Abstract
Habenulo-interpeduncular nicotinic receptors, particularly those containing α3, β4 and α5 subunits, have recently been implicated in the reinforcing effects of nicotine. Our laboratory has shown that injection of α3β4 nicotinic receptor antagonists into the medial habenula (MHb) decreases self-administration of multiple abused drugs, including nicotine (Glick et al., 2006; 2008; 2011). However, it is unclear whether blockade of MHb nicotinic receptors has a direct effect on mesolimbic dopamine. Here, we performed in vivo microdialysis in female rats. Microdialysis probes were implanted into the nucleus accumbens (NAcc) and α3β4 nicotinic receptor antagonists (18-methoxycoronaridine; 18-MC or α-conotoxin AuIB; AuIB), were injected into the ipsilateral MHb, just prior to systemic nicotine (0.4 mg/kg, s.c.). Dialysate samples were collected before and after drug administration and levels of extracellular dopamine and its metabolites were measured using HPLC. Acute nicotine administration increased levels of extracellular dopamine and its metabolites in the NAcc. Pre-treatment with intra-habenular AuIB or 18-MC prevented nicotine-induced increases in accumbal dopamine. Neither drug had an effect on nicotine-induced increases in dopamine metabolites, suggesting that α3β4 receptors do not play a role in dopamine metabolism. The effect of intra-habenular blockade of α3β4 receptors on NAcc dopamine was selective for acute nicotine: neither AuIB nor 18-MC prevented increases in NAcc dopamine stimulated by acute d-amphetamine or morphine. These results suggest the mesolimbic response to acute nicotine, but not to acute administration of other drugs of abuse, is directly modulated by α3β4 nicotinic receptors in the MHb, and emphasize a critical role for habenular nicotinic receptors in nicotine’s reinforcing effects.
1. Introduction
Conventional targets for smoking cessation pharmacotherapy have historically been nicotinic receptors concentrated in the dopaminergic mesolimbic pathway, primarily α4β2* and α6β2* subtypes (Gotti et al. 2010; Picciotto et al. 1998; Rollema et al. 2007). Recently, however, converging evidence from both human genetic and animal studies has strongly implicated nicotinic receptor subunits such as α5, α3 and β4 in human nicotine dependence and nicotine reinforcement and withdrawal in laboratory animals (Bierut et al. 2008; Chatterjee et al. 2011; Fowler et al. 2011; Frahm et al. 2011; Glick et al. 2002; Salas et al. 2009; Struthers et al. 2009). These nicotinic receptor subunits are not found in great abundance in the mesolimbic dopamine tract, but high densities are concentrated in the medial habenula (MHb) and interpeduncular nucleus (IPN) which together comprise a major cholinergic tract in mammalian brain (Clarke et al. 1985; Grady et al. 2009; Mulle et al. 1991; Quick et al. 1999).
The habenulo-interpeduncular pathway has long been known to have indirect and direct influence on the mesolimbic dopamine system (Nishikawa et al. 1986; Sutherland 1982). Yet, while the circuitry between mesolimbic structures and the lateral habenula (LHb) has been well-characterized, strongly implicating the LHb in negative reward processes (Matsumoto and Hikosaka 2007), the MHb is both pharmacologically and anatomically distinct from the LHb (Bianco and Wilson 2009). As a result, the potential interactions between the MHb and the conventional reward circuitry in the brain is much less understood. There is some evidence from anatomical studies that the MHb and IPN may directly or indirectly interact with the ventral tegmental area (VTA) (Herkenham and Nauta 1977; Kim and Chang 2005; Phillipson and Pycock 1982), but little is known about how the MHb and IPN interact functionally with the VTA or other mesolimbic structures.
Our laboratory has been investigating the role of 18-methoxycoronaridine (18-MC), a potent antagonist of α3β4 receptors, in addiction to nicotine and other drugs of abuse (Glick et al. 1996). 18-MC is an allosteric inhibitor of α3β4 receptors (IC50 = 0.75 µM) with no activity at α4β2 receptors (Pace et al. 2004). When injected either systemically or directly into the MHb, 18-MC decreases the self-administration of multiple drugs of abuse, including nicotine (Glick et al. 2006; Glick et al. 2008; Glick et al. 2011). In addition, α-conotoxin AuIB, a selective α3β4 antagonist (Luo et al. 1998), has an identical effect on drug self-administration when injected into the MHb (Glick et al. 2011). Importantly, neither 18-MC nor AuIB decreases drug self-administration when delivered into the VTA, a primary component of the mesolimbic reward pathway (Glick et al. 2006; Glick et al. 2008; Glick et al. 2011).
Recent studies have provided compelling evidence that nicotinic receptors in the MHb-IPN pathway regulate nicotine reinforcement, dependence and withdrawal (Fowler et al. 2011; Frahm et al. 2011; Glick et al. 2011; Salas et al. 2009). The goal of the present study was to determine whether modulation of nicotinic receptors in the MHb has an effect on the mesolimbic dopamine response to acute nicotine. To accomplish this, we measured changes in extracellular dopamine levels in the nucleus accumbens using in vivo microdialysis.
2. Material and methods
2.1 Animals
Naïve female Sprague–Dawley rats (250–275 g; Taconic, Germantown, NY, USA) were housed individually in a colony room on a 12-h light cycle (lights on at 7 a.m.). All animals were allowed free access to normal chow and water. The experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996).
2.2 Surgery
As performed in previously published studies (Maisonneuve and Glick 1999; Taraschenko et al. 2007), rats were implanted with one 22-gauge microinjection guide cannula (Plastics One, Roanoke, VA, USA) over the MHb, and one microdialysis guide cannula (CMA Microdialysis, North Chelmsford, MA, USA) over the ipsilateral NAcc. Left and right placement of the cannulae was alternated from rat to rat. Stereotaxic coordinates were determined according to Paxinos and Watson (1986) such that the tip of the injector was located in the MHb (in mm from Bregma: AP = −4.2; ML = ± 2.9; DV = −5.0 using a 24° angle) and the microdialysis probe was aimed at the NAcc (AP = +1.6; ML = ± 3.1; DV = −4.2 using a 14° angle). Rats were monitored daily and were allowed 3–5 days to recover before microdialysis experiments.
2.3 In vivo microdialysis
Animals were placed in the dialysis chamber on the afternoon before the experiment and a CMA dialysis probe (inner diameter, 0.5 mm; length, 2 mm; membrane, polycarbonate; cutoff, 20,000 Da) was inserted through the guide cannula. The probe was perfused with artificial cerebrospinal fluid (ACSF; 146 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2) overnight at a flow rate of 1 µl/min. The collection of brain perfusates began the following morning. Samples were collected in tubes containing 2 µl of 1.1 N perchloric acid solution (containing 1.4 mM EDTA and 2.8 mM sodium metabisulfite). After five, 20-minute baseline samples were collected, animals received an intra-habenular injection of 18-MC, AuIB, or each respective vehicle, followed 20 minutes later by an acute, subcutaneous (s.c.) injection of nicotine (0.4 mg/kg or vehicle). The dose of 0.4 mg/kg nicotine was selected as it typically elicits robust increases in accumbal dopamine overflow (Cadoni and Di Chiara 2000; Dong et al. 2010). Intra-habenular injections were administered at 1 ul over 1 minute using a 10 µl Hamilton microsyringe. To prevent backflow, the injection cannula (26 gauge) was held in place for an additional minute. Following the acute injection of vehicle or nicotine, dialysate samples were collected every 20 minutes for 3 hr.
2.3.1 Analytical procedure
The dialysate samples were analyzed for dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) using high-performance liquid chromatography (HPLC). The HPLC system with electrochemical detection was comprised of an ESA 540 autosampler (ESA, North Chelmsford, MA, USA), an ESA solvent delivery unit, an ESA column (MD-150/RP-C18; 3 µm diameter), and an ESA electrochemical detector (Coulochem II) with an ESA 5020 guard cell and an ESA 5014B analytical cell. The glassy carbon working electrode was set at a potential of 300 mV with respect to the reference electrode. The MD-TM mobile phase (ESA) was comprised of 0.075 µM sodium dihydrogenphosphate, monohydrate, 0.0017 µM 1-octanesulfonic acid, 25 µM EDTA in 10% HPLC grade acetonitrile, adjusted to a pH of 3.0 with phosphoric acid. Mobile phase was pumped at a rate of 0.53 ml/minute. Chem Station Plus software (Agilent Technologies, Wilmington, DE, USA) was used to analyze chromatograms.
2.3.2 In vitro recovery of dopamine
The afternoon prior to the microdialysis experiment, probes were placed in a standard solution containing dopamine (14 nM) and flushed with ACSF at 1 µL/minute for 20 minutes. To calculate in vitro recovery, dopamine was calculated as a percentage of the concentration in the standard. The mean recovery of dopamine obtained from the 2 mm probes was 26.3 ± 2.3%.
2.4 Histology
Following each experiment, animals were euthanized with an injection of sodium pentobarbital (50 mg/kg) and brains were rapidly removed and frozen at −80°C, then sectioned (25 µm) using a cryostat. Placement of injector cannulae and microdialysis probes were determined to be in the MHb or NAcc, using a rat brain atlas (Paxinos and Watson 1986) (Figure 1). Animals with injector cannulae outside the MHb or microdialysis probe cannulae that did not reach the NAcc shell were excluded from the study. In total, 11 animals were removed from the study: 8 were excluded due to injector cannulae being outside the MHb and 3 were excluded due to faulty microdialysis probes.
Figure 1. Location of injector cannulae (A) and microdialysis probes (B) in the MHb and NAcc, respectively.
Injector cannulae and microdialysis guide cannulae were implanted ipsilaterally; placement (right or left) was alternated from rat to rat. Injector and probe placements were examined in brain sections and verified to be in the MHb (between 4.30 and 3.80 mm posterior to bregma) or the NAcc (between 1.20 mm and 1.70 mm anterior to bregma) according to the rat brain atlas (Paxinos and Watson, 1986). Data from 8 animals were discarded due to injector cannulae implanted outside of the MHb; an additional 3 animals were excluded due to malfunctioning and/or misplaced microdialysis probes. Abbreviations: LHbL/LHbM = lateral habenula; D3V = third ventricle; MHb = medial habenula; aca = anterior commissure; AcbSh = nucleus accumbens shell; Acbc = nucleus accumbens core.
2.5 Drugs
Nicotine bitartrate (NIC; 0.4 mg/kg; dose expressed as free base) was dissolved in sterile saline, neutralized with 0.1 N NaOH and injected subcutaneously at a volume of 1 ml/kg (s.c.). D-Amphetamine was dissolved in sterile saline and injected intraperitoneally (i.p.). 18-Methoxycoronaridine (18-MC; Obiter Research, Champaign, IL, USA) was dissolved in a solution of 50% DMSO and injected in a 1 µl volume. Alpha-conotoxin AuIB (AuIB; generously provided by Dr. J. Michael McIntosh, University of Utah) was dissolved in ACSF and injected in a 1 µl volume. Unless otherwise specified, drugs were obtained from Sigma-Aldrich, St. Louis, MO, USA).
2.6 Statistical analysis
Basal extracellular concentrations of dopamine, DOPAC and HVA (expressed as pmol per µl) were analyzed separately for each pre-treatment drug (18-MC or AuIB) using repeated measures analysis of variance (ANOVA) with drug dose as the independent variable and time as the repeated measures variable. Provided that basal concentrations of dopamine and its metabolites were equivalent across treatment groups, levels of dopamine, DOPAC and HVA were then expressed as percents of the respective baseline means for all subsequent analyses. Repeated measures ANOVAs were used to analyze differences in data expressed as percent of baseline, with drug dose as the independent variable and time as the repeated measures variable. Significant repeated measures ANOVAs were followed by Bonferroni post-hoc comparisons of samples collected following drug injection.
3. Results
3.1 18-MC treatment effects
3.1.1 Basal levels of dopamine and its metabolites
Among the 18-MC-treated rats, there were no significant differences in basal concentrations of dopamine among treatment groups (F (5, 33) = 1.91, p > 0.05, using repeated measures ANOVA). Within this group of animals, mean basal dopamine was 0.02 ± 0.004 pmol/10 µl (mean ± SEM; n = 39). The mean basal levels of accumbal DOPAC and HVA in the same animals were 15.59 ± 0.17 and 8.63 ± 0.11 pmol/ 10 µl, respectively. When evaluated using repeated measures ANOVA, there were no differences in basal concentrations of the metabolites among treatment groups. For DOPAC, there was no significant main effect of group (F (5, 32) = 0.82, p > 0.05). Similarly, repeated measures ANOVA revealed no significant differences in basal levels of HVA in 18-MC-treated rats (Group effect: F (5, 32) = 1.37, p > 0.05).
3.1.2 Effect of intra-habenular 18-MC on nicotine-induced increases in accumbal dopamine
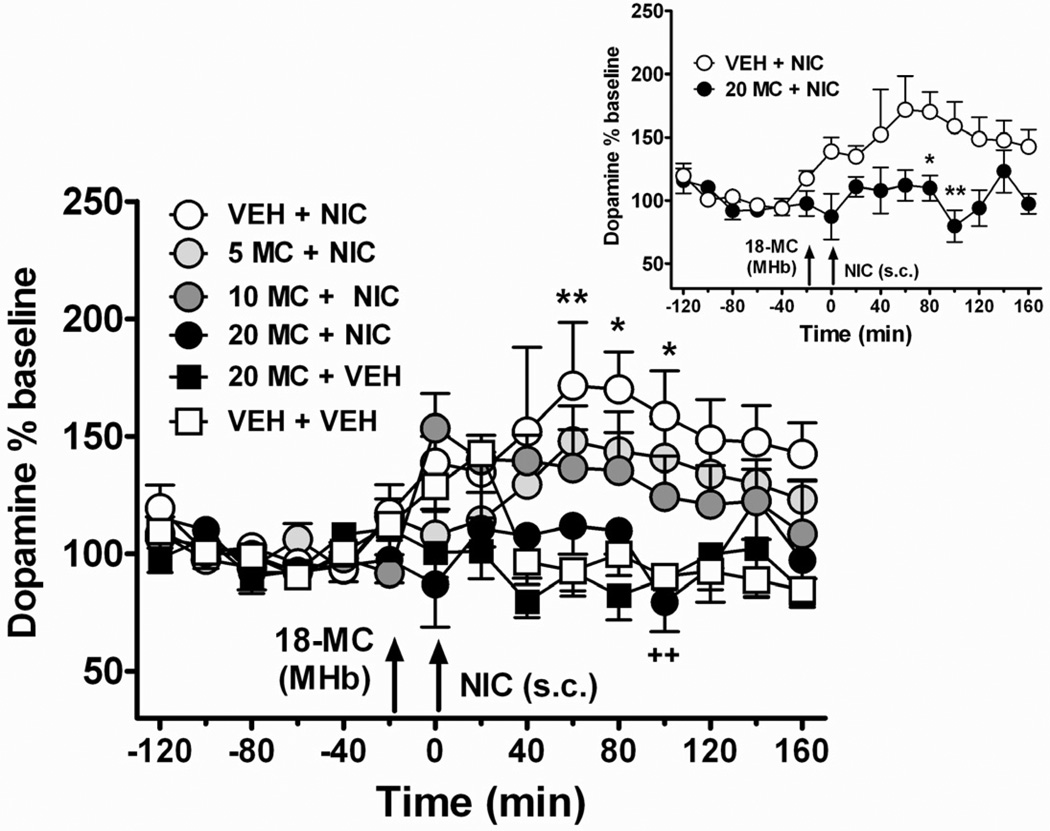
In this experiment, rats were given an intra-habenular injection of vehicle (50 % DMSO) or 18-MC (5, 10, 20 µg/µl), 20 minutes prior to an acute, systemic injection of nicotine (0.4 mg/kg; s.c.) or vehicle (saline). As indicated in Figure 2, acute nicotine administration significantly increased extracellular levels of dopamine in the NAcc in animals pre-treated with vehicle (Time effect: F (14, 168) = 2.62, p < 0.01). In this group of rats (VEH + NIC), extracellular dopamine increased by approximately 160% of baseline levels.
Figure 2. Intra-habenular pretreatment with 18-MC attenuates increases in accumbal extracellular dopamine following acute nicotine injection.
After collection of five stable, baseline samples, animals were given an intra-habenular injection of 18-MC or vehicle (at t = −20 minutes), followed by a systemic injection of nicotine or vehicle at 0 minutes (see arrows). Nicotine (0.4 mg/kg, s.c.) itself increased extracellular dopamine in the NAcc (An * indicates p < 0.05; ** indicates p < 0.01, VEH + NIC group compared to VEH + VEH group). Animals were pre-treated with an injection of either vehicle or 18-MC (5, 10, 20 µg/µl) into the MHb. 18-MC dose-dependently decreased nicotine-induced elevations in extracellular dopamine. This was significant at the highest dose of 18-MC tested, 20 µg/µl (20 MC + NIC group). ++ indicates a significant decrease in extracellular dopamine in rats treated with 20 MC + NIC (p < 0.01 compared to VEH+ NIC group). INSET: direct comparison of VEH+NIC and 20 MC+NIC groups: (* indicates p < 0.05; ** indicates p < 0.01, Bonferroni post-hoc tests following repeated measures ANOVA; n = 6–7 per group).
Intra-habenular injection with 18-MC (5, 10, 20 µg/µl) prior to nicotine injection dose-dependently attenuated increases in extracellular dopamine elicited by acute nicotine (Figure 2; main effect of Group F (5, 33) = 6.88, p < 0.01; Group by Time interaction F (40, 264) = 1.17, p > 0.05. The highest dose of 18-MC, 20 µg/µl, significantly decreased elevations in extracellular dopamine elicited by acute nicotine (Figure 2, inset). Notably, this dose of 18-MC had no effect on accumbal dopamine levels when administered into the MHb prior to a vehicle injection (F (1, 11) = 0.98, p > 0.05 compared to vehicle group).
3.1.3 Effect of intra-habenular 18-MC on nicotine-induced increases in dopamine metabolism
In addition to changes in extracellular dopamine, the metabolites DOPAC and HVA were also measured in dialysates using HPLC. As shown in Figure 3, acute nicotine elicited increases in dopamine metabolism in rats pretreated with vehicle (VEH + NIC group), as indicated by corresponding increases in both DOPAC and HVA (DOPAC Time effect: F (8, 256) = 11.07, p < 0.01; HVA Time effect = F (8, 256) = 11.07, p < 0.01). In this group of rats (VEH + NIC group), nicotine increased extracellular DOPAC to 133% above basal levels. Similarly, extracellular HVA was increased following nicotine injection, to 161% of baseline (Figure 3). We next examined nicotine-induced changes in accumbal levels of DOPAC and HVA following pretreatment with intra-habenular 18-MC (5, 10, 20 µg/µl). Unlike its effects on nicotine-induced increases in extracellular dopamine, 18-MC pretreatment did not affect nicotine elicited elevations in DOPAC at any dose of 18-MC tested (no significant effect of Group: F (3, 22) = 1.69, p > 0.05). Similarly, infusion of 18-MC into the MHb had no effect on nicotine-induced increases in HVA (no significant effect of Group: F (3, 22) = 1.79, p > 0.05).
Figure 3. Intra-habenular injection of 18-MC has no effect on nicotine induced increases in dopamine metabolism in the NAcc.
18-MC (5, 10, 20 µg/µl) or vehicle was injected into the MHb 20 minutes prior to an acute injection of nicotine (0.4 mg/kg; s.c.) or vehicle (arrows indicate intra-habenular injection of 18-MC or vehicle at −20 minutes, followed by systemic injection of nicotine or vehicle at 0 minutes). Nicotine increased extracellular NAcc levels of both DOPAC (Panel A) and HVA (Panel B; ** indicates p < 0.01 Bonferroni post-hoc test following repeated measures ANOVAs; n = 5–7 per group). However, there was no effect of 18-MC pretreatment on increases in extracellular DOPAC or HVA (p > 0.05 18-MC groups compared to VEH + NIC group).
3.2 AuIB treatment effects
3.2.1 Basal levels of dopamine and its metabolites
Among the animals in the experiments using AuIB, there were no differences in basal dopamine levels (main effect of group: F (3, 20) = 0.71, p > 0.05). In this group of animals (n = 24), the mean (± SEM) concentration of dopamine was 0.028 pmol/10 µl ± 0.001. The mean basal levels of DOPAC and HVA were 18.22 ± 0.13 and 9.19 ± 0.21 pmol/10 µl, respectively, in AuIB-treated rats. When evaluated using repeated measures ANOVA, there were no differences in basal concentrations of the metabolites among treatment groups. For DOPAC, there was no significant main effect of group (F (3, 18) = 0.71; p > 0.05). Likewise, treatment groups did not differ with respect to basal levels of HVA (F (3, 20) = 0.49, p > 0.05).
3.2.2 Effects of AuIB on nicotine-induced increases in accumbal DA overflow
After basal concentrations of dopamine and its metabolites were not found to differ, the effects of intra-habenular AuIB on nicotine-induced increases in accumbal dopamine were examined. As in the experiments with 18-MC above, rats were given an intra-habenular injection of AuIB (25 pmol/ µl) or vehicle (ACSF) 20 minutes prior to an acute, systemic injection of nicotine (0.4 mg/kg; s.c.) or vehicle (saline). As indicated in Figure 4, acute nicotine significantly increased extracellular levels of dopamine in the NAcc in rats pretreated with vehicle (main effect of Time: F (14, 168) = 3.35, p < 0.01). The increase in dopamine was approximately 172% of baseline in vehicle pretreated rats given acute nicotine (VEH + NIC group).
Figure 4. Intra-habenular AuIB blocks increases in accumbal extracellular dopamine elicited by acute nicotine but does not affect nicotine-induced increases in dopamine metabolism.
AuIB (25 pmol/ µl) or vehicle was administered into the MHb, followed by an acute injection of nicotine (0.4 mg/kg; s.c.) or vehicle, at −20 minutes and 0 minutes, respectively, as indicated by arrows. Panel A: Acute nicotine increased extracellular dopamine in the NAcc (** indicates significant difference from VEH+VEH group; p < 0.01; n = 5–8 per group). AuIB, injected into the MHb prior to nicotine treatment, completely blocked increases in accumbal dopamine elicited by systemic nicotine (++ indicates significant difference between VEH+NIC and AuIB + NIC groups; p < 0.01). Panel B: AuIB did not affect increases in extracellular DOPAC following a systemic nicotine injection. Panel C: Similarly, AuIB did not block increases in extracellular HVA elicited by acute nicotine.
Next, we examined the effect of pretreatment with AuIB on nicotine-induced increases in extracellular dopamine in the NAcc. Repeated measures ANOVA revealed a significant effect of treatment group (F (1, 12) = 8.29, p < 0.01), indicating that AuIB (25 pmol), injected into the MHb prior to nicotine administration, prevented nicotine-induced increases in extracellular dopamine (Figure 4). This dose of AuIB did not itself have an effect on dopamine levels (no main effect of treatment group comparing VEH-VEH with AUIB-VEH: (F (1, 8) =1.45, p > 0.05).
3.2.3 Effect of intra-habenular AuIB on nicotine-induced increases in dopamine metabolism
As shown in Figure 4, acute nicotine treatment increased dopamine metabolism. Extracellular DOPAC rose to 122% of baseline, which was a significant increase above basal concentrations (Time effect: F (14,154) = 2.30; p < 0.01). Similar increases in HVA were observed with acute nicotine treatment (141% of baseline; F (14,168) = 3.94; p < 0.01. When AuIB was injected into the MHb prior to nicotine treatment, it had no effect on nicotine-induced increases in extracellular DOPAC or HVA (DOPAC: Group effect: F (1,10) = 0.62; p > 0.05; HVA Group effect: F (1,12) = 0.01; p >0.05).
3.3 Effect of intra-habenular injection of α3β4 nicotinic receptor antagonists on increases in accumbal extracellular dopamine elicited by other drugs of abuse
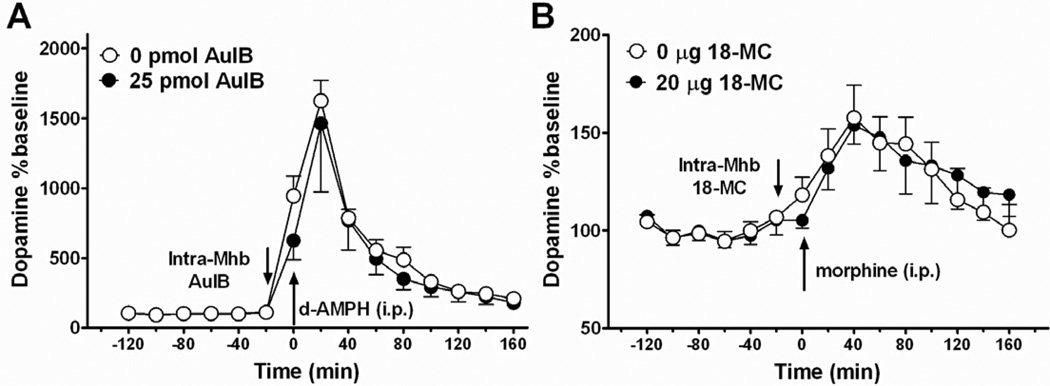
As indicated in Figure 5, when AuIB was administered into the MHb twenty minutes prior to a systemic injection of d-amphetamine, it did not prevent d-amphetamine-induced increases in accumbal dopamine (F (1,14) = 0.22; p > 0.05). As expected, d-amphetamine (1 mg/kg; i.p.) caused a marked increase in extracellular dopamine levels in the NAcc, elevating dopamine to approximately 1600% of baseline levels. Pretreatment with AuIB prior to d-amphetamine injection resulted in dopamine levels that were approximately 1460% of baseline. This lack of effect on acute d-amphetamine is in agreement with previous data showing intra-habenular infusion of 18-MC does not affect increases in dopamine elicited by acute morphine injection (Taraschenko et al. 2007).
Figure 5. Nicotinic α3β4 receptor blockade in the MHb does not affect increases in accumbal dopamine elicited by acute administration of other drugs of abuse.
A: Rats (n = 4 per group) were given an intra-habenular injection of AuIB or vehicle 20 min prior to an acute i.p, injection of d-amphetamine (1 mg/kg). d-amphetamine administration caused a robust increase in extracellular dopamine in the NAcc; this was not attenuated by AuIB pretreatment. B: 18-MC did not have an effect on extracellular dopamine levels in the NAcc following an acute injection of morphine (5 mg/kg., i.p.; 18-MC data were re-plotted from Fig. 7 of Taraschenko et al., 2007; n = 5–7 per group).
4. Discussion
Here we show that habenular blockade of nicotinic receptors modulates the mesolimbic dopamine response to acute nicotine. Intra-habenular injection of the α3β4 nicotinic receptor antagonists 18-MC and α-conotoxin AuIB blocked nicotine-induced increases in extracellular dopamine in the NAcc. These data provide further evidence that the habenulo-interpeduncular system interacts with the mesolimbic dopamine pathway. Recently, we demonstrated that intra-habenular injection of either 18-MC or AuIB decreases intravenous nicotine self-administration (Glick et al. 2011). The present results using in vivo microdialysis complement these results, and further suggest a critical role for nicotinic receptors in the MHb in nicotine reinforcement (Fowler et al. 2011; Frahm et al. 2011).
Recently, nicotinic receptor subunits in the MHb (including α3, α5 and β4 subunits) have been implicated in nicotine reinforcement, dependence and withdrawal (Fowler et al. 2011; Frahm et al. 2011; Glick et al. 2011; Salas et al. 2009). Moreover, genome wide association studies in humans have identified polymorphisms in chromosome 15 (housing the α5, α3 and β4 subunit genes) that are associated with increased risk for nicotine dependence and lung cancer (Bierut et al. 2008; Hung et al. 2008). A variety of nicotinic receptor subunits have been identified in the MHb-IPN tract but the majority of receptors in the MHb appear to be α3β4 nicotinic receptors (Grady et al. 2009; Quick et al. 1999). There is a possibility that intra-habenular 18-MC may be acting at multiple nicotinic receptor subtypes other than α3β4. While 18-MC has a 25-fold greater selectivity for α3β4 receptors (IC50 = 0.75 µM) than for α4β2 receptors, its activity at several other receptor subtypes, including those containing α5, α6 or β3 subunits, is unknown. Although 18-MC binds with low affinity (1 to 5 µM) to all three opioid receptors (Glick et al. 1999), it does not share in vivo effects characteristic of morphine and other opioid agonists. For instance, unlike morphine (1–2 nM affinity at mu opioid receptors), 18-MC has no analgesic properties (Hough et al., unpublished data), nor does it affect respiration or blood pressure (Glick et al., 1999), both of which are decreased by morphine. Moreover, it seems likely that the effect of 18-MC observed here is due to α3β4 nicotinic receptor blockade since an identical effect was seen using the selective α3β4 receptor antagonist α-conotoxin AuIB (Luo et al. 1998).
While habenular α3β4 receptor antagonism appears to reduce both nicotine self-administration (Glick et al. 2011) and nicotine-evoked increases in accumbal dopamine (present study), there are known differences between acute and chronic nicotine administration on mesolimbic dopamine (Benwell and Balfour 1992). Therefore, acute administration of nicotine may not affect mesolimbic dopamine in the same way as would chronic, self-administered nicotine. For example, decreases in NAcc dopamine overflow are detected when measured directly after chronic nicotine self-administration (Rahman et al. 2004). Further research is necessary to determine whether α3β4 receptor blockade in the MHb similarly affects mesolimbic dopamine during nicotine self-administration.
The experiments here described here pertain to α3β4 nicotinic receptor antagonist effects on acute drug. While nicotine’s effects were antagonized, we also demonstrated that there was no effect of habenular α3β4 receptor blockade on accumbal dopamine increases stimulated by acute injection of d-amphetamine. Taraschenko et al (2007) showed a similar lack of effect of intra-habenular 18-MC on increases in extracellular dopamine evoked by acute morphine, and Szumlinksi et al. (2000) showed a similar lack of effect of systemic 18-MC on increases in extracellular dopamine evoked by acute cocaine. Thus, blocking α3β4 receptors in the MHb does not appear to antagonize increases in accumbal dopamine evoked by acute administration of drugs other than nicotine. Not surprisingly, 18-MC more potently blocks the effects of nicotine than it does other drugs of abuse (Glick et al. 2000). This difference may occur because acute nicotine directly stimulates nicotinic receptors in both the MHb-IPN pathway and the mesolimbic dopamine pathway. Interactions with other drugs such as opioids and stimulants may only occur as a result of modulation of the mesolimbic pathway by the MHb-IPN, and/or after a chronic or sensitizing regimen of drug administration. This is evidenced by the fact that, while they are ineffective on acute drug responses, both 18-MC and AuIB, when injected into the MHb, decrease the chronic self-administration of methamphetamine (Glick et al. 2008) and morphine (Glick et al. 2006). Furthermore, intra-habenular 18-MC blocks the sensitized (but not the acute) response to morphine (Taraschenko et al. 2007), and systemic 18-MC blocks the sensitized (but not the acute) response to cocaine (Szumlinski et al. 2000).
In these experiments, nicotine increased dopamine metabolism in the NAcc, as observed by robust increases in both DOPAC and HVA, similar to previous experiments using acute nicotine (Benwell and Balfour 1992; Brazell et al. 1990; Janhunen and Ahtee 2004; Salminen et al. 1999; Toth et al. 1992). However, 18-MC and AuIB blocked nicotine induced increases in extracellular dopamine in the NAcc without affecting corresponding increases in dopamine metabolism. Similar results have been reported using systemic 18-MC (Glick et al. 1998). The nonselective nicotinic receptor antagonist mecamylamine has been reported to block nicotine-induced increases in both dopamine and its metabolites in vivo (Hildebrand et al. 1998; Janhunen et al. 2005; Seppa et al. 2000). A similar blockade of dopamine metabolism occurs using the selective α6β2* antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide (Rahman et al. 2008), but does not occur with the α4*-selective antagonist dihydro-β-erythroidine (Seppa et al., 2000). A conclusion can be reached that nicotinic receptors differ in their ability to modulate dopamine metabolism. These differences do not appear to be related to the mechanism of receptor blockade, but are more likely related to receptor subunit composition and/or location. For instance, α6β2* receptors, which are abundant in the mesolimbic pathway, including on dopamine cell bodies in the VTA (Gotti et al. 2010), may preferentially affect dopamine metabolism. This corresponds to an early study of the striatum (Leikola-Pelho et al. 1990) that observed that different nAChRs were involved in regulating dopamine metabolism compared to dopamine release. Our results suggest that α3β4 receptors, at least those in the MHb, do not play a role in dopamine metabolism.
In conclusion, we have demonstrated here that α3β4 receptor modulation in the MHb has a direct, albeit distal, effect on mesolimbic dopamine output. While it is still unclear precisely how nicotinic receptors in the habenulo-interpeduncular pathway modulate the mesolimbic dopamine pathway, it is likely that the MHb plays a larger role in mediating nicotine’s effects on dopamine than does the IPN. For instance, while α3β4 receptor blockade in the MHb decreases nicotine self-administration, blockade of α3β4 receptors in the IPN tends to increase self-administration (Glick et al., 2011), as does lidocaine inactivation of the IPN (Fowler et al. 2011). Anatomical studies have identified neurons in the MHb that project to the VTA and NAcc (Geisler and Zahm 2005; Phillipson and Pycock 1982), as well as a unilateral projection from the MHb to the LHb (Kim and Chang 2005), which in turn sends projections to and from the VTA (Christoph et al. 1986; Herkenham and Nauta 1977; Omelchenko et al. 2009). Therefore, it is possible that nicotine acts in the MHb to inhibit the LHb, and blockade of α3β4 nicotinic receptors in the MHb removes this inhibition. While further research is necessary to identify the specific mechanism(s) by which the MHb influences the mesolimbic dopamine pathway, our results support the utility of targeting α3β4 nicotinic receptors in the MHb for the development of smoking cessation pharmacotherapies.
Tested nicotinic α3β4 antagonists using in vivo microdialysis
Acute nicotine increased extracellular dopamine in the nucleus accumbens
Intra-habenular injection of α3β4 nicotinic antagonists blocked this effect
Habenular nicotinic receptors modulate accumbal dopamine in vivo
Acknowledgment
This study was supported by NIDA grant DA 016283.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah E. McCallum, Center for Neuropharmacology and Neuroscience, Albany Medical College, Albany, NY.
Matthew A. Cowe, Center for Neuropharmacology and Neuroscience, Albany Medical College, Albany, NY
Samuel W. Lewis, Center for Neuropharmacology and Neuroscience, Albany Medical College, Albany, NY
Stanley D. Glick, Center for Neuropharmacology and Neuroscience, Albany Medical College, Albany, NY, glicks@mail.amc.edu.
References
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazell MP, Mitchell SN, Joseph MH, Gray JA. Acute administration of nicotine increases the in vivo extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid and ascorbic acid preferentially in the nucleus accumbens of the rat: comparison with caudate-putamen. Neuropharmacology. 1990;29:1177–1185. doi: 10.1016/0028-3908(90)90042-p. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur J Pharmacol. 2000;387:R23–R25. doi: 10.1016/s0014-2999(99)00843-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE. Partial agonists of the alpha3beta4* neuronal nicotinic acetylcholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology. 2011;36:603–615. doi: 10.1038/npp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhang T, Li W, Doyon WM, Dani JA. Route of nicotine administration influences in vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J Mol Neurosci. 2010;40:164–171. doi: 10.1007/s12031-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Hough LH, Kuehne ME, Bandarage UK. (+/−)-18-Methoxycoronaridine: a novel iboga alkaloid congener having potential anti-addictive efficacy. CNS Drug Reviews. 1999;5:27–42. [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur J Pharmacol. 2002;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Visker KE, Fritz KA, Bandarage UK, Kuehne ME. 18-Methoxycoronardine attenuates nicotine-induced dopamine release and nicotine preferences in rats. Psychopharmacology (Berl) 1998;139:274–280. doi: 10.1007/s002130050716. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur J Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. Eur J Pharmacol. 2008;599:91–95. doi: 10.1016/j.ejphar.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol. 2011;669:71–75. doi: 10.1016/j.ejphar.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Janhunen S, Ahtee L. Comparison of the effects of nicotine and epibatidine on the striatal extracellular dopamine. Eur J Pharmacol. 2004;494:167–177. doi: 10.1016/j.ejphar.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Janhunen S, Mielikainen P, Paldanius P, Tuominen RK, Ahtee L, Kaakkola S. The effect of nicotine in combination with various dopaminergic drugs on nigrostriatal dopamine in rats. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:480–491. doi: 10.1007/s00210-005-1066-2. [DOI] [PubMed] [Google Scholar]
- Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- Leikola-Pelho T, Heinamaki J, Laakso I, Ahtee L. Chronic nicotine treatment changes differentially the effects of acute nicotine on the three main dopamine metabolites in mouse striatum. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:400–406. doi: 10.1007/BF00169456. [DOI] [PubMed] [Google Scholar]
- Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM. alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci. 1998;18:8571–8579. doi: 10.1523/JNEUROSCI.18-21-08571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. Eur J Pharmacol. 1999;383:15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Mulle C, Vidal C, Benoit P, Changeux JP. Existence of different subtypes of nicotinic acetylcholine receptors in the rat habenulo-interpeduncular system. J Neurosci. 1991;11:2588–2597. doi: 10.1523/JNEUROSCI.11-08-02588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. Eur J Pharmacol. 2004;492:159–167. doi: 10.1016/j.ejphar.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd edn. Academic Press, Academic Press; 1986. [Google Scholar]
- Phillipson OT, Pycock CJ. Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Some implications for habenular function. Exp Brain Res. 1982;45:89–94. doi: 10.1007/BF00235766. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. The novel nicotinic receptor antagonist N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide decreases nicotine-induced dopamine metabolism in rat nucleus accumbens. Eur J Pharmacol. 2008;601:103–105. doi: 10.1016/j.ejphar.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience. 2004;129:415–424. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Seppa T, Gaddnas H, Ahtee L. The effects of acute nicotine on the metabolism of dopamine and the expression of Fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal. J Neurosci. 1999;19:8145–8151. doi: 10.1523/JNEUROSCI.19-18-08145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppa T, Ruotsalainen M, Laakso I, Tuominen R, Ahtee L. Effect of acute nicotine administration on striatal dopamine output and metabolism in rats kept at different ambient temperatures. Br J Pharmacol. 2000;130:1147–1155. doi: 10.1038/sj.bjp.0703402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Mecamylamine, dihydro-beta-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacol Biochem Behav. 2009;94:319–328. doi: 10.1016/j.pbb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Maisonneuve IM, Glick SD. 18-Methoxycoronaridine differentially alters the sensitized behavioral and dopaminergic responses to repeated cocaine and morphine administration. Implications for sensitization in the mediation of drug addiction. Ann N Y Acad Sci. 2000;909:275–279. doi: 10.1111/j.1749-6632.2000.tb06694.x. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD. 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse. 2007;61:547–560. doi: 10.1002/syn.20396. [DOI] [PubMed] [Google Scholar]
- Toth E, Sershen H, Hashim A, Vizi ES, Lajtha A. Effect of nicotine on extracellular levels of neurotransmitters assessed by microdialysis in various brain regions: role of glutamic acid. Neurochem Res. 1992;17:265–271. doi: 10.1007/BF00966669. [DOI] [PubMed] [Google Scholar]