Abstract
Rationale
The mammalian Diaphanous-related formin, mDia1, governs microtubule and microfilament dynamics while functioning as an effector for Rho small GTP-binding proteins during key cellular processes such as adhesion, cytokinesis, cell polarity and morphogenesis. The cytoplasmic domain of the receptor for advanced glycation endproducts (RAGE) binds to the formin homology 1 (FH1) domain of mDia1; mDia1 is required for RAGE ligand-induced cellular migration in transformed cells.
Objective
As a key mechanism in vascular remodeling is the induction of smooth muscle cell migration, we tested the role of mDia1 in this process.
Methods and Results
We report that endothelial denudation injury to the murine femoral artery significantly upregulates mDia1 mRNA transcripts and protein in the injured vessel, particularly in vascular smooth muscle cells within the expanding neointima. Loss of mDia1 expression significantly reduces pathological neointimal expansion consequent to injury. In primary murine aortic smooth muscle cells, mDia1 is required for RAGE ligand-induced membrane translocation of c-Src, which leads to Rac1 activation, redox phosphorylation of AKT/GSK3β and consequent smooth muscle cell migration.
Conclusions
We conclude that mDia1 integrates oxidative and signal transduction pathways triggered, at least in part by RAGE ligands, therefore regulates pathological neointimal expansion.
Keywords: restenosis, vascular smooth muscle cell, signal transduction, formin, cellular migration
Introduction
Mammalian Diaphanous 1 (mDia1) is a member of the formin family, a group of multidomain proteins governing actin and microtubule remodeling (1–2). Formins are ubiquitously expressed and involved in a variety of fundamental cellular processes, such as adhesion, cytokinesis, polarity and morphogenesis (3–4). GTP-bound Rho proteins both directly activate and may be modulated by the mDia family of formins (5–6). Release from auto-inhibition allows the formin homology-2 (FH2) domain to nucleate and progressively elongate linear actin filaments and to stabilize microtubules in support of polarized cell division, adhesion, and migration. Whereas the role of formins in cytoskeleton modification is well established, only limited reports are available concerning their implication in disease processes (7–8).
We previously demonstrated that mDia1 binds to the intracellular domain of the Receptor for Advanced Glycation End products (RAGE) (9–10). mDia1 binds RAGE through its conserved poly-proline rich formin homology-1 (FH1) domain. RAGE is a multiligand receptor of the immunoglobulin superfamily (11) involved in a number of disease processes characterized by vascular pathology (12–17). Vascular stress leads to up-regulation of RAGE ligands, including multiple members of the S100/calgranulin family such as S100B, advanced glycation end products (AGEs), high mobility group box 1 (HMGB1), amyloid-β peptide and β-sheet fibrils, in the vessel wall, thereby facilitating their engagement of RAGE and induction of pro-inflammatory and tissue-damaging responses (17–18).
The roles of RAGE and mDia1 in fundamental mechanisms governing cellular migration prompted us to test the role of mDia1 in regulation of vascular smooth muscle cell (SMC) signal transduction and migration. In vivo, we addressed these concepts by assessing the impact of mDia1 in neointimal expansion consequent to guide wire-induced endothelial denudation in a murine model. Our findings reveal novel roles for mDia1 as a key regulator of signal transduction and oxidative stress events that control SMC migration and vascular remodeling.
METHODS
Note that detailed methods section is available in the Online Supplement.
Animals and induction of vascular injury
Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) and then bred in house for studies. Homozygous Drf1−/− mice were generated as previously described (19, 20) and were backcrossed >12 generations into C57BL/6 prior to any experimentation. All procedures were approved by the Institutional Animal Care and Use Committee of Columbia University and New York University Langone Medical Center. Male mice, age 8–12 weeks, were anesthetized and subjected to femoral artery endothelial denudation injury as previously described (17,21). All of the surgeries were performed by a single operator naive to the genotype of the animals.
Tissue analyses
Harvesting of vessel segments and their analysis was performed as previously described (17). Representative sections from the vessels were stained with antibodies to detect mDia1, α-smooth muscle actin or control IgG.
Western blotting
At least eight arteries were snap-frozen in liquid nitrogen, pooled, and stored at −80°C. Tissue samples were ground to a fine powder under liquid nitrogen and lysates subjected to SDS-PAGE and Western blotting for detection of the following antigens: RAGE, mDia1, Nox1, Nox4, p47 phox, phospho/total Akt, phospho/total GSK3β ser9, phospho/total ERK p44/42 MAP kinase and phospho/total c-Src.
Cell culture and in vitro assays on cultured SMCs
Mouse vascular SMCs were cultured from the aortas of 10-week old male mice using a modification of the procedure of Tarvo and Barret (22). SMCs were cultured following an explant protocol in accordance with institutional guidelines. Cultures were composed of more than 95% SM-α-actin positivity based on immunostaining. Human recombinant S100B was prepared as previously described (23) Endotoxin was removed following previously described protocols (24).
Small interference RNA to knockdown mDia1, Nox1 and c-Src
Small interference RNA (siRNA) duplexes against mouse mDia1, Nox1 or c-Src were synthesized by Invitrogen (Stealth RNAi®). Details of sequences and electorporation into primary smooth muscle cells are found in the Online Supplement.
Cell transfection for Dominant Negative rac and Constitutively Active GSK3
Dominant negative constructs of Rac1 (N17, Millipore), and constitutively active GSK3β and corresponding control vectors were purified following the manufacturer’s instructions and then transfected into the primary smooth muscle cells as described above (Nucleofactor). The plasmid pcDNA3-GSK3-β (S9A) was obtained from Addgene (plasmid no. 14754) and originally created by Dr. James Woodgett’s laboratory.
Cell Migration
Migration in response to RAGE ligands: S100B (10μg/ml), generously provided by Dr. Guenter Fritz, carboxymethyllysine (CML human serum albumin, 10 μg/ml), generously provided by Dr. Eric Boulanger, or general effectors (PDGF, 50ng/ml; EGF 100ng/ml, R& D systems) was assessed with wounding assay and chemotaxis assays as described in the Online Supplement.
ROS measurement: Assessment of NADPH activity
NADPH oxidase activity
NADPH dependent superoxide production was assessed by lucigenin enhanced chemiluminescence as previously described (25).
DHE
Cells (1×105) were seeded and after overnight serum starvation in DMEM without red phenol, cells were pre-incubated with 10μM of DHE for 10 minutes then stimulated with RAGE ligands (S100B, 10 μg/ml). Fluorescence was evaluated with a fluorescent plate reader excitation/emission: 370/630 at the indicated times. Results are expressed as the difference between unstimulated and stimulated conditions.
p47phox and c-Src translocation
Unstimulated and stimulated cells were used and manipulated following the recommendations of the manufacturer. After membrane preparations were prepared, detergent was removed and samples were subjected to immunoblotting for p47phox and c-Src (phospho- and total). Where indicated, immunoprecipitation experiments in smooth muscle cells were performed using rabbit polyclonal mDia1 antibody or the appropriate nonimmune IgG (Ptglab), followed by Western blotting using mouse monoclonal c-Src antibody (Cell Signaling).
Rac1 activity
Rac1 activity was assessed with a pull down assay using Pak1 as a specific ligand for activated Rac1 followed by SDS-PAGE electrophoresis and immunoblotting with Rac1 monoclonal antibody.
mDia1 rescue experiment in Drf1−/− smooth muscle cells
Plasmid pEFN. mDia1, corresponding to the murine myc-tagged derivative of mDia1 was a generous gift from Dr. John Copeland (University of Ottawa).
RNA extraction and RT-PCR
Total RNA was extracted from at least eight vessels prepared for immunoblotting as above using the micro scale RNA isolation kit. Reverse transcription was performed following the protocol suggested by the manufacturer. The primers used are indicated in the Online Supplement.
Statistical analysis
In all experiments, unless otherwise indicated, data are reported as the mean ±SEM in at least three replicates per group. Data were analyzed by post hoc comparisons using 2-tailed t test, and a p value less than or equal to 0.05 was considered significant. Statistical comparisons among groups were determined using one-way analysis of variance (ANOVA).
RESULTS
mDia1 is upregulated in vessels consequent to guide wire injury and deletion of mDia1 in mice reduces neointimal expansion
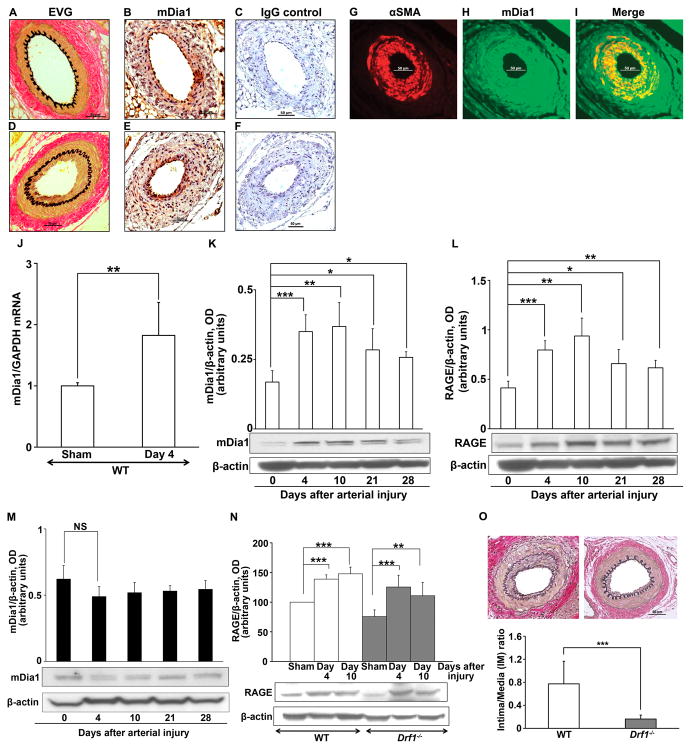
To test the role of mDia1 in vascular SMC migration and signal transduction, we employed a murine model of guide wire-induced femoral artery endothelial denudation to stimulate neointimal formation (17). Wild-type (WT) C57BL/6 mice and control or “sham” mice were subjected to the identical procedure, the latter without guide wire injury. Pathological neointimal expansion 21 days after injury was observed by Van Gieson’s elastic staining (EVG) only in the guide wire-injured mice but not the sham controls (Figure 1D vs. 1A, respectively). Immunostaining with anti-mDia1 IgG vs. isotype control IgG revealed expression of mDia1 in all the vessels studied, consistent with the ubiquitous expression of formin proteins (Figure 1B & 1E, respectively). However, injured arteries displayed enhancement of mDia1 expression particularly in cells within the expanding neointima (Figure 1E). As vascular SMCs are a chief component of the neointima, we analyzed mDia1 expression in the vessels and found that mDia1 antigen co-localized in part with α-smooth muscle actin (SMA) within the neointima. (Figure 1G, H, and I). To further study regulation of mDia1 in vascular injury, we harvested femoral arteries of WT animals on day 0, 4, 10, 21, or 28 after injury and analyzed mDia1 transcripts and protein levels by quantitative real-time PCR (qRT-PCR) and Western blot. mDia1 mRNA levels were significantly increased by day 4 after injury (p < 0.01) compared with day 0 (Figure 1J). Consistent with this finding, compared to day 0 (no injury), mDia1 protein levels were also increased by day 4 after injury (p < 0.001), and peaked at day 10 (p < 0.01) (Figure 1K). An analogous pattern of expression was found for that of RAGE antigen in the vessel tissues over this time course (Figure 1L).
Figure 1. Increased expression and impact of mDia1 after endothelial denudation injury.
Wild-type (WT), Drf1−/−, and RAGE−/− mice were subjected to femoral artery endothelial denudation or sham, and tissues analyzed at the indicated times. (A,D) Assessment of neointimal expansion by Van Gieson’s Elastic (EVG) staining on day 21 after injury in WT mice (A, sham and D, injury). (B,C,E,F) Immunostaining for mDia1 or Isotype IgG control in WT mice on day 21 after injury (E–F) or sham (B–C). (G–I) Colocalization studies: sections of injured vessels were stained for mDia1 and α-Smooth muscle cell actin (SMA). Immunofluorescence studies revealed a colocalization of the two molecules in the neointima on day 21. (J) qRT-PCR analysis of mDia1 expression. Sham and injured WT vessels (n=5/group) were harvested and subjected to RNA isolation and quantitative real-time PCR analysis for detection of mDia1 transcripts. Results are expressed in mean ± SEM. (K–L) Western blot analysis of mDia1 and RAGE in injured vessels. WT mice (n=4 per time point and per group) were subjected to femoral artery injury. At the indicated time, arteries were retrieved and pooled for preparation of lysates. These lysates were used for Western blot analysis. Each Western blot was subjected to densitometric quantification and results are expressed as the mean ± SEM of at least 3 Western blots per condition. A representative Western blot is shown. (M) mDia1 expression in RAGE−/− mice subjected to femoral artery injury was detected by Western blot analysis at the indicated times. Histogram represents the mean ± SEM of at least 3 Western blots per condition. A representative picture is shown. (N) Quantitative analysis of RAGE expression after injury in WT and Drf1−/− mice: Lysates from n=4 mice per time point and per group were prepared as in figure 1K–L, and subjected to at least 3 independent Western blot analyses for RAGE expression. Densitometric quantification was done and data are expressed as mean ± SEM. A representative Western blot is shown. (O) Intima/Media (I/M) ratio measurement based on morphometric analysis of the vessels of WT and aged-sex-matched Drf1−/− mice (n=11/group) was performed 21 days after guide wire-induced femoral artery denudation. Representative images are shown. *p<0.05, **p<0.01, ***p< 0.001.
We determined the fate of mDia1 expression in mice devoid of RAGE that were subjected to identical degrees of femoral artery injury. Interestingly, Western blotting revealed that although mDia1 was present in RAGE−/− mice vessels, its expression was not significantly altered over the same course from 0 to 28 days after guide wire injury (Figure 1M). Taken together, these results revealed that mDia1 expression is upregulated in a time- and RAGE- dependent manner in injured arterial vessels.
Prompted by these observations, we sought to establish if mDia1 contributes to injury provoked vascular remodeling. We subjected Drf1−/− mice deficient in mDia1 (19), and their age and gender-matched male WT mice to femoral artery endothelial denudation. We first analyzed RAGE expression patterns in the femoral arteries in sham and injury states and found that RAGE upregulation occurred by day 4 and day 10 in both WT and Drf1−/− mice (Figure 1N). Hence, deletion of mDia1 had no impact on RAGE expression. We performed quantitative morphometric analysis of the vessels post-injury; at three weeks, none of the sham operated arteries displayed neointimal formation (data not shown), but significant neointima was observed in WT mice. Compared to WT mice, the Intima/Media (I/M) ratio was ≈ 4.8-fold lower in mice devoid of mDia1 (Drf1−/−), 0.77±0.4 vs. 0.16±0.1, respectively; p<0.001 (Figure 1O). Consistent with this result, intimal area measurements were also highly reduced in the Drf1−/− mice and luminal area was significantly higher compared to the WT group. No significant changes were found between the two groups in the media area measurements or overall vessel size (internal elastic lamina, IEL and external elastic lamina, EEL) (Online Table I). These morphometric measurements are consistent with a reduced pathological inward remodeling in the Drf1−/− mice after guide wire injury.
mDia1 deletion blocks RAGE induced lamellipodia formation and migration in primary murine aortic smooth muscle cells
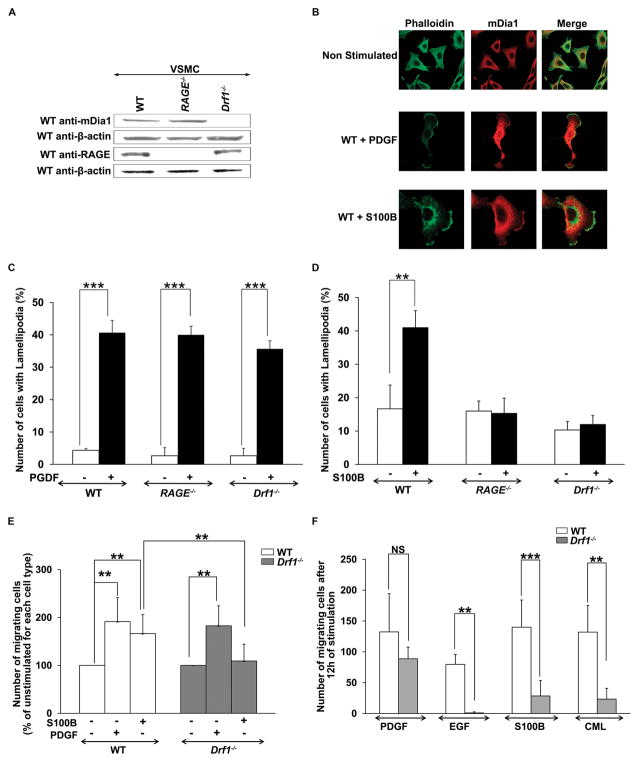
Neointimal expansion in the injured vessel wall depends on a number of factors but critically on vascular SMC migration. Actin polymerization and consequent lamellipodia formation are the initial events leading to cell migration; mDia1 has been implicated in these processes. We isolated primary SMCs from WT, RAGE−/− or Drf1−/− murine aortas and analyzed mDia1 expression. Although mDia1 protein was expressed in WT cells, as well as cells devoid of RAGE, mDia1 protein was not detected in vascular SMC isolated from Drf1−/− animals (Figure 2A).
Figure 2. mDia1 deletion blocks RAGE ligand-induced lamellipodia formation and vascular SMC migration.
(A) Western blot analysis of WT, RAGE −/− and Drf1−/− cells for mDia1 and RAGE reveal that mDia1 is expressed in both WT and RAGE −/− primary murine aortic smooth muscle cells (SMCs), while RAGE is expressed in WT and Drf1−/− cells. (B) WT SMCs were stimulated with PDGF (50 ng/ml) or S100B (10 μg/ml) for 30 minutes, then fixed and stained with Alexa fluor 488-Phalloidin and rabbit anti-mDia1 (secondary Rhodamine conjugated). Cells were then analyzed under an inverted fluorescent microscope (x40/x100). Representative images of lamellipodia scored are shown. (C–D) Quantification of the lamellipodia formation following stimulation of SMCs with PDGF (50ng/ml) or S100B (10μg/ml). (E–F) Transwell and wound healing scratch assay, respectively, were performed to assess SMC migration in response to RAGE ligands (S100B and CML, respectively 10 and 50 μg/ml) or non- RAGE ligands (PDGF and EGF, respectively 50 and 100 ng/ml) and the effects of mDia1 deletion. All data are expressed as mean ± SEM of at least 3 independent experiments. **p<0.01, *** p< 0.001.
To probe the mechanisms by which mDia1 regulated vascular SMC signal transduction and migration, cells were treated with the prototypic RAGE ligand, S100B (26), to stimulate cell surface-anchored RAGE before analysis of actin content. Unstimulated cells revealed a high content of stress fibers as evidenced by phalloidin staining. Compared to unstimulated cells, incubation of WT SMCs with S100B induced disappearance of the stress fibers, change of shape with induction of cell polarity, and the appearance of cytoplasmic protrusions enriched in both F-actin and in mDia1 (lamellipodia) (Figures 2B and D). S100B did not induce lamellipodia formation in RAGE−/− or- Drf1−/− SMCs, suggesting that mDia1 was required for the effects of RAGE ligand S100B. In contrast, stimulation of WT, RAGE−/− and Drf1−/− SMCs with a non- RAGE ligand, PDGF, induced lamellipodia formation comparably in SMCs from the three different genotypes of mice (Figure 2C and D). These data indicated that RAGE and mDia1 were required for S100B- but not for PDGF-stimulated lamellipodia formation.
To further evaluate the impact of mDia1 in RAGE-induced SMC migration, we performed chemotaxis experiments using Transwell migration chambers. Cells placed in the upper chamber migrated toward the chemoattractant placed in the bottom chamber (vehicle, PDGF 50 ng/ml, or S100B 10 μg/ml). WT cells demonstrated a significant chemotactic response to PDGF and S100B (Figure 2E). However, cells retrieved from Drf1−/− mice revealed significantly less S100B-induced migration, although chemotaxis in response to PDGF was not impaired in SMCs devoid of mDia1 (Figure 2E). These results were confirmed by an in vitro scratch wounding assay in cultured monolayers of SMCs, where stimulation with RAGE ligands, S100B or carboxymethyl-lysine (CML)-AGE human serum albumin (26–27), induced migration selectively of WT cells, but not Drf1−/− cells (Figure 2F).
Epidermal growth factor (EGF), which, like PDGF, is not a RAGE ligand, has been reported as a chemotactic factor requiring mDia1 cooperation in formation of lamellipodia in carcinoma cells (28). To confirm the role of mDia1 in EGF-dependent migration of SMCs, we stimulated WT SMCs with EGF, 100 ng/ml, and found that EGF induces significant migration in WT but not Drf1−/− cells (Figure 2F). These data indicate that mDia1-mediated induction of lamellipodia formation and migration of SMCs is both RAGE-dependent and RAGE-independent.
mDia1 deletion blocks inhibitory phosphorylation (Ser 9) of GSK3 β
We next sought to identify the signaling pathways regulated by mDia1 in vascular SMCs. It was previously shown in cultured fibroblasts that mDia1 modulated GSK3β serine 9 (ser 9) phosphorylation (29), and that GSK3β was implicated in SMC migration (30). We tested ser 9 GSK3β phosphorylation in injured arteries of WT mice and found a time-dependent increase in phospho-GSK3β ser 9 in WT arteries (Figure 3A). In contrast, in arteries retrieved from Drf1−/− mice, phospho-GSK3β ser 9 did not increase from baseline in response to injury over the same time course (Figure 3A). Of note, we previously demonstrated that guide wire-induced femoral artery injury resulted in activation of the MAP kinase ERK1/2 signaling pathway in WT mice, and that this mechanism was independent of RAGE (17). Here we found that MAP kinase ERK1/2 phosphorylation is induced equivalently at day 4 and 10 after injury in both WT and Drf1−/− animals (Figure 3B), thereby suggesting that the effects of both RAGE (17) and mDia1 were likely not mediated via ERK1/2 MAP kinases.
Figure 3. mDia1 deletion blocks inhibitory phosphorylation (ser 9) of GSK3β stimulated by RAGE ligands.
(A–B) GSK3β serine 9 phosphorylation and MAP kinase ERK1/2 phosphorylation were tested in lysates from WT or age- matched Drf1−/− mice arteries after injury. Mice (n=4 per time point and per group) were subjected to femoral artery injury and followed until the indicated day. Then, arteries were retrieved and pooled for preparation of lysates. These lysates were used for Western blot analysis. Each Western blot was subjected to densitometric quantification and results are expressed as the mean ± SEM of at least 3 Western blots per condition. A representative picture of these Western blots is shown. (C–D) Western blot analysis of AKT/GSK3β ser 9 phosphorylation in WT and Drf1−/− primary SMCs stimulated with RAGE ligand S100B (10 μg/ml). Deletion of mDia1 inhibits GSK3β serine 9 and AKT phosphorylation by S100B. Results are represented as mean ± SEM of 3 independent experiments. (E–F–G) Transfection of murine mDia1 into Drf1−/− SMCs rescues AKT phosphorylation and ser 9 phosphorylation of GSK3β by S100B. In E, Western blot for mDia1 is shown in mDia1-transfected (lane 2) and mock vector control transfected (lane 3) Drf1−/− SMCs. Lane 1 represents mDia1 expression in WT SMCs. In F, mDia1- or vector-transfected SMCs were treated with S100B (10 μg/ml) and AKT (F) and ser 9 phosphorylation of GSK3β (G) was determined at the indicated time. Results are represented as mean ± SEM of 3 independent experiments. (H) Western blot analysis of AKT and GSK3β ser 9 phosphorylation in WT and Drf1−/− primary SMCs stimulated with non-RAGE ligand PDGF (50 ng/ml). *p<0.05, **p<0.01, ***p< 0.001.
We next probed the role of mDia1 in RAGE ligand-induced GSK3β ser 9 phosphorylation in primary vascular SMCs. Deletion of mDia1 inhibited phosphorylation of AKT (a principal kinase mediating GSK3β ser 9 phosphorylation) and GSK3β (Figure 3C and D) in response to S100B stimulation. These results were confirmed by down-regulation experiments in which transfection of WT cells with mDia1 siRNA significantly inhibited AKT and GSK3β ser 9 phosphorylation in response to S100B, whereas scramble-transfected cells displayed a time dependent phosphorylation of AKT and GSK3β ser 9 (Online Figure I).
To confirm the role of mDia1 in RAGE ligand-induced GSK3β ser 9 phosphorylation, SMCs retrieved from Drf1−/− animals were transfected with full length murine mDia1 construct to restore mDia1 expression, as assessed by Western blot (Figure 3E). Compared to vector (mock)-transfected SMCs, transfection of Drf1−/− cells with cDNA expressing mDia1 restored phosphorylation of AKT and ser 9 GSK3β in response to S100B (Figure 3F and G, respectively).
Lastly, we tested the effect of a non-RAGE ligand, PDGF (17), on cellular signaling. We found that phosphorylation of AKT and GSK3β was induced similarly in wild type and Drf1−/− cells in response to PDGF (Figure 3H), supporting that these effects of PDGF, as well as formation of lamellipodia (Figure 2), do not require mDia1.
GSK3 β ser 9 inhibitory phosphorylation is required for RAGE induced vascular SMC migration
Our next step was to analyze the role of RAGE in GSK3β ser 9 phosphorylation in SMC migration. We stimulated primary WT SMCs with S100B and found a dose- and time-dependent phosphorylation of GSK3β at serine 9 (Figure 4A), which did not occur in RAGE−/− SMCs, (Figure 4B). To definitively implicate GSK3β phosphorylation in S100B-induced SMC migration, we transfected WT cells with a mutated form of GSK3β (constitutively active GSK3β, CA-GSK3β), that is unable to be phosphorylated at serine 9. Upon stimulation with S100B, migration was highly significantly reduced in CA-GSK3β-transfected cells compared to control- transfected cells (figure 4C). These data support the role of GSK3β ser 9 phosphorylation in S100B-RAGE-mediated SMC migration.
Figure 4. RAGE-induced GSK3β phosphorylation is critical for SMC migration.
(A) Western blot analysis of GSK3β serine 9 phosphorylation in primary SMCs stimulated with increasing concentrations of RAGE ligand S100B for 10 minutes. (B) Comparative analysis of GSK3β serine 9 phosphorylation in WT and RAGE−/− SMCs stimulated with S100B (10μg/ml). Results are presented as mean ± SEM of 3 independent experiments. (C) Inhibitory phosphorylation of GSK3β on Ser 9 is required for RAGE induced SMCs migration. WT primary SMCs were transfected with an empty vector or with constitutively active GSK3β plasmid (CAGSK3β), before analysis of S100B (10μg/ml)-induced migration by wound healing assay. Results are represented as mean ± SEM of 3 independent experiments. *p<0.05, **p<0.01, *** p< 0.001.
mDia1 deletion blocks reactive oxygen species (ROS) formation in vivo and in vascular SMCs
It is well established that oxidative stress is a key process implicated in vascular SMC migration and neointimal formation, (31) yet; the proximal signals triggering reactive oxygen species (ROS) formation and consequent SMC migration have yet to be fully identified. To test the hypothesis that mDia1 is required for oxidative stress in vascular SMCs, we measured NADPH oxidase activity in lysates prepared from the injured arteries of WT and Drf1−/− mice. Compared to sham operated animals, WT arteries displayed a significant increase in NADPH oxidase activity twelve hours following the injury, p<0.001, whereas no significant increases were observed in arteries retrieved from the Drf1−/− mice (Figure 5A). We next determined NADPH oxidase activity in SMCs. NADPH oxidase activity was increased by ≈2 fold in response to S100B in WT cells at 15 minutes and 30 minutes stimulation, but no significant increase was found in RAGE−/− or Drf1−/− cells (Figure 5B). We employed dihydroethidium (DHE) to measure superoxide production in response to S100B stimulation in SMC, and tested the effect of siRNA-mediated reduction of mDia1 expression (Figure 5C) and deletion of mDia1. WT cells exhibited a time-dependent increase in superoxide production in response to S100B, which was maximal at 60 minutes (≈4 fold increase compared to time zero, p < 0.01) (Figure 5D). S100B stimulation required RAGE, as RAGE−/− cells demonstrated no response to S100B (Figure 5D). Furthermore, SMCs transfected with mDia1 siRNA (Figure 5D) and Drf1−/− cells revealed a significant inhibition of S100B-induced generation of superoxide compared to scramble-transfected cells at 60 minutes stimulation (Figure 5D).
Figure 5. mDia1 deletion blocks ROS formation in vivo and in vascular SMCs.
(A) WT and Drf1−/− mice (n=3/group) were subjected to femoral artery guide wire injury. At the indicated time point, arteries were harvested and pooled prior to lysate preparation. Lysates were used for measurement of NADPH oxidase activity by chemiluminescence. Measurements were repeated in 3 independent experiments. Results are shown as plots, with a transverse bar representing the median of the data. Statistical analysis was performed on the mean + SEM. (B) NADPH oxidase activity was measured by chemiluminescence in primary vascular SMCs stimulated with S100B (10μg/ml). Results are expressed as the mean ± SEM of 3 independent experiments. (C) Western blot to demonstrate reduction in mDia1 expression in SMCs using siRNAs vs. scramble controls. (D) Measurement of superoxide production after S100B (10μg/ml) stimulation in primary SMCs in the indicated conditions using DHE. Results are expressed as the mean ± SEM of 5 independent experiments. (E–F–G) Examination of p47phox, Nox1 and Nox4 expression. Membrane fractions were prepared from unstimulated and S100B (10μg/ml)- stimulated WT, RAGE−/− and Drf1−/− SMCs and then tested by Western blot. Histograms represent mean ± SEM of the densitometric quantification of 3 independent experiments. A representative picture is shown for each Western blot. *p<0.05, **p<0.01, *** p< 0.001.
We next examined p47phox, Nox1 and Nox4, key molecules of the NADPH oxidase complex, and found that S100B stimulation of WT SMCs significantly increased the amount of p47phox at the cell membrane, whereas no translocation was found in RAGE−/− or Drf1−/− cells (Figure 5E). Further, increased expression of Nox1 was found in WT cells treated with S100B, but no increases were noted in SMCs devoid of RAGE or mDia1 (Figure 5F). In contrast, the expression of Nox4 was not modified by S100B stimulation in WT, RAGE−/− or Drf1−/− cells (Figure 5G). Note that previous studies established that upregulation of Nox1 in SMCs was accounted for by increased translation of Nox1 protein; our results are completely in line with this observation (32).
mDia1, Rac1 and Nox1 are required for RAGE ligand-induced generation of ROS and consequent cellular signaling in vascular SMCs
Rac1 is a major component of activated NADPH oxidase. To test if mDia1 impacts Rac1 activation in vascular SMCs, cells were incubated with S100B. WT but not Drf1−/− SMCs revealed activation of Rac1 at 15 minutes post-S100B stimulation (Figure 6A). Our next goal was to establish the hierarchy of vascular SMC signaling events following S100B stimulation. We first tested the relationship between RAGE induced oxidative stress and kinase phosphorylation, and examined S100B-induced GSK3 β ser9 phosphorylation in SMCs in the presence or absence of the reactive oxygen species scavenger, tyron (10 mM). Incubation of SMCs with tyron inhibited S100B-stimulated GSK3 β ser9 phosphorylation (Figure 6B); similar findings were observed with N-Acetyl cysteine (25 mM) (data not shown). These results suggest that reactive oxygen species generation was required for RAGE-mediated ser 9 GSK3 β phosphorylation.
Figure 6. mDia1, Rac1 and Nox1 are involved in RAGE induced generation of ROS and consequent cellular signaling in SMCs.
(A) Assessment of Rac1 activation after S100B (10 μg/ml) stimulation in WT and Drf1−/− primary SMCs. Histogram represents mean ± SEM of active Rac1 determined in 3 independent experiments. (B). Tyron suppresses S100B-stimulated phosphorylation of GSK3β in SMCs. WT SMCs were treated with tyron (10 mM) or vehicle followed by S100B (10 μg/ml) over the indicated time course. Western blotting for determination of phospho-ser9/total GSK3β was then performed in at least three independent experiments. Similar findings were observed with N-acetyl cysteine (25 mM; data now shown). (C–D–E) Effect of dominant negative Rac1 (DN Rac) on S100B (10 μg/ml)-stimulated superoxide production, SMCs migration and GSK3β ser 9 phosphorylation. Data are presented as mean ± SEM of 3 independent experiments. (F) Western blot indicates Nox1 expression after transfection with Nox1 siRNA vs. scramble siRNA in SMCs stimulated with S100B (10 μg/ml). (G–H) Effect of Nox1 down regulation in S100B (10 μg/ml)-induced superoxide production and GSK3β ser 9 phosphorylation in SMCs. *p<0.05, **p<0.01, ***p<0.001.
To explore the specific mechanisms linking RAGE and mDia1 to reactive oxygen species generation, we hypothesized that Rac1 and Nox1 activation were essential for both S100B-stimulated reactive oxygen species generation and kinase activation in vascular SMCs. We employed dominant negative Rac1 (DN Rac) and Nox1 siRNA to test these concepts. Compared to cells transfected with the control vector, cells transfected with DN Rac revealed significantly less generation of superoxide and migration in response to S100B (Figure 6C and D). DN Rac inhibited AKT (Online Figure II) and GSK3β ser 9 phosphorylation (Figure 6E). To confirm the role of RAGE-induced oxidative stress in GSK3 β ser9 phosphorylation, we transfected WT cells with Nox1 siRNA or scramble siRNA (Figure 6F). Downregulation of Nox1 significantly inhibited superoxide production and consequent phosphorylation of GSK3β ser 9, whereas scramble transfection had no effect on the impact of S100B (Figure 6G–H). Together, these data implicate Rac1 and Nox1 in RAGE ligand-induced ROS generation and signaling in SMCs.
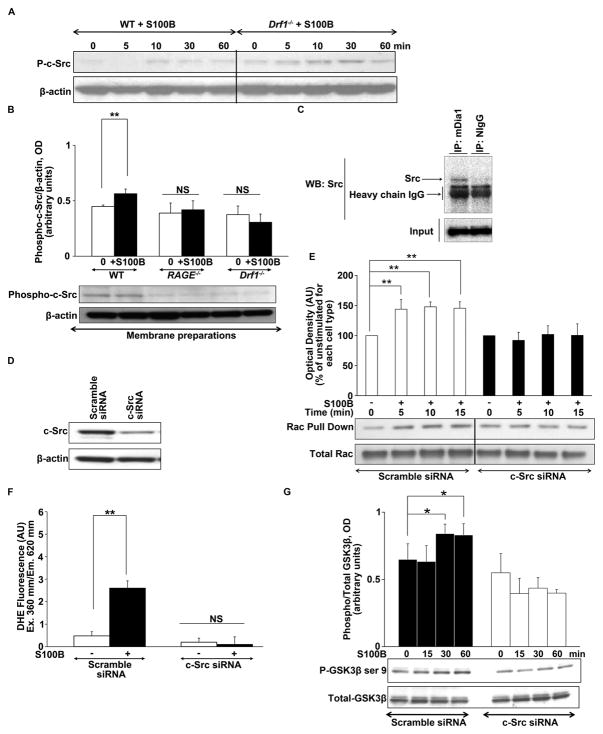
mDia1 is critical for RAGE-induced c-Src translocation to the membrane and consequent Redox signaling
It has been shown that RAGE activates c-Src kinases in SMCs (33) and that c-Src kinases bind to mDia1 and act as downstream effectors of diaphanous proteins (34–35). We tested RAGE-induced c-Src phosphorylation in WT and in Drf1−/− SMCs. Analysis of total lysates found that stimulation with S100B induced phosphorylation of c-Src kinases in both WT and Drf1−/− cells (Figure 7A). However, analysis of the membrane fractions revealed that membrane translocation of phospho-c-Src in response to S100B was observed in WT cells, but not RAGE−/− or Drf1−/− SMCs, (Figure 7B). To further confirm the interaction of c-Src with mDia1 we performed co-immunoprecipitation studies. Immunoprecipitation using anti-mDia1 IgG, but not nonimmune IgG, from S100B-stimulated SMC lysates resulted in immunoprecipitation of c-Src, as revealed by Western blot (Figure 7C). Anti-mDia1 antibody immunoprecipitated mDia1 by Western blot as well (data not shown).
Figure 7. mDia1 regulates RAGE-induced c-Src translocation to the membrane and consequent Rac1 activation, ROS generation and cellular signaling in SMCs.
(A) Western blot analysis of S100B induced phosphorylation of c-Src in total lysates of WT and Drf1−/− SMCs reveals identical degrees of c-Src phosphorylation in both cell types. (B) Western blot analysis of S100B (10μg/ml)-induced c-Src translocation to the membrane in WT, RAGE −/− and Drf1−/− primary SMCs. Membrane fractions were prepared from unstimulated and stimulated SMC and then used for Western blots for Phospho-c-Src and total c-Src. Histograms represent mean ± SEM of the densitometric quantification of 3 independent experiments. A representative picture is shown for each Western blot. (C) Immunoprecipitation studies. Lysates obtained from WT SMCs were stimulated with S100B and subjected to immunoprecipitation using rabbit polyclonal anti-mDia1 IgG or isotype nonimmune control. Immunoprecipitated products were subjected to Western blot analysis of c-Src. C-Src immunoprecipitates with mDia1 antibody, but not with the isotype control. Input is Western blot for total c-Src. (D) c-Src protein expression after transfection with c-Src siRNA vs. scramble siRNA. (E) c-Src is involved in RAGE induced Rac activation. Cells were transfected with c-Src siRNA prior to stimulation with S100B (10μg/ml) and pulldown of activated rac1 was performed at the indicated time. Results are expressed as mean ± SEM of 3 independent experiments; **p<0.01. (F–G) Effect of c-Src down regulation in S100B (10μg/ml)-induced superoxide production and GSK3β ser 9 phosphorylation is shown. In G, histograms represent mean ± SEM of the densitometric quantification of 3 independent experiments. A representative picture is shown for each Western blot. *p<0.05, **p<0.01, ***p< 0.001.
We next delineated the role of c-Src in S100B–mediated downstream events. Reduction of c-Src expression with siRNA (Figure 7D) significantly inhibited S100B-induced Rac 1 activation (Figure 7E), and reactive oxygen species production in SMCs (Figure 7F), whereas scramble siRNA had no effect. Finally, down regulation of c-Src expression significantly inhibited RAGE ligand-induced AKT phosphorylation (Online Figure III) and GSK3β ser 9 phosphorylation (Figure 7G).
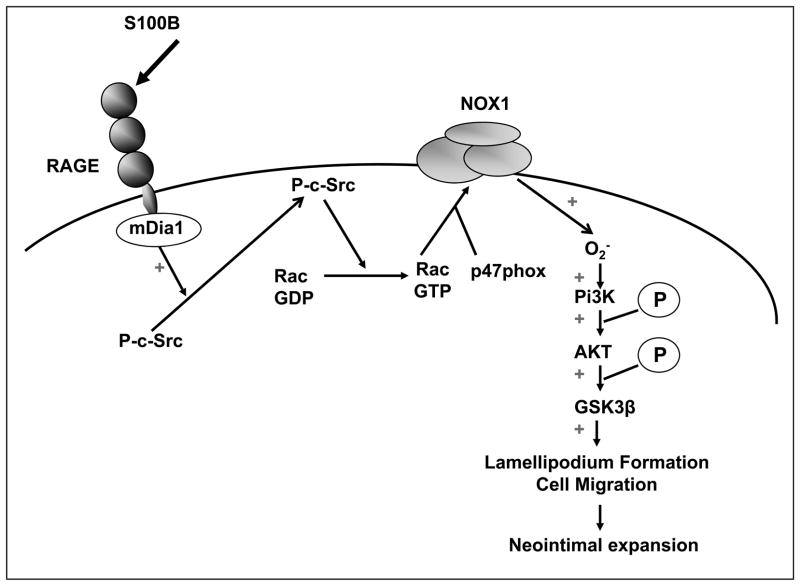
Taken together, these results establish a critical role for mDia1 in transducing the effects of RAGE ligands on c-Src, Rac1 and Nox1 activation in consequent phosphorylation of AKT and GSK3β ser 9, processes essential for RAGE ligand-induced vascular SMC migration. As the initial signaling events are dependent on RAGE and mDia1, these findings integrate for the first time the role of this axis in regulating oxidative and cell signaling mechanisms controlling SMC migration and neointimal formation (Figure 8).
Figure 8. Proposed mechanism of the role of mDia1 in RAGE-induced redox signaling SMC migration and neointimal expansion.
Collectively, the findings in this manuscript establish a critical role for mDia1 in transducing the effects of RAGE ligands on c-Src, Rac1 and Nox1 activation in consequent phosphorylation of AKT/GSK3β ser 9, processes essential for RAGE ligand-induced vascular SMC migration.
DISCUSSION
Formins are effectors and modulators of Rho-GTPase signal transduction involved in regulation of actin and microtubule rearrangements during cell migration and cytokinesis (1, 3). mDia1 is one of the best characterized members of this family, but only limited reports have implicated mDia1 directly in disease processes (7–8, 36–37). As neointimal expansion triggered by denuding arterial injury is a well established stress used to identify factors involved in acute vascular remodeling (21), we tested the role of mDia1 and report for the first time significant upregulation of mDia1 mRNA and protein levels in the neointima of injured arterial vessels compared to sham-treated arteries. Moreover, genetic deletion of mDia1 protected animals against neointimal hyperplasia. As invading and proliferating SMCs express mDia1 and are the main constituents of the neointima, our data link mDia1 to fundamental smooth muscle cell responses to injury.
RAGE and RAGE-ligands are up-regulated in the vessel wall after guide wire injury, leading not only to SMC activation, migration and proliferation, but also to extracellular matrix accumulation (17, 38). Blockade of RAGE by use of soluble truncated receptor or genetic deletion significantly reduces neointimal hyperplasia in murine and rat models of injury (17, 38). mDia1 is an intracellular binding partner for RAGE, critical for RAGE-induced C6 glioma cell migration and RAGE induced Egr1 expression in hypoxic macrophages (9, 10). In the latter case, we showed that hypoxia results in rapid release of RAGE ligand AGEs into the supernatants of cultured cells (39), hence linking hypoxia directly to activation of RAGE signaling via these AGE ligands. Here we show that in injured vessels, deletion of mDia1 mimics the protective effect of RAGE blockade, suggesting that reduction of the neointimal hypertrophy in Drf1−/− mice is at least in part through blockade of the impact of RAGE. In line with this hypothesis, we found that mDia1 deletion inhibits RAGE ligand-induced signaling and cell activation in isolated smooth muscle cells.
Ligand binding to RAGE induces production of reactive oxygen species largely by the NADPH oxidase system (40–41), but the proximate mechanisms involved in this process are not fully identified. Our data reveal the novel finding that mDia1 is required for RAGE-induced oxidative stress generation and consequent signaling events. Furthermore, our findings highlight seminal insights into the important role of RAGE-dependent NADPH oxidase activation as the initial key event leading to superoxide production, AKT and GSK3β serine 9 phosphorylation, and SMC migration. Activation of NADPH oxidase is an ordered multistep process, including protein phosphorylation, GTPase activation and translocation of certain cytosolic proteins to the plasma membrane (42). Our data reveal that in mDia1 deficient cells, membrane translocation of c-Src and p47phox, activation of Rac1, and upregulation of Nox1 upon RAGE ligand stimulation are inhibited, suggesting that RAGE-induced NADPH oxidase activation requires the cooperation of mDia1.
As underlying mechanisms included a possible role for mDia1 in Rac1 activation (43), we investigated the role of c-Src kinase. Src family kinases are potent activators of Rac1 (44–45). They are also important effectors of the formin family of proteins (44). Additionally, recent reports highlighted the role of mDia1 in v-Src kinase membrane translocation and in c-Src mediated activation of Rac1 upon EGF stimulation (46–47). In our studies, RAGE ligand-induced c-Src translocation to the membrane was defective in primary aortic SMCs isolated from Drf1−/− or RAGE-−/− mice. Our data also demonstrate key roles for c-Src in RAGE-dependent reactive oxygen species generation and signaling, as siRNA-mediated reduction of c-Src expression inhibited Rac 1 activation, superoxide production and consequent AKT and GSK3β phosphorylation. These results are strengthened by previous reports suggesting a critical role for Src kinases in RAGE-dependent ROS production (33). Moreover, here we identified mDia1 as a key molecule regulating RAGE dependent c-Src translocation to the membrane and consequent redox signaling.
Previous reports have linked mDia1 and c-Src interaction to actin polymerization and cell protrusion. It was shown that mDia1 interaction with c-Src and DIP (mDia Interacting Protein) upon EGF stimulation and Vav2 activation led ultimately to Rac1 activation (46). Our results are in complete agreement with these reports and illustrate this relationship in vascular SMCs. Indeed, the potential participation of Vav2 in RAGE-induced Rac1 activation is a subject of future investigation.
It is well established that RAGE engagement potentially activates numerous signaling pathways depending on the cell type stimulated (18). Here we show that RAGE ligand S100B impacts the GSK3β phosphorylation state of serine 9. Unlike most kinases, GSK3β is constitutively active in its unphosphorylated form. Phosphorylation of GSK3β at serine 9 inhibits its activity and consequently induces glycogen synthesis, mitochondrial protection and inhibition of the ubiquitin-proteasome system leading to cell survival and protein stability (48). Increased vascular SMC survival mediated by GSK3 β ser 9 phosphorylation has been reported by Park and colleagues as a mechanism leading to neointimal expansion after balloon injury of the carotid artery in rats (30). Our data reveal that RAGE activation induces GSK3β ser 9 mediated migration of vascular SMCs, therefore suggesting that the phosphorylation status of GSK3β is important for two processes critically involved in neointimal expansion, SMC survival and migration.
Importantly, we found that mDia1 deletion prevented RAGE induced phosphorylation of GSK3β and consequent SMC migration. mDia1 was previously linked to GSK3β phosphorylation during lysophosphatidic acid (LPA) induced microtubule stabilization, in a manner dependent on a novel PKC isoform which was required to phosphorylate and inhibit GSK3β (29). We report that mDia1 is essential for RAGE induced c-Src translocation to the membrane and Rac1 activation. These signaling events account for RAGE induced reactive oxygen species production and consequent downstream AKT and GSK3β phosphorylation, however, a direct activation of PI3Kinase pathway by membrane bound Src cannot be excluded.
It is essential to note that although mDia1 is required for the actions of RAGE ligands and EGF on lamellipodia formation, mDia1 was not required for PDGF-mediated formation of lamellipodia nor for PDGF-stimulated phosphorylation of AKT and ser 9 GSK3β in SMCs. We previously found that knockdown of mDia1 expression in C6 glioma cells blocked CML-AGE RAGE ligand-mediated cellular migration, but that reduced mDia1 expression had no effect on migration induced by a general stimulus, fetal bovine serum (10). Importantly, EGF is not a ligand for RAGE. Hence, mDia1 actions are, as expected, both RAGE-dependent and RAGE independent. These concepts have parallels in the exquisite regulation of distinct mechanisms mediating cellular migration via RhoA vs. rac1-dependent processes. It has been shown that active RhoA is present in the forward movement-provoking cellular protrusions known as membrane ruffles (49). In human umbilical vein endothelial cells, exposure to thrombin resulted in rapid activation of RhoA and inhibition of rac1; in contrast, stimulation of these cells with the bioactive phospholipid sphingosine-1-phospate (S1P) resulted in very modest and delayed activation of RhoA with strong activation of rac1. In those endothelial cells, Src and rac1 were essential for recruitment of the F-actin-binding protein cortactin to sites of actin polymerization at the rim of membrane ruffles and consequent cellular migration (50). Our data link RAGE ligands to rapid activation of c-Src and rac1 in smooth muscle cells, and downstream signaling mechanisms that regulate lamellipodia formation and cellular migration in an mDia1-dependent manner. Hence, taken together, these considerations suggest defined but not unrestricted roles for mDia1 in transducing the effects of distinct mediators of signal transduction and migration cues in smooth muscle and other cell types.
Finally, our recent work has provided further support for the interaction of the cytoplasmic domain of RAGE with the FH1 domain of mDia1. Shekhtman and colleagues showed that in the RAGE cytoplasmic domain, amino acids R5/Q6 were essential for the mDia1 (FH1 domain) interaction; mutation of these amino acids blocked RAGE ligand-stimulated signaling, migration and proliferation in primary murine aortic SMCs. These data, along with the published solution structure of the RAGE cytoplasmic domain with mDia1, buttress the concept that RAGE signaling requires this interaction with mDia1 to facilitate engagement of a broader range of downstream signaling effectors (51).
In summary, our findings implicate for the first time the engagement of the RAGE-mDia1 axis as a critical signaling pathway for smooth muscle cell function and vascular remodeling. This axis integrates a series of key events essential for aberrant neointimal expansion; we show that mDia1 is required for RAGE–induced c-Src membrane translocation and Rac1 activation, the key molecular events leading to redox phosphorylation of AKT and GSK3β and smooth muscle cell migration. As deletion of mDia1 protected against aberrant vascular remodeling, blockade of RAGE-mDia1 may be a newly-discovered therapeutic approach to limit pathological inward remodeling in human vascular diseases.
Supplementary Material
Novelty and Significance.
What is Known?
Ligand engagement by receptor for advanced glycation endproducts (RAGE) triggers oxidative stress in vascular smooth muscle cells.
Deletion of RAGE is highly protective in a murine model of femoral artery endothelial denudation injury.
The cytoplasmic domain of RAGE binds to the formin mammalian diaphanous-related formin (mDia1); in transformed cells, mDia1 is required for RAGE-activated cell migration and signaling.
What is New?
mDia1 is essential for RAGE ligand-mediated generation of oxidative stress.
Expression of the formin mDia1 is elevated in the arterial tissue of wild-type but not RAGE-null mice after femoral artery endothelial denudation injury.
mDia1 is required for RAGE ligand-stimulated generation of Rac and lamellipodia, the recruitment of c-Src to the plasma membrane, activation of Akt and GSK-3β serine 9, and the in vivo migration of vascular smooth muscle cells following femoral artery endothelial denudation injury.
One paragraph summary.
The transmembrane RAGE molecule lacks intrinsic signaling activity despite strong evidence for its ability to induce tyrosine signaling and cell migration in response to stress. Previous studies identified the formin mDia1 as a binding partner that interacts directly with the cytoplasmic tail of RAGE. mDia1 is a canonical Rho GTPase member of the Diaphanous-related formin family. mDia1 generates linear actin filaments and stabilizes microtubule dynamics and is known to dock with Src family non-receptor tyrosine kinases. Using genetically-deficient mice or cells lacking either RAGE or mDia1, we tested the hypothesis that mDia1 is a key intermediate in RAGE signaling. We report that injury to the femoral artery significantly upregulates mDia1 gene and protein expression and is RAGE-dependent. Loss of mDia1 expression significantly impairs pathological neointimal expansion consequent to injury that is known to activate RAGE. In primary murine aortic smooth muscle cells, mDia1 is required for RAGE ligand-induced membrane translocation of c-Src, Rac1 activation, redox phosphorylation of AKT and GSK3β, and migration. Hence, for the first time we have illustrated key roles for mDia1 in smooth muscle cell migration and signal transduction. Taken together, these data illuminate a novel RAGEmDia1 signaling axis that mediates pathobiological neointimal formation in the cardiovasculature in response to injury.
Acknowledgments
The authors gratefully acknowledge Dr. John W. Copeland (University of Ottawa) for sharing cDNA to express murine mDia1 and Ms. Latoya Woods for her expert assistance in the preparation of the manuscript.
SOURCES OF FUNDING.
This work is supported by grants from; “Sociéte de Néphrologie” and “Fondation Transplantation” (to F.T.), the Deutsche Forschungsgemeinschaft (FR 1488/3-1 and FR 1488/5-1, to G.F.), Van Andel Foundation (to A.S.A) and by funds from the United States Public Health Service, HL60901 (to A.M.S) and by funds from the JDRF (to A.M.S).
Non-standard Abbreviations
- AGEs
advanced glycation endproducts
- CA
constitutively active
- CML
carboxy methyl lysine
- DHE
dihydroethidium
- DIP
mDia interacting protein
- EEL
external elastic lamina
- EGF
epidermal growth factor
- EVB
Van Gieson’s elastic staining
- GSK
glycogen synthase kinase
- HMGB1
high mobility group box 1
- IEL
internal elastic lamina
- I/M ratio
intima/media ratio
- LPA
lysophosphatidic acid
- MAP kinase
mitogen activated protein kinase
- mDia1
mammalian diaphanous-related formin
- NADPH
nicotinamide adenine dinucleotide phosphate
- PDGF
platelet derived growth factor
- RAGE
receptor for advanced glycation endproducts
- S1P
sphingosine 1 phosphate
- SMA
smooth muscle actin
- SMC
vascular smooth muscle cell
Footnotes
DISCLOSURES.
There are no relationships to disclose.
References
- 1.Baarlink C, Brandt D, Grosse R. SnapShot: Formins. Cell. 2010;142:172, 172, e171. doi: 10.1016/j.cell.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta. 2010;1803:164–173. doi: 10.1016/j.bbamcr.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 4.Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13:435–446. doi: 10.1016/s0962-8924(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 5.Habas, Kato Y, He X. Wnt/frizzled activation of Rho regulates vertebrate gastrulation and requires a novel formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 6.Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG and RhoA regulates cell morphology and invastion. Genes Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch ED, Lee MK, Morrow JE, Welcsh PL, Leon PE, King MC. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science. 1997;278:1315–1318. [PubMed] [Google Scholar]
- 8.DeWard AD, Eisenmann KM, Matheson SF, Alberts AS. The role of formins in human disease. Biochim Biophys Acta. 2010;1803:226–233. doi: 10.1016/j.bbamcr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Toure F, Qu W, Lin L, Song F, Shen X, Rosario R, Garcia J, Schmidt AM, Yan SF. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285:23233–23240. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 12.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 13.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 14.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 15.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Gröne HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hämmerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng J, Kitchen SM, West RA, Sigler R, Eisenmann KM, Alberts AS. Myeloproliferative defects following targeting of the Drf1 gene encoding the mammalian diaphanous related formin mDia1. Cancer Res. 2007;67:7565–7571. doi: 10.1158/0008-5472.CAN-07-1467. [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Wallar BJ, Flanders A, Swiatek PJ, Alberts AS. Disruption of the Diaphanousrelated formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol. 2003;13:534–545. doi: 10.1016/s0960-9822(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 21.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20:335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 22.Tarvo P, Barret G. Differences in proliferation of primary cultures of vascular smooth muscle cells taken from male and female rats. Blood Vessels. 1980;17:110–116. doi: 10.1159/000158240. [DOI] [PubMed] [Google Scholar]
- 23.Ostendorp T, Leclerc E, Galichet A, Koch M, Demling N, Weigle B, Heizmann CW, Kroneck PM, Fritz G. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 2007;26:3868–3878. doi: 10.1038/sj.emboj.7601805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Tobias R, McClure S, Styba G, Shi Q, Jackowski G. Removal of endotoxin from recombinant protein preparations. Clin Biochem. 1997;30:455–463. doi: 10.1016/s0009-9120(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 25.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 27.Kislinger TK, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation endproducts that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 28.Sarmiento C, Wang W, Dovas A, Yamaguchi H, Sidani M, El-Sibai M, Desmarais V, Holman HA, Kitchen S, Backer JM, Alberts A, Condeelis J. WASP family members and formin proteins coordinate regulation of cell protrusions in carcinoma cells. J Cell Biol. 2008;180:1245–1260. doi: 10.1083/jcb.200708123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eng CH, Huckaba TM, Gundersen GG. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol Biol Cell. 2006;17:5004–5016. doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park KW, Yang HM, Youn SW, Yang HJ, Chae IH, Oh BH, Lee MM, Park YB, Choi YS, Kim HS, Walsh K. Constitutively active glycogen synthase kinase-3beta gene transfer sustains apoptosis, inhibits proliferation of vascular smooth muscle cells, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol. 2003;23:1364–1369. doi: 10.1161/01.ATV.0000081633.53390.B4. [DOI] [PubMed] [Google Scholar]
- 31.Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 32.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression and regulation. Am J Physiol - Regul, Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 33.Reddy MA, Li SL, Sahar S, Kim YS, Xu ZG, Lanting L, Natarajan R. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–13693. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- 34.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanousrelated formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 35.Gasman S, Kalaidzidis Y, Zerial M. RhoD regulates endosome dynamics through Diaphanous-related Formin and Src tyrosine kinase. Nat Cell Biol. 2003;5:195–204. doi: 10.1038/ncb935. [DOI] [PubMed] [Google Scholar]
- 36.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem. 2007;282:25152–25158. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- 37.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N, Narumiya S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Wang K, Penn MS, Marso SP, Lauer MA, Forudi F, Zhou X, Qu W, Lu Y, Stern DM, Schmidt AM, Lincoff AM, Topol EJ. Receptor for AGE (RAGE) mediates neointimal formation in response to injury. Circ. 2003;107:2238–2243. doi: 10.1161/01.CIR.0000063577.32819.23. [DOI] [PubMed] [Google Scholar]
- 39.Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102:905–913. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 40.Guo J, Ananthakrishnan R, Qu W, Lu Y, Reiniger N, Zeng S, Ma W, Rosario R, Yan SF, Ramasamy R, D’Agati V, Schmidt AM. RAGE mediates podocyte injury in adriamycin-induced glomerulosclerosis. J Am Soc Nephrol. 2008;19:961–972. doi: 10.1681/ASN.2007101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wautier JL, Wautier MP, Schmidt AM, Anderson GM, Hori O, Zoukourian C, Capron L, Chappey O, Yan SD, Brett J, Guillausseau PJ, Stern D. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci U S A. 1994;91:7742–7746. doi: 10.1073/pnas.91.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 44.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta. 2010;1803:183–190. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Tanji M, Ishizaki T, Ebrahimi S, Tsuboguchi Y, Sukezane T, Akagi T, Frame MC, Hashimoto N, Miyamoto S, Narumiya S. mDia1 targets v-Src to the cell periphery and facilitates cell transformation, tumorigenesis, and invasion. Mol Cell Biol. 2010;30:4604–4615. doi: 10.1128/MCB.00197-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng W, Numazaki M, Takeuchi K, Uchibori Y, Ando-Akatsuka Y, Tominaga M, Tominaga T. DIP (mDia interacting protein) is a key molecule regulating Rho and Rac in a Src-dependent manner. Embo J. 2004;23:760–771. doi: 10.1038/sj.emboj.7600095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satoh S, Tominaga T. mDia-interacting protein acts downstream of Rho-mDia and modifies Src activation and stress fiber formation. J Biol Chem. 2001;276:39290–39294. doi: 10.1074/jbc.M107026200. [DOI] [PubMed] [Google Scholar]
- 48.Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–14. doi: 10.1016/s0959-437x(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 49.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 50.Vouret-Craviari V, Bourcier C, Boulter E, van Obberghen-Schilling E. Distinct signals via Rho GTPases and Src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells. J Cell Sci. 2002;115:2475–2484. doi: 10.1242/jcs.115.12.2475. [DOI] [PubMed] [Google Scholar]
- 51.Rai V, Maldonado AY, Burz DS, Reverdatto S, Yan SF, Schmidt AM, Shekhtman A. Signal transduction in receptor for advanced glycation endproducts (RAGE): solution structure of C terminal RAGE (ct-RAGE) and its binding to mDia1. J Biol Chem. 2012;287:5133–5144. doi: 10.1074/jbc.M111.277731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.