Background
Caffeine is a naturally occurring stimulant found in coffee, tea, chocolate, and used as an additive in other beverages and adjuvant analgesic in some pain medications [1,2]. It has long been known that genetic variation influences caffeine responses, indeed caffeine has been used as a probe drug for phenotyping CYP1A2 activity [3]. This brief summary highlights the candidate genes involved in the caffeine metabolism pathway and discusses the pharmacogenomic (PGx) variants, both pharmacokinetic and pharmacodynamic, their interaction with caffeine and its impact on disease risk. Caffeine is the most widely used drug in the world [4]. Since its use is so widespread it can be hard to assess the effect of the drug in isolation, and often PGx associations have been studied on the basis of intake of coffee or caffeinated beverages. These are also discussed here. Caffeine acts through multiple mechanisms, the most important of which is the antagonism of adenosine receptors (ADORA1 and ADORA2A) [2]. Recent studies have suggested a role for caffeine in neuroprotection and as a potential treatment for Parkinson disease (PD) [5]. For caffeine to be applied as a therapeutic agent in genomic medicine, it is important to examine the evidence and limitations of the current knowledge of caffeine PGx.
Metabolism
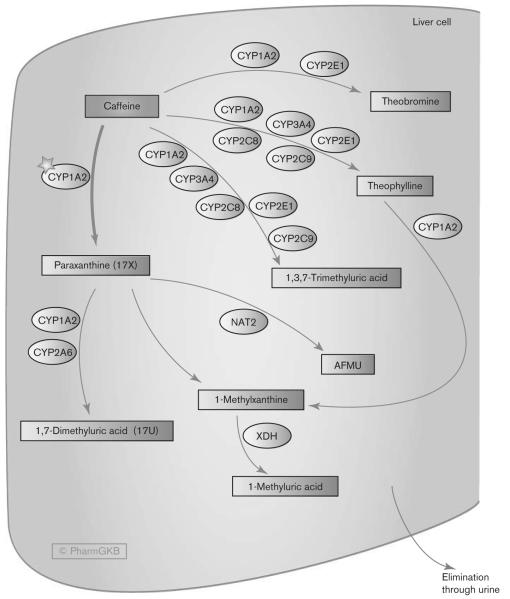
Caffeine is almost completely metabolized with 3% or less being excreted unchanged in urine [3,6]. The main route of metabolism in humans (70–80%) is through N-3-demethylation to paraxanthine also known as 1,7-dimethylxanthine or 17X [3,6,7] (see Fig. 1). This reaction is carried out by CYP1A2 in the liver [6]. Experiments with human liver microsomes estimate that 1-N-demethylation to theobromine accounts for approximately 7 to 8% of caffeine metabolism with 7-N-demethylation to theophylline also around 7 to 8% [8]. The remaining 15% of caffeine undergoes C-8 hydroxylation to form 1,3,7-trimethyluric acid [8].
Fig. 1.
Stylized liver cell depicting candidate genes involved in the pharmacokinetics of caffeine. The major route of metabolism to paraxanthine is highlighted by a bold arrow, and the key enzyme, CYP1A2, by a star. A fully interactive version is available online at http://www.pharmgkb.org/pathway/PA165884757. AFMU, 5-acetylamino-6-formylamino-3-methyluracil; CYP, cytochrome P450; NAT2, N-acetyl transferase 2; 17U, 1,7-dimethyluric acid; 17X, 1,7-dimethylxanthine; XDH, xanthine dehydrogenase.
CYP1A2 is responsible for more than 95% of the primary metabolism of caffeine [9]. Therefore, caffeine is used as a probe drug for CYP1A2 activity with the relative ratios of urinary metabolites used as an indicator of the flux through different parts of the pathway [6]. Other than paraxanthine, the major metabolites of caffeine in urine are 1-methylxanthine (1X), 1-methyluric acid (1U), 5-acetylamino-6-formylamino-3-methyluracil (AFMU), and 1,7-dimethyluric acid (17U) [6]. These are formed by the secondary metabolism of paraxanthine by cytochrome P450 (CYP)1A2, CYP2A6, N-acetyltransferase 2, and xanthine dehydrogenase (also known as xanthine oxidase) [6]. In vitro studies in cell lines show involvement of CYP2E1 in the formation of theobromine and theophylline, whereas studies of recombinant proteins in microsomes do not support this but instead suggest that it contributes to the formation of 1,3,7-trimethyluric acid [8,10]. Microsome experiments have shown that CYP2C8, CYP2C9, and CYP3A4 also participate in the primary metabolism of caffeine [3,8,10]. The intrinsic clearance of other CYPs compared with CYP1A2 is lower for all routes of metabolism and lower by a factor of 10 when compared with the 3-demethylation reaction of CYP1A2. However, when comparing the intrinsic clearance for the other branches of the pathway, several are of the same order as CYP1A2 (see Table 2 in Kot and Daniel [8]). Although the impact of candidate genes other than CYP1A2 may have limited relevance in the majority of cases, in situations where CYP1A2 activity is altered, such as coadministration of other CYP1A2-metabolized drugs, the relative flux through other parts of the pathway and other polymorphic enzymes may become more important.
Caffeine has a half-life of 4 to 5 h, which may be prolonged in patients with hepatic diseases, infants and neonates (up to 100 h), or during pregnancy [6]. Smoking increases clearance of caffeine because of its actions on CYP1A2 [11] (see below and PharmGKB CYP1A2 VIP at http://www. pharmgkb.org/search/annotatedGene/cyp1a2/index.jsp).
Pharmacogenomics
There have been several studies that examined the PGx of caffeine (see Table 1 for summary). Most have looked at the role of variants in CYP1A2 with several also considering those in ADORA2A and have found associations with various phenotypes (discussed below).
Table 1.
Summary of pharmacogenomic studies of caffeine indicating variants or alleles tested, phenotypes associated, and details of type of caffeine and population
| Allele/genotype | Associated phenotype | Reference | Study parameters |
|---|---|---|---|
|
CYP1A2 rs762551
| |||
| *1F/*1F haplotype, rs762551 Genotype AA |
Increased caffeine metabolism compared with non-*1F carriers |
17370067 [12] | Caffeine intake: 100 mg caffeine dose Study number and race: Swedish smokers, n= 35 (the effect was not seen in Swedish nonsmokers, or in a cohort of Korean smokers, n =28 with low *1F allele frequency) |
| rs762551 Genotype AA |
Increased caffeine metabolism | 20390257 [13] | Caffeine intake: heavy coffee consumers (the association was not seen in nonheavy consumers) Study number and race: Swedish, n= 42, Serbian, n=17 |
| rs762551 Allele C |
Not associated with habitual consumption of caffeine |
17616786 [14] | Caffeine intake: habitual consumption of caffeine Study number and race: Hispanic Americans (n= 2735) |
| rs762551 Genotype CC |
Decreased risk of Parkinson’s disease in coffee drinkers compared with genotype AA |
21281405 [15] | Caffeine intake: association was seen in coffee consumers compared with those who never consume coffee Study number and race: mixed population, n= 948 cases, n= 1286 controls |
| rs762551 | Not associated with caffeine-related protection from Parkinson’s disease |
18759349 [16] | Caffeine intake: coffee drinking habits assessed Study number and race: mixed population, n= 782 matched case–control pairs |
| rs762551 | Not associated with caffeine-related protection from Parkinson’s disease |
18075470 [17] | Caffeine intake: caffeine consumption Study number and race: Asian, n= 418 cases, n =468 controls |
| rs762551 Genotype CC and AC |
Increased risk of myocardial infarction in coffee consumers |
16522833 [18] | Caffeine intake: coffee intake questionnaire Study number and race: Hispanic Americans, n=2104 cases, n=2104 controls |
| rs762551 Genotype CC and AC |
Decreased risk of breast cancer, in carriers of BRCA1 mutations |
17507615 [19] | Caffeine intake: coffee consumption (either caffeinated or decaffeinated), compared with individuals who have never consumed coffee Study number and race: White, n=89 cases and n= 49 controls (coffee consumers) vs. n= 30 controls and n= 36 cases (never consumed coffee) |
| rs762551 | Not associated with risk of ovarian cancer and caffeine, coffee, or tea intake |
18941913 [20] | Caffeine intake: high vs. low caffeine consumption Study number and race: n =1354 cases, n =1851 controls (unknown race) |
| rs762551 | Not associated with risk of bladder cancer and coffee consumption |
18798002 [21] | Caffeine intake: coffee consumption (cups per day) Study number and race: Spanish, n= 1034 cases, n= 911 controls |
| rs762551 Genotype CC |
Increased risk of recurrent pregnancy loss | 15849225 [22] | Caffeine intake: maternal coffee consumption Study number and race: Japanese, n= 58 cases, n= 148 controls |
| rs762551 Genotype CC and AC |
Increased risk for neural tube defects | 20641098 [23] | Caffeine intake: maternal coffee consumption Study number and race: mixed population, n= 306 cases, n= 669 controls |
|
CYP1A2 Alleles
| |||
| *1K allele (Key SNP: –730C>T rs12720461) |
Reduced caffeine metabolism, compared with *1A, *1F, or *1J alleles |
12920202 [24] | Caffeine intake: 100 mg caffeine dose Study number and race: Ethiopian, n =173 (association only seen in nonsmokers, n =153) |
| *1A, *1F, *1J | No significant effect on caffeine metabolism | 12920202 [24] | Caffeine intake: 100 mg caffeine dose Study number and race: Ethiopians, n =173 (nonsmokers: n= 153, smokers, n= 20) |
| *1A, *1D, *1L, *1V, *1W alleles |
No significant effect on caffeine metabolism | 17370067 [12] | Caffeine intake: 100 mg caffeine dose Study number and race: Swedes, n =114 (nonsmokers), n= 35 (smokers), Koreans, n =121 (nonsmokers), n= 28 (smokers) |
|
Additional CYP1A2 variants
| |||
| rs2470890 Genotype CC |
Decreased risk of Parkinson’s disease in coffee drinkers |
21281405 [15] | Caffeine details: coffee consumption Study number and race: mixed population, n= 941 cases, n= 1264 controls |
| rs35694136 | Not associated with caffeine-related protection from Parkinson’s disease |
18759349 [16] | Caffeine intake: coffee drinking habits assessed Study number and race: mixed population, n= 910 matched case–control pairs |
| rs2470893 | Associated with increased coffee consumption |
21490707 [25] | Caffeine intake: coffee drinking habits assessed Study number and race: White, n = 47341 |
| rs2472297 Allele T |
Associated with increased coffee consumption |
21357676 [26] | Caffeine intake: coffee drinking habits assessed Study number and race: mixed population, n= 6611 |
|
ADORA2A rs5751876
| |||
| rs5751876 Genotype TT |
Decreased caffeine consumption | 17616786 [14] | Caffeine intake: habitual consumption of caffeine Study number and race: Hispanic American, association found in n =2735 (full cohort), n = 1767 (nonsmoker subset), and n =968 (current smokers subset) |
| rs5751876 Genotype TT |
Increased anxiety in response to caffeine | 12825092 [27] | Caffeine intake: 150 mg caffeine administration Study number and race: mixed population, n= 100 |
| rs5751876 Genotype TT |
Increased anxiety in response to caffeine | 18305461 [28] | Caffeine intake: 150 mg caffeine administration Study number and race: mixed population, n= 102 (not significant in the European-American subset in this cohort, n= 62) |
| rs5751876 Genotype TT |
Increased anxiety in response to caffeine | 20520601 [29] | Caffeine intake: initial 100 mg caffeine administered and then 150mg 90min later, or a placebo administered at both time points (capsules) Study number and race: mixed population, n= 379 [predominantly (95%) White European] |
| rs5751876 | No increase in anxiety in response to caffeine |
22012471 [30] | Caffeine intake: 150 mg caffeine administration Study number and race: White, n=110 |
| rs5751876 Genotype CC |
Increased likelihood of being sensitive to caffeine compared with genotype TT |
17329997 [31] | Caffeine intake: questionnaire of caffeine sensitivity and sleeping habits Study number: 58 cases (self-rated caffeine sensitive), 84 controls (self-rated caffeine insensitive) (race unknown) |
| rs5751876 Genotype CC |
Increased likelihood of insomnia when exposed to caffeine |
17329997 [31] | Caffeine intake: 2 doses of 200 mg caffeine or placebo, at 11 and 23 h of wakefulness Study number and race: n =19 (race unknown) |
| rs5751876 Genotype TT and CC |
Not associated with vasodilator response | 17558310 [32] | Caffeine intake: administration of adenosine, followed by caffeine (90 mg/min/dl) for 10 min Study number and race: n= 20 (race unknown) |
|
Additional ADORA2A variants
| |||
| rs2298383 Genotype CC |
Increased anxiety in response to caffeine | 18305461 [28] | Caffeine intake: 150mg caffeine administration Study number and race: European-Americans, n=62 |
| rs4822492 Genotype CC |
Increased anxiety in response to caffeine | 18305461 [28] | Caffeine intake: 150mg caffeine administration Study number and race: European-Americans, n=62 |
| rs3761422 Genotype TT |
Increased anxiety in response to caffeine | 20520601 [29] | Caffeine intake: initial 100 mg caffeine administered and then 150mg 90min later, or a placebo administered at both time points (capsules) Study number and race: mixed population, n =379 [predominantly (95%) White European] |
| rs35320474 Genotype TT |
Increased anxiety in response to caffeine | 12825092 [27] | Caffeine intake: 150mg caffeine administration Study number and race: mixed population, n =100 |
| rs3032740 | Not associated with caffeine-related protection from Parkinson’s disease |
18759349 [16] | Caffeine intake: coffee drinking habits assessed Study number and race: mixed population, n =1100 matched case–control pairs |
| rs5751862 | Not associated with increased anxiety in response to caffeine |
20520601 [29] | Caffeine intake: initial 100 mg caffeine administered and then 150mg 90min later, or a placebo administered at both time points (capsules) Study number and race: mixed population, n =379 [predominantly (95%) White European] |
| rs5760405 | Not associated with increased anxiety in response to caffeine |
20520601 [29] | Caffeine intake: initial 100 mg caffeine administered and then 150mg 90min later, or a placebo administered at both time points (capsules) Study number and race: mixed population, n =379 [predominantly (95%) White European] |
| rs11704959 | Not associated with increased anxiety in response to caffeine |
20520601 [29] | Caffeine intake: initial 100 mg caffeine administered and then 150mg 90min later, or a placebo administered at both time points (capsules) Study number and race: mixed population, n =379 [predominantly (95%) White European] |
| rs2298383 | Not associated with increased anxiety in response to caffeine (in multiple testing) |
20520601 [29] | Caffeine intake: initial 100 mg caffeine administered and then 150mg 90min later, or a placebo administered at both time points (capsules) Study number and race: mixed population, n =379 [predominantly (95%) White European] |
| rs2267076 | Not associated with increased anxiety in response to caffeine |
20520601 [29] | Caffeine intake: initial 100 mg caffeine administered and then 150mg 90min later, or a placebo administered at both time points (capsules) Study number and race: mixed population, n =379 [predominantly (95%) White European] |
|
CYP1A1 variants
| |||
| rs4646421 | Not associated with risk of bladder cancer and coffee consumption |
18798002 [21] | Caffeine intake: coffee consumption (cups per day) Study number and race: Spanish, n= 1002 cases, n= 892 controls |
| rs2198843 | Not associated with risk of bladder cancer and coffee consumption |
18798002 [21] | Caffeine intake: coffee consumption (cups per day) Study number and race: Spanish, n= 1018 cases, n= 919 controls |
| rs2472299 | Not associated with risk of bladder cancer and coffee consumption |
18798002 [21] | Caffeine intake: coffee consumption (cups per day) Study number and race: Spanish, n= 1000 cases, n= 890 controls |
|
CYP2A6 alleles
| |||
| *4/*9 and *9/*9 | Reduced metabolite ratio of 17U/17X | 20155256 [33] | Caffeine intake: 100 mg caffeine administration Study number and race: Serbian, n= 100 |
|
CYP2E1 variants
| |||
| rs2070676 | Not associated with risk of bladder cancer and coffee consumption |
18798002 [21] | Caffeine intake: coffee consumption (cups per day) Study number and race: Spanish, n= 1018 cases, n= 918 controls |
| rs8192766 | Not associated with risk of bladder cancer and coffee consumption |
18798002 [21] | Caffeine intake: coffee consumption (cups per day) Study number and race: Spanish, n= 1017 cases, n= 919 controls |
|
AHR variants
| |||
| rs4410790 | Associated with increased coffee consumption |
21490707 [25] | Caffeine intake: coffee drinking habits assessed Study number and race: White, n=47341 |
| rs6968865 Allele T |
Associated with increased coffee consumption |
21357676 [26] | Caffeine intake: coffee drinking habits assessed Study number and race: mixed population, n =6611 |
|
GRIN2A variants
| |||
| rs4998386 Allele T |
Decreased risk of Parkinson’s disease in heavy coffee drinkers |
21876681 [34] | Caffeine intake: heavy vs. light caffeine consumption Study number and race: White, n= 1458 cases, n= 931 controls |
The CYP1A2*1F allele is the most commonly studied variant with respect to caffeine. The variant that defines this haplotype is rs762551 (CYP1A2: – 163C > A) [24]. A study of CYP1A2*1F, where other haplotypes containing rs762551 were excluded, showed that rs762551AA was associated with increased metabolism of caffeine in Swedish smokers as well as in Swedish and Serbian heavy coffee consumers [12,13]. Other haplotypes that included rs762551 (CYP1A2*1L, *1V, and *1W) did not have significantly altered metabolism of caffeine [12]. A previous study reported association of the CYP1A2*1K allele with significantly reduced CYP1A2 activity in nonsmokers compared with *1A or *1F, using caffeine as a probe substrate [24]. To date, no studies have shown the mechanism by which the intronic rs762551 variant influences CYP1A2 activity and it may be that other variants in linkage with this locus may be responsible for the phenotypes (for more details on CYP1A2 see http://www.pharmgkb.org/vip/PA27093).
Poor metabolizer variants in CYP2A6, which is the major enzyme responsible for the formation of 17U, influence the ratio of 17U to 17X [33]. Nonsmoking individuals with two inactive alleles (CYP2A6*9 homozygotes and *4/*9 heterozygotes) had lower values of 17U/17X than those with one inactive allele, whose values were lower than those with two active alleles (CYP2A6*1A, *1B1, or *1B1 × 2) [33]. The effect was less pronounced in smokers, although this may be because of smaller sample numbers [33].
Studies examining the effects of variants in the adenosine receptor ADORA2A and caffeine-related behavior and responses have had mixed results. ADORA2A rs5751876 TT is associated with decreased habitual consumption of caffeine as compared with genotypes CC + CT, and this association is more pronounced in smokers [14]. ADOR-A2A rs5751876 TT, rs2298383 CC, and rs4822492 CC were all associated with increased anxiety in response to caffeine in a healthy population that did not routinely consume much caffeine [28]. When the analysis was restricted to European-Americans it lacked sufficient power and no association was seen [28]. The association with ADORA2A rs5751876 TT and increased caffeine-induced anxiety were also seen in a mostly White European nonsmoking or light smoking population [29], and a cohort of American college students with relatively low routine caffeine intake [27]. Although these studies were relatively small, the association did hold up to multiple testing and false discovery correction. A recent study in White healthy volunteers did not replicate this association, although the authors state that this may have been because of differences between the American and German anxiety measurement assessment scales or dose of caffeine [30]. They did observe qualitative differences in startle reflex that were more pronounced in women and involved the interaction of rs5751876 genotype, caffeine, and type of stimuli, although they did not compare genotype groups directly [30]. ADORA2A rs5751876 is not associated with vasodilator response when exposed to adenosine and caffeine [32]. Conversely, the CC genotype for ADORA2A rs5751876 is associated with increased likelihood of being sensitive to caffeine and increased likelihood of insomnia when exposed to caffeine [31].
The heritability of coffee consumption has been estimated at around 50% [33]. Recent independent genome wide association studies (GWAS) have identified variants in CYP1A2 and AHR that influence caffeine intake [25,26]. In a meta-analysis of four large GWAS studies from Europe and the USA (totaling 6611 subjects), an effect of approximately 0.2 cups a day per allele was observed for rs2472297 T in the regulatory region of CYP1A2 and rs6968865 T in AHR [26]. Another large meta-analysis of GWAS (47 341 White individuals) associated single nucleotide polymorphisms rs2472304 between CYP1A2 and CYP1A1 and rs4410790 near AHR with habitual caffeine intake [25]. The polymorphic sites identified in the coffee consumption GWAS were not in linkage with any known functional variants. However, the regions where these variants are located are involved in the transcriptional regulation of AHR and CYP1A2, therefore the variants may have a functional consequence or be linked to functional variants, however, these regions have not been fully characterized.
Gene-drug-disease relationships
Some studies have looked at the relationship between variants, caffeine, and disease risk. Most have looked at PD, a few studies have looked at cancer risk, some looked at risk for pregnancy complications and one study investigated risk for cardiovascular events. It should be noted that the associations seen in these studies have not been replicated. See Table 1 for a list of variants tested and study information.
In the Parkinson’s Epidemiology and Genetic Associations Studies in the United States, the CYP1A2 rs762551 genotype CC and rs2470890 genotype CC were associated with decreased risk of PD in coffee drinkers [15]. Although two variants in ADORA2A were associated with reduced risk for PD, there was no caffeine interaction for ADORA2A noted in this study [15]. As mentioned above, the CC genotype of rs762551 is not the genotype associated with inducibility of CYP1A2 in response to smoking or heavy coffee drinking, which may suggest that caffeine is processed more slowly and has a greater effect in these individuals. Subsequent studies, however, have failed to replicate these associations. None of the variants tested in ADORA2A (rs3032740) or CYP1A2 (rs35694136 and rs762551) were associated with caffeine-related protection from PD in a study of people from Midwest USA with mostly European ancestry [16]. A study in an Asian population also failed to find any interaction between CYP1A2 rs762551, caffeine, and PD, although a significant association was seen between moderate-to-high caffeine intake and lower risk for PD [17]. Since it is likely that the rs762551 variant in CYP1A2 is not the functional variant but only in linkage with it, the different haplotype structures in the different populations may have affected the ability to reproduce the association. A recent GWAS identified a new candidate gene for PD [34]. The GRIN2A rs4998386 T variant carriers had lower risk for PD among heavy coffee drinkers compared with the CC genotype [34]. The association was not seen in those who drink no or less than the median intake of coffee [34]. GRIN2A encodes an NMDA-glutamate-receptor subunit and regulates excitatory neurotransmission in the brain [34]. Caffeine has also been proposed as a modulator of Alzheimer disease and other dementias but no studies have yet reported the role of genomic variants in this effect [35].
In a study of patients with breast cancer-predisposing BRCA1 variants, the C allele of CYP1A2 rs762551 was associated with decreased risk for breast cancer in coffee drinkers compared with those who never consumed coffee [19]. This protective effect of coffee was not seen in the rs762551 AA homozygotes [19]. This was a very small study and has not been replicated. Studies of CYP1A2 and coffee consumption and risk for ovarian cancer [20] or bladder cancer [21] found no association. In-vitro studies suggest that the protective effects of caffeine against cancer may be because of growth inhibition through phosphatase and tensin homolog and the phosphatidyl inositol 3-kinase/protein kinase B pathway [36]. Phosphatase and tensin homolog, phosphatidyl inositol 3-kinase, and protein kinase B are part of the Ras signaling pathway involved cellular growth and are the targets of several new anticancer drugs [37].
Studies of the effects of caffeine on pregnancy outcomes have shown increased risk for spontaneous pregnancy loss in women with high caffeine intake particularly among smokers [38,39]. Early studies that used metabolite phenotyping rather than genotyping suggested that women with low activity of caffeine metabolizing enzymes xanthine dehydrogenase or N-acetyltransferase 2 might be at increased risk for recurrent pregnancy loss, but results for CYP1A2 were unclear [40]. However, another study found that high CYP1A2 activity was associated with risk for pregnancy loss and risk was increased with increasing caffeine intake [41]. Studies examining the role of CYP1A2 variants showed an association between recurrent pregnancy loss, homozygous CYP1A2*1F, and caffeine intake above 100 mg per day, with an even greater risk for intake above 300 mg/day [22]. Another recent study showed that infant CYP1A2*1F genotype and maternal caffeine intake were associated with risk for neural tube defects [23].
In a study of South Americans, the authors reported that ‘slow’ caffeine metabolizers, than those with the CYP1A2 rs762551 C allele, had increased risk of myocardial infarction [18]. However, this has not been validated. This study was criticized by others because the slow caffeine metabolizer phenotype for rs762551 C has only been observed in the context of smoking or heavy coffee consumption and this was not addressed in the study [42].
Conclusion
Since the use of caffeine is so widespread, knowledge of its pharmacokinetics and pharmacogenomics, the genes and variants that impact its metabolism and effects, is of importance for public health. Although there may be some beneficial effects of caffeine or coffee intake for particular individuals in the prevention of diseases, for others caffeine use may be associated with increased risk of disease, drug interactions, adverse events, and harm. Current studies have failed to validate clear relationships between gene variants, caffeine intake, and phenotypes. Work is needed to better define the functional variants that are involved in caffeine response. In addition, the components of coffee in addition to caffeine should be considered as these may have confounding effects in their actions on AHR and CYP1A2.
Acknowledgements
This work was supported by the NIH/NIGMS (R24 GM61374).
Footnotes
Conflicts of interest There are no conflicts of interest.
References
- 1.Mandel HG. Update on caffeine consumption, disposition and action. Food Chem Toxicol. 2002;40:1231–1234. doi: 10.1016/s0278-6915(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 2.Sawynok J. Methylxanthines and pain. Handb Exp Pharmacol. 2011;200:311–329. doi: 10.1007/978-3-642-13443-2_11. [DOI] [PubMed] [Google Scholar]
- 3.Kot M, Daniel WA. Caffeine as a marker substrate for testing cytochrome P450 activity in human and rat. Pharmacol Rep. 2008;60:789–797. [PubMed] [Google Scholar]
- 4.Donovan JL, DeVane CL. A primer on caffeine pharmacology and its drug interactions in clinical psychopharmacology. Psychopharmacol Bull. 2001;35:30–48. [PubMed] [Google Scholar]
- 5.Prediger RD. Effects of caffeine in Parkinson’s disease: from neuroprotection to the management of motor and non-motor symptoms. J Alzheimers Dis. 2010;20(Suppl 1):S205–S220. doi: 10.3233/JAD-2010-091459. [DOI] [PubMed] [Google Scholar]
- 6.Begas E, Kouvaras E, Tsakalof A, Papakosta S, Asprodini EK. In vivo evaluation of CYP1A2, CYP2A6, NAT-2 and xanthine oxidase activities in a Greek population sample by the RP-HPLC monitoring of caffeine metabolic ratios. Biomed Chromatogr. 2007;21:190–200. doi: 10.1002/bmc.736. [DOI] [PubMed] [Google Scholar]
- 7.Benowitz NL, Jacob P, III, Mayan H, Denaro C. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin Pharmacol Ther. 1995;58:684–691. doi: 10.1016/0009-9236(95)90025-X. [DOI] [PubMed] [Google Scholar]
- 8.Kot M, Daniel WA. The relative contribution of human cytochrome P450 isoforms to the four caffeine oxidation pathways: an in vitro comparative study with cDNA-expressed P450s including CYP2C isoforms. Biochem Pharmacol. 2008;76:543–551. doi: 10.1016/j.bcp.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53:503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- 10.Gu L, Gonzalez FJ, Kalow W, Tang BK. Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenetics. 1992;2:73–77. doi: 10.1097/00008571-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76:178–184. doi: 10.1016/j.clpt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Ghotbi R, Christensen M, Roh HK, Ingelman-Sundberg M, Aklillu E, Bertilsson L. Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype-phenotype relationship in Swedes and Koreans. Eur J Clin Pharmacol. 2007;63:537–546. doi: 10.1007/s00228-007-0288-2. [DOI] [PubMed] [Google Scholar]
- 13.Djordjevic N, Ghotbi R, Jankovic S, Aklillu E. Induction of CYP1A2 by heavy coffee consumption is associated with the CYP1A2 -163C > A polymorphism. Eur J Clin Pharmacol. 2010;66:697–703. doi: 10.1007/s00228-010-0823-4. [DOI] [PubMed] [Google Scholar]
- 14.Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86:240–244. doi: 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- 15.Popat RA, Van Den Eeden SK, Tanner CM, Kamel F, Umbach DM, Marder K, et al. Coffee, ADORA2A, and CYP1A2: the caffeine connection in Parkinson’s disease. Eur J Neurol. 2011;18:756–765. doi: 10.1111/j.1468-1331.2011.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Facheris MF, Schneider NK, Lesnick TG, de Andrade M, Cunningham JM, Rocca WA, et al. Coffee, caffeine-related genes, and Parkinson’s disease: a case–control study. Mov Disord. 2008;23:2033–2040. doi: 10.1002/mds.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan EK, Chua E, Fook-Chong SM, Teo YY, Yuen Y, Tan L, et al. Association between caffeine intake and risk of Parkinson’s disease among fast and slow metabolizers. Pharmacogenet Genomics. 2007;17:1001–1005. doi: 10.1097/FPC.0b013e3282f09265. [DOI] [PubMed] [Google Scholar]
- 18.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295:1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- 19.Kotsopoulos J, Ghadirian P, El-Sohemy A, Lynch HT, Snyder C, Daly M, et al. The CYP1A2 genotype modifies the association between coffee consumption and breast cancer risk among BRCA1 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2007;16:912–916. doi: 10.1158/1055-9965.EPI-06-1074. [DOI] [PubMed] [Google Scholar]
- 20.Kotsopoulos J, Vitonis AF, Terry KL, De Vivo I, Cramer DW, Hankinson SE, et al. Coffee intake, variants in genes involved in caffeine metabolism, and the risk of epithelial ovarian cancer. Cancer Causes Control. 2009;20:335–344. doi: 10.1007/s10552-008-9247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva CM, Silverman DT, Murta-Nascimento C, Malats N, Garcia-Closas M, Castro F, et al. Coffee consumption, genetic susceptibility and bladder cancer risk. Cancer Causes Control. 2009;20:121–127. doi: 10.1007/s10552-008-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sata F, Yamada H, Suzuki K, Saijo Y, Kato EH, Morikawa M, et al. Caffeine intake, CYP1A2 polymorphism and the risk of recurrent pregnancy loss. Mol Hum Reprod. 2005;11:357–360. doi: 10.1093/molehr/gah175. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt RJ, Romitti PA, Burns TL, Murray JC, Browne ML, Druschel CM, et al. Caffeine, selected metabolic gene variants, and risk for neural tube defects. Birth Defects Res A Clin Mol Teratol. 2010;88:560–569. doi: 10.1002/bdra.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aklillu E, Carrillo JA, Makonnen E, Hellman K, Pitarque M, Bertilsson L, et al. Genetic polymorphism of CYP1A2 in Ethiopians affecting induction and expression: characterization of novel haplotypes with single-nucleotide polymorphisms in intron 1. Mol Pharmacol. 2003;64:659–669. doi: 10.1124/mol.64.3.659. [DOI] [PubMed] [Google Scholar]
- 25.Cornelis MC, Monda KL, Yu K, Paynter N, Azzato EM, Bennett SN, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet. 2011;7:e1002033. doi: 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulem P, Gudbjartsson DF, Geller F, Prokopenko I, Feenstra B, Aben KK, et al. Sequence variants at CYP1A1-CYP1A2 and AHR associate with coffee consumption. Hum Mol Genet. 2011;20:2071–2077. doi: 10.1093/hmg/ddr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–1702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- 28.Childs E, Hohoff C, Deckert J, Xu K, Badner J, de Wit H. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2008;33:2791–2800. doi: 10.1038/npp.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers PJ, Hohoff C, Heatherley SV, Mullings EL, Maxfield PJ, Evershed RP, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology. 2010;35:1973–1983. doi: 10.1038/npp.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domschke K, Gajewska A, Winter B, Herrmann MJ, Warrings B, Muhlberger A, et al. ADORA2A gene variation, caffeine, and emotional processing: a multi-level interaction on startle reflex. Neuropsychopharmacology. 2011:253. doi: 10.1038/npp.2011.253. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 32.Riksen NP, Franke B, van den Broek P, Smits P, Rongen GA. The 1976C > T polymorphism in the adenosine A2A receptor gene does not affect the vasodilator response to adenosine in humans in vivo. Pharmacogenet Genomics. 2007;17:551–554. doi: 10.1097/FPC.0b013e32803fb78f. [DOI] [PubMed] [Google Scholar]
- 33.Djordjevic N, Carrillo JA, Gervasini G, Jankovic S, Aklillu E. In vivo evaluation of CYP2A6 and xanthine oxidase enzyme activities in the Serbian population. Eur J Clin Pharmacol. 2010;66:571–578. doi: 10.1007/s00228-010-0785-6. [DOI] [PubMed] [Google Scholar]
- 34.Hamza TH, Chen H, Hill-Burns EM, Rhodes SL, Montimurro J, Kay DM, et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson’s disease modifier gene via interaction with coffee. PLoS Genet. 2011;7:e1002237. doi: 10.1371/journal.pgen.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 1):S117–S126. doi: 10.3233/JAD-2010-091249. [DOI] [PubMed] [Google Scholar]
- 36.Miwa S, Sugimoto N, Shirai T, Hayashi K, Nishida H, Ohnari I, et al. Caffeine activates tumor suppressor PTEN in sarcoma cells. Int J Oncol. 2011;39:465–472. doi: 10.3892/ijo.2011.1051. [DOI] [PubMed] [Google Scholar]
- 37.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez-Rojas V, de Juanes-Pardo JR, Astasio-Arbiza P, Ortega-Molina P, Gordillo-Florencio E. Spontaneous abortion in a hospital population: are tobacco and coffee intake risk factors? Eur J Epidemiol. 1994;10:665–668. doi: 10.1007/BF01719278. [DOI] [PubMed] [Google Scholar]
- 39.Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. 2003;82:182–188. doi: 10.1034/j.1600-0412.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 40.Fenster L, Quale C, Hiatt RA, Wilson M, Windham GC, Benowitz NL. Rate of caffeine metabolism and risk of spontaneous abortion. Am J Epidemiol. 1998;147:503–510. doi: 10.1093/oxfordjournals.aje.a009477. [DOI] [PubMed] [Google Scholar]
- 41.Signorello LB, Nordmark A, Granath F, Blot WJ, McLaughlin JK, Anneren G, et al. Caffeine metabolism and the risk of spontaneous abortion of normal karyotype fetuses. Obstet Gynecol. 2001;98:1059–1066. doi: 10.1016/s0029-7844(01)01575-7. [DOI] [PubMed] [Google Scholar]
- 42.Ingelman-Sundberg M, Sim SC, Nebert DW. Coffee, myocardial infarction, and CYP nomenclature. JAMA. 2006;296:764–765. doi: 10.1001/jama.296.7.764-b. author reply 765–766. [DOI] [PubMed] [Google Scholar]