Abstract
Normophosphatemic familial tumoral calcinosis, characterized by ectopic mineralization of skin, is caused by mutations in the SAMD9 gene located on human chromosome 7q21, next to a paralogous gene, SAMD9-like (SAMD9L). Mouse does not have a SAMD9 orthologue, Samd9, because it has been deleted during evolution due to genomic rearrangements. It has been suggested that the mouse Samd9l gene serves as a functional paralogue of human SAMD9. In this study we examined Samd9l knockout mice with respect to ectopic mineralization. We also crossed these mice with Abcc6tm1JfK mice, a model system to study pseudoxanthoma elasticum, to see if the absence of the Samd9l gene modifies the mineralization process. Necropsy analysis of Samd9ltm1Homy mice revealed no evidence of ectopic mineralization, and deletion of the Samd9l gene in mice failed to modify the mineralization process on the Abcc6tm1JfK background. Collectively, the results suggest that mouse Samd9l is not a functional paralogue of human SAMD9.
Keywords: Ectopic connective tissue mineralization, mouse models of skin diseases, familial tumoral calcinosis, pseudoxanthoma elasticum
Background
Familial tumoral calcinosis (MIM610455) is a group of heritable disorders manifesting with ectopic mineralization of connective tissues (1). The normophosphatemic variant (NFTC) is characterized by extensive mineralization of the skin and mucous membranes, associated with preceding inflammation. We have recently demonstrated that mutations in the sterile alpha motif domain-containing 9 gene (SAMD9) underlie NFTC (2,3). The SAMD9 gene is located in the human genome in chromosomal region 7q21, and next to it in head-to-tail orientation is a paralogous gene, SAMD9-like (SAMD9L). Both genes have similar structures consisting of a large, ~5–6 kb coding exon (4). Both human genes encode respective proteins of ~170–190 kD, with 56% sequence homology.
An intriguing observation is that an orthologous gene of human SAMD9 has been deleted in mouse genome as a result of unique genomic rearrangement during evolution (4). However, mice do have the orthologue of human SAMD9L, but relatively little is known of the functional relationships of the SAMD9/SAMD9L gene/protein interactions either in human or mouse. Since the SAMD9 orthologue has been deleted in mouse, it has been suggested that the SAMD9L paralogue might functionally substitute for the SAMD9 gene (4,5). Thus, one could postulate that the deletion of the Samd9l gene in mouse would result in ectopic mineralization similar to that noted in NFTC patients.
Questions addressed
To explore the possibility that mouse Samd9l could serve as a functional paralogue of human SAMD9, the gene mutated in NFTC, we have now examined targeted mutant Samd9l (“knockout”, KO) mouse, Samd9ltm1Homy. These KO mice were first examined with respect to tissue mineralization up to eight months of age. The Samd9ltm1Homy mice, as well as their heterozygous or wild type counterparts were also crossed with Abcc6tm1JfK mice, an animal model for another ectopic mineralization disorder, pseudoxanthoma elasticum (PXE) (6), to examine whether the absence of Samd9l functional protein may accelerate the mineralization noted in Abcc6tm1JfK mice.
Experimental design
Samd9ltm1Homy mouse model was developed by homologous recombination resulting in replacement of part of exon 2 in the Samd9l gene by the LacZ gene (7). These mice were maintained on the pure C57BL/6N background (N10). Complete necropsies were performed at approximately 8 months of age (8).
In another set of experiments, Samd9ltm1Homy mice were crossed with Abcc6tm1JfK mice, which have been shown to develop ectopic connective tissue mineralization at ~5–6 weeks of age (9–11). The Abcc6tm1JfK mice were congenics on C57BL/6J background (N10). The targeted mutant Samd9l mice (Samd9ltm1Homy), the corresponding heterozygote carriers (Samd9l+/tm1Homy) or their wild type counterparts (Samd9l+/+), all on Abcc6tm1JfK background, were placed at weaning (4 weeks of age) on diet that was previously shown to accelerate the ectopic mineralization process in Abcc6tm1JfK mice (12,13). Biopsies from the muzzle skin containing the vibrissae were obtained at 4 and 8 weeks after initiation of the acceleration diet, and mineralization of the connective tissue sheath of vibrissae, an early site of mineralization which serves as a biomarker of the entire mineralization process, was determined either semi-quantitatively by histopathologic examination or quantitatively by chemical assay of calcium and phosphate (10–13).
Results
Necropsy of Samd9ltm1Homy mice – no evidence of ectopic mineralization
Homozygous (Samd9ltm1Homy) and heterozygous (Samd9l+/tm1Homy) mice, together with their wild type counterparts, were subjected to complete necropsy approximately at the age of 8 months, with focus on ectopic mineralization of soft connective tissues. Careful examination of histopathologic sections failed to reveal any evidence of ectopic mineralization in these mice. The pathology in the homozygous and heterozygous KO mice consisted primarily of age associated changes similar to those noted in wild type mice, including focal areas of granulomatous inflammation of the skin, mild focal dermal fibrosis, alopecia and dermatitis. However, one of the heterozygous mice had signs of alterations in the hematopoetic system, consistent with lymphoma. It should be noted that both the heterozygous and homozygous mice naturally develop acute myeloid leukemia at high frequency after the mice have reached 20 months of age (7).
Deletion of the Samd9l gene does not accelerate ectopic mineralization in Abcc6tm1JfK mice
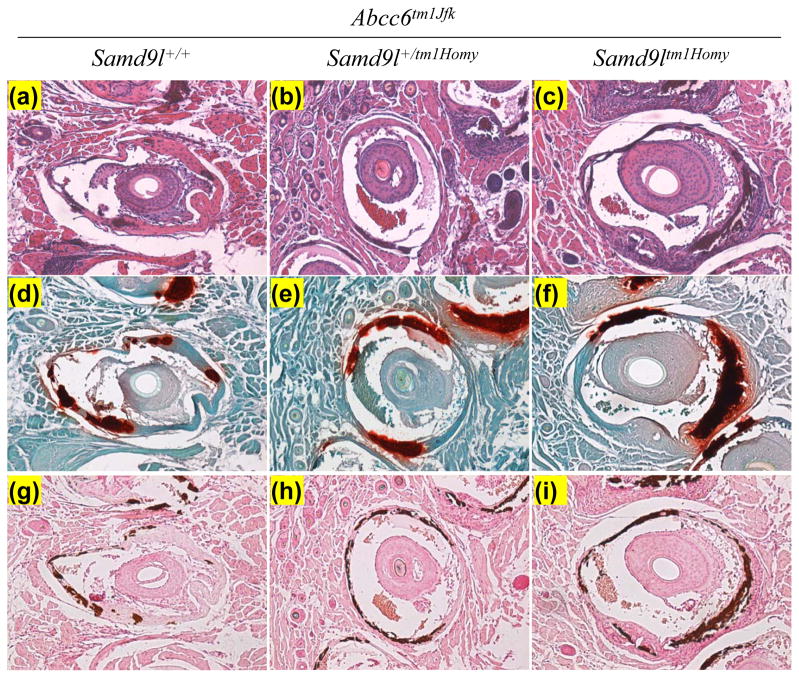
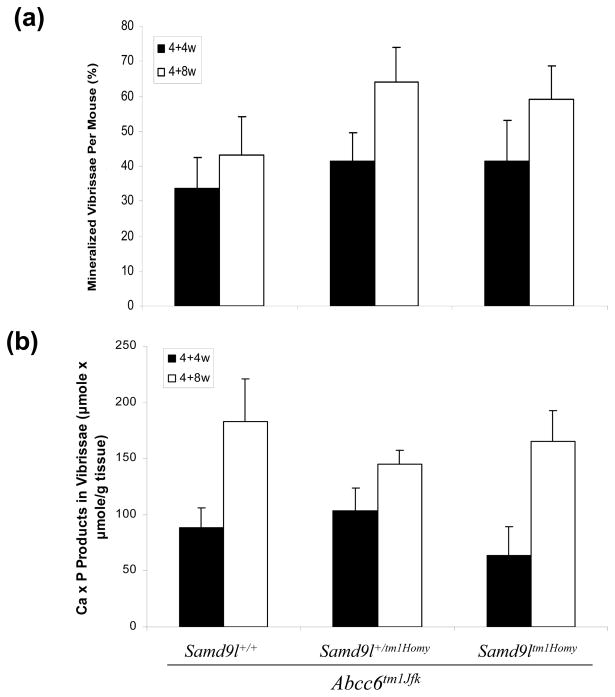
Abcc6tm1JfK mice serve as a model for PXE, a heritable mineralization disorder, and these mice develop progressive mineralization from ~5–6 weeks on. To examine the possibility that the Samd9l gene product might play a role in modulating this mineralization process, Samd9l KO mice were crossed with Abcc6tm1JfK mice, and Samd9l+/+, Samd9l+/tm1Homy and Samd9ltm1Homy mice, all on Abcc6tm1JfK background, were then generated and examined for mineralization of the connective tissue sheath of vibrissae as well as a number of internal organs. Histopathologic examination of muzzle skin at 8 and 12 weeks of age (4 weeks and 8 weeks after the initiation of the acceleration diet) revealed progressive mineralization of connective tissue sheath of vibrissae by routine hematoxylin-eosin stain, as well as special stains revealing calcium deposits (Alizarin Red and von Kossa), similar to that previously described for the Abcc6tm1JfK mice (Fig. 1). However, deletion of the Samd9l gene, either in homozygous or heterozygous state, did not influence the degree of mineralization at these time points. These histopathologic findings were confirmed by determining quantitatively the percent of vibrissae that were mineralized in each group (Fig. 2a). Finally, skin biopsies from the muzzle skin were used to determine chemically the calcium and phosphate content, a reflection of the mineralization of vibrissae (Fig. 2b). No statistically significant differences either at 4 weeks or 8 weeks on the acceleration diet were noted in mineralization between the genotypically different groups of mice.
Figure 1.
Histopathologic examination of mineralization of the connective tissue sheath of vibrissae in Samd9ltm1Homy and Samd9l+/tm1Homy mice in comparison to their wild type counterpart (Samd9l+/+), all on Abcc6tm1JfK background. The stains used were hematoxylin-eosin (a–c), Alizarin Red (d–f), and von Kossa (g–i).
Figure 2.
Quantitation of mineralization of the connective tissue sheath of vibrissae in the group of mice shown in Fig. 1 by the count of mineralized vibrissae over total number examined (%) (a), and by chemical assay of calcium and phosphate in the skin biopsy specimens (b), at 4 weeks (4+4w) and 8 weeks (4+8w) of feeding with a diet accelerating the mineralization process in Abcc6tm1JfK mice. The values are mean + S.E., n = 6–13 mice per group.
A number of internal organs were also examined for evidence of ectopic mineralization in the same mice by histopathologic examination. Aberrant mineralization was noted, in addition to vibrissae, in the kidneys, heart, and the eyes. No evidence for increased mineralization in mice with allelic deletion of the Samd9l gene was noted (Supplementary Table 1). No mineralization was noted in any groups of mice in aorta, spleen or lung (not shown).
Conclusion
The results of this study indicate that deletion of the Samd9l gene does not result in ectopic mineralization of mouse tissues nor does it accelerate the mineralization that takes place in Abcc6tm1JfK mice. Collectively, our observations suggest that the mouse Samd9l gene does not serve as a functional paralogue for human SAMD9, the gene harboring mutations in NFTC.
Supplementary Material
Acknowledgments
We thank Alix Grand-Pierre for technical assistance. Carol Kelly assisted in preparation of this manuscript. This study was supported by the National Institutes of Health, Grants R01 AR28450 and R01 AR55225. Dr. Li is recipient of the Dermatology Foundation Career Development Award.
Dr. Li performed the research and analyzed the data; Dr. Guo performed the research; Drs. Matsui, Honda and Inaba contributed essential reagents and tools; Dr. Sundberg performed research and analyzed the data; Dr. Sprecher designed the research study; Dr. Uitto designed the research study, analyzed the data and wrote the paper.
Abbreviations
- NFTC
normophosphatemic familial tumoral calcinosis
- PXE
pseudoxanthoma elasticum
- KO
knockout
Footnotes
Conflict of interest
The authors have declared no conflicting interests.
References
- 1.Sprecher E. J Invest Dermatol. 2010;130:652–660. doi: 10.1038/jid.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topaz O, Indelman M, Chefetz I, et al. Am J Hum Genet. 2006;79:759–764. doi: 10.1086/508069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chefetz I, Ben Amitai D, Browning S, et al. J Invest Dermatol. 2008;128:1423–1429. doi: 10.1038/sj.jid.5701203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CF, MacDonald JR, Wei RY, et al. BMC Genomics. 2007;8:92–109. doi: 10.1186/1471-2164-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Q, Quaynor B, Sun A, et al. J Invest Dermatol. 2011;131:1428–1434. doi: 10.1038/jid.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uitto J, Li Q, Jiang Q. J Invest Dermatol. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui H, Nagamachi A, Ozaki Y, et al. ASH Annual Meeting and Exposition; New Orleans, LA, USA. 2009. p. Abstr. 2963. [Google Scholar]
- 8.Seymour R, Ichiki T, Mikaelian I, et al. Necropsy Methods. In: Hedrich HJ, Bullock GR, editors. The Laboratory Mouse. London: Academic Press; 2004. pp. 495–516. [Google Scholar]
- 9.Klement JF, Matsuzaki Y, Jiang QJ, et al. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Q, Li Q, Uitto J. J Invest Dermatol. 2007;127:1392–1402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Uitto J. Exp Dermatol. 2011;20:452–454. doi: 10.1111/j.1600-0625.2010.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaRusso J, Jiang Q, Li Q, et al. Exp Dermatol. 2007;17:203–207. doi: 10.1111/j.1600-0625.2007.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Uitto J. J Mol Med (Berl) 2010;88:173–181. doi: 10.1007/s00109-009-0522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.