Abstract
OBJECTIVES
To test the main and interactive effects of activities derived from the Need-driven Dementia-compromised Behavior model for responding to behavioral symptoms in nursing home residents. Activities tailored to functional level and personality style of interest were hypothesized to improve behavioral outcomes to a greater extent than partially- tailored or non-tailored activities.
DESIGN
Randomized clinical trial, double-blind.
SETTING
Nine community-based nursing homes.
PARTICIPANTS
One hundred and twenty eight cognitively impaired residents randomly assigned to activities tailored to: functional level (FL) (n= 32); personality style of interest (PSI) (n= 33); functional level and personality style of interest (FL+PSI) (n= 31); or active control (AC) (n= 32).
INTERVENTION
Three weeks of activities provided twice daily.
MEASUREMENTS
Agitation, passivity, engagement, affect, and mood assessed from video-recordings and real time observations during baseline, intervention, random times outside of intervention, and one week post-intervention.
RESULTS
Compared to baseline all treatments improved outcomes during intervention except mood which worsened under AC. During intervention the PSI group demonstrated greater engagement, alertness, and attention than the other groups; the FL+PSI group demonstrated greater pleasure. During random times, engagement returned to baseline levels except in the FL group where it decreased. There was also less agitation and passivity in groups with a tailored to personality style of interest component. One week post intervention mood, anxiety and passivity improved over baseline; there was significantly less pleasure displayed following withdrawal of treatment.
CONCLUSION
The hypothesis was partially supported. Personality style of interest is a critical component of individualized activity prescription.
Keywords: Behavioral symptoms, dementia, nursing home, activity intervention, personality style of interest
INTRODUCTION
Behavioral symptoms such as agitation and passivity are common among nursing home residents with dementia.[1] As dementia progresses these behaviors often co-occur[2] and are associated with decline in physical functioning[3], use of chemical restraints[4], and increased risk of abuse[5]. Pharmacological treatments have not demonstrated strong efficacy[6], and may have serious adverse effects in frail older nursing home residents[7]. Safe and efficacious interventions are needed to improve residents’ quality of life while reducing the burden and high cost of behavioral symptoms in the nursing home[8].
Non-pharmacological interventions are recommended as a first line of treatment for behavioral symptoms [9]. Overall, these treatments demonstrate small to moderate effects with a short or unknown duration of action [10–12]. The within-group variation in response to treatment suggests that effects may be improved by tailoring interventions to individual preferences and needs.
Algase and colleagues were among the first to conceptualize agitation and passivity as symptoms of unmet needs[13]. In their Need-driven Dementia-compromised Behavior (NDB) model, behavioral symptoms result from the interaction of: background/risk factors (neuropathology, cognitive deficits, physical function, and premorbid personality); and proximal/precipitating factors (qualities of the physical and social environment, and physiological and psychological need states). Failure to understand the need communicated by the behavior may lead to inappropriate and ineffective treatment.
Persons with dementia often experience unmet needs because they lack the internal and external resources to meet them. One basic human need is for activity. Most nursing homes provide activities, but few adequately individualize them[14]. Residents are often excluded from activities because of their behavioral symptoms[15] and as a result are unoccupied for long periods of time. Both agitation[16] and passivity[17] occur under these circumstances. Because life in the nursing home often lacks appropriate stimulation, almost any type of personal attention demonstrates some relief of behavioral symptoms[18]. Unknown are the individual components of treatments that are most responsible for effects and the duration of those effects. This study aimed to fill that gap.
Methods to identify activities that engage residents, such as the Pleasant Events Schedule[19] or use of self-identity roles[20] have been developed. In general, they rely on informant report of activities or roles enjoyed in the past. It can be difficult, however, to identify meaningful activities for residents who never participated in leisure pursuits or who lost the functional ability to engage in activities they once enjoyed. Qualitative data suggest that residents with dementia, staff and family caregivers have different views about what is meaningful activity for the resident [21]. Given the vast differences in preference for activity, it is obvious that “one size will not fit all.” Background factors in the NDB model may help to individualize activities for this population.
Activities are meaningful when they meet needs related to personality style of interest[22], i.e., an individual’s long-standing disposition to gratify activity needs in a particular manner[23]. In the Five-Factor Model (FFM)[24], personality traits are hierarchically organized with the five major domains of Neuroticism, Extraversion, Openness to Experience, Agreeableness and Conscientiousness at the top level, and below are the more important, narrower traits, or facets, that define each domain. In the FFM, which is currently the leading framework for the study of personality, the domains of extraversion and openness define personality style of interest and are associated with preference for leisure activities[25, 26]. The NDB model, which considers both personality style of interest and functional abilities, offers an alternative framework for assessment of activity preferences in nursing home residents with dementia.
The purpose of this double-blind randomized clinical trial (RCT) was to test the efficacy of activities derived from the NDB model for reducing agitation and passivity and improving engagement, affect and mood in nursing home residents with dementia. NDB-derived activities were tailored to the resident’s functional level (cognitive and physical) and personality style of interest. The main and interactive effects of these treatment components were investigated. Repeated measures of behavioral outcomes were obtained during treatment, at random times outside of treatment and one-week post-treatment to assess duration of effect. Activities tailored to both functional level and personality style of interest were hypothesized to improve behavioral outcomes compared to activities tailored to functional level only, personality style of interest only or active control. These activities are designed to enhance precision of prescription by meeting needs originating from premorbid personality, and cognitive and physical functioning-all risk factors for behavioral symptoms identified in the NDB model.
METHODS
Study Setting and Participants
The protocol for this RCT (ClinicalTrials.gov NCT00388544) was approved by the university Institutional Review Board and had a Data Safety and Monitoring Committee that convened annually. Residents of nine community-based nursing homes in Pennsylvania were approached for the study. The nursing homes provided the investigators with contact information for the legally authorized representatives of residents who agreed to be contacted by the investigators.
Consented residents underwent screening to determine eligibility by a research nurse and the project director, a certified recreational therapist. Inclusion criteria were English speaking; 65 years of age or older; diagnosis of dementia according to DSM-IV criteria[27]; a Mini-mental State Exam (MMSE)[28] score of 8 or greater but less than 24; no new psychoactive drugs prescribed from pre-baseline through final observation as verified by a weekly chart review; and presence of behavioral symptoms as reported by staff and documented in the Minimum Data Set (MDS). Exclusion criteria were delirium or a progressive, unstable medical, metabolic, or neurological illness; and history of Parkinson’s disease, Huntington’s disease, seizure disorder, stroke, alcoholism, drug abuse, head trauma with loss of consciousness, or psychiatric illness preceding the onset of memory loss. Participants were assessed for physical function using the physical capacity subscale of the Psychogeriatric Dependency Rating Scale (PGDRS), a 7-item Likert-type scale that includes items on hearing, vision, mobility, dressing, toileting, speech and hygiene [29]. A knowledgeable informant (usually a spouse or adult child) provided personality data. Form R of the Revised NEO Personality Inventory (NEO-PI-R) [30], a 240-item Likert-type scale adapted for observer ratings, was used. The NEO-PI-R allows a comprehensive assessment of adult personality in the five domains of neuroticism, extraversion, openness, agreeableness, and conscientiousness and the six more specific facets that comprise each domain. Observers were considered “knowledgeable informants” if they had monthly contact with the subject for at least 3 years during the subject’s adult life[31]. Coefficient alphas for observer ratings on the domain scales range from .89 to .96[32]. Studies using samples from the general population, as well as samples with dementia, give evidence that close acquaintances are accurate raters of an individual’s personality[30, 33].
Sample Size
Estimates of means for treatment and control conditions were available from our previous study [34]. Based on those results, power of the proposed study was calculated for a two-way analysis of variance, assuming a medium effect size. A very conservative approach to power analysis was used, in which the subject was considered as the unit of analysis, with no contribution of information from the multiple observations per subject that were to be obtained in the actual study. The analysis approach, based on mixed models and least-squares adjustment of means, allowed incorporation of all available information for each subject, regardless of whether complete data were available. A total sample size of 128 subjects, or 32 per group, provided 80% power.
PROCEDURE
The study had three phases: baseline (one week); intervention (three weeks); and post intervention (one week). We took measures of outcomes during all phases. The time for observation and intervention during these phases was individually selected for each participant based on staff report of high behavioral symptom time and a pre-baseline observation period where subjects were observed every hour for 5-minutes (7am to 7pm) for three days using the Cohen-Mansfield Agitation Inventory (CMAI)[35] and the Passivity in Dementia Scale (PDS)[36]. We identified each subject’s high behavioral symptom time by visually inspecting these data. One daily observation/intervention session was scheduled within two hours of that time and the second daily session was scheduled plus or minus four hours from that point so that all sessions occurred between the hours of 9am and 5pm to accommodate morning care and meal times. During the intervention phase we also took measures of outcomes at random times outside of treatment to capture duration of effect throughout the day. Baseline, intervention and post-intervention sessions were video-recorded to improve the reliability of measures.
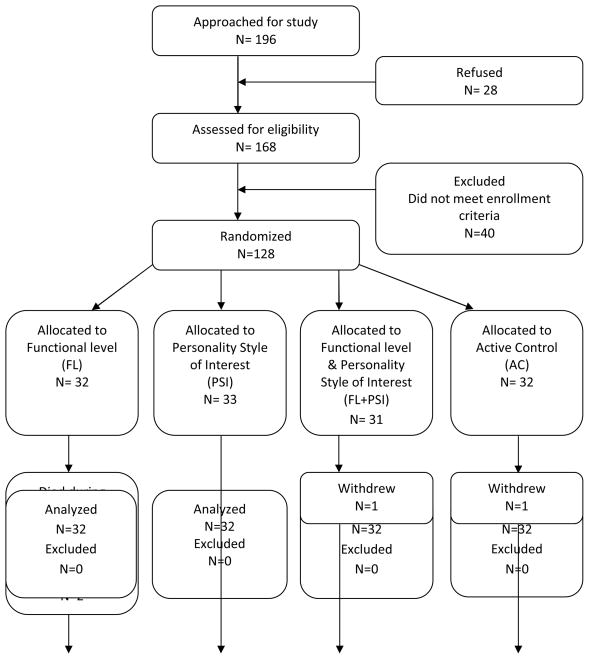
Participants were recruited, enrolled and completed the protocol in one nursing home at a time. Figure 1 depicts the flow of participants through the course of the study. Participants were randomized into one of four groups: activities tailored to functional level (FL) (n= 32); activities tailored to personality style of interest (PSI) (n= 33); activities tailored to both functional level and personality style of interest (FL+PSI) (n= 31); and active control (AC) (n= 32). Participants’ group assignment was determined by the statistician using a random number generator with random block sizes to ensure equal assignment across the four groups at the completion of the study and approximately equal assignments throughout the study to control for unknown temporal effects. Group assignment was concealed until after all screening data were collected. The project director obtained the assignment from a secured central location after verifying that the participant qualified for the study. Because all participants received some type of activity we were able to blind the interventionists, data collectors, video raters, nursing home staff and the participants.
Figure 1.
Flow diagram of recruitment, enrollment, intervention delivery and number of participants who contributed data to the analysis.
The demographic and baseline clinical characteristics of the sample by group assignment are in Table 1. The PSI group had a higher MMSE than the FL+PSI group and more years of education than the other three groups. There were no other statistically significant differences among the groups.
Table 1.
Demographic and Baseline Clinical Characteristics by Group*
| Demographic Characteristic Mean (±sd)/(%) | FL | PSI | FL+PSI | AC |
|---|---|---|---|---|
| N= 32 | N= 33 | N= 31 | N= 32 | |
| Age in years | 85.34 (±6.1) | 87.21 (±5.9) | 85.96 (±7.1) | 85.87 (±4.9) |
| Gender (% Female) | 75.00 | 75.76 | 74.19 | 81.25 |
| Race (% Caucasian) | 87.50 | 90.91 | 93.55 | 81.25 |
| Years of Education | 11.59 (±2.8) | 13.87 (±3.1) | 11.64 (±3.0) | 11.84 (±3.2) |
| MMSE† | 15.06 (±4.2) | 15.78 (±4.9) | 12.68 (±3.3) | 13.22 (±4.6) |
| PGDRS‡ | 13.03 (±7.6) | 10.87 (±7.4) | 13.64 (±6.4) | 14.43 (±8.0) |
| Baseline Clinical Characteristic | ||||
| Least Square Means (95% CI) | ||||
| Controlling for Multiple | ||||
| Measures Across Subjects | ||||
| Engagement§ | 2.30 (2.1, 2.5) | 2.43 (2.3, 2.6) | 2.39 (2.2, 2.6) | 2.41 (2.2, 2.6) |
| ARS¶ | ||||
| Pleasure | 2.23 (1.9, 2.5) | 2.20 (1.9, 2.5) | 2.07 (1.8, 2.3) | 2.06 (1.8, 2.3) |
| Anxiety | 1.79 (1.5, 2.1) | 2.21 (1.9, 2.6) | 2.02 (1.7, 2.4) | 1.99 (1.7, 2.3) |
| Alert | 4.44 (4.2, 4.7) | 4.58 (4.4, 4.8) | 4.40 (4.2, 4.6) | 4.36 (4.1, 4.6) |
| Attends | 1.51 (1.3, 1.8) | 1.13 (0.9, 1.4) | 1.09 (0.8, 1.4) | 1.18 (0.9, 1.4) |
| DMPT# | 9.73 (9.1, 10.4) | 10.05 (9.4, 10.7) | 9.56 (8.9, 10.2) | 9.80 (9.1, 10.5) |
| CMAI** | 1.62 (0.9, 2.4) | 2.46 (1.7, 3.2) | 1.86 (1.1, 2.6) | 1.88 (1.1, 2.6) |
| PDS†† | 16.68(13.4, 19.9) | 18.53(15.3, 21.7) | 16.29(12.9, 19.6) | 16.24(13.0, 19.5) |
FL = activities tailored to functional level
PSI = activities tailored to personality style of interest
FL+PSI= activities tailored to functional level and personality style of interest
AC = active control
NB: PSI had higher MMSE than FL+PSI and higher education than FL, FL+PSI or AC.
Mini-mental State Exam: range 0–30 (higher scores indicate greater cognitive function)
Psychogeriatric Dependency Rating Scale: range 0–34 (higher scores indicate greater dependency)
Engagement Rating Form: 1= asleep; 2= doing nothing; 3= activity (formal & informal)
Affective Rating Scale: range 1–5 (higher score indicates greater display of the affect)
Dementia Mood Picture Test: range 0–12 (higher scores indicate more positive mood)
Cohen Mansfield Agitation Inventory: range 0–29 (higher scores indicate greater agitation)
Passivity in Dementia Scale: range (−16) – (+40) (higher scores indicate less passivity)
Together the principal investigator, co-investigator, and the project director selected participants’ activities from a large base of activities that were previously tested and used in nursing homes[37]. Cognitive status (MMSE), physical function (PGDRS), and personality style of interest (NEO-PI-R) were the participant characteristics used to prescribe the activities. The method for prescription was published[38] and is briefly described below. Appendix A lists examples.
Basically, the study design is a traditional 2×2 factorial study design that involves two factors and two levels within each factor: 1. Function, with the two levels of tailored-to or difficult; and 2. Personality style of interest, with the two levels of tailored-to or opposite. Thus, to test the main and interactive effects of the two treatment components (functional level and personality style of interest), participants were randomized to one of four groups: (1) function-tailoring and PSI opposite (FL), (2) personality style of interest- tailoring and FL difficult (PSI), (3) both function- and personality style of interest-tailoring (FL+PSI), and (4) function difficult and personality style of interest opposite (AC).
Participants in the FL group were prescribed activities that were specifically tailored to their skill level but opposite their personality style of interest. The selection of activities was determined by their physical and cognitive capabilities and the scores they received on the NEO-PI-R for certain facets that comprise the domains of extraversion (gregariousness, assertiveness, activity, excitement seeking) and openness (fantasy, aesthetics, feelings, ideas). We focused on those facets that were most prominent (low or high), as they reveal the individual’s distinct pattern within each domain and allow specificity in prescription. For example, if a participant was capable of fine motor activity but scored low on gregariousness and aesthetics they might be prescribed an art/craft activity in a small group setting. This activity is easily within their functional ability but not consistent with their style of interest.
Participants in the PSI group were prescribed activities that were specifically tailored to their personality style of interest and deliberately selected to be functionally challenging for the participant. For example, if a participant had limited range of motion in their upper body and scored high on excitement seeking and gregariousness they might be prescribed a competitive tether ball game with two or three other individuals. This activity is consistent with their style of interest, but would be difficult given their limited range of motion.
Participants in the FL & PSI group were prescribed activities that were specifically tailored to their functional skill level and personality style of interest. For example, if a participant had intact speech and scored low on activity and high on feelings they might be prescribed a one-on-one feeling cube activity, an activity that requires no physical activity.
Participants in the AC group were prescribed activities that were functionally challenging and opposite their personality style of interest. For example, if a participant had difficulty with orientation and scored low on fantasy, they might be prescribed the game of “Where Am I?”
Participants received their assigned activity for up to 20 minutes twice per day (morning and afternoon) five days each week for three consecutive weeks. The intervention schedule and dosage were based on the results of preliminary work [34]. In that study, agitation, anxiety and mood did not improve with a once daily activity schedule; the schedule was increased to twice daily for the RCT in an attempt to improve these outcomes.
An important component of implementation was to address potential confounding precipitating factors such as pain, thirst or poor environmental conditions prior to each activity session. Resident reports of discomfort were brought to the attention of the nursing staff who were asked to intervene prior to the activity session. Poor environmental conditions, such as glare and noise, were addressed by RAs turning off loud TVs and adjusting lighting levels. We developed a treatment fidelity plan to help ensure reliability in the field[39]. Treatment fidelity checks were conducted on 10% of all intervention sessions. Re-training took place if the intervention was not implemented according to protocol. We had only one protocol deviation; we attribute this high rate of fidelity to the close supervision provided interventionists in the field.
Major Outcome Measures
During the baseline and intervention phases trained video raters obtained measures of agitation, passivity and affect from video recordings of each session; engagement and mood were measured at each session in real time through direct observation by trained data collectors. To assess the duration of treatment effect, measures of agitation, passivity and engagement were taken in real time on two randomly selected days per week of intervention. Two 20-minute observational points were randomly selected for each of these days at times other than treatment (one each from the available am and pm hours). To further assess for duration of effect, measures of agitation, passivity, and affect were taken from video recordings and measures of engagement were taken in real time during two daily 20-minute observational points on two randomly selected days one week post intervention. Inter-rater reliability was performed on 10% of all video and real time measures; re-training was instituted when reliability fell below .80.
Agitation was measured using the Cohen-Mansfield Agitation Inventory (CMAI)[35], a questionnaire that consists of 29 behaviors, modified for direct observation [40]. Higher scores indicate greater agitation. We obtained inter-rater reliabilities (ICC) ranging from .64 for measures taken from video recordings to .99 for those obtained in real time.
Passivity was measured using the Passivity in Dementia Scale (PDS)[36] an observational scale consisting of 40 behaviors: 11 passive items that are scored in the negative and 29 active items that are scored in the positive. Lower scores indicate greater passivity. Inter-rater reliabilities (ICC) ranged from .79 for measures taken from video recordings to .98 for those taken in real time.
Engagement during intervention encompassed two measures: the time in minutes and seconds that the subject participated in an activity (time on task) and the intensity of participation. We used a stopwatch for measurement and obtained a percentage agreement of 93.6 and a weighted Kappa of .91 for time on task. Intensity of participation was measured using a scale developed by Kovach and Magliocco[41] and was rated from 0 to 3 with higher scores indicating greater participation. We obtained a percentage agreement of 98.2 and a weighted Kappa of .96 for level of participation.
Engagement during baseline, random times outside of treatment and during post intervention was measured by direct observation using a modified version of Nolan, Grant and Nolan’s[42] molar coding scheme. The instrument has descriptors for behaviors that depict time use: asleep, doing nothing, and activity (formal and informal). The one behavior exhibited by the participant that was predominate over the observation period (i.e., occurred for more than 50% of the time) was selected. We obtained percentage agreements ranging from .92 to .97, and weighted Kappas ranging from .94 to .97 for this measure of engagement.
Affect was measured using the Philadelphia Geriatric Center Affect Rating Scale (ARS)[43]. This observational scale has descriptive indicators for six affective states: pleasure, anger, anxiety, depression/sad, alert, and attends. Higher scores on each subscale indicate greater display of that affect. Inter-rater reliability (ICC) for the subscales was: .60 (pleasure); .58 (anxiety); .88 (alert); and .94 (attends). We did not use data for anger or depression because of our inability to obtain adequate reliability for the measure of these affects.
Mood was measured using the Dementia Mood Picture Test (DMPT)[44], an instrument that measures self-reported positive and negative moods (bad, good, angry, sad, happy, worried). Each mood can receive a potential score of 0–2 in intensity with higher scores representing more positive mood. Mood was measured immediately after each observation and intervention session. Inter-rater reliability (ICC) was .99.
Analysis
Intention-to-treat analysis was used. Measures from both direct observation and video recordings were used in analysis. Sample distributions of all dependent measures were evaluated, and statistical models appropriate to the observed distributions were implemented. Analysis of variance (ANOVA) was used for dependent variables that were approximately normally-distributed. Analyses of continuous variables showing skewed distributions were based on log-or rank-transformed data. Categorical outcome variables were analyzed using multinomial models implemented with generalized estimating equations (GEE).
Demographic variables were compared among the study groups using one-way analysis of variance (ANOVA) for continuous variables and the chi-square test for categorical variables. Baseline measurements were compared among the four treatment groups by one-way mixed model ANOVA. Mixed-model analyses including a subject term as a random effect were used to account for correlations due to multiple measurements of the outcome variables for each study subject. Using mixed model analyses, baseline measurements were also compared with measurements at intervention, random and post intervention phases by treatment group controlling for MMSE, PGDRS and years of education with period as a fixed effect and subject as a random effect. The primary statistical analytic model utilized a two-factor layout, reflecting the study design with four treatment groups defined by two factors and two levels within each factor.
Total intervention dose was calculated as the product of time on task and intensity of participation for each day. MMSE, PGDRS, years of education and total dose were included as covariates in the analyses of treatment effects.
Results are presented as least-squares means, in order to adjust for unequal numbers of observations per subject and per treatment; 95% confidence intervals were calculated for the least squares means. All statistical analyses were conducted using SAS® statistical software, release 9.2 (copyright 2002–2008 by SAS Institute, Inc., Cary, NC, USA).
RESULTS
Recruitment began in August, 2005 and follow up ended in November, 2008. Figure 1 shows the flow of participants through the course of the study. Participants were approximately 86 years of age, Caucasian and female (Table 1). Overall they had moderate to severe cognitive and physical impairments. At baseline, participants displayed little affect, approximately 2 agitated behaviors, and more passive than active behavior, over 20 minute behavior streams. We experienced no adverse events related to the protocol.
Participants varied in the dose of intervention they received, with total dose ranging from 4.9 to 1800 units. There were no significant differences, however, in mean total dose among the groups (p=0.122).
A within-person comparison of the major outcomes at intervention with those at baseline was done. All outcomes demonstrated improvement during intervention regardless of group assignment (data not shown) with the exception of mood which became more negative in AC (9.80 {9.1, 10.5} vs. 9.52 {8.8, 10.2}; p=.04).
Table 2 lists the major outcomes by treatment group during intervention. Participants randomized to PSI or FL+PSI activities demonstrated greater engagement (time on task and intensity of participation), more alertness and more attention than participants randomized to FL or AC activities. Pleasure was observed statistically more often in participants randomized to FL+PSI activities. Agitation (full scale score), passivity, anxiety and self-reported mood did not differ by group.
Table 2.
Least Square Means and 95% CI for Major Outcomes by Group*
| Outcome | FL | PSI | FL+PSI | AC | Two-factor ANOVA
|
||
|---|---|---|---|---|---|---|---|
| Interest tailoring | Function tailoring | Inter-action | |||||
|
Engagement1
| |||||||
| Time on Task† | 16.75 | 18.48 | 18.74 | 17.12 | |||
| 15.6, 17.9 | 17.3, 19.6 | 17.5, 19.9 | 16.0, 18.3 | .005 | .928 | .605 | |
|
| |||||||
| Participation‡ | 2.62 | 2.86 | 2.90 | 2.65 | |||
| 2.5, 2.7 | 2.7, 3.0 | 2.8, 3.0 | 2.5, 2.8 | .000 | .573 | .687 | |
|
Affect (ARS)§2
| |||||||
| Pleasure | 2.57 | 2.45 | 2.96 | 2.67 | |||
| 2.3, 2.8 | 2.2, 2.7 | 2.7, 3.2 | 2.4, 2.9 | .548 | .158 | .035 | |
|
| |||||||
| Anx/Fear | 1.59 | 1.74 | 1.46 | 1.64 | |||
| 1.3, 1.9 | 1.4, 2.0 | 1.2, 1.8 | 1.3, 1.9 | .903 | .269 | .452 | |
|
| |||||||
| Alert | 4.76 | 4.90 | 4.93 | 4.79 | |||
| 4.7, 4.8 | 4.8, 5.0 | 4.8, 5.0 | 4.7, 4.9 | .003 | .909 | .545 | |
|
| |||||||
| Attends | 4.60 | 4.80 | 4.84 | 4.65 | |||
| 4.4, 4.88 | 4.6, 5.0 | 4.6, 5.0 | 4.5, 4.8 | .024 | .845 | .580 | |
|
| |||||||
| Mood (DMPT)¶2 | 9.77 | 10.15 | 9.94 | 9.93 | |||
| 9.1, 10.1 | 9.5, 10.8 | 9.3, 10.6 | 9.2, 10.6 | .689 | .688 | .894 | |
| Behavior2 | |||||||
|
| |||||||
| CMAI# | 1.16 | 1.71 | 1.46 | 1.10 | |||
| .3, 2.0 | .9, 2.5 | .6, 2.3 | .3, 1.9 | .607 | .339 | .923 | |
|
| |||||||
| PDS** | 33.38 | 34.82 | 37.38 | 34.22 | |||
| 30.6, 36.1 | 32.1, 37.5 | 34.6, 40.2 | 31.5, 37.0 | .101 | .538 | .233 | |
FL= activities tailored to functional level
PSI= activities tailored to personality style of interest
FL+PSI= activities tailored to functional level and personality style of interest
AC= active control
Time on Task- range 0–20 minutes
Participation- range 0–3; higher scores indicate more active engagement
Affective Rating Scale: range 1–5 (higher score indicates greater display of the affect)
Dementia Mood Picture Test: range 0–12 (higher scores indicate more positive mood)
Cohen Mansfield Agitation Inventory: range 0–29 (higher scores indicate greater agitation)
Passivity in Dementia Scale: range (−16) – (+40) (higher scores indicate less passivity)
Models account for MMSE, PGDRS and YrsEd
Models account for MMSE, PGDRS, YrsEd and Total Intervention Dose
To assess duration of effect we conducted within-person comparisons of outcomes at random times during the intervention phase and one week post intervention with those at baseline. During random times, engagement returned to baseline except in the FL group where participants became less engaged {2.15 (2.0, 2.3) vs. 2.30 (2.1, 2.5), p=.009}. Participants randomized to PSI activities demonstrated less agitation {1.81 (1.2, 2.4) vs. 2.46 (1.7, 3.2), p=.007}, and participants randomized to FL+PSI activities demonstrated less passivity {18.42 (15.8, 21.1) vs. 16.29 (12.9, 19.6), p=.025} than at baseline, indicating some extended benefits of these activities throughout the day. There was, however, increased agitation in the AC {2.57(1.9, 3.2) vs. 1.88 (1.1, 2.6), p=.046} and FL+PSI {2.55 (1.9, 3.2) vs. 1.86 (1.1, 2.6), p=.003} groups. No other significant changes were observed.
Most outcomes returned to baseline levels one week post-intervention with a few exceptions: mood improved in the FL+PSI group {9.78 (9.0, 10.6) vs. 9.56 (8.9, 10.2), p=.017} and anxiety improved in the PSI group {1.91 (1.5, 2.3) vs. 2.21 (1.9, 2.6), p=.016}. Greater passivity was noted in the FL group {11.82 (8.4, 15.2) vs. 16.68 (13.4, 19.9), p<.0001}, and there was a significant decrease in pleasure in the FL {1.84 (1.6, 2.1) vs. 2.23 (1.9, 2.5), p <. 0001} and PSI {1.92 (1.7, 2.4) vs. 2.20 (1.9, 2.5) p= .001} groups.
DISCUSSION
Behavioral symptoms are caused by many factors, including several we were not able to control for in this study, such as staff turn-over and quality of care. The intervention phase was three weeks and it is unclear if a longer treatment period may have demonstrated greater, more durable effects. Our hypothesis that activities tailored to functional level and personality style of interest would have greater benefit than either personality style of interest- tailored activities or functional level-tailored activities or non-tailored activities was not confirmed. However, NDB-derived activities resulted in statistically significant improvements in several behavioral symptoms associated with dementia during intervention; duration of effect varied by outcome. We also observed negative outcomes when activities were not individualized, and when withdrawn in post-intervention. Some of the observed improvements were small but, unlike pharmacological interventions which also show modest clinical effects (6), we experienced no adverse effects. Our findings underscore the clinical utility of activities as a first line of treatment for the behavioral symptoms of dementia.
Any type of activity improved outcomes over baseline; this finding has been reported by others and was not unexpected [11]. Passivity and attention were notably improved over baseline, and demonstrate that simple activities can promote resident engagement in the nursing home. The only exception to improvement over baseline was mood, which worsened when activities were not tailored on either treatment component. These data demonstrate the importance of individualizing activities to prevent possible negative outcomes of poorly selected activities. Recreational therapists are key members of the interdisciplinary team who can individualize activities so they achieve therapeutic benefits for the resident.
We investigated the components of treatment that were responsible for effects during intervention, an identified research priority in support of evidence-based practice[45]. We found that personality style of interest is the activity component that produced the most efficacious results for improving engagement, capturing attention and resident alertness during treatment itself. This was in spite of a lack of tailoring to functional level. There was no further gain in any other outcome by tailoring to both treatment components as we initially hypothesized, except pleasure. This latter finding is supported by data that indicate when residents are engaged in skill-appropriate activities they experience positive emotions [46]. Our overall findings during intervention are similar to those we obtained in preliminary work [34] and are supported by the work of others who found an advantage in tailoring interventions to personal characteristics of the resident [11, 20, 47]. This study adds to that literature by indicating that individualizing activities on personality style of interest provides an advantage for engaging nursing home residents with dementia. Intrinsic motivation is supplied by activities that individuals find personally interesting [48], and motivation may explain our findings. Cognitively impaired residents can be difficult to engage and consequently are at high risk for functional decline. The clinical benefits of improving engagement, attention, and alertness on a daily basis could be substantial over time, and may help slow the cognitive and physical decline associated with dementia. To our knowledge this is the first study to demonstrate the effects of unique components of activities on behavioral symptoms.
No treatment component demonstrated an advantage for reducing the negative affects of agitation, anxiety or improving mood and passivity during intervention. Negative emotions may be impacted by disease progression to a greater extent than positive emotion[49] and may explain why psychosocial interventions which improved positive affect had no effect on negative affect[50]. Our sample had moderate to severe cognitive impairments, which may explain why the fully-tailored (FL+PSI) activity improved pleasure, but could not reduce negative affect.
We found extended benefits of activities outside of treatment times. Less passivity was noted throughout the day in the FL+PSI group. These activities provide opportunity for greater involvement and may be needed to sustain activation throughout the day. Agitation improved in the PSI group but increased in AC, possibly reflecting frustration with unappealing activities. Agitation also increased, surprisingly, in the FL+PSI group. We are not able to fully explain this later finding; it may be due to unmeasured confounders. Overall, our findings indicate that activities tailored to include a personality style of interest component may help reduce agitation and passivity throughout the day and not just during treatment.
Most outcomes returned to baseline during the post intervention phase with the exception of mood and anxiety which improved, pleasure which declined, and passivity which increased in different groups. Mood and anxiety are enduring affects requiring more intense treatment and may not show improvement until after several weeks of activities that include a tailored to PSI component, as we found here. When successfully treated, mood and anxiety may show improvement for some time. The withdrawal of activities had a negative effect on the expression of pleasure, a less enduring affect, in the tailored to FL and PSI groups. We also observed greater passivity in FL group. Residents may come to expect the stimulation that is provided by activities, and suffer poor outcomes when these activities are no longer provided. In this regard, daily programming may contribute to quality of life by way of improving pleasure and reducing poor mood, anxiety and passive behavior.
Across treatment phases, personality style of interest was the activity component responsible for most of the demonstrated behavioral benefits. Our findings lend support to the importance of personality as a background factor in the NDB model and give direction for the prescription of activities in the nursing home. The selection of activities based on personality style of interest has the potential to complement other methods for individualizing activities with the goal of improving quality of life in the nursing home.
Acknowledgments
Funding sources and related paper presentations:
This research was supported by NIH grant R01 NR008910 awarded to the first author. The funding organization had no role in the design and conduct of the study; analysis and interpretation of data; or preparation, review and approval of the manuscript.
Sponsor’s Role: None
The authors thank Susan Rutchauskas, Mary Pape, Mark Kolanowski, and Lindsey Rowan for their assistance in video coding. The authors also thank the research participants for their participation in this study.
Appendix A
Activities That Meet Needs By NEO Facets
| Openness Facet | Score | Need expressed | Activity |
|---|---|---|---|
| Fantasy | |||
| High | Need interesting inner world | Guided imagery; relaxation tapes; creative writing; water color painting | |
| Low | Need to keep mind on task | Sewing; baking; building a birdhouse; practicing the piano | |
| Aesthetics | |||
| High | Need for art & beauty | Arts & crafts; flower arranging; gourmet cooking; poetry reading | |
| Low | No sensitivity to art or beauty | Avoid arts & crafts; play dart game or toss activity; Price is Right Game | |
| Feelings | |||
| High | Need to express inner feelings | Feeling cube; poetry; reminiscence; pet therapy | |
| Low | Feeling states not important | Bowling; cognitive games (i.e., identify old movie stars); building projects or woodworking; | |
| Open to action | |||
| High | Need for variety | Learn new dance steps; offer new games, activities on a regular basis | |
| Low | Need for tried-and-true | Keep to familiar activities; hang the laundry; table ball game | |
| Open to ideas | |||
| High | Need to explore new areas | Look-inside purse/fishing box; scavenger hunt; brain teaser | |
| Low | No need for exploration | Avoid new or unconventional activities, use traditional home type activities | |
| Extraversion Facet | Score | Need Expressed | Activity |
|---|---|---|---|
| Gregariousness | |||
| High | Need for greater social stimulation | Use small-group activity with interaction; cooking club; parties; team activities | |
| Low | Need for less social stimulation | Use one-on-one or independent activity; listen to radio; solitary art/craft activity | |
| Assertiveness | |||
| High | Need to lead and be dominant | Whack-a-mole; war card game; or put person in charge of activity | |
| Low | Need to stay in background | Avoid putting in spotlight or putting in charge of activity | |
| Activity | |||
| High | Need for physical movement | Exercise to music; dancing; tetherball | |
| Low | Need for more leisurely pace | Leisurely walk; table games; discussion | |
| Excitement seeking | |||
| High | Need for stimulation, bright colors, sounds | Betting on horse races; wheelchair biking; snoozelen; sensory stimulation with vivid colors and sounds; watch exciting competitive sports games | |
| Low | No need for thrills | Use even-tempo games; reading or listening to books on tape or music | |
Reprinted with permission from SLACK Incorporated: Kolanowski A, Buettner L. Prescribing activities that engage passive residents: An innovative method. Journal of Gerontological Nursing 2008; 34(1): 13–18.
Footnotes
Conflict of Interest
AK was supported by NIH grant R01 NR008910
Paul T. Costa receives royalties from the NEO-PI-R and the NEO-FFI and was supported in part by NIH Grant DAO26652
Author Contributions:
Kolanowski: led the study concept and design, obtained funding, collaborated in the analysis and interpretation of data; and drafted the manuscript.
M. Litaker: collaborated on the study design, conducted the data analysis, contributed to the interpretation of data, and had critical input to the manuscript.
L. Buettner: collaborated on the activity intervention, contributed to the interpretation of data, and had input to the manuscript.
J. Moeller: collaborated on the conduct of the study and the activity intervention, acquired the data, contributed to the interpretation of data, and had input to the manuscript.
Paul T. Costa: collaborated on the personality style of interest classification of activities and had input to the manuscript.
Contributor Information
Ann Kolanowski, School of Nursing, Pennsylvania State University, University Park, Pa.
Mark Litaker, School of Dentistry, University of Alabama, Birmingham, AL.
Lin Buettner, School of Health and Human Performance, University of North Carolina at Greensboro, Greensboro, North Carolina.
Joyel Moeller, Quality of Life Project, Pennsylvania State University, University Park, PA.
Paul T. Costa, Jr., Dept. of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
References
- 1.Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: The Cache County Study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 2.Rubin EH, Morris JC, Berg L. The progression of personality changes in senile dementia of the Alzheimer’s type. J Am Geriatr Soc. 1987;35(8):721–5. doi: 10.1111/j.1532-5415.1987.tb06349.x. [DOI] [PubMed] [Google Scholar]
- 3.Tractenberg RE, Weiner MF, Patterson MB, et al. Emergent psychopathology in Alzheimer’s disease patients over 12 months associated with functional, not cognitive, changes. J Geriatr Psychiatry Neurol. 2002;15:110–117. doi: 10.1177/089198870201500210. [DOI] [PubMed] [Google Scholar]
- 4.Ballard C, Corbett A, Chitramohan R, et al. Management of agitation and aggression associated with Alzheimer’s disease: Controversies and possible solutions. Curr Opin Psychiatry. 2009;6:532–540. doi: 10.1097/YCO.0b013e32833111f9. [DOI] [PubMed] [Google Scholar]
- 5.Dyer CB, Pavlik VN, Murphy KP, et al. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48:205–208. doi: 10.1111/j.1532-5415.2000.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 6.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 7.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. J Am Med Assoc. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 8.Murman DL, Chen Q, Powell MC, et al. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59:1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- 9.American Geriatrics Society, American Association for Geriatric Psychiatry. 2003 The American Geriatrics Society and American Association for Geriatric Psychiatry Recommendations for Policies in Support of Quality Mental Health Care in U. S Nursing Homes. J Am Geriatr Soc. 2003;51:1299–1304. doi: 10.1046/j.1532-5415.2003.51416.x. [DOI] [PubMed] [Google Scholar]
- 10.Kong E, Evans LK, Guevara J. Nonpharmacological intervention for agitation in dementia: A systematic review and meta-analysis. Aging Ment Health. 2009;13:512–520. doi: 10.1080/13607860902774394. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor D, Ames D, Gardner B, et al. Psychosocial treatments of psychological symptoms in dementia: A systematic review of reports meeting quality standards. Int Psychogeriatr. 2009;21:241–251. doi: 10.1017/S1041610208008223. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor D, Ames D, Gardner B, et al. Psychosocial treatments of behavioral symptoms in dementia: A systematic review of reports meeting quality standards. Int Psychogeriatr. 2009;21:225–240. doi: 10.1017/S1041610208007588. [DOI] [PubMed] [Google Scholar]
- 13.Algase DL, Beck C, Kolanowski A, et al. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. Am J Alzheimers Dis Other Demen. 1996;11:10–19. [Google Scholar]
- 14.Buettner L, Fitzsimmons S. Activity calendars for older adults with dementia: What you see is not what you get. Am J Alzheimers Dis Other Demen. 2003;18:215–226. doi: 10.1177/153331750301800405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voelkl JE, Fries BE, Galecki AT. Predictors of nursing home residents’ participation in activity programs. Gerontologist. 1995;35:44–51. doi: 10.1093/geront/35.1.44. [DOI] [PubMed] [Google Scholar]
- 16.Sloane PD, Mitchell CM, Preisser JS, et al. Environmental correlates of resident agitation in Alzheimer’s disease special care units. J Am Geriatr Soc. 1998;46:862–869. doi: 10.1111/j.1532-5415.1998.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 17.Zeisel J, Silverstein NM, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43:697–711. doi: 10.1093/geront/43.5.697. [DOI] [PubMed] [Google Scholar]
- 18.Garland K, Beer E, Eppingstall B, et al. A comparison of two treatments of agitation in nursing home residents with dementia: Simulated presence and preferred music. Am J Geriatr Psychiatry. 2007;15:514–521. doi: 10.1097/01.JGP.0000249388.37080.b4. [DOI] [PubMed] [Google Scholar]
- 19.Teri L, Logsdon R. Identifying pleasant activities for Alzheimer’s disease patients: The Pleasant Events Schedule-AD. Gerontologist. 1991;31:124–127. doi: 10.1093/geront/31.1.124. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Mansfield J, Parpura-Gill A, Golander H. Utilization of self-identity roles for designing interventions for persons with dementia. J Gernotol B Psychol Sci Soc Sci. 2006;61B:202–212. doi: 10.1093/geronb/61.4.p202. [DOI] [PubMed] [Google Scholar]
- 21.Harmer B, Orrell M. What is meaningful activity for people with dementia living in care homes? A comparison of the views of older people with dementia, staff and family carers. Aging Mental Health. 2008;12:548–558. doi: 10.1080/13607860802343019. [DOI] [PubMed] [Google Scholar]
- 22.Tinsley H, Eldredge B. Psychological benefits of leisure participation: A taxonomy of leisure activities based on their need-gratifying properties. J Counsel Psychol. 1995;42:123–132. [Google Scholar]
- 23.Costa P, McCrae R. Manual supplement for the NEO-4. Odessa, FL: Psychological Assessment Resources; 1998. [Google Scholar]
- 24.Digmon J. Personality structure: Emergence of the five-factor model. Annu Rev Psychol. 1990;41:417–440. [Google Scholar]
- 25.Holland J. Why interest interventions are also personality inventories. In: Savickos M, Spokane A, editors. Vocational Interests. Palo Alto: Davis-Black Publishing; 1999. [Google Scholar]
- 26.Piedmont R. The revised NEO personality inventory: clinical and research applications. New York: Plenum Press; 1998. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. Washington, DC: American Psychiatric Press Inc; 1994. [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson IM, Graham-White J. Psychogeriatric dependency rating scales (PGDRS): A method of assessment for use by nurses. Br J Med Hypn. 1980;137:558–565. doi: 10.1192/bjp.137.6.558. [DOI] [PubMed] [Google Scholar]
- 30.Costa P, McCrae R. Revised NEO personality inventory and NEO five-factor inventory: professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 31.Ritchie K, Fuhrer R. The validity of informant screening test for irreversible cognitive decline in the elderly: Performance characteristics within a general population sample. Int J Gen Psychiatry. 1996;11:149–156. [Google Scholar]
- 32.Kurtz J, Lee P, Sherker J. Internal and temporal reliability estimates for informant ratings of personality using the NEO PI-R and IAS. Assessment. 1999;6:103–113. doi: 10.1177/107319119900600201. [DOI] [PubMed] [Google Scholar]
- 33.Strauss M, Pasupathi M, Chatterjee A. Concordance between observers in description of personality change in Alzheimer’s disease. Psychol Aging. 1993;8:475–480. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- 34.Kolanowski AM, Litaker M, Buettner L. Efficacy of theory-based activities for behavioral symptoms of dementia. Nurs Res. 2005;54(4):219–28. doi: 10.1097/00006199-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Mansfield J, Billig N. Agitated behaviors in the elderly. I: A conceptual review. J Am Geriatr Soc. 1986;34:711–721. doi: 10.1111/j.1532-5415.1986.tb04302.x. [DOI] [PubMed] [Google Scholar]
- 36.Colling KB. Passive behaviors in Alzheimer’s disease: A descriptive analysis. Am J Alzheimers Dis. 1999;14:27–40. [Google Scholar]
- 37.Buettner L. Simple pleasures: A multilevel sensorimotor intervention for nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 1999;14:41–52. [Google Scholar]
- 38.Kolanowski A, Buettner L. An innovative method for prescribing activities that engage residents who are passive. J Gerontol Nurs. 2008;34:13–18. doi: 10.3928/00989134-20080101-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolanowski A, Buettner L, Moeller J. Treatment fidelity plan for an activity intervention designed for persons with dementia. Am J Alzheimer Dis Other Demen. 2006;21:326–332. doi: 10.1177/1533317506291074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chrisman M, Tabar D, Whall AL, et al. Agitated behavior in the cognitively impaired elderly. J Gerontol Nurs. 1991;17:9–13. doi: 10.3928/0098-9134-19911201-04. [DOI] [PubMed] [Google Scholar]
- 41.Kovach CR, Magliocco JS. Late-stage dementia and participation in therapeutic activities. Appl Nurs Res. 1998;11:167–173. doi: 10.1016/s0897-1897(98)80285-1. [DOI] [PubMed] [Google Scholar]
- 42.Nolan M, Grant G, Nolan J. Busy doing nothing: Activity and interaction levels amongst differing populations of elderly patients. J Adv Nurs. 1995;22:528–538. doi: 10.1046/j.1365-2648.1995.22030528.x. [DOI] [PubMed] [Google Scholar]
- 43.Lawton MP, Van Haitsma K, Klapper J. Observed affect in nursing home residents with Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 1996;51:3–14. doi: 10.1093/geronb/51b.1.p3. [DOI] [PubMed] [Google Scholar]
- 44.Tappen RM, Barry C. Assessment of affect in advanced Alzheimer’s disease: The Dementia Mood Picture Test. J Gerontol Nurs. 1995;21:44–46. doi: 10.3928/0098-9134-19950301-09. [DOI] [PubMed] [Google Scholar]
- 45.Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging. 2007;22:28–36. doi: 10.1037/0882-7974.22.1.28. [DOI] [PubMed] [Google Scholar]
- 46.Orsulic-Jeras S, Judge KS, Camp CJ. Montessori-based activities for long-term care residents with advanced dementia: Effects on engagements and affect. Gerontologist. 2000;40:107–111. doi: 10.1093/geront/40.1.107. [DOI] [PubMed] [Google Scholar]
- 47.Gitlin L, Winter L, Earland T, et al. The tailored activity program to reduce behavioral symptoms in individuals with dementia: Feasibility, acceptability, and replication potential. Gerontologist. 2009;49:428–439. doi: 10.1093/geront/gnp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi J, Medalia A. Factors associated with a positive response to cognitive remediation in a community psychiatric sample. Psychiatr Serv. 2005;56:602–604. doi: 10.1176/appi.ps.56.5.602. [DOI] [PubMed] [Google Scholar]
- 49.Kolanowski A, Hoffman L, Hofer SM. Concordance of self-report and informant assessment of emotional well-being in nursing home residents with dementia. J Gerontol B Psychol Sci Soc Sci. 2007;62:20–27. doi: 10.1093/geronb/62.1.p20. [DOI] [PubMed] [Google Scholar]
- 50.Beck C, Vogelpohl TS, Rasin JH, et al. Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nurs Res. 2002;51:219–228. doi: 10.1097/00006199-200207000-00002. [DOI] [PubMed] [Google Scholar]