Abstract
Obesity and its attendant metabolic disorders represent the great public health challenge of our time. Recent evidence suggests that onset of inflammation in metabolic tissues pathogenically links obesity to insulin resistance and type 2 diabetes. In this review, we briefly summarize the extant literature with special attention to the central role of the tissue-associated macrophage in the initiation of metabolic inflammation. We argue that rather than simple inflammatory disease, obesity and metabolic syndrome represent derangements in macrophage activation with concomitant loss of metabolic coordination. As such, the sequelae of obesity are as much products of the loss of positive macrophage influences as the presence of deleterious inflammation. The therapeutic implications of this conclusion are profound because they suggest that pharmacologic targeting of macrophage activation, rather than purely inflammation, might be efficacious in treating this global epidemic.
Keywords: obesity, insulin resistance, inflammation, diabetes, PPAR
INTRODUCTION
Modern humans represent a great survival success story: in an evolutionary blink of an eye, we sprung from a natural state of chronic starvation and activity to one of caloric plenty and leisure. The precipitate rush from savannah to Subway™, however, left little time for our metabolic physiology to catch up; with a physiology shaped by ~200 million years of starving mammals—and ~200,000 years of starving humans—we are ill-adapted to our new-found dietary riches and inactivity. We are, in effect, living a McDonald’s™ culture with a Pleistocene metabolism.
The obvious, but no less lamentable, denouement of this mismatch is an epidemic of obesity and its accompanying metabolic disorders. At last count, over one in three adults in the United States was obese and nearly 75% were overweight (1). The mortality burden associated with these figures is staggering: 300,000 – 400,000 deaths per year in the United States alone are directly attributable to obesity, putting it on par with smoking with regards to its public health impact (2; 3). Indeed, Olshanksy and colleagues predict that the unabated increase in obesity, especially in children, would lead to a plateau or decrease in US life expectancy in the first half of this century (4).
Much of the mortality attributed to obesity is due to the metabolic disorders of insulin resistance, glucose intolerance, dyslipdemia, and hypertension—collectively known as metabolic syndrome—and their well-documented cardiovascular sequelae (5); however, more recent evidence has implicated obesity as a major risk factor in more than a dozen different cancers, hepatic and renal failure, thrombotic disease, and many infectious diseases (6). These findings suggest that obesity’s toll on our health, already known to be fearsome, is greatly understated.
Recognition of obesity’s catastrophic health sequelae has engendered intense interest regarding its pathophysiology and led to the description of dozens of genes, scores of environmental and physiological factors, and literally hundreds of dietary and behavioral modifiers that affect the disease process in one way or another (7–9). However, despite the daunting number of variables, chronic inflammation appears to function as a common etiologic mechanism, contradicting the established view of obesity and metabolic syndrome as purely metabolic disorders (10–13). As such, much investigation has focused on the etiology and effectors of inflammation. Studies completed in the past decade have highlighted the central role of tissue-associated bone marrow-derived macrophages in coordinating both the metabolic and inflammatory aspects of metabolic syndrome (14; 15). However, despite the recent progress made in elucidating the role of these cells, relatively little is known about their homeostatic functions beyond their role in disease.
In this review, we will briefly summarize the literature regarding inflammatory insulin resistance in metabolic syndrome, discuss the protective role played by alternatively activated macrophages (AAMs), and discuss the therapeutic implications there of. We argue that inflammation is only a portion of the larger functionality of the tissue macrophage and that metabolic syndrome is thus a derangement of macrophage activation characterized by both a deleterious state of chronic inflammation and loss of positive trophic signals.
FUELING IMMUNITY
To fully appreciate the complex dialogue between the immune system and metabolic tissues, we must first review the mechanisms by which the immune system protects the host against potential pathogens. In vertebrates, a layered immune defense system protects against unwanted intrusion (16). Passive barriers and recruited microbial allies comprise the outer curtain of defense—epithelial and mucus barriers, commensal competition, and chemically inhospitable gateways (e.g. the stomach) dissuade the vast majority of unwanted guests (17). For intruders that penetrate these barriers, the active immune response, comprising both innate and adaptive responses, presents an inner defense (16). The innate immune response, comprised of a host of cell types including neutrophils, eosinophils, mast cells, and macrophages, is the first of the active defenses that respond to any intrusion (17; 18). These cells are present throughout the entire organism—with special attention paid to those passive barriers mentioned above—continuously sampling their local tissue microenvironments for evidence of potential pathogens. To stand ready for any one of the myriad forms of invader, vertebrate genomes encode a variety of pathogen-associated molecular pattern (PAMP) receptors, e.g. Toll-like receptors (TLRs), which recognize and categorize threats into broad classes and mount the appropriate responses appropriate (17). For the small, highly replicative bacterial trespasser, robustly phagocytic neutrophils armed with toxic reactive oxygen and nitrogen species mount a localized, intense, and highly destructive response to quickly halt the rapid spread of these organisms. This response, however, would be entirely ineffective against larger pathogens such as helminths, with their own passive barriers, large size, and relative indifference towards toxic insult. In this instance, PAMP-driven innate responses tend towards eosinophil-coordinated mucus barriers and gastrointestinal/respiratory expulsion (19). While these responses are generally successful, they are often incapable of completely eradicating the offending organism. The continued persistence of an antigenic stimulus triggers the activation and deployment of the adaptive immune response. In contrast to the immediate and semi-specific responses of the innate immune response, the adaptive immune system takes 4–7 days of gene rearrangement and somatic hypermutation to fully deploy and results in highly-specific antibody and effector lymphocyte responses against specific molecular epitopes on offending organisms that persist for years after the interloper’s eradication (16; 18; 20).
The spectrum of immune response evinces an impressive range of intensity, from the hours-long half-life of neutrophils to the decades-long persistence of memory lymphocytes. Until recently, this spectrum was viewed as just another curiosity in an impressive list of immune system statistics; however, recent work has defined that unexpectedly complex metabolic programs are necessary for these divergent roles. Moreover, these studies have also defined an instructional role for these same “provisional” metabolic programs in guiding and shaping the immune response, establishing a paradigm in which cellular metabolism and immune activation are intimately linked, both requiring the other for full execution (21–24). Indeed, the curious anatomic juxtaposition of immune and metabolically critical cell types, recognized for decades but unexplained by host defense theory, provides an architectural framework for direct communication between the two putatively unrelated yet evolutionarily fundamental systems (11). For example, lymph nodes—the local command and operation centers for the adaptive immune system—are embedded in depots of white adipose tissue (perinodal adipose tissue or PAT), which provide a dedicated source of metabolic substrate for fueling immune activation (25). Interestingly, studies of single lymph nodes have demonstrated that the composition of lipids provided by the PAT differs tremendously from those provided by neighboring adipose tissue just distal to the node (26). Specifically, the PAT of resting lymph nodes is dominated by polyunsaturated fatty acids, whereas their concentration is much less in neighboring adipose tissue. Under conditions of prolonged inflammatory activation of the lymph node, however, the lipid composition shifts to provide large amounts of saturated fatty acids and glycerol, metabolic substrates for which inflammatory cells have a known predilection. Intriguingly, in vitro studies have demonstrated the capacity of these same lipid mixtures to induce the quiescent or inflammatory profiles with which they are associated in vivo, independent of any immune signals. These observations define a mutual coordinated network of instructive and provisional signals between immune and metabolic cell types.
The anatomic and functional juxtaposition of immunity and metabolism is obvious in central metabolic tissues as well: the liver and white adipose tissue—the two primary players in mammalian energy homeostasis—are densely populated by representatives of both the adaptive and innate immune systems whose activation states and behaviors vary with varying metabolic circumstance (11). For instance, obesity is associated with a dramatic decrease in the number of natural killer T (NKT) cells in the liver (27), while the same metabolic variance is accompanied by an increase in activated T cells in white adipose tissue (28). However, despite the presence and functional contributions of lymphocytes, neutrophils, eosinophils, mast cells, and even the enigmatic basophil to metabolic tissue homeostasis, it is the macrophage that is both numerically and functionally dominant.
MACROPHAGE ACTIVATION
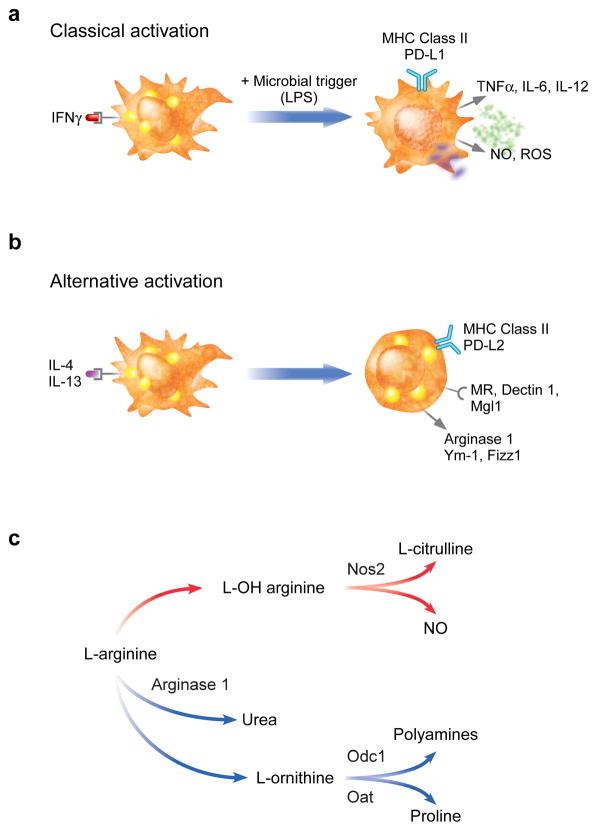
Macrophages are highly heterogeneous hematopoietic cells found in nearly every tissue in the body (29). Canonically, these cells have been defined as the sentinels of the innate immune system, monitoring the varied tissue milieu for early signs of infection or tissue damage. In this role, the macrophage is responsible for sensing, integrating, and appropriately responding to a bewildering array of stimuli from its local microenvironment. Despite the daunting array of inputs, macrophage responses are coordinated through two distinct and mutually exclusive activation programs, termed classical (or M1) and alternative (or M2) (30; 31). These activation programs were initially defined by their antimicrobial activities: classical activation occurs in response to products derived from or associated with bacterial infections, such as lipopolysaccharide (LPS) and interferon gamma (IFNγ), and results in highly inflammatory macrophages with high phagocytic and bactericidal potential (30; 32) (Figure 1a). In contrast, alternative activation occurs in response to products derived from or associated with parasitic infections, such as Schistosoma egg antigen and interleukins (IL)-4 and -13, and promotes antiparasitic functionalities as well as those involved in tissue repair and remodeling (33) (Figure 1b). Both programs promote differentiation of neighboring macrophages to their same activation state and potently inhibit maturation of the other. A large number of differentially expressed markers have been identified by which the two activation states can be discriminated; however, the differential metabolism of arginine is perhaps the most well-defined and reliable of them (33). In the classically activated macrophage (CAM), arginine is catabolized to bactericidal nitric oxide and citrulline via the induction of inducible nitric oxide synthase (Nos2), whereas the alternatively activated macrophage (AAM), by contrast, upregulates arginase 1, which produces the polyamine precursor urea and ornithine, necessary for collagen synthesis and cellular proliferation, respectively (34; 35) (Figure 1c).
Figure 1. Classical and alternative macrophage activation.
Macrophage activation comprises a broad spectrum of activities coordinated in response to specific environmental stimuli. While in reality a continuum, these responses can be separated into two basic patterns: classical, or M1, and alternative, or M2. a) Classical activation is a pro-inflammatory state purposed for the rapid destruction of bacterial invaders. Classically activated macrophages generate induce reactive oxygen species (ROS) and nitric oxide (NO) for their microbicidal actions, and secrete pro-inflammatory cytokines, such as TNFα and IL-12, to enhance cell mediated immunity. b) In contrast, alternative activation represents a more sustained response such as that typified by infection with parasites. While the induction of MHC class II and co-stimulatory molecules (PD-L2) indicate these macrophages are activated, they express a distinct repertoire of cell surface receptors (mannose receptor, Mrc1; dectin-1, Clec7a; and Mgl1, Clec10A), and secreted products (Ym-1, Chi3l3; and FIZZ1, Retnla). c) Differential metabolism of l-arginine in classically and alternatively activated macrophages by inducible nitric oxide synthase (Nos2) and arginase 1, respectively. Odc1: ornithine decarboxylase; Oat: ornithine aminotransferase.
While these activation states were defined initially by their function in host defense, further study has revealed additional roles for them in health and disease. As mentioned previously, the alternative phenotype has been implicated in wound healing, tissue remodeling, atopic disease states, and apoptotic cell disposal, whereas the classical phenotype has been shown to play a causal role in inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and atherosclerosis (30). One of the most interesting areas in which the dichotomy between classical and alternative activation has been studied, however, is that of energy homeostasis and metabolic disease (23).
INFLAMMATION AND INSULIN RESISTANCE
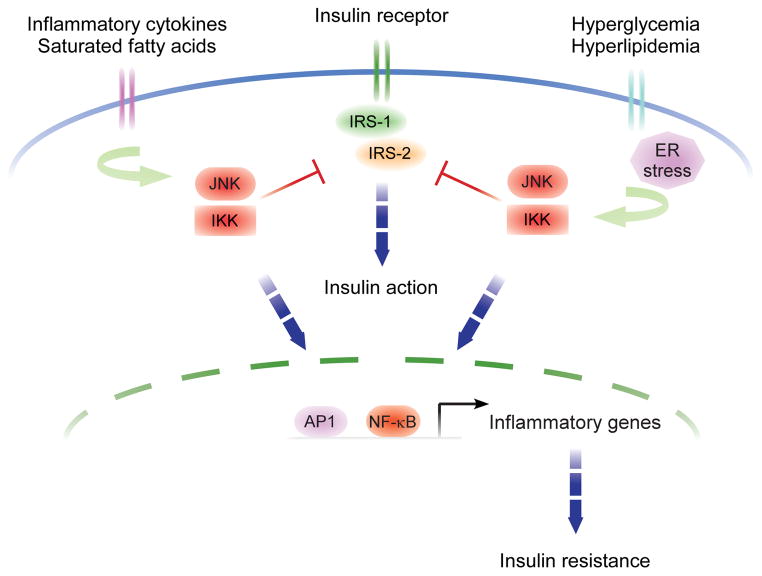
As mentioned in the introduction, metabolic diseases, including obesity, insulin resistance and type 2 diabetes, are driven by a chronic, low-grade inflammatory state that contributes directly to their induction, maintenance, and escalation (12; 13; 36). This state is characterized by elevated serum levels of proinflammatory cytokines (e.g. IL-1, IL-6, IL-8, IL-12, and TNFα), chemokines (e.g. MCP-1, RANTES, and MIP-1), acute phase reactants (e.g. C-reactive protein, serum amyloid A, and ferritin), insulin resistance-associated adipokines (e.g. retinol binding protein-4 and resistin), procoagulative factors (e.g. PAI-1), and hypertensive agents (e.g. angiotensinogen) and with decreased serum levels of the so-called “negative” acute phase reactants (e.g. transcortin, and transferrin), insulin sensitivity-associated adipokines (e.g. adiponectin, visfatin, omentin, and vaspin) (12; 13; 36). Indeed, infusion of proinflammatory mediators, such as TNFα, alone is sufficient to recreate the insulin resistant phenotype observed in animal models fed a high-fat diet and to promote obesity (37), whereas genetic deletion of these same mediators greatly attenuate the diet’s deleterious effects (38). Congruent with these findings, central regulators of inflammation, including NF-κB and jun N-terminal kinase (JNK1), are chronically activated in adipose tissue from obese and insulin resistant subjects (12; 13; 36). Interestingly, these same tissues show abnormal inhibitory serine phosphorylation of critical insulin receptor substrates, suggesting a possible mechanism by which kinases in inflammatory signaling pathways could directly impinge upon insulin signaling. Indeed, JNK1 and inhibitor of NF-κB-kinase β (IKKβ, an NF-κB-activating kinase) both directly phosphorylate serine residues on insulin receptor substrate 1 (IRS1), the major downstream effector of the insulin receptor, in response to TNFα stimulation (37; 39; 40) (Figure 2).
Figure 2. Intracellular mechanisms of inflammatory insulin resistance.
Insulin action is transduced from the cell surface to cytoplasmic and nuclear responses via tyrosine phosphorylation of insulin receptor substrate (IRS)-1 and -2. However, serine phosphorylation of these same substrates by JNK1 and IKKβ, the central mediators of stress and inflammatory responses, potently inhibits insulin action, thereby directly linking these responses to insulin resistance. In addition, the transcriptional activation of inflammatory genes by JNK1 and IKKβ induces insulin resistance in an autocrine and paracrine manner in tissues. Moreover, in states of obesity, JNK1 and IKKβ signaling pathways are activated by increased influx of free fatty acids and glucose.
These observations provide a direct mechanism by which inflammation subverts insulin signaling in a self-sustaining manner, but they fail to explain exactly how well-known behavioral and dietary indiscretions initiate this inflammatory brew. The well-described acquisition of an inflammatory phenotype somewhere along the trajectory to obesity suggests the appearance of at least one inflammatory stimulus during the process. Indeed, there is evidence for multiple sources that contribute to both initiation and propagation of inflammation within liver and white adipose tissue. For example, Flier et al astutely recognized the structural similarities between saturated fatty acids, a known dietary risk factor for insulin resistance, and immunogenic components of bacterially-derived lipids such as LPS. They hypothesized that the elevated levels of saturated fatty acids present in obesity are able to activate TLR4, the cell-surface receptor activated by LPS, and initiate an inflammatory response (41). Indeed, high concentrations of dietary saturated fatty acids are able to activate TLR4 in vitro and in vivo, and animals lacking TLR4 are protected from high-fat diet-induced insulin resistance (41–43). Furthermore, infusion of saturated fatty acids is sufficient to induce insulin resistance in lean animals in a TLR4-dependent manner. Interestingly, ligation of TLR4 directly activates the JNK1 and NFκB signaling pathways, providing a direct mechanistic link between diet and insulin resistance vis-à-vis elevated saturated fatty acids (41; 42; 44; 45) (Figure 23).
A second link between dietary excess and insulin resistance was uncovered through study of the endoplasmic reticulum (ER) stress response (11). During conditions of high circulating nutrient levels, such as those which occur post-prandially, glucose and fatty acids are stored as triglycerides in the adipocyte for later use. However, in chronically over-fed states, this storage/buffer capacity is exceeded, and excess free-fatty acids leak into the cytoplasm of the adipocyte and into circulation. Hotamisligil and colleagues demonstrated that elevated intracellular concentrations of these free fatty acids lead to ER stress and activation of downstream signaling pathways, including JNK1, that contribute to the development of insulin resistance (46; 47) (Figures 2, 3). Furthermore, administration of chemical chaperones known to reduce ER stress alleviates diet-induced insulin resistance (48; 49); whereas genetic inactivation of the ER stress response leads to its exacerbation (47). These findings provide a second mechanism linking inflammatory insulin resistance with caloric excess (Figure 3).
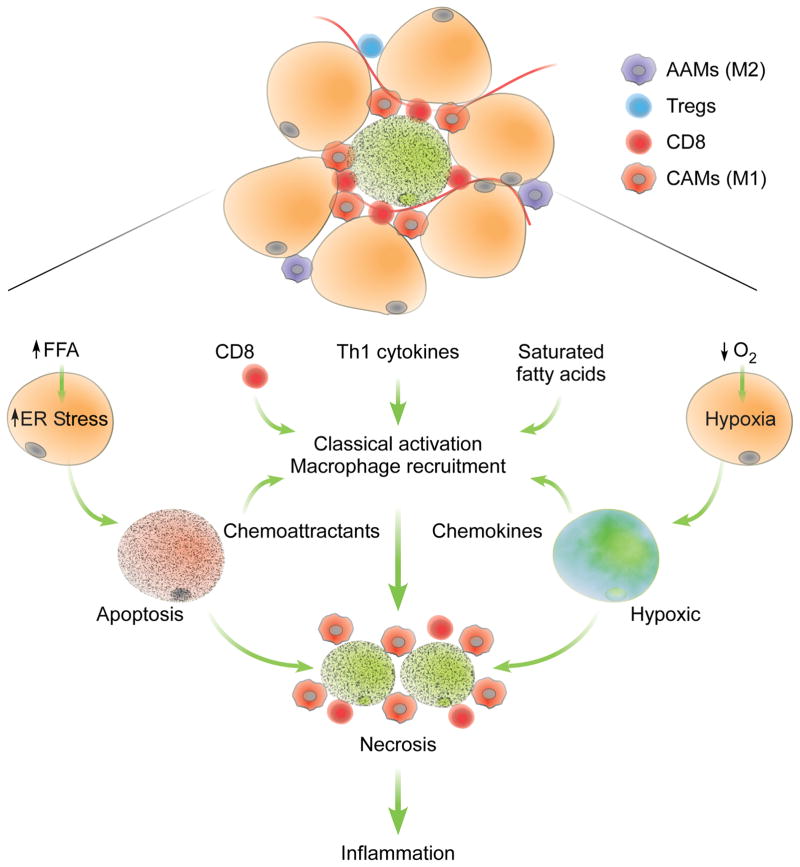
Figure 3. Factors controlling recruitment and classical activation of ATMs.
Increased intake of diets rich in saturated fatty acids induces obesity, resulting in recruitment of CCR2+ monocytes that differentiate into classically activated macrophages. The inflammatory milieu that promotes classical activation (M1) of ATMs in obese animals includes recruitment of CD8 cells, reduction in numbers of regulatory T cells (Tregs), and increased production of Th1-type cytokines. In addition, adipocyte hypertrophy induces ER stress and hypoxia, factors that eventually lead to cellular necrosis. In obese animals, CD8 cells and CAMs form crown-like structures around necrotic adipocytes. The dramatic increase in CAMs in obese adipose tissue negates the anti-inflammatory and homeostatic functions of AAMs, resulting in increased inflammation. AAMs: alternatively activated macrophages; CAMs: classically activated macrophages.
Thirdly, the robust hypertrophy of adipocytes in obesity is also accompanied by adipose tissue hyperplasia. Unlike the exercise-associated corollary in skeletal muscle, however, adipose tissue hyperplasia does not take place in a highly vascularized tissue nor does angiogenesis keep pace with the process. As such, the rapidly-expanding visceral adipose depots out-strip their vascular supply, resulting in hypoxia that synergizes with ER-stress to lead to increased rates of adipocyte necrosis (50; 51). As opposed to immunologically silent apoptosis, necrotic cell death is highly phlogistic, triggering inflammatory activation of macrophages in addition to other related cell types (52) (Figure 3). Even in those cells that survive, hypoxia activates HIF-1α, which results in the elaboration of the same pro-inflammatory mediators implicated in insulin resistance.
CLASSICAL MACROPHAGE ACTIVATION PROMOTES INSULIN RESISTANCE
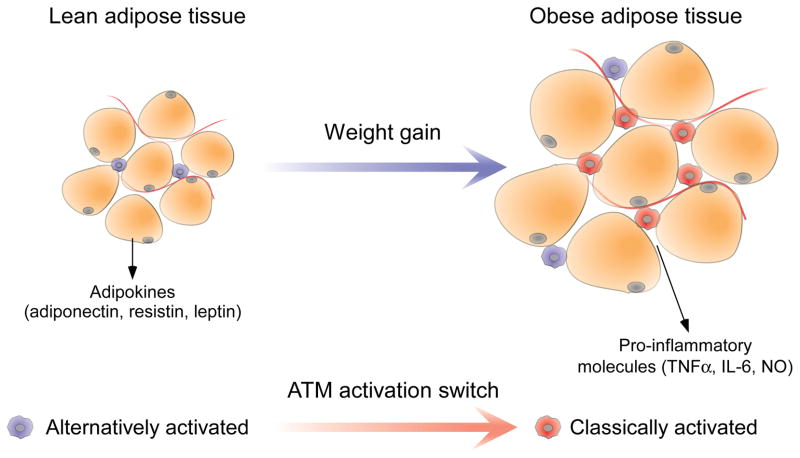
While elevated saturated fatty acids levels, ER stress, and tissue hypoxia necessarily originate with the adipocyte, the tissue macrophage is the primary cell responsible for mounting and maintaining the inflammatory response and, as such, represents the nexus of adipose tissue inflammation (Figure 3). Ferrante, Chen and colleagues were the first to observe that macrophage representation within white adipose tissue increases with increasing adiposity, indicating that recruitment and infiltration of new, bone marrow-derived macrophages into the adipose tissue also increased with adiposity (14; 15) (Figure 4). The expansion in representation from 10% to over 50% of total cell number, when accompanied by 5-fold or more increase in overall adipose tissue mass that necessarily accompanies obesity, dictates that nearly all adipose tissue macrophages (ATMs) in obese individuals are recruited after the onset of weight gain. Ferrante and colleagues suggested that blocking the recruitment and infiltration of pro-inflammatory blood monocytes into adipose tissue would then selectively impair ATM inflammatory activation without affecting other myeloid populations or tissue beds. Previous work had demonstrated that C-C motif chemokine receptor-2 (CCR2)-positive blood monocytes are selectively recruited to sites of inflammation where these cells, but not CCR2− monocytes, differentiate into pro-inflammatory tissue macrophages (53). Studies with CCR2 deficient mice demonstrated that recruitment of pro-inflammatory monocytes to adipose tissue was sufficient to protect mice against diet-induced insulin resistance (54). By contrast, animals overexpressing MCP1 in the adipocyte exhibit increased susceptibility to insulin resistance and obesity (55; 56). Thus, the systemic metabolic and inflammatory improvements observed in these models confirm the role of inflammatory ATMs in paracrine and endocrine metabolic regulation.
Figure 4. Functional differences between lean and obese adipose tissue.
Adipose tissue from lean individuals comprises small, insulin-sensitive adipocytes that secrete large amounts of insulin-sensitizing adipokines under the influence of alternatively (M2)-biased adipose tissue macrophages (ATMs). In contrast, adipose tissue from obese individuals displays an expanded and prominently classically (M1)-biased population of ATMs, which secrete a variety of potent inflammatory mediators, together with hypertrophic, insulin-resistant adipocytes.
Further evidence supporting the central role of tissue macrophages was provided by global and lineage-specific genetic ablation of transcriptional mediators of inflammation. Consistent with its pivotal roles in inflammatory insulin resistance, global deletion of Mapk8 (the gene encoding JNK1) resulted in marked protection from diet-induced obesity and insulin resistance (57). Interestingly, hepatic deletion of Ikbkb (the gene encoding IKKβ, a kinase required for NF-κB activation) prevented the development of inflammation and insulin resistance solely in this depot, suggesting that the systemic inflammatory milieu encountered in obese subjects is the product of multiple local phenomena rather than a coordinated systemic condition (58; 59).
In addition to providing definitive evidence of inflammation’s role in insulin resistance, these studies also provide an avenue for distinguishing the contribution of macrophage inflammation from that of adipocytes and hepatocytes as well as from that of other infiltrating leukocytes. Using reciprocal adoptive transfer to create chimeric animals, Karin et al were able to demonstrate that JNK1 deletion from nonhematopoietic cells is sufficient to protect mice from diet-induced obesity and, indirectly, from concomitant insulin resistance (60). By contrast, deletion of JNK1 from the hematopoietic compartment decreases hepatic and adipose tissue inflammation and improves insulin sensitivity without affecting adiposity, suggesting that diet-induced inflammation, not obesity, is directly responsible for insulin resistance and mediated primarily by bone marrow-derived cells (60). Furthermore, myeloid-specific deletion of IKKβ utilizing a cre-lox approach is sufficient to dramatically reduce inflammation, similar to loss of JNK1 from the entire hematopoietic compartment (58). Given the relative paucity of other myeloid lineages in adipose tissue and the liver, these data strongly suggest macrophages as the primary source of diet-induced inflammation in these tissues.
ALTERNATIVE MACROPHAGE ACTIVATION ENHANCES INSULIN ACTION
These studies define much of inflammatory insulin resistance and place the macrophage in the pathogenic role of inflammatory instigator. Several lines of evidence, however, suggest that this is an overly simplified model. For example, while macrophage representation in adipose tissue increases with increasing adiposity, representation in the liver does not, nor is the adipose tissue of lean individuals bereft of macrophages. Moreover, despite the marked phenotype, ATM numbers are only moderately reduced in the CCR2−/− animals that lack the ability to recruit inflammatory macrophages. Indeed, the non-linear relationship between macrophage number and behavior suggests that tissue macrophages have a greater functional repertoire than simple inflammation.
The first evidence to suggest diversity in the metabolic tissue-associated macrophage pool came from differential profiling studies of adipose tissue from lean and obese mice. Saltiel and colleagues demonstrated that ATMs from lean mice, rather than being quiescent or mildly inflammatory, are activated along the alternative pathway (61) (Figure 4). Similarly, Kupffer cells (the resident tissue macrophages of the liver) from lean animals express high levels of alternative markers, which are swapped for an inflammatory profile in obesity (62; 63).
Based on the ability of AAMs to restrain their classically activated brethren, their presence in the adipose tissue and liver of lean animals strongly suggests that they play a role in preventing inflammatory insulin resistance. Despite this promise, the paucity of knowledge regarding the biology of AAMs hindered its exploration.
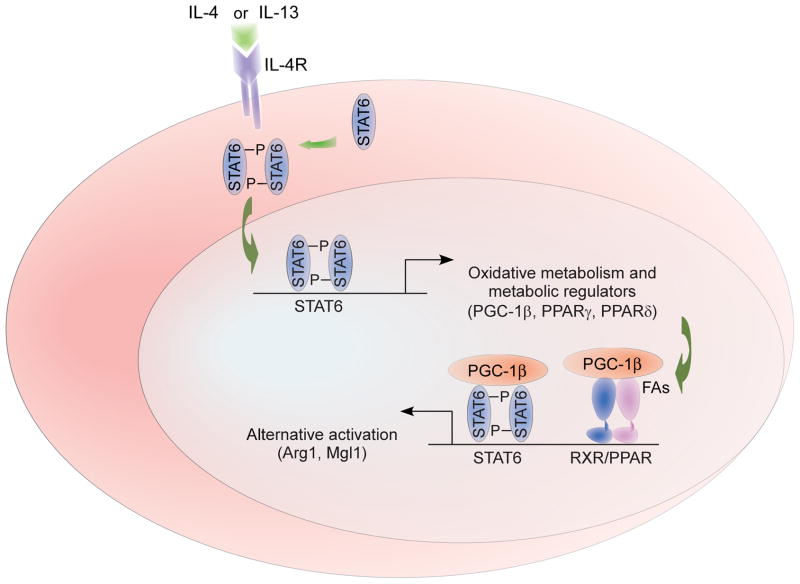
To explore the biological relevance of these findings, our laboratory exploited the overlapping provisional and instructive roles of metabolism in immune activation to identify transcriptional machinery necessary for alternative activation. Unlike classical activation, where functions are executed over hours to days, alternative activation is purposed for long-term activities with functional lifetimes of months. The dramatic difference in time scale suggests distinct metabolic provisional requirements. Indeed, while classical activation of macrophages induces a glycolytic program capable of rapidly providing the energy and reducing equivalents necessary for intense, short-lived bactericidal activity (64; 65), alternative activation opts for a more efficient program of fatty acid oxidation and oxidative glucose metabolism capable of being sustained for long periods of time (65). Furthermore, blocking oxidative metabolism not only selectively abrogates the cell’s ability to undergo alternative activation but also potentiates expression of the classical program. Conversely, forcing the macrophage down the oxidative route by overexpressing PGC-1β, a key transcriptional proponent of oxidative metabolism, potentiates alternative activation and prevented classical (65). As such, metabolism operates as both a provisional and instructive cue for macrophage activation (Figure 5).
Figure 5. Transcriptional mechanisms controlling alternative (M2) activation.
The canonical pathway of alternative macrophage activation involves activation of STAT6 by the Th2 cytokines IL-4 and -13. STAT6 activation initiates a transcriptional program comprising both immunologic effector responses and metabolic adaptations necessary to sustain them. Among the metabolic targets of STAT6 are PPARγ and δ as well as related coactivator PGC-1β. The transcriptional synergy between these metabolic regulators and STAT6 serves to sustain the immune effector response as well as direct metabolic adaptation for oxidative metabolism.
Not surprisingly, these approaches identified activation of STAT6, IL-4’s well-studied transcriptional effector, as the critical initiating event in macrophage alternative activation (65); however, peroxisome proliferator-activated receptor (PPAR)-γ and -δ, the body’s fatty acid sensors, were identified as responsible for sustaining it (62; 63; 66) (Figure 5). These proteins were both shown to bind to and directly regulate the activity of genes critical to alternative activation in an overlapping but non-redundant manner. While PPARγ directly regulates its associated metabolic program (66), PPARδ coordinates the immunologic effector functions of alternative activation, including expression of pattern-recognition receptors and co-stimulatory molecules, as well as suppression of the inflammatory response (62; 63). Importantly, unsaturated dietary fatty acids such as oleic acid synergize with IL-4 to drive the alternative response by acting as metabolic substrates as well as transcriptionally activating PPARδ (63) (Figure 6). These findings complement those mentioned previously in which unsaturated fatty acids possessed anti-inflammatory properties, providing a mechanism by which unsaturated fatty acids promote macrophage alternative activation and antagonism of the classical phenotype. Additionally, these findings potentially explain why dietary shifts from foods rich in unsaturated fatty acids to those rich in saturated are associated with the acquisition of an inflammatory phenotype within lipid-rich tissues.
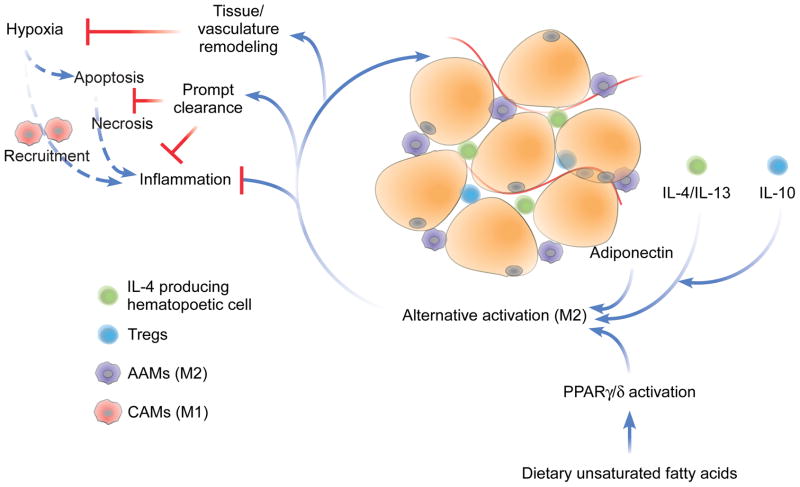
Figure 6. Effects of alternatively activated (M2) macrophages on adipose tissue.
Dietary intake of unsaturated fatty acids and maintenance of lean body mass results in alternative activation (M2) of ATMs via activation of PPARγ/δ, local production of Th2 cytokines IL-4, IL-13, and IL-10, and release of trophic adipokines, such as adiponectin. The alternative phenotype protects against IR through the direct suppression of inflammation as well as through positive homeostatic maintenance activities, including remodeling of adipose tissue to prevent hypoxia and prompt clearance of apoptotic cells to prevent cellular necrosis. AAMs: alternatively activated macrophages; CAMs: classically activated macrophages.
Utilizing these insights, we developed two mouse models in which macrophage alternative activation was severely compromised: in the first, PPARγ was selectively deleted from the myeloid compartment utilizing cre-lox technology (PPARγ-LysMCre); in the second, PPARδ−/− bone marrow was adoptively transferred into irradiated wild-type mice to generate animals lacking PPARδ in hematopoietic cells only (PPARδ-BMT). Bone marrow- and tissue-derived macrophages from both strains of mice are markedly impaired in alternative activation as assessed by in vivo transcriptional profiling, ex vivo stimulation, and in vivo functionality (63; 66). Importantly, both strains of mice display significantly higher expression of inflammatory markers, increased weight gain, and reduced insulin sensitivity than their wild-type counterparts when fed a high-fat diet (62; 63; 66; 67). While the systemic effects of these interventions are similar, there is an interesting discordance in tissue specificity: the inflammatory changes present in PPARγ-LysMCre mice are most marked in visceral white adipose tissue, while those of PPARδ-BMT mice were most prominent in the liver (63; 66). The discrepancy between these results may reflect divergent roles for PPARγ and δ in Kupffer cell and ATM biology; however, it may also be explained by differences in strain (Balb/cJ vs. Sv129/J, respectively) and experimental design (cre-lox vs. adoptive transfer) as cre-lox abrogation of myeloid PPARδ produced a phenotype more similar to that of the PPARγ-LysMCre mouse than that of the PPARδ-BMT (62). Additionally, Tontonoz et al observed no significant difference between mice lacking myeloid PPARγ, PPARδ, or both and wild-type controls on the more M1-prone C57/B6 background, further supporting a role for strain dependence (68).
REGULATION OF ALTERNATIVE MACROPHAGE ACTIVATION IN METABOLIC TISSUES
These studies identify a critical homeostatic role for macrophage alternative activation in metabolic tissues. Additionally, they provide insight into mechanisms by which this protective role is effected. Since its identification as a distinct functional state, alternative activation has been largely defined by its ability to antagonize and inhibit classical activation and the associated phlogistic sequelae (30; 31; 69). As these sequelae include insulin resistance, the functional aspect of macrophage alternative activation that has received the most attention has been its anti-inflammatory properties (Figure 6). Indeed, multiple studies have demonstrated that any impairment of macrophage alternative activation exacerbates expression of inflammatory markers within liver and adipose tissue (61–63; 66; 70). Additionally, in vitro and ex vivo studies have demonstrated the ability of AAMs to inhibit inflammatory mediator expression in both classically activated macrophages (CAMs) and other leukocytes, as well as in adipocytes and hepatocytes themselves. Complementary to these findings, a recent study demonstrated that IL-10-producing AAMs also restrain diet-induced adipose tissue inflammation through the induction of regulatory T cells (71).
Additionally, AAMs have been implicated in a number of tissue maintenance roles that function to limit cellular damage, which would otherwise result in inflammation and, concomitantly, insulin resistance (Figure 6). For example, adipose tissue undergoes striking hyperplasia during the development of obesity. Alternatively activated macrophages are crucial for the vascular and structural remodeling necessary to prevent ischemia and overcrowding (30; 31). Indeed, chronically inflamed adipose tissue demonstrates larger hypoxic zones and higher levels of expression of hypoxia-associated genes including those that promote inflammatory activation, such as HIF-1α (50; 51; 72). Complementary studies have demonstrated that PPARδ, which controls alternative macrophage activation, is also critically involved in immunologically silent disposal of those cells that do die, whether from hypoxia or other causes (73). Indeed, inflamed adipose tissue contains inflammatory cells associated with dying adipocytes—so-called crown-like structures—whereas these complexes are not observed in adipose tissue from lean insulin-sensitive animals (74; 75).
While studies of the anti-inflammatory properties of AAMs have yielded much information regarding their protective role in diet-induced insulin resistance, the view of AAMs as simple antagonists of their classically activated counterparts, or even of leukocyte- and parenchyma-derived inflammation alike, is overly simplistic. Indeed, several lines of evidence suggest that there are roles for AAMs unrelated to inflammation (76). For example, obesity is defined by massive expansion of white adipose tissue depots, a process that necessarily involves extensive remodeling of not only the tissue vasculature, as discussed above, but also the adipose tissue itself. Without proper hypertrophy of white adipose tissue, chronic overnutrition rapidly leads to lipodystrophy, a condition of ectopic and non-specific lipid deposition throughout the body (72). Congruent with these findings, lipodystrophy is strongly correlated in both mice and humans with an absence of alternative activation signatures in white adipose tissue accompanied by an over-expression of classical phenotype (23).
The hepatic response to high-fat diets similarly evinces a homeostatic role for macrophage alternation activation. Under eucaloric conditions, the liver packages and exports dietary lipids for peripheral use or storage in white adipose tissue. When fed a high-fat diet, this export capacity can become overwhelmed, resulting in dystrophic lipid storage within the hepatocytes themselves. As in adipocytes, dystrophic lipid storage results in elevated cytoplasmic free fatty acids and activation of inflammatory pathways through the ER stress response or TLR ligation (77; 78). If the dietary stress is not relieved, the liver develops non-alcoholic steatohepatitis (NASH, a condition marked by hepatic inflammation and hepatocytes necrosis), which can progress to cirrhosis and, rarely, hepatocellular carcinoma (79). Animal models with impairments in macrophage alternative activation (e.g. PPARδ−/− BMT, and PPARδ-LysMCre mice) develop more severe NASH than wild-type controls when exposed to a high-fat diet (62; 63). Interestingly, however, ablation of the entire Kupffer cell population causes mice to develop a similar phenotype despite a reduction in hepatic inflammation (80–82). These data demonstrate that while macrophage-derived inflammation may drive steatohepatitis, the alternative macrophage phenotype is necessary for proper hepatocellular lipid handling (83).
The prevalence of AAMs in metabolic tissues and the importance of their homeostatic roles raise a key question: what drives this alternative polarization in lean animals? While the answer to this question remains unknown, several lines of evidence suggest that it is an integration of immunologic and metabolic signals. First, while saturated fatty acids dominate in obese, insulin-resistant individuals, unsaturated fatty acids are the predominant free lipid in both metabolic tissues and in the circulation of lean subjects (84). These same unsaturated fatty acids activate PPARδ, while rising saturated fatty acids concentrations inhibit PPARδ transcriptional activity even as they activate antagonistic, pro-inflammatory pathways via ligation of TLR4 (41; 44; 63) (Figures 3 and 6). Furthermore, specific unsaturated fatty acids (e.g. n-3 PUFA) contribute to the formation of resolvins and protectins, lipid-based antagonists of inflammatory activation (85). As described above, a similar transition in free fatty acid composition takes place between lymph node lymphocytes and perinodal adipocytes following chronic inflammatory activation.
A second, compatible mechanism for biasing resident macrophages is suggested by studies of the adipokine adiponectin and its effects in macrophages (86) (Figure 6). Early studies of adiponectin describe its ability to promote oxidative metabolism in target cells (87; 88), including the macrophage (89). This induction of oxidative metabolism, as discussed above, effectively polarizes the macrophage towards alternative activation via dual-role transcriptional mediators such as PPARγ and δ.
While much of the alternative phenotype may be due to non-canonical signals through metabolic lines of communication, direct stimulation by Th2 cytokines form a third contributing factor. Lee et al have reported low-level expression of both IL-4 and -13 in adipocytes and hepatocytes and correlated this with lean, insulin sensitive phenotypes (62). While this expression level is not sufficient to be detected in circulation, these cytokines function in a paracrine manner to influence macrophages within the local microenvironment. Addition studies utilizing IL4-reporter mice are necessary to identify the cellular sources of IL-4 in liver and adipose tissue (90) (Figure 6).
THERAPEUTIC IMPLICATIONS
Our increased understanding of the complex regulatory relationship between immune and metabolic cell types has engendered interest in exploiting this relationship to address the mounting threat of metabolic disease. Undeniably, the most effective manipulation of this relationship is accomplished by lifestyle modification. Dietary replacement of saturated fats and refined sugars by unsaturated fats and complex carbohydrates, as seen in Mediterranean diets for example, replaces a pro-inflammatory stimulus with an anti-inflammatory one, as outlined above, and is, unsurprisingly, associated with improvements in inflammation and insulin resistance (91–93). Normalization of caloric intake is similarly associated with improvement in insulin resistance, largely through decreased adiposity. Exercise also operates through weight reduction, though it additionally promotes anti-inflammatory tone and suppresses chronic inflammation independent of weight loss (94; 95).
Despite the relative simplicity and undeniable efficacy of lifestyle interventions, persistent public disinterest in this approach has refocused attention on pharmacologic adjuncts. Unfortunately, the redundant and positively reinforcing quagmire that is metabolic syndrome—not to mention our desire for high-calorie food and lots of it—has largely thwarted initial efforts. These efforts can be divided into four main categories: 1) intake inhibition (e.g. appetite suppressants, malabsorptive agents, bariatric surgery), 2) output augmentation (e.g. stimulants), 3) immunosuppression, and 4) immunomodulation. The first two categories are solely based on thermodynamic arguments and as such will not be discussed here. Instead we will focus on the two latter approaches, both of which exploit the relationship between immune function and metabolism.
Not surprisingly, the first therapies targeting the immune component of obesity and metabolic syndrome were designed to broadly inhibit immune function. Our rudimentary early understanding of inflammation’s role in insulin resistance suggested that removing immune influences over metabolic tissues would prevent their detrimental influence. Without knowledge of the pathophysiologic mechanisms of metabolic disease, Williamson and Lond first demonstrated more than a century ago that high-dose salicylate treatment reduced the severity of glycosuria in diabetic patients (96). Fifty years later, Reid and colleagues further demonstrated that aspirin treatment improved oral glucose tolerance test results in diabetic patients (97). The modern interpretation of these experiments is that the salicylates operate through direct inhibition of IKKβ, a known mediator of inflammatory insulin resistance (98–100). While the adverse effects of the salicylate doses required for meaningful pharmacological effect prevent their clinical adoption, targeted biologics have revived interest in this approach. Systemic TNFα blockade with biologics such as infliximab and entanercept have shown promising results in animal models; however, results have been disappointing in early prospective trials in diabetic patients and mixed in retrospective studies of patients taking the drugs for inflammatory conditions such as psoriasis and rheumatoid arthritis (101; 102). These mixed results may be the result of a failure of these interventions to adequately neutralize inflammation in the liver and adipose tissue microenvironments. Alternatively, their failure may be understood in the context of our discussion above regarding the complexity of macrophage function in metabolic syndrome—metabolic health is not defined solely by the absence of systemic inflammation. Inhibition of pathologic inflammation alone may not be sufficient to replace the positive trophic influences lost with the alternatively activated macrophage pool.
Therapies directed at biasing the immune system rather than inhibiting it, also known as immunomodulation, represent a more nuanced approach, integrating the needs to both blunt pathologic classical activation and promote positive alternative activation. Though not as developed as traditional anti-inflammatory approaches, immunomodulatory therapies have demonstrated promise. Although unrecognized at the time, the first large-scale use of an immunomodulatory agent occurred with the introduction of the statins. Originally targeted at lowering cholesterol levels (103), statins have subsequently been shown to possess potent anti-inflammatory properties stemming from their unique ability to bias the immune response towards alternative activation via interference in isoprenoid biosynthesis (104; 105). Indeed, statin treatment of isolated dendritic cells in vitro leads to the cell autonomous upregulation of alternative markers, whereas treatment of uncommitted CD4+ helper T cells promotes Th2 maturation and the capacity to further alternatively bias uncommitted monocytes (106; 107). While their cholesterol-lowering properties are certainly important, their immunomodulatory capacity is thought to underlie much of the statins’ cardioprotective properties (108–110). Indeed, statins have been shown to be effective in ameliorating inflammatory diseases as varied as multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, and graft-versus-host-disease (104). Observational studies have even described a chemopreventative effect for statins in the development of inflammatory-driven colorectal malignancies similar to that of non-steroidal anti-inflammatory drugs (111). Importantly, in contrast to the well-described adverse effects of current anti-inflammatory biologics, there is no evidence of any significant immunocompromise associated with their use. While most studies have focused on their cardioprotective effects, numerous studies have described mixed effects on insulin sensitivity despite reproducible improvements in inflammatory markers. Importantly, analysis of these reports demonstrates that certain statins (e.g. pravastatin, rosuvastation, fluvastatin) reproducibly improve insulin sensitivity independent of lipid lowering in obese subjects with and without diabetes (112–116), while others (e.g. simvastatin) consistently fail to do so despite similarly improvements in inflammatory markers (112; 115; 117). Interestingly, pravastatin therapy had no effect in lean, insulin sensitive patients, a population where previous studies have shown the alternative macrophage phenotype to be dominant (118). When taken together, these findings suggest that statins may exert their effects at least partly through the promotion of tissue macrophage alternative activation.
Another unintended foray into immunomodulatory therapy has been described in the murky field of nutrition and supplements. It has been long-appreciated that diets rich in unsaturated fatty acids are correlated with reduced chronic inflammation and its sequelae (91; 119), and recent studies discussed above (i.e. TLR4 ligation by saturated fatty acids and PPARδ ligation by unsaturated) have lent mechanistic validity to them. Large numbers of both cross-sectional and prospective studies have examined this question and while the results are somewhat mixed, the data generally support the conclusion that unsaturated fatty acids, especially n-3 polyunsaturated fatty acids, improve global insulin sensitivity, tend to decrease adiposity, and significantly lower levels of serum inflammatory markers (91; 119; 120). These data, when taken together with the mechanistic data discussed above, suggest that the long-appreciated dietary wisdom serves, at least in part, an immunomodulatory function by promoting alternative immune responses.
While immunomodulation may exert its efficacy through macrophage deviation as a final common pathway, Dosch et al recently capitalized on the ability of T lymphocytes to direct macrophage function through a novel lymphocyte-directed approach (71). Recent studies have described a transition in the white adipose tissue-associated T lymphocyte population from protective Th2-biased helper cells to pathogenic Th1-biased helper and cytotoxic cells during the development of diet-induced obesity (71; 121; 122). This shift parallels the alternative-to-classical transition in macrophage activation, and together they describe a shift in the tissue microenvironment from anti-inflammatory to inflammatory with all of its attendant metabolic sequelae (Figure 3). Dosch and colleagues astutely recognized that eradication of adipose tissue-related lymphocytes from insulin resistant animals would reset the lymphocyte population and potentially redistribute the Th1/Th2 bias (71). Indeed, treatment with an anti-CD3 antibody erases the Th1-skewed T cell repertoire, and subsequent repopulation restores the T cell balance to something approximating that seen in lean animals. Furthermore, the reintroduction of regulatory and Th2 T cells re-directs the inflammatory macrophage profile to the alternative phenotype and results in dramatically decreases tissue inflammatory markers and results in sustained normalization of insulin sensitivity (71; 121; 123).
The evidence is clear that diet-induced insulin resistance is largely a disorder of deranged macrophage activation; however, efforts focused solely on inhibiting or correcting that derangement have fallen short of expectations. This is perhaps because once established, the metabolic derangements are self-sustaining and rely less upon continued inflammatory instigation, or alternatively, that these derangements provide their own inflammatory stimulation independent of immune function (e.g. high levels of saturated fatty acids can perpetuate insulin resistance in muscle and adipose tissue directly without intermediary leukocyte amplification). Regardless, it is likely that for any therapy to be effective in reversing established insulin resistance, it must target not only the driving mechanism of immune deviation but also the effector mechanisms of deranged skeletal muscle, liver, and adipose tissue metabolism. It is exactly this approach that is suggested by the discussion above: metabolic and immune functions not only communicate but also share certain transcriptional regulators, e.g. the PPARs, which are natural pharmacologic targets. Indeed, studies of synthetic PPARδ agonists have demonstrated great potential for use in metabolic syndrome: PPARδ activation not only promotes alternative macrophage activation and suppresses tissue inflammation (62; 63; 124), but also directly increases oxidative lipid catabolism in skeletal and cardiac muscle, adipose tissue, and liver; improves serum lipid profiles and global insulin sensitivity; and promotes weight loss in obesity (125).
PPARs.
The peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear receptor superfamily. There are three PPARs (α, δ, and γ) in mice and humans, which control nearly all aspects of fatty acid metabolism. Rate-limiting enzymes involved in transport, synthesis, storage, mobilization, activation or oxidation of fatty acids are all regulated by PPARs. In a tissue- and stimulus-dependent manner, PPARs regulate distinct programs of fatty acid metabolism. PPARα regulates β- and ω-oxidation of fatty acids in the liver, whereas PPARδ regulates their oxidation in other peripheral tissues, including white adipose tissue, skeletal muscle and heart. In contrast, PPARγ is essential for long-term storage of fatty acids as triglycerides in adipocytes. However, in macrophages, PPARγ is required for β-oxidation of fatty acids in response to IL-4.
PPARs regulate expression of their target genes through association with corepressor and coactivator proteins. Ligand binding results in a conformation change in the receptor, resulting in release of corepressor and recruitment of coactivator proteins. Endogenous ligands for PPARs include native and modified fatty acids, prostaglandins, leukotrienes and phospholipids. Fibric acids and thiazolidinediones are synthetic activators of PPARα and γ, and are currently used clinically to treat hypertriglyceridemia and type 2 diabetes mellitus, respectively.
Additionally, synthetic PPARγ ligands have been in clinical use for decades as insulin-sensitizing agents (126). These drugs were originally thought to operate through direct insulin sensitization of adipocytes (127–129); however, recent studies utilizing myeloid-specific deletion of PPARγ have demonstrated that the macrophage is an important site of action where they inhibit inflammation as well as promote the elaboration of insulin-sensitizing signals (67).
CONCLUSIONS
The rapid rise of humans from near-chronic starvation and universal parasitic infection to our current state of dietary excess and immunologic leisure has left our physiology feeling a bit dated. Not surprisingly, evolution paid little attention to the management of caloric excess and instead adapted to optimize caloric efficiency (130). Equally unexpected is that this optimal efficiency would be associated with the alternative immune phenotype associated with chronic infection with Th2-biasing parasites. Indeed, it seems that the twin threats of starvation and infection drove the co-evolution of the immune and metabolic systems into a single interconnected network with overlapping regulation and function. This system maintains homeostasis by harnessing the exquisite sensitivity and specificity of the immune system to regulate metabolic circumstance. As such, the alternative phenotype necessary to control chronic parasitic infections provides a basal stimulus directing optimally efficient oxidative metabolism. When the threat of an invading pathogen is detected, the inflammatory activation of the previously alternatively activated tissue macrophages rapidly induces an insulin-resistant metabolic state intended to shunt large amounts of glucose and other metabolic substrates into the periphery for unfettered use by the immune system to quickly eradicate the offending organism (23; 131).
In context of metabolic disease, the eradication of parasitic infections by modern sanitation has removed the basal alternative stimulus while our newfound caloric wealth and sedentary lifestyle provide novel stimuli misinterpreted as classical activation cues. The maladaptive constellation of responses mounted by our elegant yet somewhat anachronistic immune and metabolic systems is the metabolic syndrome.
This evolutionary perspective immediately suggests therapeutic strategies aimed at returning the immune and metabolic systems to their homeostatic states. Frequent exercise and balanced, eucaloric diets dominated by unsaturated fatty acids are the most obvious interventions as well as those most well-supported by experimental evidence. Rather than reintroducing parasitic infections, pharmacologic interventions such as those discussed above would serve to re-establish the alternative phenotype within inflamed tissues. Regardless of the approach, an appreciation of metabolic syndrome as a derangement of tissue macrophage activation will help guide future therapeutic strategies.
SUMMARY POINTS.
Obesity and metabolic syndrome are diseases of macrophage activation in adipose tissue and liver.
Chronic inflammation is the common mechanism by which obesity and metabolic syndrome are initiated and sustained.
Obesity and metabolic syndrome are accompanied by a shift in macrophage activation from the protective alternative state to the maladaptive classical response.
Oxidative metabolism fuels alternative macrophage activation, a metabolic adaptation that is transcriptionally controlled by STAT6, PPARγ and PGC-1β.
While IL-4/STAT6 signaling provides the instruction for alternative activation, PPARγ and δ signaling sustains this program of macrophage activation in metabolic tissues.
Disruption of PPARγ or δ in myeloid cells impairs alternative activation in adipose tissue and liver, and worsens diet-induced metabolic disease.
Obesity and metabolic syndrome are as much products of the loss of alternatively activated macrophages as the presence of classically activated macrophages.
Pharmacologic approaches must address both the re-establishment of protective alternative functions as well as the inhibition of deleterious classical activities.
Acknowledgments
We thank A. Loh for valuable comments on this review. This work was supported by grants from: NIH (DK076760, HL076746, DK081405), American Diabetes Association, Larry L. Hillblom Foundation Network Grant and a NIH Director’s Pioneer Award (1DP1OD006415) to A.C.
ACRONYMS
- AAMs
alternatively activated macrophages
- CAMs
classically activated macrophages
- LPS
lipopolysaccharide
- TLR
toll-like receptor
- BMT
bone marrow transplantation
- ATMs
adipose tissue macrophages
- PAMP
pathogen associated molecular patterns
- TNFα
tumor necrosis factor α
- NF-κB
nuclear factor κB
- JNK
Jun N-terminal kinase
- IKK
inhibitor of κB kinase
- PPAR
peroxisome proliferator activated receptor
- ER
endoplasmic reticulum
- Th
T helper
Footnotes
MINI-GLOSSARY
The mini-glossary of key terms is the same as the acronym list.
DISCLOSURE STATEMENT
The authors declare that they have no competing financial interests, memberships or funding that affected the objectivity of this review.
LITERATURE CITED
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Correction: actual causes of death in the United States, 2000. JAMA. 2005;293:293–4. doi: 10.1001/jama.293.3.293. [DOI] [PubMed] [Google Scholar]
- 4.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 5.Reaven GM. Multiple CHD risk factors in type 2 diabetes: beyond hyperglycaemia. Diabetes Obes Metab. 2002;4(Suppl 1):S13–8. doi: 10.1046/j.1462-8902.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Metabolic complications of obesity. Endocrine. 2000;13:155–65. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- 7.O’Rahilly S, Farooqi IS. Human obesity as a heritable disorder of the central control of energy balance. Int J Obes (Lond) 2008;32(Suppl 7):S55–61. doi: 10.1038/ijo.2008.239. [DOI] [PubMed] [Google Scholar]
- 8.Pollex RL, Hegele RA. Genetic determinants of the metabolic syndrome. Nat Clin Pract Cardiovasc Med. 2006;3:482–9. doi: 10.1038/ncpcardio0638. [DOI] [PubMed] [Google Scholar]
- 9.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–44. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 10.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–5. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 12.Olefsky J, Glass C. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol. 2010;72:1–28. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 13.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Barnes GT, Yang Q, Tan G, Yang D, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–3. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 17.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 18.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–3. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 19.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–77. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 20.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 21.Cramer T, Johnson RS. A Novel Role for the Hypoxia Inducible Transcription Factor HIF-1alpha: Critical Regulation of Inflammatory Cell Function. Cell Cycle. 2003;2:192–3. [PubMed] [Google Scholar]
- 22.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 23.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–26. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight SC. Specialized perinodal fat fuels and fashions immunity. Immunity. 2008;28:135–8. doi: 10.1016/j.immuni.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Westcott E, Windsor A, Mattacks C, Pond C, Knight S. Fatty acid compositions of lipids in mesenteric adipose tissue and lymphoid cells in patients with and without Crohn’s disease and their therapeutic implications. Inflamm Bowel Dis. 2005;11:820–7. doi: 10.1097/01.mib.0000179213.80778.9a. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–5. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 28.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, et al. T-lymphocyte Infiltration in Visceral Adipose Tissue. A Primary Event in Adipose Tissue Inflammation and the Development of Obesity-Mediated Insulin Resistance. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 29.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 30.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 32.Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, et al. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222–6. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- 33.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 34.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–4. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 35.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–7. [PubMed] [Google Scholar]
- 36.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–8. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 38.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 39.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 40.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–7. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 41.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–29. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–9. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 45.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 46.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 47.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 48.Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–63. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 49.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276:5738–46. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 52.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol. 2001;2:627–33. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 53.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–18. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 56.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 58.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 59.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–97. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–95. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–69. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marathe C, Bradley MN, Hong C, Chao L, Wilpitz D, et al. Preserved glucose tolerance in high-fat-fed C57BL/6 mice transplanted with PPARgamma−/−, PPARdelta-/−, PPARgammadelta−/−, or LXRalphabeta−/− bone marrow. J Lipid Res. 2009;50:214–24. doi: 10.1194/jlr.M800189-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen- presenting cells. Immunity. 1999;10:137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 70.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–74. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winer S, Chan Y, Paltser G, Truong D, Tsui H, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009 doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 76.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–57. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 78.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22(Suppl 1):S20–7. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 79.Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med. 2009;87:679–95. doi: 10.1007/s00109-009-0464-1. [DOI] [PubMed] [Google Scholar]
- 80.Clementi AH, Gaudy AM, van Rooijen N, Pierce RH, Mooney RA. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim Biophys Acta. 2009;1792:1062–72. doi: 10.1016/j.bbadis.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–57. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neyrinck AM, Cani PD, Dewulf EM, De Backer F, Bindels LB, Delzenne NM. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochem Biophys Res Commun. 2009;385:351–6. doi: 10.1016/j.bbrc.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 83.Diehl AM. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1–5. doi: 10.1152/ajpgi.00384.2001. [DOI] [PubMed] [Google Scholar]
- 84.Kien CL. Dietary interventions for metabolic syndrome: role of modifying dietary fats. Curr Diab Rep. 2009;9:43–50. doi: 10.1007/s11892-009-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–83. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 86.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, et al. Adiponectin Promotes Macrophage Polarization toward an Anti-inflammatory Phenotype. J Biol Chem. 2010;285:6153–60. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 88.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 89.Neumeier M, Weigert J, Schaffler A, Wehrwein G, Muller-Ladner U, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–8. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 90.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 91.Bergouignan A, Momken I, Schoeller DA, Simon C, Blanc S. Metabolic fate of saturated and monounsaturated dietary fats: the Mediterranean diet revisited from epidemiological evidence to cellular mechanisms. Prog Lipid Res. 2009;48:128–47. doi: 10.1016/j.plipres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–46. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 93.Funaki M. Saturated fatty acids and insulin resistance. J Med Invest. 2009;56:88–92. doi: 10.2152/jmi.56.88. [DOI] [PubMed] [Google Scholar]
- 94.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc Immunol Rev. 2009;15:6–41. [PubMed] [Google Scholar]
- 96.Williamson RT. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br Med J. 1901;1:760–2. doi: 10.1136/bmj.1.2100.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reid J, Macdougall AI, Andrews MM. Aspirin and diabetes mellitus. Br Med J. 1957;2:1071–4. doi: 10.1136/bmj.2.5053.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 99.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 100.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 101.Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-alpha blockade on metabolic syndrome components in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61–73. doi: 10.1111/j.1529-8019.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 102.Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–25. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 103.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–13. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 104.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6:358–70. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 106.Arora M, Chen L, Paglia M, Gallagher I, Allen JE, et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:7777–82. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med. 2005;15:202–6. doi: 10.1016/j.tcm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 110.Schonbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109:II18–26. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- 111.Xiao H, Yang CS. Combination regimen with statins and NSAIDs: a promising strategy for cancer chemoprevention. Int J Cancer. 2008;123:983–90. doi: 10.1002/ijc.23718. [DOI] [PubMed] [Google Scholar]
- 112.Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2010;87:98–107. doi: 10.1016/j.diabres.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Furuya DT, Poletto AC, Favaro RR, Martins JO, Zorn TM, Machado UF. Anti-inflammatory effect of atorvastatin ameliorates insulin resistance in monosodium glutamate-treated obese mice. Metabolism. 2010;59:395–9. doi: 10.1016/j.metabol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 114.Kanno H, Iwai M, Inaba S, Senba I, Nakaoka H, et al. Improvement of glucose intolerance by combination of pravastatin and olmesartan in type II diabetic KK-A(y) mice. Hypertens Res. 2009;32:706–11. doi: 10.1038/hr.2009.81. [DOI] [PubMed] [Google Scholar]
- 115.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis. 2009;204:483–90. doi: 10.1016/j.atherosclerosis.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Naples M, Federico LM, Xu E, Nelken J, Adeli K. Effect of rosuvastatin on insulin sensitivity in an animal model of insulin resistance: evidence for statin-induced hepatic insulin sensitization. Atherosclerosis. 2008;198:94–103. doi: 10.1016/j.atherosclerosis.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 117.Hwu CM, Kwok CF, Chen HS, Shih KC, Lee SH, et al. Lack of effect of simvastatin on insulin sensitivity in Type 2 diabetic patients with hypercholesterolaemia: results from a double-blind, randomized, placebo-controlled crossover study. Diabet Med. 1999;16:749–54. doi: 10.1046/j.1464-5491.1999.00113.x. [DOI] [PubMed] [Google Scholar]
- 118.Gannage-Yared MH, Azar RR, Amm-Azar M, Khalife S, Germanos-Haddad M, et al. Pravastatin does not affect insulin sensitivity and adipocytokines levels in healthy nondiabetic patients. Metabolism. 2005;54:947–51. doi: 10.1016/j.metabol.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 119.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- 120.Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20:379–85. doi: 10.1097/MOL.0b013e32832fa5c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 123.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302:453–7. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 125.Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–7. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–6. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 127.Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–61. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 128.He W, Barak Y, Hevener A, Olson P, Liao D, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–4. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 130.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 131.Diangelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]