Abstract
BACKGROUND
The etiology of non-syndromic craniosynostosis remains elusive. While compressive forces have been implicated in premature suture fusion, conclusive evidence of force-induced craniosynostosis is lacking. The purpose of this study was to determine if cyclical loading of the murine calvarium could induce suture fusion.
METHODS
Calvarial coupons from post-natal day 21, B6CBA wild-type mice (n = 18) were harvested and cultured. A custom appliance capable of delivering controlled, cyclical, compressive loads was applied perpendicular to the sagittal suture within the coupon in vitro. Nine coupons were subjected to 0.3g of force for 30 minutes each day for a total of 14 days. A control group of nine coupons was clamped in the appliance without loading. Analysis of suture phenotype was performed using alkaline phosphatase and H&E staining techniques, as well as in situ hybridization analysis using Bone Sialoprotein (BSP).
RESULTS
Control group sagittal sutures—which normally remain patent in mice—showed their customary histological appearance. In contradistinction, sagittal sutures subjected to cyclic loading showed histological evidence of premature fusion (craniosynostosis). In addition, alkaline phosphatase activity and BSP expression was observed to be increased in the experimental group when compared to matched controls.
CONCLUSIONS
An in vitro model of forced-induced craniosynostosis has been devised. Premature fusion of the murine sagittal suture was induced with the application of controlled, cyclical, compressive loads. These results implicate abnormal forces in the development of non-syndromic craniosynostosis, which supports our global hypothesis that epigenetic phenomena have a crucial role in the pathogenesis of craniosynostosis.
Since Julius Wolff published his classical treatise in 1892,1 the impact of biomechanical forces on bone adaptation has been repeatedly demonstrated. Moss extended Wolff's law with the concept of the functional matrix model for craniofacial growth.2 He emphasized the role of soft tissue forces in skull development. Enlow's “depository and resorptive fields” were extensions of the functional matrix model.3 Enlow proposed that because of native forces, certain regions of the skull were intrinsically inclined to be either osteoblastic or osteoclastic. The exquisite responsiveness of bone to external force has been clinically exploited by Illizarov's principles, which form the basis of distraction osteogenesis.4 Osseous distraction is an important tool now commonly utilized for orthognathic management in the congenitally hypoplastic craniofacial skeleton.5 Numerous investigators have described cellular signaling cascades that occur under conditions of bone mechano-response. For example, Ingber, in reporting about his theory of tensegrity, links bone remodeling to the process of mechanotransduction, whereby genes are transcribed in response to forces.6 In cranial sutures, one transcription factor, Tbx2, has been observed to increase three-fold after mechanical loading.7 A connection between such force-induced gene expression and craniosynostosis, however, has yet to be established.
Sutures are one of the essential growth sites of the craniofacial complex. The craniofacial skeleton is made up of intramembranous bones that demonstrate growth based on two intrinsic physiological forces: organ-induced expansion (i.e. from the eye and brain)8 and mastication.9 Altered masticatory force has been shown to induce craniosynostosis, as osteopetrotic mice display premature fusion of the sagittal suture,10 and rats on soft diets exhibited premature fusion of the internasal suture.11 Brain expansion results in both translational and transformational cranial growth, as described by Enlow.12 The absence of brain expansile forces is evidenced in fetuses born with anencephaly and microcephaly, by demonstrating complete absence or severely reduced cranial growth patterns. Conversely, the presence of extrinsic pathological forces can also have an impact on skull development. Multiple births,13 low pelvic station,14 and late-term presentation15 have all been associated with the development of non-syndromic craniosynostosis. Increased intrauterine forces—secondary to constraint—have been cited as a common etiology.16 Furthermore, experimentally induced increased intrauterine compression utilizing cervical cerclage to delay births of mice pups by three days led to a higher incidence of premature suture fusion.17 In a separate set of studies, Moss identified that rat sutures which normally fuse failed to do so after the falx cerebri were severed, suggesting a role for altered force transmission in suture biology.18 The impact of these dural attachments to skull morphology has been reproduced in other animal models.19–21 All of these studies and observations implicate an abnormal mechanical environment in the pathogenesis of craniosynostosis.
Because of its relatively high frequency and significant biomedical burden, the causative factor(s) in non-syndromic craniosynostosis are of great clinical interest. Previously, the role of soluble factors—such as transforming growth factor-β(TGF-β)—have been given central importance in the pathogenesis of craniosynostosis, down-playing or even negating the contribution of force.22 It is our contention that force plays a key regulatory role in the timing and magnitude of premature suture fusion. We postulate that biomechanical forces may function to activate intra- and extracellular signaling cascades, and in so doing, alter gene expression. This study attempts to isolate the impact of force alone, and its independent effects on the induction of craniosynostosis.
Materials and Methods
Mechanical Loading
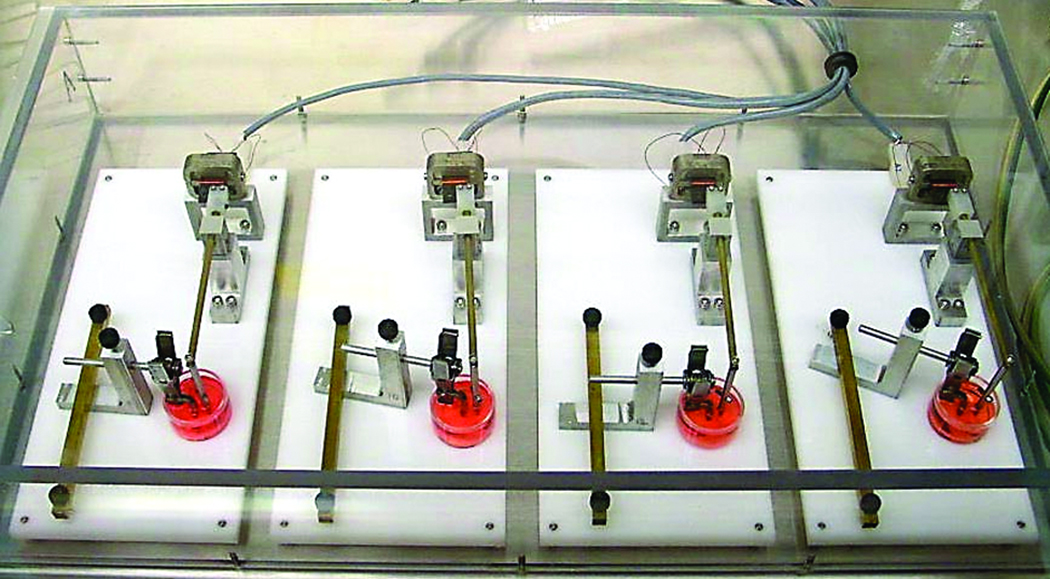
All experiments were approved by the University of Michigan Committee on the Use and Care of Animals. Postnatal day 21, B6CBA F1/J wild-type mice were obtained (Jackson Laboratories, Bar Harbor, ME, USA). In order to apply a controlled, cyclical, compressive load to the specimen samples, a custom, novel explant loading system was designed and fabricated.23 The force application device was comprised of two aluminum alloy clamps attached to a force sensor (figure 1 and figure 2).
Figure 1.
Force application device with loaded calvarial coupon in culture medium.
Figure 2.
Series of devices used to deliver controlled, cyclical, compressive loads to the calvarial coupons.
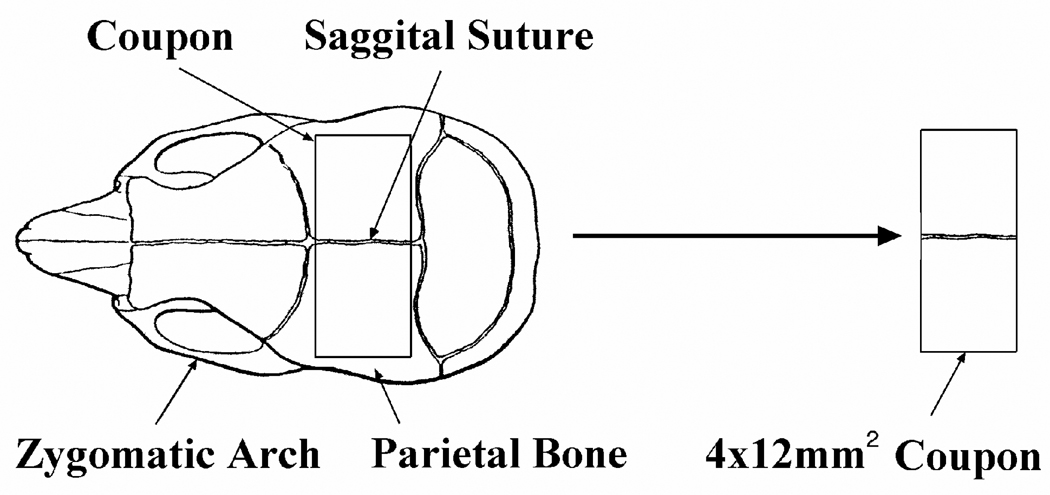
The sagittal sutures of mouse calvaria (n = 18) were harvested as 4×12 mm2 coupons (figure 3) and rinsed of debris using phosphate-buffered saline (PBS). The underlying dura was preserved. The sagittal suture was used because it normally remains patent in the murine model throughout their lifetime. Other sutures, such as the frontonasal, coronal, and nasomaxillary suture, have also been used experimentally.24–25 The calvarial coupons were cultured in serum-free media for 14 days at 37 °C, 98% humidity, and 5% CO2. Medium was formulated based on Opperman et al.,26 and contained Dulbecco’s modified eagle medium supplemented with: 1 µg/ml gentamycin, 2 mM glutamine, 1 mM non-essential amino acids, 100U/ml penicillin G sodium, 100ug/ml streptomycin sulfate, 0.25 mg/ml Fungizone (Invitrogen Corp, Carlsbad, CA), 1 mM ITS+ (Collaborative Biomedical Products, Bedford, MA), and 3 mM inorganic phosphate (Sigma, St. Louis, MO).
Figure 3.
Calvarial coupons containing the sagittal suture were harvested from the murine skull.
Nine specimens were loaded in the uniaxial compression device, while nine specimens remained unloaded, serving as controls. Loaded sutures were subjected to the aforementioned compressive loading regimen. Three separate iterations of the experiment were required for optimization of alignment, compression frequency and magnitude of force. In the first experimental iteration, coupons misaligned along the loading axis caused the bony plates to override each other. Tearing at the sutures was also visualized. Coupons from mice younger than 3 weeks old were prohibitively small and difficult to clamp in the device; these were subsequently discarded. Meticulous attention to alignment within the loading device was maintained on subsequent experiments. In the second experimental iteration, the application of force was optimized. When compression frequencies of 2Hz or greater were used, tearing and overriding of the sutures resulted. Similar outcomes were observed with forces of greater than 0.5g. Optimization was reached with a cyclic load of 0.3 gram-force (2.94 mN, yielding 1.2% average peak strain) for 30 minutes per day for a total of 14 days (trapezoidal waveform with 20% ramp and 20% plateau, 1 Hz frequency).
Following the loading phase, all coupons were fixed in 100% ethanol for 30–60 minutes, soaked in 95% ethanol for 20 minutes, and transferred to 70% ethanol for storage at 4°C.
Histomorphometry
All specimens were embedded in paraffin and cut into 1-mm coronal sections (i.e. perpendicular to the long axis of the suture). Hematoxylin and eosin (H&E) staining was performed using the standard staining protocol as defined by Thompson.27 Specimens were then embedded in Paraplast (The Kendall Company, Mansfield, MA) at 58°C, and serially sectioned at 7µm.
Two additional staining protocols were implemented to assess for bone specific markers: Bone Sialoprotein (BSP), a component of bone extracellular matrix, and alkaline phosphatase, a hydrolase enzyme expressed in osteoblast cells.
Non-radiolabeled BSP in situ hybridization was performed using Bianco’s method.28 Briefly, Single-stranded digoxigenin(DIG)-labeled sense and anti-sense RNA probes were generated using in vitro transcription of linearized plasmids. Slides were deparaffinized, rehydrated in diethylpyrocarbonate (DEPC) treated water, permeabilized, and prehybridized. Hybridization was performed overnight under humid conditions with hybridization mix and then washed with 2x saline sodium citrate (SCC) containing 50% formamide. Hybridization was visualized as a blue/purple precipitate via incubation with alkaline-phosphatase conjugated anti-digoxygenin antibody followed by NBT / BCIP color development according to the manufacturer protocol (Kit 1175041, Roche).
Coupons from each group were also stained for alkaline phosphatase activity using a p-nitrophenyl conversion assay as per the manufacturer’s protocol (Sigma Kit #245). Coupons were immersed in 5% polyvinyl alcohol (Sigma) for 1 min, blotted, snap frozen in isopentane (Sigma), embedded in Tissue-Tek O.C.T. (Thermo Fisher Scientific Inc, Pittsburgh, PA), and cryosectioned at 10 µm.
All specimens were examined under light microscopy, whereby control and experimental groups were compared. The histological sections were assessed for BSP expression and alkaline phosphatase activity using quantitative methods. A suture was determined to be fused by the presence of contiguous osteoid deposition across the adjacent parietal bones.
Statistical Analysis
A one-tailed Fisher’s exact test was used to assess for statistical significance between the experimental and control groups with regard to suture fusion. If contiguous osteoid deposition was observed in the histological specimen, the suture was considered to be fused. A null hypothesis was established as the following: the experimental and control groups will demonstrate suture fusion with equal frequency. Statistical significance was set with an alpha value of 0.05 per convention.
Results
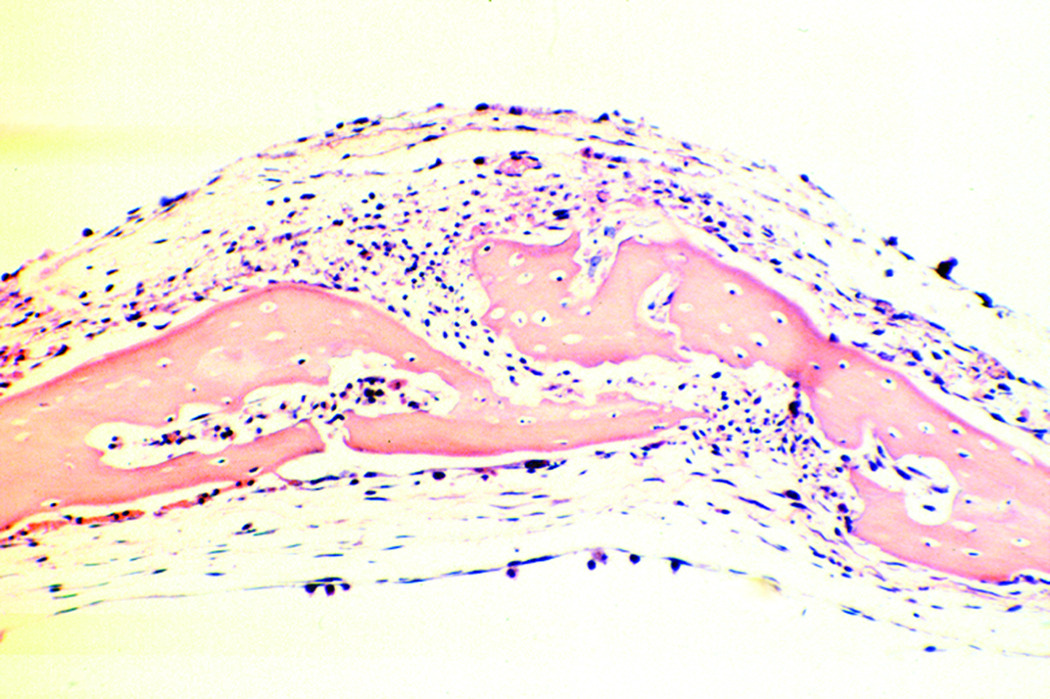
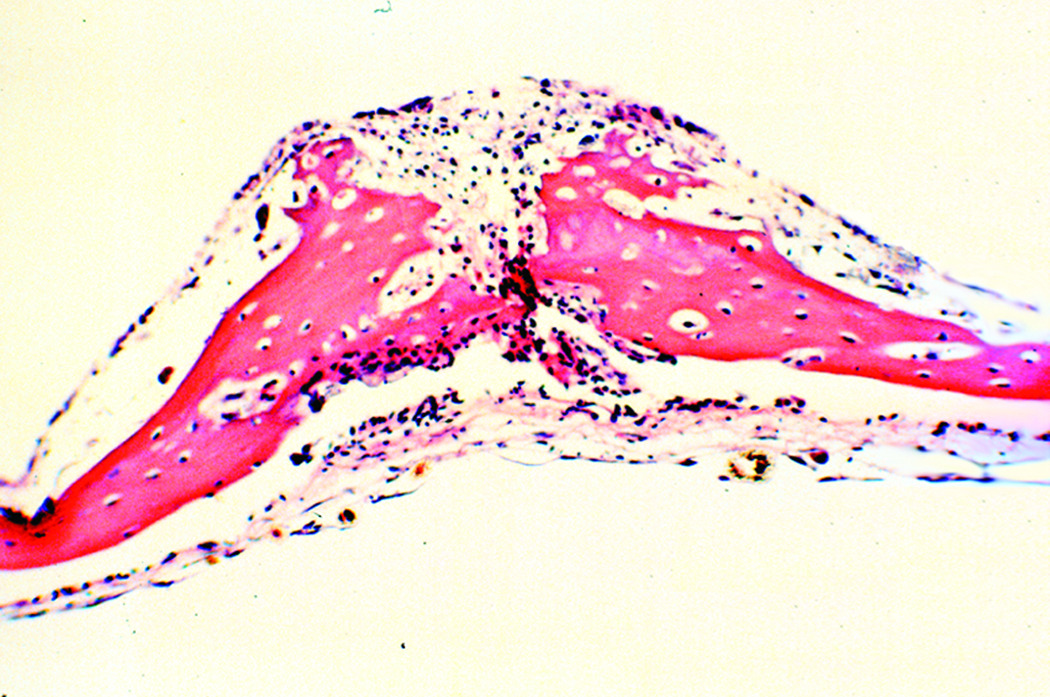
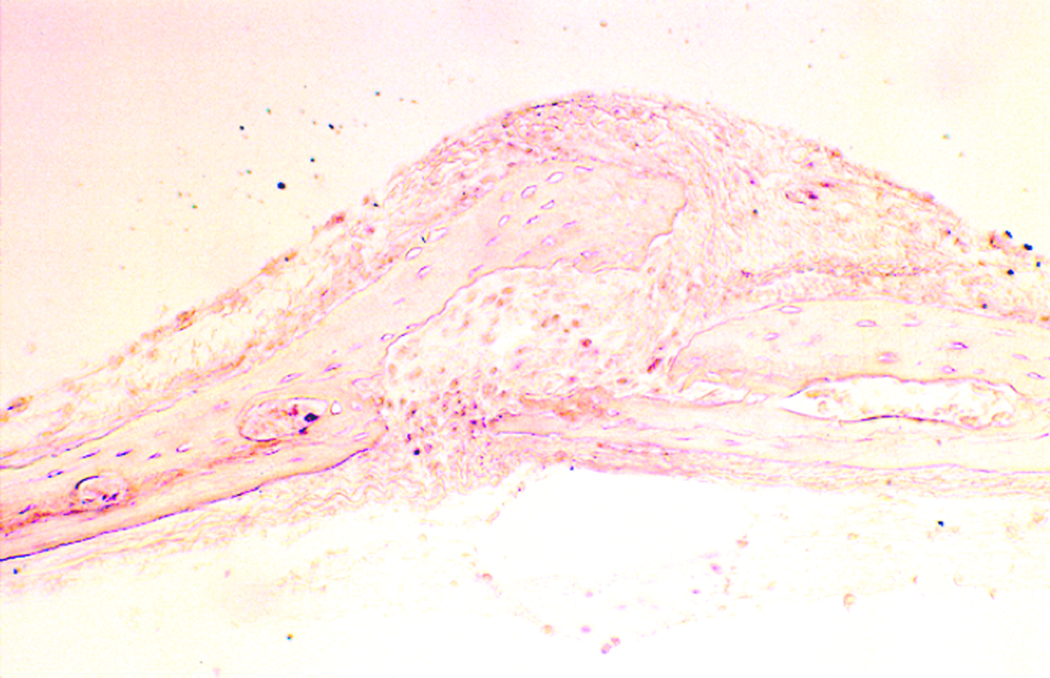
In the third experimental iteration, the sagittal sutures of unloaded coupons remained patent (figure 4), while the mechanically loaded calvarial coupons demonstrated evidence of sagittal suture fusion on light microscopy (figure 5). Histological examination of the control coupons demonstrated discontinuity between the parietal bone sections on light microscopy. Fronts of fibrous tissue and extracellular matrix were present at the suture line. Conversely, the experimental coupons revealed osseous bridging across the suture, with osteoblast populations present in continuity. Bone matrix deposition was visualized in a continuous fashion. Osteoid production was increased in the loaded sutures, as demonstrated by increased eosinophilc staining.
Figure 4.
Sagittal suture of unloaded calvarial coupon demonstrates physiologic appearance of parietal bones and patent suture (20× magnification, H&E stain).
Figure 5.
Sagittal suture of loaded calvarial coupon demonstrates rich staining of osteoid, indicative of bone growth. Contiguous osteoid deposition demonstrates force-induced craniosynostosis (20× magnification, H&E stain).
Among the experimental group, all 9 sagittal sutures demonstrated contiguous osteoid deposition, with bridging across the suture line. None of the control groups demonstrated this finding, as all 9 control sutures remained patent. Fisher’s exact test was implemented to assess for significance, and a p-value of 0.00002 was reached. The null hypothesis was therefore rejected.
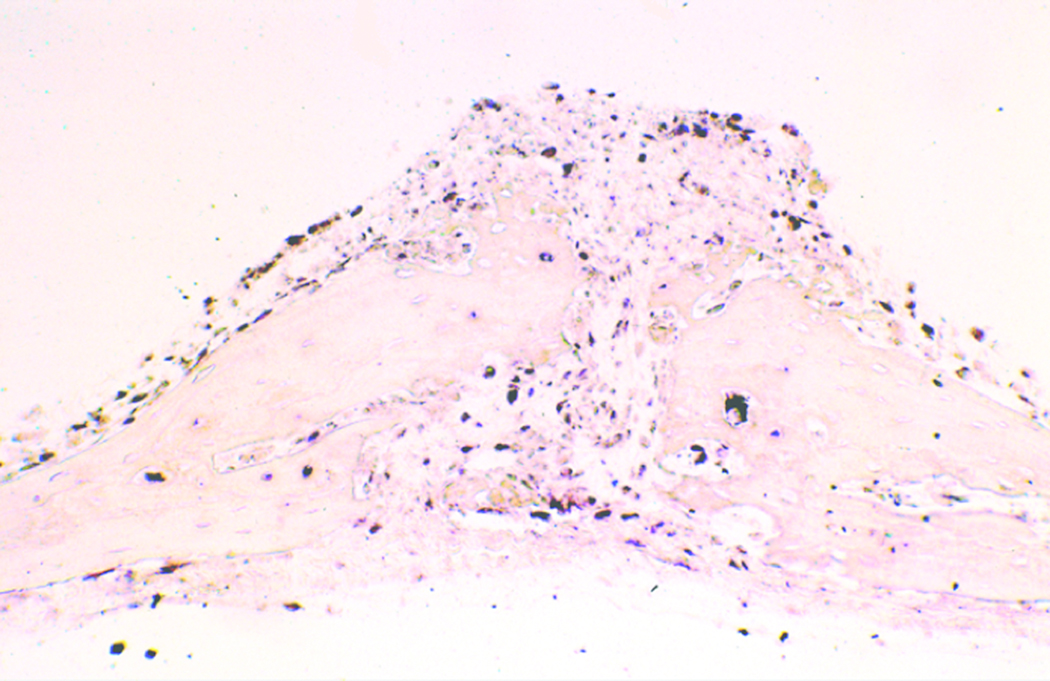
BSP mRNA expression was utilized as a marker for bone cell differentiation and early bone formation. A qualitative analysis commenced. While relatively absent in unloaded coupons (figure 6), BSP in situ hybridization revealed increased BSP expression in loaded coupons (figure 7). This was seen as the result of augmented osteogenic differentiation of bone lining and suture cells. Cells expressing high transcript levels were also found in regions far from the suture line in the loaded specimens. The majority of mRNA expression was visualized in the apical and basal regions of the parietal bones (figure 7). In addition, a preponderance of BSP expression was seen along the suture line.
Figure 6.
In situ hybridization analysis of unloaded calvarial coupon demonstrates near complete absence of Bone Sialoprotien (BSP) expression, evidenced by the lack of black pigmentation. Patency of the sagittal suture is also visualized (20× magnification, non-radiolabeled BSP immunostain).
Figure 7.
Augmented BSP expression is demonstrated on in situ hybridization analysis of loaded calvarial coupons. Cells expressing high BSP transcript levels (black staining) were found in regions far from the suture line in the loaded specimens, indicating diffuse bone growth. Note the fused sagittal suture (20× magnification, non-radiolabeled BSP immunostain).
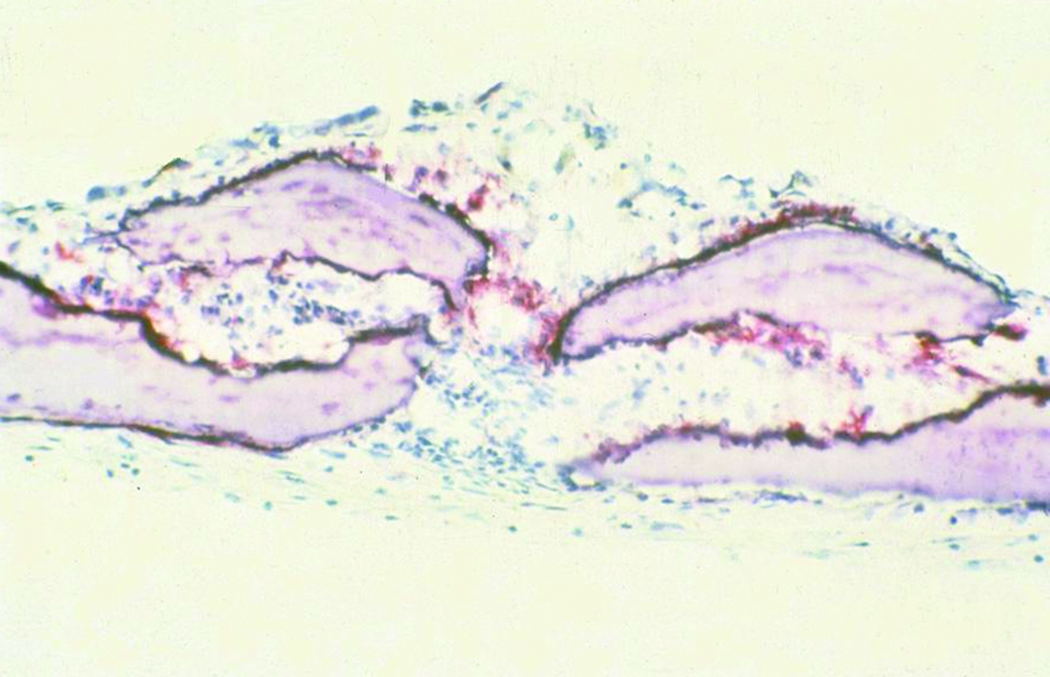
Alkaline phosphatase activity was also utilized as a marker of mature osteoblastic activity. In corroboration with the BSP results, alkaline phosphatase activity was observed to be upregulated in the experimental group. Alkaline phosphatase activity was demonstrated to be focally active at the suture line, indicating a front of developing bone in preparation for fusion (figure 8).
Figure 8.
Sutures subjected to cyclic compression demonstrated bridging of alkaline phosphatase along the synostosing suture on selected histological sections (figure 8). This “bridging” effect was found adjacent to areas of suture fusion within the specimen. These findings support the induction of new bone formation at the sagittal suture in response to mechanical force (20× magnification, alkaline phosphatase stain).
Discussion
Since Rudolph Virchow first described craniosynostosis in 1851,29 there has been an increased interest in all aspects of suture growth and development. Craniosynostosis, or premature fusion of the cranial sutures, occurs in as many as 1/2500 births and often requires the combined efforts of the neurosurgeon and the craniofacial plastic surgeon in order to achieve correction. The overwhelming majority of craniosynostoses arise sporadically. Understanding the conditions that give rise to these cases will be of paramount importance in determining the etiology of premature suture fusion. Previous studies in our laboratory examining human sagittal craniosynostotic sutures using scanning electron microscopy and microcomputed tomography demonstrated ultra-structural manifestations consistent with a substantial influence of biomechanical forces on suture morphology.30,31 Specifically, the studies reported a statistically significant decrease in trabecular size, organization, and polarization, a decrease in mineralized bone density, and an increase in the number of marrow spaces of the complete craniosynostotic suture when compared to the open portion of the craniosynostotic suture.32 Our findings were consistent with a scenario whereby biomechanical forces acting upon the open region of the synostotic sutures resulted in specific and quantifiable microarchitectural changes.
Clinical associations of craniosynostosis, including post-term pregnancy and low pelvic station, have further prompted inquiry into the role of incremental intra-uterine force and premature suture fusion. Twinning, in addition, has been identified as a potential risk factor for craniosynostosis.13 In fact, midline craniosynostosis has been documented to occur more frequently in twins compared to singletons.33 Experimental studies utilizing animal models of uterine constraint have demonstrated both a higher incidence of craniosynostosis and substantial phenotypic changes at the murine spheno-occipital synchondrosis.23–24 In the squirrel monkey, constant compressive forces were applied to the developing maxillae, resulting in cessation of growth at the sutures with eventual obliteration.24 While these clinical associations and experimental studies implicate compressive forces in premature suture fusion, conclusive evidence of force-induced craniosynostosis is lacking. In particular, a causal relationship between abnormal mechanical forces and premature suture fusion has remained elusive.34
The purpose of our research is to determine if cyclical loading of the murine calvarium can induce suture fusion. Our global hypothesis is that force is a critical epigenetic phenomenon, which plays a crucial role in the regulation and modification of gene expression in craniosynostosis. In this study, we have successfully induced suture fusion in vitro with the application of controlled, cyclical, compressive loads and have been able to observe qualitative changes in gene expression and protein product in suture-associated cells. Histological evidence of suture fusion has been demonstrated across sagittal sutures which would otherwise have remained patent (figure 5). Statistical significance at the 0.05 level was reached, as force-induced synostosis was demonstrated in all experimental sutures and no control sutures.
In addition, alkaline phosphatase, a non-specific bone maker of osteoblastic activity, was qualitatively observed to be upregulated. Sutures subjected to cyclic compression demonstrated bridging of alkaline phosphatase along the synostosing suture on selected histological sections (figure 8). This “bridging” effect was found adjacent to areas of suture fusion within the specimen. These findings support the induction of new bone formation at the sagittal suture in response to mechanical force.
Augmented BSP expression was also found in sutures subjected to cyclic compression when compared to unloaded sutures (figure 6 and figure 7). This serves to further corroborate the mechanism of force induced osteogenesis in the pathogenesis of craniosynostosis, as BSP is known to be a significant component of extracellular matrix in newly forming bone.35
Our findings at the tissue level are in keeping with a number of molecular studies whereby abnormal forces on the cranial vault have demonstrated changes in genetic expression and cell signaling.36–38 Collectively known as mechanotransduction, this process converts mechanical loads into cellular signals. Specifically, changes in calcium homeostasis and permeability have been isolated,39 in addition to apoptotic induction,40 and collagen-matrix deposition.41 Similarly, many discreet phenotypic changes in calvarial morphology have been observed in response to mechanical loads, including: increased bone volume and mineral apposition rate,42, 43–44 augmented osteoprogenitor45 and fibroblast cell numbers,46 increased alkaline phosphatase activity,47,48 praline incorporation47,48 and collagen matrix production.44,49 In light of our results, these well-documented structural changes lend credence to the potential role of mechanotransduction in non-syndromic craniosynostosis.
The cyclical nature of loading in this experiment may have been effective in replicating physiological development in utero, with mimicry of uterine muscle activity and brain pulsations. Cyclical loading, by definition, implies intermittent relief from external forces. In this study, we utilized an explant loading system that could produce cyclical, compressive forces on the experimental coupons.23 This method is more controlled than previous attempts at mechanical loading, including circular mount devices,50 synthetic adhesives,25 helical springs,39, 51, 52, 53 and mastication-related experiments.54–55 Nonetheless, it must be emphasized that the specific cyclical loading regimen used in this experiment was chosen by the necessity of bony failure; other regimens of loading caused disruption of the calvarial coupons. This experiment is an in vitro approximation of forces that we believe to be pathophysiological. These forces may not truly mimic those occurring in the aforementioned natural examples.
Recent studies advancing the role of soluble factors in regulating the closure of calvarial sutures have dismissed the role of force as an etiologic factor in the development of craniosynostosis.56,57 These studies, and others,58 site the ability to induce suture fusion in tissue culture devoid of forces. Those experiments, however, do not negate a regulatory or etiologic role of force in premature suture fusion, just as our results do not rule out the role for soluble factors in the pathophysiology of craniosynostosis. Force may well play a key regulatory role in the timing and magnitude of the expression of soluble factors acting to influence suture morphogenesis.
Genetic mutations of the fibroblast growth factor receptor-1 (FGFR1), the TWIST gene, and others have been strongly implicated in syndromic craniosynostosis.59 These soluble factors have been shown to induce premature suture fusion both in vitro and in vivo. While the association between discreet genetic abnormalities and syndromic craniosynostosis is clear, the causative factors involved in non-syndromic craniosynostosis are less evident. Although it may be possible to override normal regulatory mechanisms to induce premature suture fusion with force alone, we postulate that the etiology of the majority of non-syndromic craniosynostoses is neither purely genetic nor force-induced. Rather, the development of non-syndromic synostosis may relate to a genetic predisposition, with activation dependent on external forces, and other epigenetic phenomena.
Conclusions
An in vitro model of forced-induced craniosynostosis has been devised. Premature fusion of the murine sagittal suture was induced with the application of controlled, cyclical, compressive loads alone. The induced forces were associated with an increase in BSP gene expression and alkaline phosphatase production. These results implicate abnormal forces in the development of non-syndromic craniosynostosis, which supports our global hypothesis that epigenetic phenomena play a crucial role in the pathogenesis of craniosynostosis. Additional research is required to better understand the complex interaction between genes and their environment during craniofacial development.
Acknowledgment
Sources of Funding:
This study was supported by a National Institutes of Health Training grant-T32DM08616-02 (S.T.R., S.R.B.), a Maxillofacial Surgeons Foundations Research Grant from the American Society of Maxillofacial Surgeons (S.R.B.) and a Plastic Surgery Educational Foundation Grant (S.R.B.)
The authors wish to acknowledge Juan Taboas, Ph.D., for his research on this subject.
Footnotes
Robert H. Ivy Society Award Winner, The 68th Annual Meeting of the American Society of Plastic and Reconstructive Surgeons (ASPRS); New Orleans, Louisiana. October, 1999
Statement of Financial Interest:
The authors have no commercial associations, financial disclosures, or other conflicts of interest to report with regard to this manuscript.
Products/Devices Used:
Fungizone (Invitrogen Corp, Carlsbad, CA)
Tissue-Tek O.C.T. (Thermo Fisher Scientific Inc, Pittsburgh, PA)
ITS+ (Collaborative Biomedical Products, Bedford, MA)
Paraplast (The Kendall Company, Mansfield, MA)
References
- 1.Wolff J. Das Gesertz der Transformation der Knochen. Berlin: Springer; 1892. [Google Scholar]
- 2.Moss M. Facial growth: The functional matrix concept. In: Grabb WC, et al., editors. Cleft Lip and Palate. 1971. pp. 97–107. [Google Scholar]
- 3.Enlow D. The Handbook of Facial Growth. Piladelphia: WB Saunders; 1982. [Google Scholar]
- 4.Ilizarov G, Lediaev V, Shitin V. The course of compact bone reparative regeneration in distraction osteosynthesis under different conditions of bone fragment fixation. Eksperimentalnaia Khirurgiia i Anesteziologiia. 1969;14(6):3–12. [PubMed] [Google Scholar]
- 5.McCarthy J, Schreiber J, Karp N, et al. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1. [PubMed] [Google Scholar]
- 6.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 7.Borke J, Yu JC, Isales CM, Wagle N, Do NN, Chen JR, et al. Tension-induced reduction in connexin 43 expression in cranial sutures is linked to transcriptional regulation by TBX2. Ann Plast Surg. 2003;51:499–504. doi: 10.1097/01.SAP.0000067964.14122.3E. [DOI] [PubMed] [Google Scholar]
- 8.Huggare J, Ronning O. Growth of the cranial vault: influence of intracranial and extracranial pressures. Acta Odontol Scand. 1995;53:192–195. doi: 10.3109/00016359509005971. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, Lee E, Herring SW. Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawata T, Tokimasa C, Fujita T, Kawasoko S, Kaku M, Sugiyama H, Tanne K. Midpalatal suture of osteopetrotic (op/op) mice exhibits immature fusion. Exp Anim. 1998;47:277–281. doi: 10.1538/expanim.47.277. [DOI] [PubMed] [Google Scholar]
- 11.Kiliaridis S. Masticatory muscle function and craniofacial morphology: An experimental study in the growing rat fed a soft diet. Swed Dent J. 1986 Suppl 36:1–55. [PubMed] [Google Scholar]
- 12.Enlow D. Handbook of Facial Growth. Philadelphia: WB Saunders; 1982. [Google Scholar]
- 13.van Aalst JA, S G, Eppley BL. Craniosynostosis anomalies in twins. J Craniofac Surg. 2005;16(4):696–699. doi: 10.1097/01.scs.0000157248.24203.85. [DOI] [PubMed] [Google Scholar]
- 14.Graham JM, Richard JB, Smith DW. Coronal Craniostenosis: Fetal Head Constraint as One Possible Cause. Pediatrics. 1980;65(5):995–999. [PubMed] [Google Scholar]
- 15.Hunenko O, Karmacharya J, Ong G, Kirschner RE. Toward an understanding of non-syndromic craniosynostosis: altered patterns of TGF-[beta] receptor and FGF receptor expression induced by intrauterine head constraint. Annals of Plastic Surgery. 2001;46(5):546–554. doi: 10.1097/00000637-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Jaskoll T, Melnick M. The effects of long-term fetal constraint in vitro on the cranial base and other skeletal components. American Journal of Medical Genetics. 1981;12(3):289–300. doi: 10.1002/ajmg.1320120307. [DOI] [PubMed] [Google Scholar]
- 17.Smartt J, Karmacharya J, Gannon FH, et al. Intrauterine Fetal Constraint Induces Chondrocyte Apoptosis and Premature Ossification of the Cranial Base. Plast Reconstr Surg. 2004;16:1363–1369. doi: 10.1097/01.prs.0000182224.98761.cf. [DOI] [PubMed] [Google Scholar]
- 18.Moss M. The pathogenesis of premature cranial synostosis in man. Acta Anat (Basel) 1959;37:351–370. doi: 10.1159/000141479. [DOI] [PubMed] [Google Scholar]
- 19.Opperman L, Chhabra A, Nolen AA, Bao Y, Ogle RC. Dura mater maintains rat cranial sutures in vitro by regulating suture cell proliferation and collagen production. J Craniofac Genet Dev Biol. 1998;18:150–158. [PubMed] [Google Scholar]
- 20.Opperman L, Sweeney TM, Redmon J, Persing JA, Ogle RC. Tissue interactions with underlying dura mater inhibit osseous obliteration of developing cranial sutures. Dev Dyn. 1993;198:312–322. doi: 10.1002/aja.1001980408. [DOI] [PubMed] [Google Scholar]
- 21.Opperman L, Passarelli RW, Morgan EP, Reintjes M, Ogle RC. Cranial sutures require tissue interactions with dura mater to resist osseous obliteration in vitro. J Bone Miner Res. 1995;10:1978–1987. doi: 10.1002/jbmr.5650101218. [DOI] [PubMed] [Google Scholar]
- 22.Opperman LA, N A, Ogle RC. TGF-beta 1, TGF-beta 2, and TGF-beta 3 exhibit distinct patterns of expression during cranial suture formation and obliteration in vivo and in vitro. J Bone Miner Res. 1997;12(3):301–310. doi: 10.1359/jbmr.1997.12.3.301. [DOI] [PubMed] [Google Scholar]
- 23.Taboas JM. Department of Biomedical Engineering. University of Michigan: Ann Arbor; 2004. Mechanobiologic regulation of skeletal progenitor cell differentiation. [Google Scholar]
- 24.Droschl H. The effect of heavy orthopedic forces on the sutures of the facial bones. Angle Orthod. 1975;45:26–33. doi: 10.1043/0003-3219(1975)045<0026:TEOHOF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Persson K, Roy WA, Persing JA, Rodeheaver GT, Winn HR. Craniofacial growth following experimental craniosynostosis and craniectomy in rabbits. J Neurosurg. 1979;50:187–197. doi: 10.3171/jns.1979.50.2.0187. [DOI] [PubMed] [Google Scholar]
- 26.Opperman LA, Passarelli RW, Nolen AA, Gampper TJ, Lin K, Ogle RC. Dura mater secretes soluble heparin-binding factors required for cranial suture morphogenesis. Journal In Vitro Cellular & Developmental Biology - Animal. 1996;32(10):627–632. [Google Scholar]
- 27.Thompson SW. Selected Histochemical and Histopathological methods. Springfield, IL: Chalrels C. Thomas; 1996. [Google Scholar]
- 28.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of Bone Sialoprotein (BSP) in Developing Human Tissues. Calcified Tissue International. 1991;49:421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- 29.Persing JA, Jane JA, Shaffrey M. Virchow and the pathogenesis of craniosynostosis: a translation of his original work. Plastic & Reconstructive Surgery. 1989;83(4):738–742. doi: 10.1097/00006534-198904000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Sherick DG, Buchman SR, Goulet RW, Goldstein SA. A new technique for the quantitative analysis of cranial suture biology. The Cleft Palate-Craniofacial Journal. 2000;37(1):5–11. doi: 10.1597/1545-1569_2000_037_0005_antftq_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 31.Buchman SRS, Sherick DG, Goulet RW, Goldstein SA. Use of Microcomputed Tomography Scanning as a New Technique for the Evaluation of Membranous Bone. Journal of Craniofacial Surgery. 1998;9(1):48–54. doi: 10.1097/00001665-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Ozaki W, Buchman SR, Muraszko KM, Coleman D. Investigation of the influences of biomechanical force on the ultrastructure of human sagittal craniosynostosis. Plastic & Reconstructive Surgery. 1998;102(5):1385–1394. doi: 10.1097/00006534-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Lejeunie E, C D, Arnaud E, Renier D. Genetic considerations in non-syndromic midline craniosynostoses: as stuy of twins and their families. J Neurosurg. 2005;103(4 Suppl):353–356. doi: 10.3171/ped.2005.103.4.0353. [DOI] [PubMed] [Google Scholar]
- 34.Herring S, editor; Hanken J HB, editor. Epigenetic and functional influences on skull growth. The Skull. Chicago, IL: Univ. of Chicago Press; 2000. pp. 153–206. [Google Scholar]
- 35.Fisher L, McBride OW, Termine JD, Young MF. Human bone sialoprotein. Deduced protein sequence and chromosomal localization. J Biol Chem. 1990;265:2347–2351. [PubMed] [Google Scholar]
- 36.Bourret L, Rodan GA. The role of calcium in the inhibition of cAMP accumulation in epiphyseal cartilage cells exposed to physiological pressure. J Cell Physiol. 1976;88:353–361. doi: 10.1002/jcp.1040880311. [DOI] [PubMed] [Google Scholar]
- 37.Norton L, Rodan GA, Bourret LA. Epiphyseal cartilage cAMP changes produced by electrical and mechanical perturbations. Clin Orthop Relat Res. 1977:59–68. [PubMed] [Google Scholar]
- 38.Rodan G, Bourret LA, Harvey A, Mensi T. Cyclic AMP and cyclic GMP: mediators of the mechanical effects on bone remodeling. Science. 1975;189:467–469. doi: 10.1126/science.168639. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Lucas JH, Fryberg K, Borke JL. Extrinsic tension results in FGF-2 release, membrane permeability change, and intracellular Ca++ increase in immature cranial sutures. J Craniofac Surg. 2001;12:391–398. doi: 10.1097/00001665-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Goga Y, Chiba M, Shimizu Y, Mitani H. Compressive force induces osteoblast apoptosis via caspase-8. J Dent Res. 2006;85:240–244. doi: 10.1177/154405910608500307. [DOI] [PubMed] [Google Scholar]
- 41.Meikle M, Heath JK, Hembry RM, Reynolds JJ. Rabbit cranial suture fibroblasts under tension express a different collagen phenotype. Arch Oral Biol. 1982;27:609–613. doi: 10.1016/0003-9969(82)90078-4. [DOI] [PubMed] [Google Scholar]
- 42.Parr J, Garetto LP, Wohlford ME, Arbuckle GR, Roberts WE. Sutural expansion using rigidly integrated endosseous implants: an experimental study in rabbits. Angle Orthod. 1997;67:283–290. doi: 10.1043/0003-3219(1997)067<0283:SEURIE>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Parr J, Garetto LP, Wohlford ME, Arbuckle GR, Roberts WE. Implant-borne suture expansion in rabbits: A histomorphometric study of the supporting bone. J Biomed Mater Res. 1999;45:1–10. doi: 10.1002/(sici)1097-4636(199904)45:1<1::aid-jbm1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi I, Mizoguchi I, Nakamura M, Sasano Y, Saitoh S, Kagayama M, Mitani H. Effects of expansive force on the differentiation of midpalatal suture cartilage in rats. Bone. 1996;18:341–348. doi: 10.1016/8756-3282(96)00012-9. [DOI] [PubMed] [Google Scholar]
- 45.Zahrowski J, Turley PK. Force magnitude effects upon osteoprogenitor cells during premaxillary expansion in rats. Angle Orthod. 1992;62:197–202. doi: 10.1043/0003-3219(1992)062<0197:FMEUOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Steenvoorden G, van de Velde JP, Prahlandersen B. The effect of duration and magnitude of tensile mechanical forces on sutural tissue in vivo. Eur J Orthod. 1990;12:330–339. doi: 10.1093/ejo/12.3.330. [DOI] [PubMed] [Google Scholar]
- 47.Miyawaki S, Forbes DP. The morphologic and biochemical effects of tensile force application to the interparietal suture of the Sprague-Dawley rat. Am J Orthod Dentofacial Orthop. 1987;92:123–133. doi: 10.1016/0889-5406(87)90367-2. [DOI] [PubMed] [Google Scholar]
- 48.Southard K, Forbes DP. The effects of force magnitude on a sutural model: A quantitative approach. Am J Orthod Dentofacial Orthop. 1988;93:460–466. doi: 10.1016/0889-5406(88)90074-1. [DOI] [PubMed] [Google Scholar]
- 49.Yen E, Yue CS, Suga DM. Effect of force level on synthesis of type III and type I collagen in mouse interparietal suture. J Dent Res. 1989;68:1746–1751. doi: 10.1177/00220345890680120501. [DOI] [PubMed] [Google Scholar]
- 50.Meikle M, Reynolds JJ, Sellers A, Dingle JT. Rabbit cranial sutures in vitro: a new experimental model for studying the response of fibrous joints to mechanical stress. Calcif Tissue Int. 1979;28:137–144. doi: 10.1007/BF02441232. [DOI] [PubMed] [Google Scholar]
- 51.Nanda R, Hickory W. Zygomaticomaxillary suture adaptations incident to anteriorly-directed forces in rhesus monkeys. Angle Orthod. 1984;54:199–210. doi: 10.1043/0003-3219(1984)054<0199:ZSAITA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Hirukawa K, Miyazawa K, Maeda H, Kameyama Y, Goto S, Togari A. Effect of tensile force on the expression of IGF-I and IGF-I receptor in the organ-cultured rat cranial suture. Arch Oral Biol. 2005;50:367–372. doi: 10.1016/j.archoralbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Ikegame M, Ishibashi O, Yoshizawa T, Shimomura J, Komori T, Ozawa H. Tensile stress induces bone morphogenetic protein 4 in preosteoblastic and fibroblastic cells, which later differentiate into osteoblasts leading to osteogenesis in the mouse calvariae in organ culture. J Bone Miner Res. 2001;16:24–32. doi: 10.1359/jbmr.2001.16.1.24. [DOI] [PubMed] [Google Scholar]
- 54.Byron C, Borke J, Yu J, Pashley D, Wingard CJ, Hamrick M. Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:676–684. doi: 10.1002/ar.a.20055. [DOI] [PubMed] [Google Scholar]
- 55.Hinton R. Response of the intermaxillary suture cartilage to alterations in masticatory function. Anat Rec. 1998;220:376. doi: 10.1002/ar.1092200406. [DOI] [PubMed] [Google Scholar]
- 56.Bradley JP, Shahinian H, Levine JP, Rowe N, Longaker MT. Growth Restriction of Cranial Sutures in the Fetal Lamb Causes Deformational Changes, Not Craniosynostosis. Plastic & Reconstructive Surgery. 2000;105(7):2416–2423. doi: 10.1097/00006534-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 57.Stelnicki EJ, Vanderwall K, Hoffman WY, Harrison MR, Glowacki J, Longaker MT. A New in Utero Sheep Model for Unilateral Coronal Craniosynostosis. Plastic & Reconstructive Surgery. 1998;101(2):278–286. doi: 10.1097/00006534-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Roth DA, Gold LI, Han VK, McCarthy JG, Sung JJ, Wisoff JH, Longaker MT. Immunolocalization of Transforming Growth Factor [beta]1, [beta]2, and[beta]3 and Insulin-Like Growth Factor I in Premature Cranial Suture Fusion. Plastic & Reconstructive Surgery. 1997;99(2):300–309. doi: 10.1097/00006534-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Hehr U, Muenke M. Craniosynostosis syndromes: From genes to premature fusion of skull bones. Mol Genet Metab. 1999;68:139–151. doi: 10.1006/mgme.1999.2915. [DOI] [PubMed] [Google Scholar]