Abstract
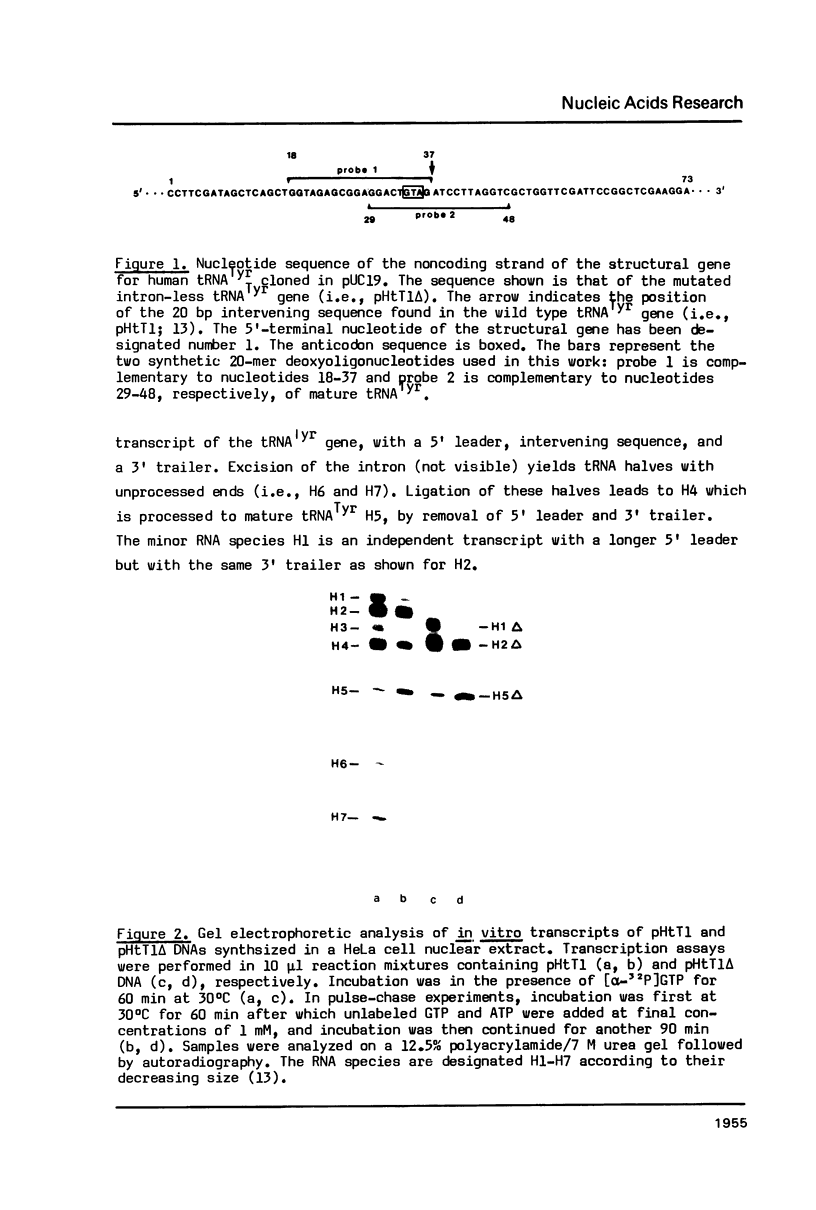
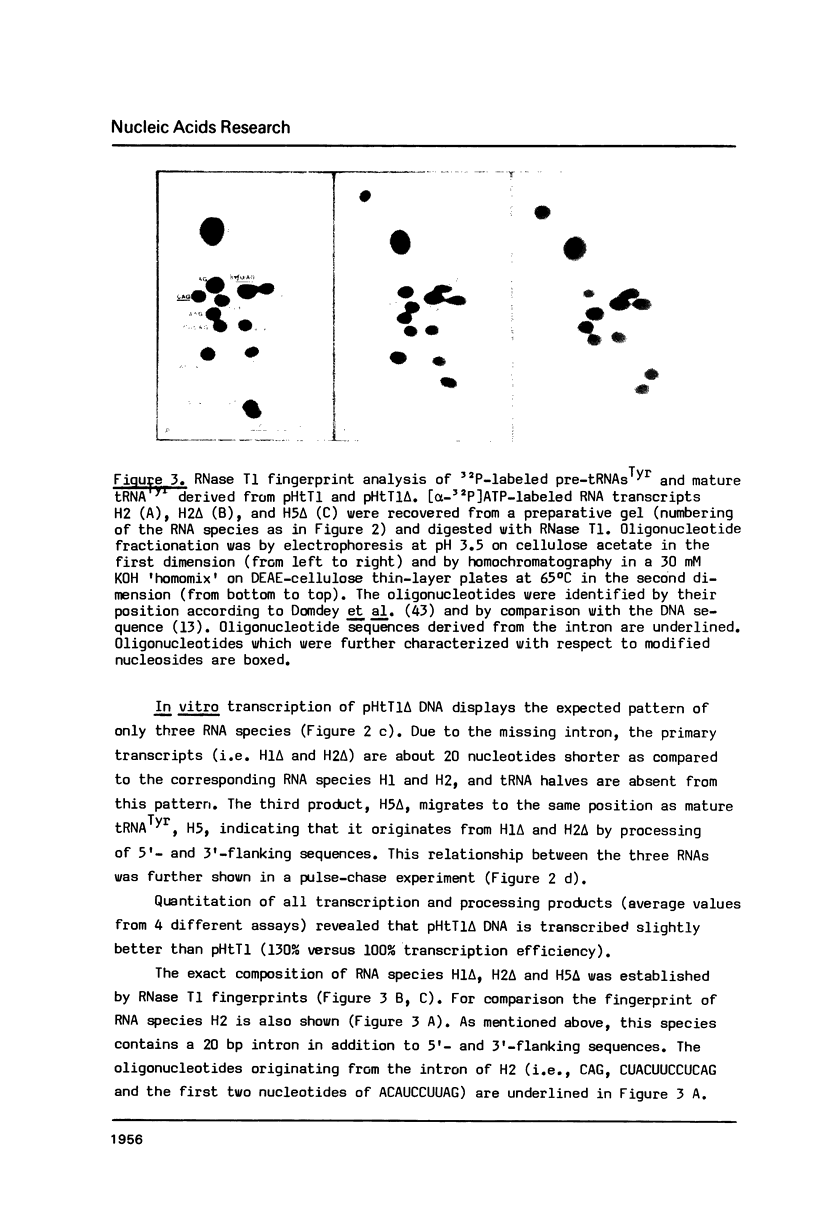
Two synthetic oligonucleotides, one specific for the 5' exon, the other spanning the splice junction, were used to show that (a) the human haploid genome contains at least 12 independent gene loci for tRNATyr, and (b) that all of them carry an intron. From one of the cloned human tRNATyr genes (pHtT1) the 20 bp intron was deleted to generate pHtT1 delta. Homologous in vitro transcription, fingerprint analyses of the products and elucidation of their nucleoside composition revealed that the pseudouridine (psi 35) in the center of the anticodon of tRNATyr was synthesized in the intron-containing precursor whereas this U to psi modification did not take place in precursors or mature tRNATyr derived from pHtT1 delta. On the basis of these results and of studies from other laboratories we suggest that the evolutionary pressure for maintaining introns in eukaryotic tRNAsTyr is this strict intron-requirement for psi 35 synthesis. Taking into account that all eukaryotic cytoplasmic tRNAsTyr contain a psi 35 we discuss here a special need for this modified nucleoside in stabilizing codon-anticodon interactions involving (a) classical base pairing upon translation of tyrosine codons and (b) unconventional interactions during UAG amber codon suppression by tRNATyrG psi A in eukaryotic cells.
Full text
PDF



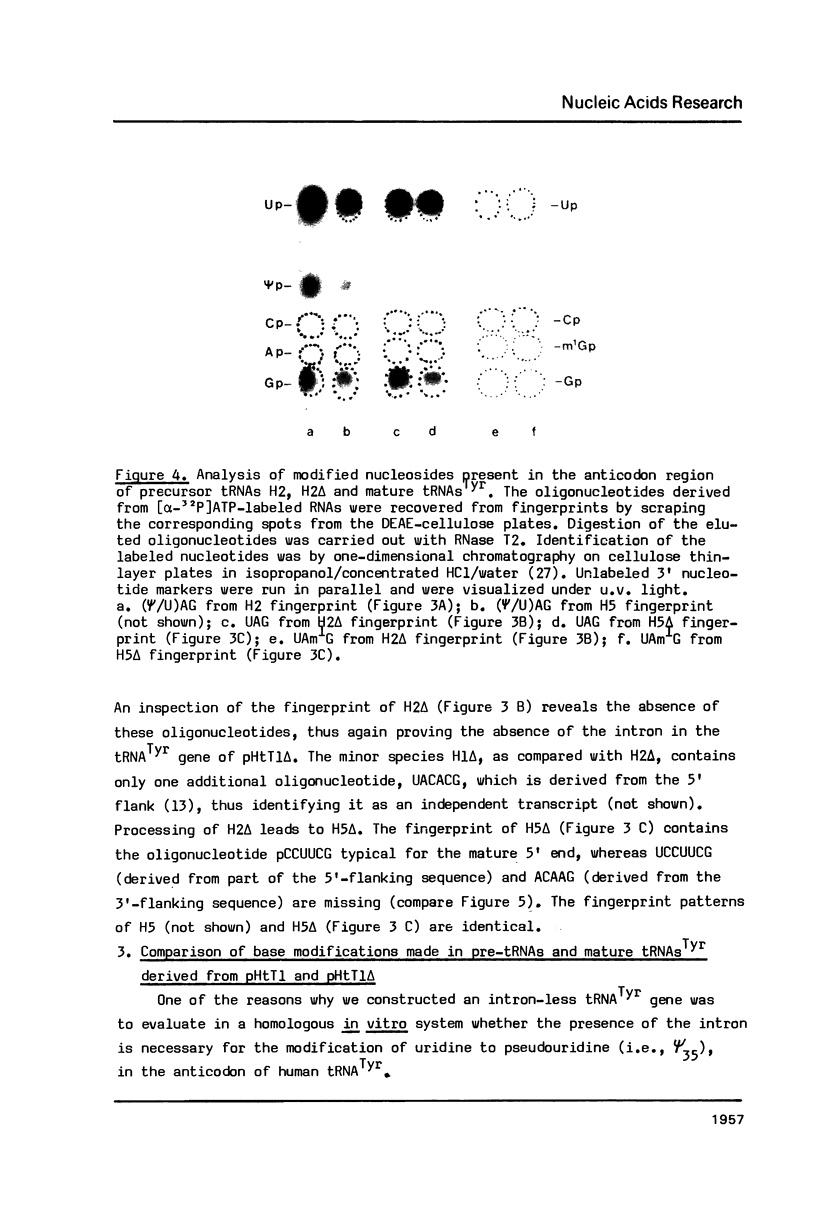

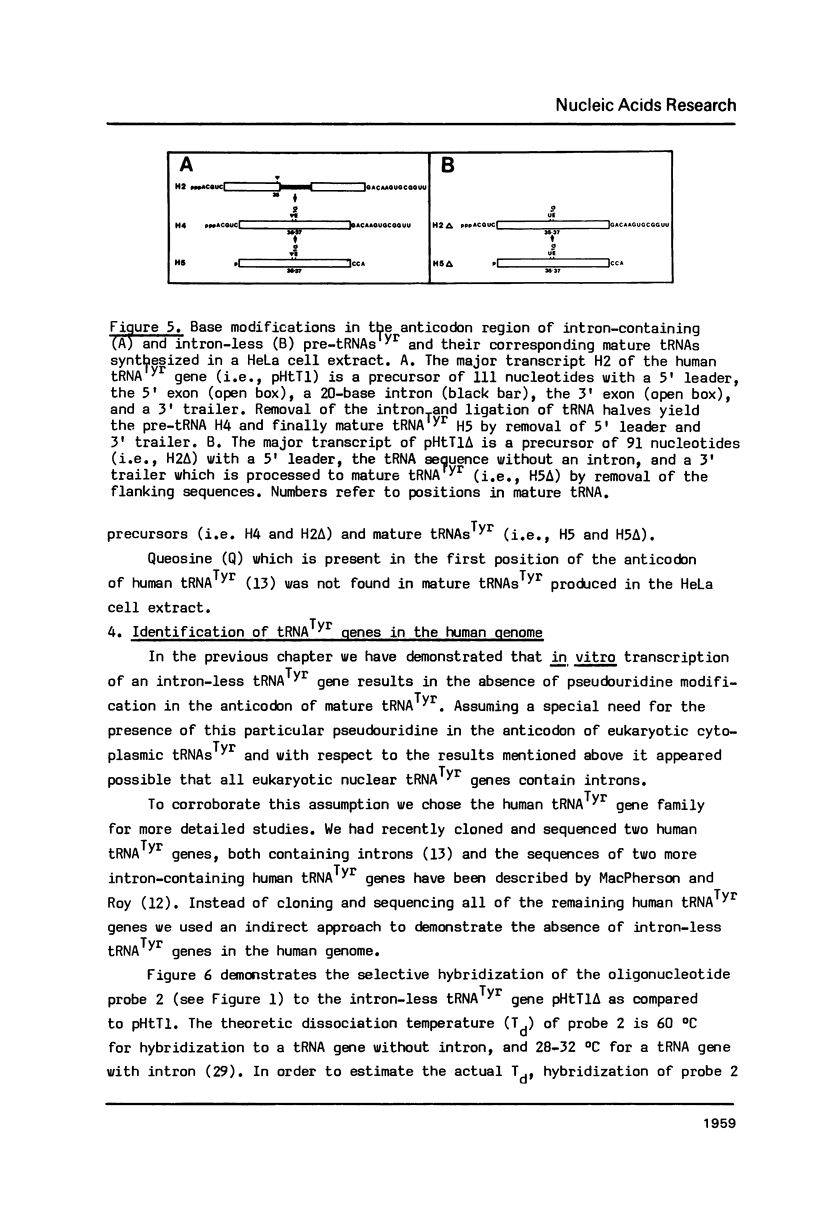







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Sickinger H. D. The molecular basis for the differential translation of TMV RNA in tobacco protoplasts and wheat germ extracts. EMBO J. 1984 May;3(5):1091–1096. doi: 10.1002/j.1460-2075.1984.tb01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Pan C. J., Marzluff W. F. Methylation of ribonucleic acid in a cell-free system from mouse myeloma cells. Biochemistry. 1982 Aug 31;21(18):4303–4310. doi: 10.1021/bi00261a020. [DOI] [PubMed] [Google Scholar]
- Colby D. S., Schedl P., Guthrie C. A functional requirement for modification of the wobble nucleotide in tha anticodon of a T4 suppressor tRNA. Cell. 1976 Nov;9(3):449–463. doi: 10.1016/0092-8674(76)90090-8. [DOI] [PubMed] [Google Scholar]
- Debuchy R., Brygoo Y. Cloning of opal suppressor tRNA genes of a filamentous fungus reveals two tRNASerUGA genes with unexpected structural differences. EMBO J. 1985 Dec 16;4(13A):3553–3556. doi: 10.1002/j.1460-2075.1985.tb04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domdey H., Jank P., Sänger L., Gross H. J. Studies on the primary and secondary structure of potato spindle tuber viroid: products of digestion with ribonuclease A and ribonuclease T1, and modification with bisulfite. Nucleic Acids Res. 1978 Apr;5(4):1221–1236. doi: 10.1093/nar/5.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghtedarzadeh M. K., Henikoff S. Use of oligonucleotides to generate large deletions. Nucleic Acids Res. 1986 Jun 25;14(12):5115–5115. doi: 10.1093/nar/14.12.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew L., Lo R., Reece T., Nichols M., Söll D., Bell J. The nucleotide sequence, localization and transcriptional properties of a tRNALeuCUG gene from Drosophila melanogaster. Gene. 1986;44(2-3):307–314. doi: 10.1016/0378-1119(86)90195-2. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilloud E., Clarkson S. G. A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem. 1986 Jan 5;261(1):486–494. [PubMed] [Google Scholar]
- Griffey R. H., Davis D., Yamaizumi Z., Nishimura S., Bax A., Hawkins B., Poulter C. D. 15N-labeled Escherichia coli tRNAfMet, tRNAGlu, tRNATyr, and tRNAPhe. Double resonance and two-dimensional NMR of N1-labeled pseudouridine. J Biol Chem. 1985 Aug 15;260(17):9734–9741. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hopper A. K., Banks F. A yeast mutant which accumulates precursor tRNAs. Cell. 1978 Jun;14(2):211–219. doi: 10.1016/0092-8674(78)90108-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Abelson J. The yeast tRNATyr gene intron is essential for correct modification of its tRNA product. Nature. 1983 Apr 21;302(5910):681–687. doi: 10.1038/302681a0. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Alzner-DeWeerd B., RajBhandary U. L., Sharp P. A. Expression of a X. laevis tRNATyr gene in mammalian cells. Nucleic Acids Res. 1982 Aug 11;10(15):4609–4626. doi: 10.1093/nar/10.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Fire A. Z., RajBhandary U. L., Sharp P. A. Characterization of tRNA precursor splicing in mammalian extracts. J Biol Chem. 1983 Oct 10;258(19):11974–11980. [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- MacPherson J. M., Roy K. L. Two human tyrosine tRNA genes contain introns. Gene. 1986;42(1):101–106. doi: 10.1016/0378-1119(86)90155-1. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Lee M. C., Knapp G. Transfer RNA splicing in Saccharomyces cerevisiae: defining the substrates. Nucleic Acids Res. 1984 Dec 21;12(24):9367–9382. doi: 10.1093/nar/12.24.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. V., Page G. S., Sentenac A., Piper P. W., Worthington M., Weiss R. B., Hall B. D. Only one of two closely related yeast suppressor tRNA genes contains an intervening sequence. Nature. 1981 Jun 11;291(5815):464–469. doi: 10.1038/291464a0. [DOI] [PubMed] [Google Scholar]
- Robinson R. R., Davidson N. Analysis of a drosophila tRNA gene cluster: two tRNALeu genes contain intervening sequences. Cell. 1981 Jan;23(1):251–259. doi: 10.1016/0092-8674(81)90289-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Meissner F., Moll J., Vorderwülbecke T. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1987;15 (Suppl):r53–188. doi: 10.1093/nar/15.suppl.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Beier H. A cell-free plant extract for accurate pre-tRNA processing, splicing and modification. EMBO J. 1987 Sep;6(9):2811–2818. doi: 10.1002/j.1460-2075.1987.tb02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Beier H. A gene for the major cytoplasmic tRNATyr from Nicotiana rustica contains a 13 nucleotides long intron. Nucleic Acids Res. 1986 Nov 11;14(21):8691–8691. doi: 10.1093/nar/14.21.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel M. C., Abelson J. Effect of intron mutations on processing and function of Saccharomyces cerevisiae SUP53 tRNA in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2663–2673. doi: 10.1128/mcb.6.7.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Stiles J. I., Tye B. K., Chiu P., Sherman F., Wu R. Hybridization with synthetic oligonucleotides. Methods Enzymol. 1979;68:419–428. doi: 10.1016/0076-6879(79)68031-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson P. F., Tanaka S., Schöld M., Itakura K., Abelson J. Directed deletion of a yeast transfer RNA intervening sequence. Science. 1980 Sep 19;209(4463):1396–1400. doi: 10.1126/science.6997991. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M., Mendenhall M. D., Cummins C. M., Culbertson M. R., Knapp G. Splicing of a yeast proline tRNA containing a novel suppressor mutation in the anticodon stem. J Mol Biol. 1986 Nov 5;192(1):49–63. doi: 10.1016/0022-2836(86)90463-8. [DOI] [PubMed] [Google Scholar]
- van Tol H., Stange N., Gross H. J., Beier H. A human and a plant intron-containing tRNATyr gene are both transcribed in a HeLa cell extract but spliced along different pathways. EMBO J. 1987 Jan;6(1):35–41. doi: 10.1002/j.1460-2075.1987.tb04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]