptides with antiangiogenic activity inhibited tumor growth in Tyr-tag mice. Thus, modulation of TSP1 expression or its antiangiogenic mimetic peptides may provide a novel approach for treatment of uveal melanoma and inhibition of tumor growth.
Angiogenesis plays an important role in tumor growth, invasion, and eventually metastasis. Although the most emphasis has been placed on identifying factors that promote angiogenesis, the alteration in expression of agents that normally inhibit angiogenesis has gained significant interest and, is shown to be critical in progression of many solid tumors. Antiangiogenic strategies, including use of TSP1 and its peptides, have been proven to be a promising approach for clinical therapy of a variety of solid tumors (10). However, changes in TSP1 expression during uveal melanoma progression, as well as its potential therapeutic utility, have not been previously evaluated. Although the role of tumor angiogenesis in pathogenesis of uveal melanoma has been recognized for quite some time it has been only recently that antiangiogenic therapies have been attempted to prevent tumor growth (27). Anti-VEGF and pigmented epithelial derived factor are effective in halting tumor growth (16, 18).
TSP1 expression in uveal melanomas was examined previously, and shown to be attenuated in most human uveal melanomas (3). This is consistent with our results where, very limited staining of TSP1 was observed in mature tumors of Tyr-Tag mice. Together these results support the notion that changes in TSP1 expression occur during progression of uveal melanoma, and that administration of TSP1 and/or its anti-angiogenic mimetic peptides can effectively halt tumor progression and metastasis. These conclusions are consistent with up-regulation of TSP1 by the tumor suppressor gene p53 and its down-regulation by oncoproteins such as Myc and Ras, whose alterations have been linked to the pathogenesis of uveal melanoma (28–30).
TSP1 may impact uveal melanoma growth in Try-tag mice by a direct effect on neoplastic cells. TSP1 can induce direct tumor cell apoptosis via the CD36/caspase pathway in some leukemic cells (33), and a similar mechanism may operate in other tumors such as breast carcinoma (34). Although suppression of tumor growth in transgenic mice that over-express TSP1 suggest a direct role for TSP1 on tumor cells, the direct impact of TSP1 on uveal melanoma cells needs further investigation. Alternatively, TSP1 may also have an indirect antitumor effect by inhibiting angiogenesis. TSP1 inhibits angiogenesis through direct effects on endothelial cell migration and survival, and through effects on vascular endothelial cell growth factor bioavailability. Together our results suggest TSP1 could be a novel therapeutic target for treatment of uveal melanoma, which requires further validation in human uveal melanoma cells and other in vivo melanoma models.
The molecular and cellular mechanisms which contribute to the pathogenesis of uveal melanoma have been subject of numerous studies. A number of studies have attempted to address the potential contribution of mutations in P53, a gene mutated in more than half of human tumors, to the pathogenesis of uveal melanoma. A genetic link between P53 mutation and uveal melanoma has been previously reported (31). Other studies of the role of P53 in uveal melanoma have focused on its role in apoptosis and/or enhanced proliferation of tumor cells. The decreased P53 expression in a low percentage of uveal melanomas is reported to be associated with increased proliferation (32). In addition, other studies have failed to detect P53 protein in uveal melanomas (28) or reported very low expression (29), despite the report of infrequent loss of heterozygosity at P53 locus in uveal melanomas (33). Thus, a role for P53 downstream pathways has been proposed in the pathogenesis of uveal melanomas. However, the impact of these changes on TSP1 expression and tumor vascularization needs further investigation. The presence of microcirculation patterns in uveal melanomas associated with lack of P53 expression (34) further supports a role for decreased expression of P53 and TSP1 promoting angiogenesis and tumor growth.
Figure 2.
Suppression of tumor growth in Tyr-Tag mice over expressing TSP1. Tyr-Tag transgenic mice which over-express TSP1 in their eye were generated by crossing Tyr-Tag mice with a line of transgenic mice which express high level of TSP1 in their eyes (Tyr-Tag; TSP1). A slower progression of tumors was observed in Tyr-Tag; TSP1 mice compared to parental Tyr-Tag mice. Histological evaluation of tumors in 8 week-old mice showed a significant decrease in size of tumor in Tyr-Tag; TSP1 mice compared to Tyr-Tag mice. The magnification for the two upper panels was x40 and for the lower panel was x400. The tumor areas were evaluated as described in Methods. Please note that tumor volume in Tyr-Tag; TSP1 mice is approximately 10-fold lower than Tyr-Tag mice (P<0.05; n=10). Although the tumor cells looked similar in two groups, Tyr-Tag; TSP1mice tumors showed less mitotic figures compared to Tyr-Tag mice.
Acknowledgments
This work was supported by grants EY16995, EY18179, EY21357 (NS), and P30-EY16665, from the National Institutes of Health and an unrestricted departmental award from Research to Prevent Blindness. NS is a recipient of a Research Award from American Diabetes Association, 1-10-BS-160 and Retina Research Foundation. CMS is supported by a grant from American Heart Association, 0950057G. NS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005;18:75–84. viii. doi: 10.1016/j.ohc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Sheibani N, Albert DM. Angiogenesis and ocular tumorigenesis. In: Tombran-Tank J, Barnstable CJ, editors. Ocular angiogenesis: Diseases, Mechanisms, and Therapies. Totowa, NJ: Humana Press Inc; 2006. pp. 161–171. [Google Scholar]
- 3.Ordonez JL, Paraoan L, Hiscott P, et al. Differential expression of angioregulatory matricellular proteins in posterior uveal melanoma. Melanoma Res. 2005;15:495–502. doi: 10.1097/00008390-200512000-00003. [DOI] [PubMed] [Google Scholar]
- 4.van Ginkel PR, Gee RL, Shearer RL, et al. Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer Res. 2004;64:128–134. doi: 10.1158/0008-5472.can-03-0245. [DOI] [PubMed] [Google Scholar]
- 5.Missotten GS, Notting IC, Schlingemann RO, et al. Vascular endothelial growth factor a in eyes with uveal melanoma. Arch Ophthalmol. 2006;124:1428–1434. doi: 10.1001/archopht.124.10.1428. [DOI] [PubMed] [Google Scholar]
- 6.Boyd SR, Tan D, Bunce C, et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol. 2002;86:448–452. doi: 10.1136/bjo.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 8.Good DJ, Polverini PJ, Rastinejad F, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 10.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 12.Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem Biophys Res Commun. 2000;267:257–261. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn. 2006;235:1908–1920. doi: 10.1002/dvdy.20837. [DOI] [PubMed] [Google Scholar]
- 15.el Filali M, Missotten GS, Maat W, et al. Regulation of VEGF-A in uveal melanoma. Invest Ophthalmol Vis Sci. 2010;51:2329–2337. doi: 10.1167/iovs.09-4739. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Jager MJ, Grossniklaus HE. Bevacizumab suppression of establishment of micrometastases in experimental ocular melanoma. Invest Ophthalmol Vis Sci. 2010;51:2835–2842. doi: 10.1167/iovs.09-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Xu Z, Iuvone PM, Grossniklaus HE. Angiostatin decreases cell migration and vascular endothelium growth factor (VEGF) to pigment epithelium derived factor (PEDF) RNA ratio in vitro and in a murine ocular melanoma model. Molecular Vision. 2006;12:511–517. [PubMed] [Google Scholar]
- 18.Yang H, Grossniklaus HE. Constitutive overexpression of pigment epithelium-derived factor inhibition of ocular melanoma growth and metastasis. Invest Ophthalmol Vis Sci. 2010;51:28–34. doi: 10.1167/iovs.09-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syed NA, Windle JJ, Darjatmoko SR, et al. Transgenic mice with pigmented intraocular tumors: tissue of origin and treatment. Invest Ophthalmol Vis Sci. 1998;39:2800–2805. [PubMed] [Google Scholar]
- 20.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson DW, Volpert OV, Pearce SF, et al. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol. 1999;55:332–338. doi: 10.1124/mol.55.2.332. [DOI] [PubMed] [Google Scholar]
- 22.Garside SA, Henkin J, Morris KD, Norvell SM, Thomas FH, Fraser HM. A Thrombospondin-Mimetic Peptide, ABT-898, Suppresses Angiogenesis and Promotes Follicular Atresia in Pre- and Early-Antral Follicles in Vivo. Endocrinology. 2010;151:5905–5915. doi: 10.1210/en.2010-0283. [DOI] [PubMed] [Google Scholar]
- 23.Wilkerson CL, Darjatmoko SR, Lindstrom MJ, Albert DM. Toxicity and dose-response studies of 1,25-(OH)2-16-ene-23-yne vitamin D3 in transgenic mice. Clin Cancer Res. 1998;4:2253–2256. [PubMed] [Google Scholar]
- 24.Grostern RJ, Bryar PJ, Zimbric ML, et al. Toxicity and dose-response studies of 1alpha-hydroxyvitamin D2 in a retinoblastoma xenograft model. Arch Ophthalmol. 2002;120:607–612. doi: 10.1001/archopht.120.5.607. [DOI] [PubMed] [Google Scholar]
- 25.Dawson DG, Gleiser J, Zimbric ML, et al. Toxicity and dose-response studies of 1-alpha hydroxyvitamin D2 in LH-beta-tag transgenic mice. Ophthalmology. 2003;110:835–839. doi: 10.1016/S0161-6420(02)01934-6. [DOI] [PubMed] [Google Scholar]
- 26.Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev Dyn. 1999;214:44–54. doi: 10.1002/(SICI)1097-0177(199901)214:1<44::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Stitt AW, Gardiner TA. Anti-angiogenic therapy for uveal melanoma--more haste, less speed. Br J Ophthalmol. 2002;86:368–369. doi: 10.1136/bjo.86.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chana JS, Wilson GD, Cree IA, et al. c-myc, p53, and Bcl-2 expression and clinical outcome in uveal melanoma. Br J Ophthalmol. 1999;83:110–114. doi: 10.1136/bjo.83.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brantley MA, Jr, Harbour JW. Deregulation of the Rb and p53 pathways in uveal melanoma. Am J Pathol. 2000;157:1795–1801. doi: 10.1016/s0002-9440(10)64817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edmunds SC, Kelsell DP, Hungerford JL, Cree IA. Mutational analysis of selected genes in the TGFbeta, Wnt, pRb, and p53 pathways in primary uveal melanoma. Invest Ophthalmol Vis Sci. 2002;43:2845–2851. [PubMed] [Google Scholar]
- 31.Jay M, McCartney AC. Familial malignant melanoma of the uvea and p53: a Victorian detective story. Surv Ophthalmol. 1993;37:457–462. doi: 10.1016/0039-6257(93)90142-t. [DOI] [PubMed] [Google Scholar]
- 32.Kishore K, Ghazvini S, Char DH, Kroll S, Selle J. p53 gene and cell cycling in uveal melanoma. Am J Ophthalmol. 1996;121:561–567. doi: 10.1016/s0002-9394(14)75431-5. [DOI] [PubMed] [Google Scholar]
- 33.Scholes AG, Liloglou T, Maloney P, et al. Loss of heterozygosity on chromosomes 3, 9, 13, and 17, including the retinoblastoma locus, in uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:2472–2477. [PubMed] [Google Scholar]
- 34.Chowers I, Folberg R, Livni N, Pe’er J. p53 Immunoreactivity, Ki-67 expression, and microcirculation patterns in melanoma of the iris, ciliary body, and choroid. Curr Eye Res. 2002;24:105–108. doi: 10.1076/ceyr.24.2.105.8166. [DOI] [PubMed] [Google Scholar]