Abstract
We report that females of the broad-horned flour beetle, Gnathocerus cornutus, can plastically adjust the sex ratio in their broods in response to environmental quality. Specifically, females reared in nutritionally poor environments produce broods that are 65% female, on average, with the degree of female-bias in some broods approaching 95%. In addition, females reared in nutritionally poor environments lay significantly more eggs than do females reared on standard medium, which produce broods with an even sex ratio. These effects of the mother's environment on size and sex ratio in broods are manifest even when oviposition occurs in the standard nutritional environment; indeed, the degree of female-bias increases with advancing female age despite the availability of nutritional resources to females at the time of egg laying. Our studies rule out sex-specific differences in viability early in larval development as the mechanism for the bias in sex-ratio of broods, since females reared in nutritionally poor environments have broods with hatchability and larval viability comparable to those of nonstressed females. Our studies also rule out an effect of the sire on the sex ratio in broods, since all male mates were reared on standard medium. We discuss our results in the context of theories for the evolution of plastic sex-ratios in the face of environmental deterioration and discuss how plasticity can resolve a long-standing question about the conditions underlying the evolution of biased sex ratios.
Introduction
The observation of 1:1 sex ratios among most dioecious organisms is consistent with the prediction from evolutionary theory that there should be equal investment into male and female offspring due to rare sex fitness advantage (due to negative frequency dependent selection) (Fisher 1930; Bodmer and Edwards 1960; Colwell 1981). However, biased sex ratios can evolve in a number of circumstances including local mate competition (Hamilton 1967), sex-linked genes causing unequal production of X or Y gametes in the heterogametic sex (Shaw 1958; Hamilton 1967), age, or environmental variation (Trivers and Willard 1973; Charnov and Bull 1989), or in genetically subdivided populations, in which local mate competition is important and female-biased sex ratios increase local mean fitness and are favored by among-group selection (Wilson and Colwell 1981). Facultative adjustment of sex ratio has also been observed in many systems, especially in insects with haplo-diploid sex determination (Werren 1980; Mueller 1991) and in mammals and birds with chromosomal sex determination (Wolff 1988; Kruuk et al. 1999; Kohlman 1999; Nager et al. 1999; Olsent and Cockburn 1991). These latter examples of biased sex ratio in broods are consistent with Trivers' and Willard's (1973) hypothesis (TW) that maternal condition affects the success of their sons in reproductive competition. Specifically, females in poor condition are predicted to produce a preponderance of daughters, while females in good condition are expected to produce male-biased broods.
We describe facultative adjustment of sex ratio in the broad-horned flour beetle Gnathocerus cornutus. Gnathocerus species are closely related to flour beetles in the genus, Tribolium (Angelini and Jockusch 2008), which have chromosomal sex determination (Smith and Brower 1974), and are easy to rear and manipulate in the laboratory. Adult females resemble T. castaneum males and females, but males have distinctive characteristics including modified mandibular horns, flattened dorsal projections on the head, and a pair of small cephalic horns (Fig. 1). Many species of sexually dimorphic beetles use horns in competitions to gain access to mates (e.g., Moczek and Emlen 2000; Karino et al. 2005). Likewise, male Gnathocerus beetles use their mandibular horns to compete with conspecific males for reproductive access to females (Okada et al. 2006; Okada and Miyatake 2009).
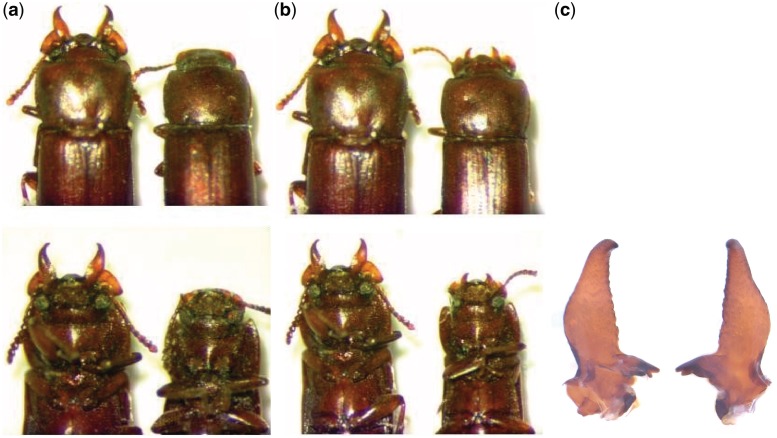
Fig. 1.
(a) Male (left) and female (right) G. cornutus [David Craig]. (b) Dorsal (top) and ventral (bottom) views of differences in horn size of male G. cornutus [David Craig]. (c) Image of a dissected mandible of a male [Franck Simmonet].
Two observations indicate that males reared in poor environments are at a disadvantage during reproductive competition relative to males reared under better conditions. First, males reared under poor environmental conditions develop smaller mandibles than do those reared in standard medium (controlling for body size and maternal condition, see “Results” section and Okada and Miyatake [2010]). Similarly, Demuth et al. (2012) showed that beetles infected with the tapeworm parasite, Hymenolepis diminuta, developed shorter horns than did uninfected beetles. Second, males with larger mandibles win fights and mate for longer durations when paired with relatively smaller-horned males (Okada and Miyatake 2009; Demuth et al. 2012). Taken together, males reared in poor environments are likely to lose bouts of reproductive competition when they encounter males from good environments. The TW hypothesis further requires that sons of mothers in poor condition develop smaller mandibles (or the environment of the expected offspring matches the environment their mother experienced). Finally, Katsuki et al. (2012) have recently shown that Gnathocerus females alter the sex ratio according to female condition (and independent of rearing environment).
In G. cornutus, variation in the environment leads to both high variance in mandible size among males and to variation in sex ratio among families. We describe this plastic variation in sex ratio in G. cornutus; it is repeatedly inducible in the laboratory and we show that it is determined by maternal condition. We also describe the effect of maternal condition on the mean and distribution of male mandibular horns and on the average size of broods. More broadly, we emphasize the importance of plasticity for the traits of groups and describe how plasticity can resolve a long-standing question about the evolution of biased sex ratios.
Materials and methods
A G. cornutus stock was obtained from the Tribolium Stock Center (Manhattan, Kansas [Kathy Leonard]) and maintained in a laboratory at Indiana University Bloomington for 5 years. During and prior to this study, beetles were reared on a 12-h day–night cycle at 27.5°C and ∼70% relative humidity. Standard medium (our “good” resource treatment) consisted of 85% organic flour, 10% fishmeal, and 5% yeast. Our experimentally manipulated poor-quality resource consisted of standard medium adulterated with 15% indigestible α-cellulose. This level was chosen arbitrarily because preliminary experiments in which females were reared in 5, 25, and 50% α-cellulose indicated that this level of nutritional severity biased the sex-ratio in broods to the same magnitude.
Small larvae (1–2 instars) from the G. cornutus stock were reared independently in standard or poor-quality medium. Because pupation is inhibited at high population density in this species (Tsuda and Yoshida 1985), beetle larvae were reared to adulthood individually in 24-well tissue-culture plates. Adults were sexed and length of the body and mandible measured to determine the effect of environment on adult phenotype when maternal condition is not manipulated. Measurements on these beetles revealed the direct effects of good and poor quality of food on body size, mandible length of males, and sex ratio.
We then used the dams from both standard medium and poor-quality medium to assess whether the sex ratio in broods is affected by the rearing condition of dams. We assessed the sex ratio of eggs and the sex ratio of the adults that develop from those eggs to determine whether differential survival contributes to bias of the sex-ratio in populations of adults. First, we mated dams from each rearing environment (standard medium, n = 10; poor quality medium, n = 20) singly with sires from standard medium (environment of good quality) so that only the dams differed in the quality of rearing environment. Under standard rearing conditions, development from egg to adult occurs within 47–57 days (Ntifo and Nowosielski-Slepowron 1973). Accordingly, after 3 months, we examined the adult offspring of each brood and assessed the density, sex ratio, mandible size, and body size (thorax width). This allowed us to assess properties of broods when offspring were allowed to develop to adulthood as a group.
We also determined whether the sex ratio of newly hatched larvae was already biased, to rule out differential survival during development accounting for deviations from a 1:1 sex ratio later in life. In order to effectively separate eggs from flour, we did not supplement flour with fish meal in this experiment, so that the medium consisted of 95% organic flour and 5% yeast. We mated each of 10 males reared in standard medium with three females reared in standard medium and three females reared in poor-quality medium (total n = 60 females); the sequence of mating was varied; half of the males were mated to females reared on standard medium first and the other half were mated to females reared in poor quality medium first. After 3 days of mating, we separated the females and placed them into individual vials from which we collected eggs by sifting every 3 days. Larvae emerging from these eggs were reared to adulthood individually in 24-well tissue-culture plates. For each dam, we recorded egg count, sex ratio in the brood, size of males' mandibles (measured as the length of the inside edge from tip to tip), and thorax width.
Due to the correlation between body size (thorax width) and mandible size, we analyzed raw mandible data as well as the residual of males' horn size regressed on thorax width (to control for body size). We also considered two measurements of the sex ratio in broods—the proportion of females per brood, and the difference between number of males and number of females for each brood. The effect of sire on sex ratio in the brood was determined by fitting a linear mixed-effects model to our data using environment as a fixed effect, with or without a random effect of sire; we used the lmer function in the lme4 package in R (R Development Core Team 2004). We compared the fit of the models using the likelihood ratio test statistic to compute P-values (using the chi-squared approximation to the distribution).
Results
Sex ratio
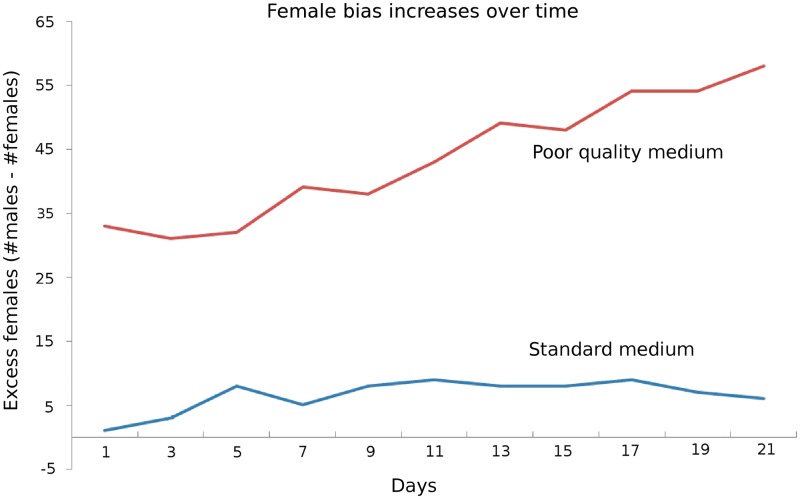
Brood sex ratio varied with maternal environmental quality. Progeny of dams reared on poor resources were female-biased, in contrast to the progeny of dams reared on standard medium, which produced progeny in an approximately 1:1 sex ratio. Dams raised on poor-quality medium (but mated with sires raised on standard medium) produced progeny that were biased toward daughters (60–64% females on average). This difference between good and bad maternal environments was apparent both in the sex ratio of broods allowed to develop to adults as a group (t = 3.38, P = 0.003, average group size = 31; Table 1) and in that of individually reared larvae, separated as eggs (t = 3.389, P = 0.002; in addition, female bias increases over time [Table 1 and Fig. 2]). The survival rate of eggs (the proportion of eggs that hatch) was equal to 80% for both maternal environments. This congruence indicates that neither differential survival of larvae during development nor competition among siblings is an important factor affecting female-biased broods. In data from the half-sib design, we found no effect of sire on broods' sex ratio when we fitted a linear mixed-effects model to our data with environment as a fixed effect, with or without a random effect of sire (P < 0.001).
Table 1.
Number eggs, larvae, and pupae per female after 60 days and proportion female for dams reared on good or poor quality medium
| Good-quality medium | Poor-quality medium | |
|---|---|---|
| Mean (SE) | Mean (SE) | |
| 60 day counts | ||
| Number of eggs laid per female, 60 days | 29.1 (3.8) | 48.0 (5.3) |
| Number of larvae (eggs hatched) | 20.3 (3.0) | 35.6 (4.3) |
| Number of pupae | 12.0 (1.8) | 25.0 (2.7) |
| Proportion female | ||
| Eggs separated, reared individually | 0.47 | 0.6 |
| Broods reared to adulthood as a group | 0.49 | 0.64 |
All comparisons are significantly different (P < 0.01) using two-sample t-tests.
Fig. 2.
Sex ratio when offspring are separated as eggs and reared independently is significantly higher for dams reared in a poor-quality environment compared with dams reared in standard medium; this difference increases over time. Total numbers of male and female offspring are summed across all dams.
In two of the 20 families of females reared in the poor environment, with broods reared as a group, the sex-ratio bias of the brood was extreme: >95% of offspring were female. This extreme bias co-occurs with a corresponding 10-fold increase in number of offspring (309 and 276 individuals in these two instances, compared with an average of 30.5 for all other families). Dams producing extremely female-biased broods may lay an unusually large total number of eggs, or produce eggs that hatch and survive to adulthood at a higher rate. We are unable to distinguish between these alternative explanations, especially given the rarity of such extreme bias. However, an earlier unpublished study by D. Craig and M. J. Wade of 15 families reared in 25 g of standard medium documented a similar case of the co-occurrence of extreme female-bias (97% females) at extreme offspring density.
Effect of environment on mandibular size
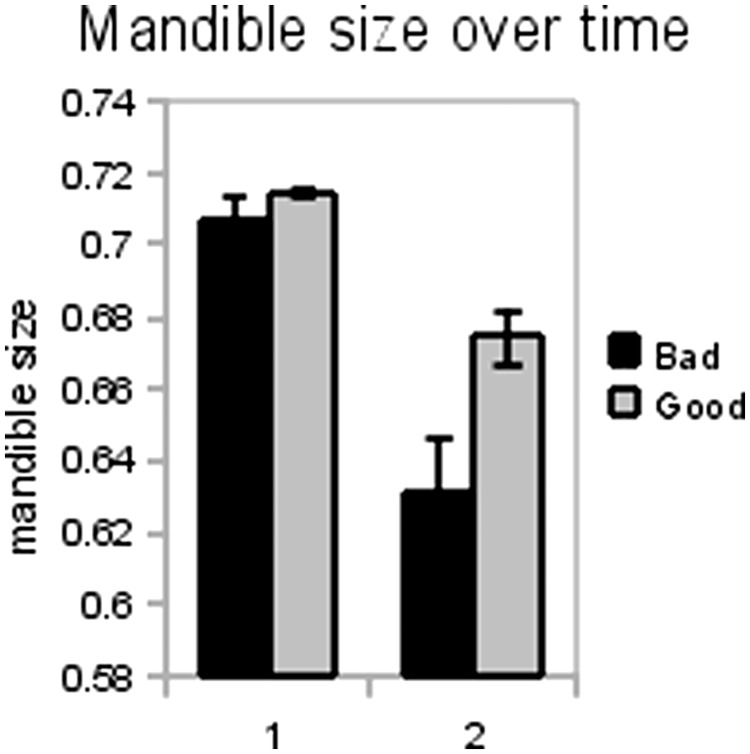
Males reared in poor-quality medium (from dams reared in standard medium) developed 15% smaller mandibles on average than did males reared in standard medium. In addition, the mean and distribution of the sizes of males' mandibles differed depending on maternal condition (Kolmogorov–Smirnov test; D = 0.405, P < 0.001 for raw mandibular sizes; for D = 0.424, P < 0.0001 controlling for body size). The magnitude of the maternal effect on size of mandible is much weaker compared with the direct effect of the rearing environment; however, the variance in sizes of mandibles is comparatively greater. We also measured the differences in males' mandible size between the first 24 eggs laid (unit chosen because offspring were separated into 24-well plates as eggs were laid) and later offspring (25th egg laid and beyond). We found that average mandibular size of sons is significantly larger for the first group of eggs collected, compared with later collections of eggs (t = 5.665, P < 0.001 controlling for body size; t = 7.021, P < 0.001 using raw data; Fig. 3). This finding demonstrates an effect of maternal age on the mandibular size of male offspring; older mothers have sons with proportionately smaller mandibles.
Fig. 3.
Mean mandibular size for the first 24 offspring produced (1) is significantly greater than that of later offspring (2).
Although not directly related to our analyses, we estimated heritability as twice the slope of the father–offspring regression of mandible sizes (standard errors obtained by jackknifing). To account for the correlation between males' horn size and thorax width (body size), we used the residuals of mandible size regressed on body size (see Demuth et al. 2012). We found a significantly nonzero heritability of male mandible size, h2 = 0.210 ± 0.006. Our estimate is consistent with previous estimates of realized heritability (the regression coefficient of selection response on cumulative selection differential) conducted by Okada and Miyatake (2009) and which ranged from 0.13 to 0.26 across replicated lines artificially selected for large and small mandibles in males.
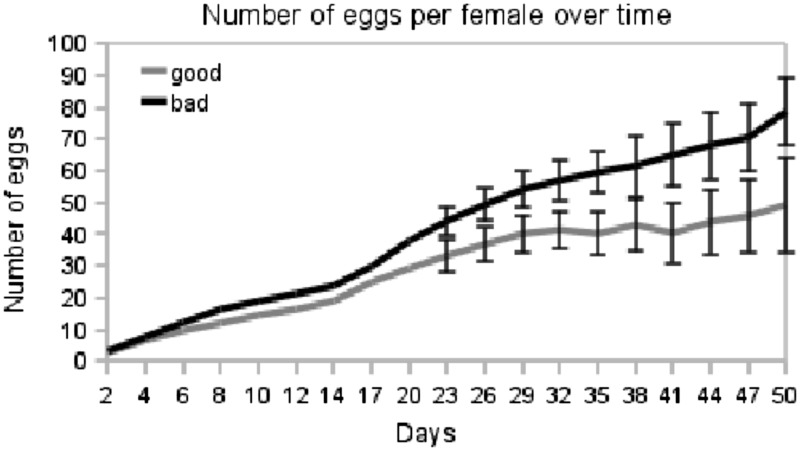
Effect of maternal condition on number of eggs
Dams reared on poor-quality medium laid more eggs per day (and 40% more total eggs) compared with dams reared on standard medium (Fig. 4). Just as there is no effect of sire on sex ratio in broods, there is no effect of sire on number of eggs laid. Specifically, we fit linear mixed effects models to our data with environment as a fixed effect, with and without a random effect of sire. We compared the fit of these models to our data using AIC scores and a likelihood ratio test. We found that a model that does not include sire as a random effect was significantly better than one that did include sire effect (P < 0.001). However, this may be due to the low sample size of sires (nsire = 10); there is a very weak and nonsignificant negative correlation between the mandibular size of sires and the number of eggs laid per day (r2 = −0.104).
Fig. 4.
Gnathocerus cornutus dams reared in a poor-quality environment (n = 30) lay more eggs per day and more total eggs, compared with dams raised in standard medium (n = 30). Error bars indicate standard errors.
Discussion
We are able to induce female-biased sex ratios in broods by experimentally manipulating the environmental condition experienced by the mother. Although we have not determined the precise mechanism underlying facultative adjustment of sex ratio in Gnathocerus, our observations allow us to eliminate a number of obvious potential explanations. First, our data exclude sex-specific demographic changes due to mortality of eggs or larvae during development; females reared under nutritionally good or poor conditions exhibit no differences in hatching rate of eggs or in larval survival. We eliminated any potential cannibalism by rearing offspring independently in some of our studies and found sex ratios identical to those observed in broods reared as a group. We can also exclude some nondemographic mechanisms. Gnathocerus have XY chromosomal sex determination and they are not parthenogenic (single virgin females do not lay eggs over the course of their lifetime). Finally, while bacterial endosymbionts are common in insects (Werren and Windsor 2000) and distort sex ratios even in species with chromosomal sex determination, we have been unable to detect a sex-ratio distorting strain of Wolbachia using PCR in Gnathocerus. Therefore, mothers appear to be directly altering their broods' sex ratio in response to the conditions the mother experiences, perhaps via interactions with male accessory gland proteins or differential storage or survival of X and Y sperm. These possibilities will be pursued in later studies.
The maternal plasticity of brood sex ratio that we observed is consistent with the Trivers–Willard sexual-selection hypothesis.
Under this hypothesis, mothers in poor condition are expected to produce female-biased progeny, whenever poor maternal condition results in sons with poor reproductive competitive ability. Our data show that mothers reared under nutritionally poor conditions produce smaller sons with smaller mandibles. Such males in this species are poor reproductive competitors relative to males with larger mandibles. We found that mothers reared under nutritionally poor conditions not only produce smaller sons, but also more daughters, as predicted by the TW hypothesis. This condition appears to be reversible, at least between generations, since the daughters of poor-condition mothers produce broods with even sex ratios if reared on standard medium. Katsuki et al. (2012) found that genetic quality of G. cornutus females correspond with sex ratio, indicating that manipulation of the sex ratio does occur in response to other factors in addition to rearing environment of dams.
Our data are also consistent with the refinement of the TW hypothesis introduced by Wade et al. (2003), who showed that the relationship between maternal condition and the bias in broods' sex ratio should be asymmetric. Using population genetic models, Wade et al. (2003) showed that sex ratio biasing is opposed by Fisherian sex ratio selection. Furthermore, because female-biased sex ratios increase the strength of sexual selection acting on males while male-biased sex ratios diminish it (Shuster and Wade 2003; Wade et al. 2003), Wade et al. showed that the conditions favoring the evolution of female-biased sex ratios in broods are more easily satisfied than is the reciprocal. In contrast, the TW hypothesis says that mothers in good condition will bias their broods' sex ratios toward males. We observed only the female-biased broods of mothers in poor condition; females in good condition produced broods with even sex ratios and not with the male-biased sex ratios predicted by the TW hypothesis. However, it is possible that our good quality flour can be improved further, for instance, by increasing the yeast content; this may cause a male bias.
An alternative explanation for our data is the Wilson–Colwell (WC) group-selection model. In that model, a 1:1 sex ratio is favored within groups, but groups producing female-biased sex ratios have greater rates of population growth, dispersal, and colonization of new patches of resource than do groups with even sex ratios. Many beetles appear to have plastic developmental traits uniquely suited for escaping diminishing resources and over-crowding. For example, some species of bruchid beetles do not develop wings when resources are abundant and crowding is minimal (Utida 1972; Appleby and Credland 2007). However, under conditions of severe crowding, females mature at a smaller size and develop wings and disperse to other, less crowded patches of resource. We propose that under crowded conditions, females produce larger broods with female-biased sex ratios, since inseminated daughters are better colonizers than are sons. Notably, we found that the earliest sons produced by poor-condition females are the largest, perhaps guaranteeing an ability to inseminate large numbers of females prior to dispersal. Our data are consistent with the interpretation that both larger broods and female-biased broods are developed on deteriorating resources, thereby maximizing the number of potential colonizers, as predicted by the WC hypothesis.
The female-biased broods exhibit the same skew in sex ratio, i.e., 15% increase, on average (mean proportion female = 0.64368 ± 0.0772) that has been observed when Gnathocerus stocks have been allowed to reproduce in nonexperimentally manipulated poor-quality medium. This includes cultures that developed mold at high humidity (three of which exhibited extreme sex ratios, >90% female) and six families that were maintained in flour that was not replaced over the course of 3–4 years (one which had a sex ratio >95% female). Furthermore, a female-biased sex ratio >95% was observed previously in an experiment that lasted 18 months without replacing the resource, indicating a rather rapid and extreme shift in sex ratio (D. Craig and M. J. Wade, unpublished data). This latter observation resembled our results in two other ways: (1) Craig and Wade observed an unusually high density of beetles in the culture with the most extreme sex ratio (four-fold higher than the average); and (2) they also observed a shift in the distribution of males' mandibular sizes similar to the one we observed. The infrequency of the extreme biases towards females makes them difficult to study—manipulating the α-cellulose content of the flour does not appear to have as great an effect on sex ratios in broods as the presence of mold, but we do not yet have a reliable means for manipulating mold in the laboratory.
Our observations in this model system suggest a number of obvious follow-up studies, chiefly testing the remaining assumptions and predictions of the Trivers–Willard hypothesis. For instance, adaptive biasing of sex ratio requires a positive covariance between sex ratio and reproductive fitness of sons (Shuster and Wade 2003; Wade et al. 2003). Previous studies indicate that male reproductive success may depend upon environmental conditions. Males develop relatively small mandibles in poor-quality environments, and these males are ineffective competitors with relatively larger-horned males (Okada and Miyataki 2009). The tractability and biology of Gnathocerus beetles is well-suited to direct tests of male reproductive success as a function of the sex ratio in broods. More generally, G. cornutus may be an effective study-system for broad questions related to maternal effects, sex-ratio biasing, sexual selection, and the adaptive plasticity of individuals.
Funding
NIH grant 5R01GM65414-4 to M. J. W. The symposium was generously funded by a grant from the National Science Foundation Division of Integrated Organismal Systems (IOS# 1153657).
References
- Angelini DR, Jockusch EL. Relationships among pest flour beetles of the genus Tribolium (Tenebrionidae) inferred from multiple molecular markers. Mol Phylogenet Evol. 2008;46:127–41. doi: 10.1016/j.ympev.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby JH, Credland PF. The role of temperature and larval crowding in morph determination in a tropical beetle, Callosobruchus subinnotatus. J Insect Physiol. 2007;53:983–93. doi: 10.1016/j.jinsphys.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Bodmer WF, Edwards AWF. Natural selection and the sex ratio. Ann Hum Genet. 1960;24:239–44. doi: 10.1111/j.1469-1809.1960.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Bull JJ. The primary sex ratio under environmental sex determination. J Theoret Biol. 1989;139:431–36. doi: 10.1016/s0022-5193(89)80063-3. [DOI] [PubMed] [Google Scholar]
- Colwell RK. Group selection is implicated in the evolution of female-biased sex ratios. Nature. 1981;290:401–04. [Google Scholar]
- Demuth JP, Naidu A, Mydlarz LD. Sex, war and disease: the role of parasite infection on weapon development and mating success in a horned beetle (Gnathocerus cornutus) PloS One. 2012;7:e28690. doi: 10.1371/journal.pone.0028690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Oxford: Oxford University Press; 1930. The Genetical Theory of Natural Selection. [Google Scholar]
- Hamilton WD. Extraordinary sex ratios. Science. 1967;156:477–88. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- Karino K, Niiyama H, Chiba M. Horn length is the determining factor in the outcomes of escalated fights among male Japanese horned beetles, Allomyrina dichotoma L. (Coleoptera: Scarabaeidae) J Insect Behav. 2005;18:805–15. [Google Scholar]
- Katsuki M, Harano T, Miyatake T, Okada K, Hosken DJ. Intralocus sexual conflict and offspring sex ratio. Ecol Lett. 2012;15:193–7. doi: 10.1111/j.1461-0248.2011.01725.x. [DOI] [PubMed] [Google Scholar]
- Kohlman SG. Adaptive fetal sex allocation in elk: evidence and implications. J Wildl Manag. 1999;63:1109–17. [Google Scholar]
- Kruuk LEB, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE. Population density affects sex ratio variation in red deer. Nature. 1999;399:459–61. doi: 10.1038/20917. [DOI] [PubMed] [Google Scholar]
- Moczek AP, Emlen DJ. Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim Behav. 2000;59:459–66. doi: 10.1006/anbe.1999.1342. [DOI] [PubMed] [Google Scholar]
- Mueller UG. Haplodiploidy and the evolution of facultative sex ratios in a primitively eusocial bee. Science. 1991;254:442–44. doi: 10.1126/science.254.5030.442. [DOI] [PubMed] [Google Scholar]
- Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci USA. 1999;96:570–73. doi: 10.1073/pnas.96.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntifo SEA, Nowosielski-Slepowron BJA. Developmental period and mortality of Gnathocerus maxillosus (F.) (Coleoptera, Tenebrionidae) under various conditions of temperature and humidity. J Stored Prod Res. 1973;9:51–9. [Google Scholar]
- Okada K, Miyanoshita A, Miyatake T. Intra-sexual dimorphism in male mandibles and male aggressive behavior in the broad-horned flour beetle Gnatocerus cornutus (Coleoptera: Tenebrionidae) J Insect Behav. 2006;19:457–67. [Google Scholar]
- Okada K, Miyatake T. Effect of losing on male fights of broad-horned flour beetle, Gnatocerus cornutus. Behav Ecol Sociobiol. 2009;64:361–9. [Google Scholar]
- Okada K, Miyatake T. Plasticity of size and allometry in multiple sexually selected traits in an armed beetle Gnatocerus cornutus. Evol Ecol. 2010;24:1339–51. [Google Scholar]
- Olsent PD, Cockburn A. Female-biased sex allocation in peregrine falcons and other raptors. Behav Ecol Sociobiol. 1991;28:417–23. [Google Scholar]
- Park T, Mertz DB, Grodzinski W, Prus T. Cannibalistic predation in populations of flour beetles. Physiol Zool. 1965;38:289–321. [Google Scholar]
- R Development Core Team. Vienna: R Foundation for Statistical Computing; 2004. A language and environment for statistical computing. [Google Scholar]
- Shaw RF. The theoretical genetics of the sex ratio. Genetics. 1958;43:149–63. doi: 10.1093/genetics/43.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster SM, Wade MJ. Princeton: Princeton University Press; 2003. Mating Systems and Strategies. [Google Scholar]
- Smith SG, Brower JH. Chromosome numbers of stored-product Coleoptera. J Kansas Entomol Soc. 1974;47:317–28. [Google Scholar]
- Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–2. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Yoshida T. Population biology of the broad-horned flour beetle Gnathocerus cornutus (F.). II. Crowding effects of larvae on their survival and development. Res Popul Ecol. 1985;27:77–85. [Google Scholar]
- Utida S. Density dependent polymorphism in the adult of Callosobruchus maculatus (Coleoptera, Bruchidae) J Stored Prod Res. 1972;8:111–25. [Google Scholar]
- Wade MJ, Shuster SM, Demuth JP. Sexual selection favors female-biased sex ratios: the balance between the opposing forces of sex-ratio selection and sexual selection. Am Nat. 2003;162:403–14. doi: 10.1086/378211. [DOI] [PubMed] [Google Scholar]
- Werren JH. Sex ratio adaptations to local mate competition in a parasitic wasp. Science. 1980;208:1157–60. doi: 10.1126/science.208.4448.1157. [DOI] [PubMed] [Google Scholar]
- Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc Biol Sci. 2000;267:1277–85. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Colwell RK. Evolution of sex ratio in structured demes. Evolution. 1981;35:882–97. doi: 10.1111/j.1558-5646.1981.tb04952.x. [DOI] [PubMed] [Google Scholar]
- Wolff JO. Maternal investment and sex ratio adjustment in American bison calves. Behav Ecol Sociobiol. 1988;23:127–33. [Google Scholar]