Abstract
Acute liver failure (ALF) is a rare but challenging clinical syndrome with multiple causes; a specific etiology cannot be identified in 15% of adult and 50% of pediatric cases. The course of ALF is variable and the mortality rate is high. Liver transplantation is the only therapy of proven benefit, but the rapidity of progression and the variable course of ALF limit its use. Currently in the United States, spontaneous survival occurs in approximately 45%, liver transplantation in 25%, and death without transplantation in 30% of adults with ALF. Higher rates of spontaneous recovery (56%) and transplantation (31%) with lower rates of death (13%) occur in children. The outcome of ALF varies by etiology, favorable prognoses being found with acetaminophen overdose, hepatitis A, and ischemia (≈60% spontaneous survival), and poor prognoses with drug-induced ALF, hepatitis B, and indeterminate cases (≈25% spontaneous survival). Excellent intensive care is critical in management of patients with ALF. Nonspecific therapies are of unproven benefit. Future possible therapeutic approaches include N-acetylcysteine, hypothermia, liver assist devices, and hepatocyte transplantation. Advances in stem cell research may allow provision of cells for bioartificial liver support. ALF presents many challenging opportunities in both clinical and basic research.
Acute liver failure (ALF) is a dramatic but rare clinical syndrome marked by the sudden loss of hepatic function in a person with no prior history of liver disease. The causes of ALF include viral hepatitis, drug-induced and toxin-induced liver disease, metabolic errors, ischemia, and miscellaneous rare causes. Currently, there are no specific therapies of proven benefit except for emergency liver transplantation. The many challenges in understanding and management of ALF led to the organization of a 2-day research workshop held on December 4–5, 2006 in Bethesda, Maryland, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) with support from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and the Office of Rare Diseases of the National Institutes of Health (NIH). This manuscript summarizes the presentations at that meeting and the recommendations arising therefrom.
Overview of ALF
Epidemiology and Etiology in Adults
The sudden loss of hepatic function in a person without preexisting liver disease defines ALF.1,2 The most reliable signs of severe acute liver injury are the presence of coagulopathy (international normalized ratio [INR] ≥ 1.5) and any degree of hepatic encephalopathy, the length of illness being considered anything ≤ 24 weeks. Many patients evolve to coma within 1 week or less. The term “acute liver failure” is preferable to fulminant hepatic failure1 or acute hepatic necrosis.2 ALF is rare and represents a syndrome rather than a specific disease, having multiple causes that vary in course and outcome.
The rarity of ALF and its unpredictable, severe course make it a challenging entity for prospective studies. ALF is difficult to identify in its early stages, resulting in frequent delays in initiation of treatment. Deliberate decision-making is often impossible. For these reasons, the U.S. ALF Study Group was established with pilot funding from the Food and Drug Administration (FDA) and full support from the NIH.3–6 Its two aims were to utilize a database for prospective, standardized collection of clinical information, serum, and DNA and tissue samples and to develop controlled trials of innovative therapies.
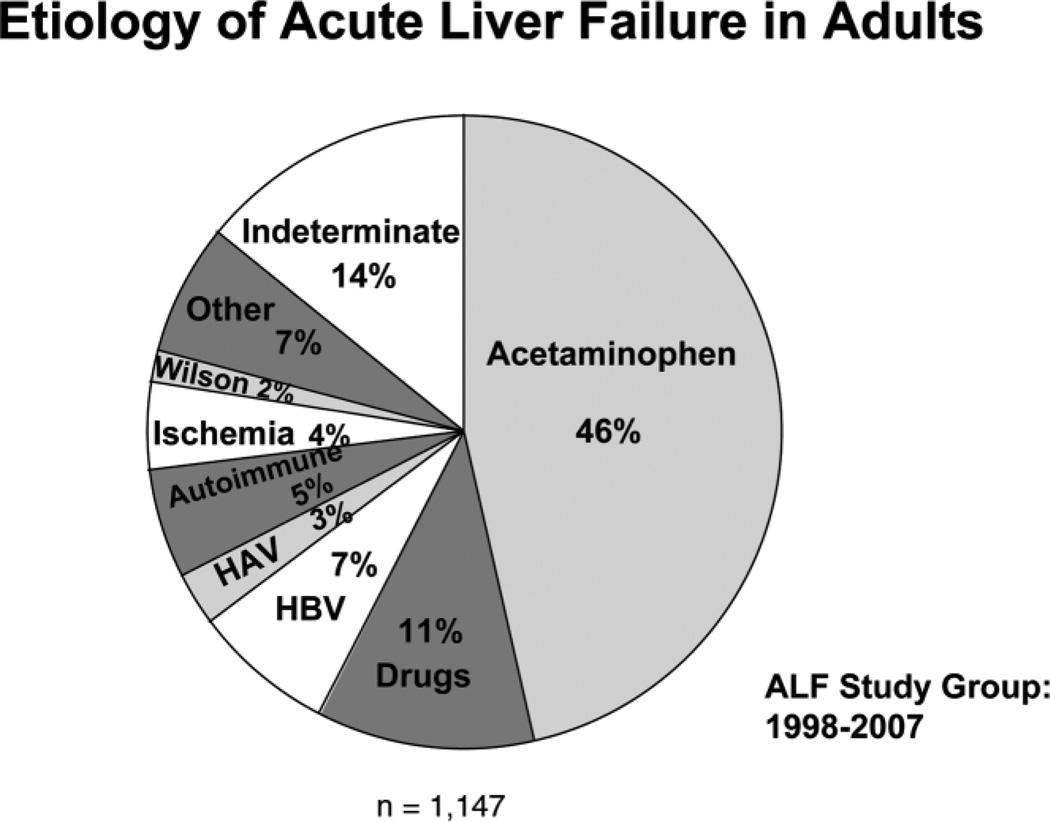
Between January 1998 and July 2007, the adult ALF Study Group enrolled 1,147 patients at 23 clinical sites. Overall, 67% of cases were women and the average age was 38 years (range 17–79 years). The most frequent single cause of ALF was acetaminophen overdose (Fig. 1) accounting for 46% of cases. Over the 9-year period, there was a gradual increase in the proportion of cases of ALF due to acetaminophen overdose, and somewhat decreasing rates of hepatitis A and B. The distribution of etiologies differs around the world. In Africa and Asia, viral hepatitis is the leading cause of ALF, and cases of hepatitis E as well as hepatitis A and B are common.7
Fig. 1.
Etiology of ALF in the 1,147 adult patients who were enrolled in the U.S. ALF Study Group database between January 1998 and July 2007.
In the U.S. ALF Study Group database, the overall outcomes included spontaneous recovery without need for transplantation in 45% of the patients. Of the 44% of patients who were listed for transplantation, 25% of the overall group received a graft; of the remaining 19%, 10% died on the waiting list while 9% recovered. Overall, 30% died. Outcome rates are far better than in the era before transplants when mortality rates were 80% or higher. Changing patterns of etiology accounted for some outcome differences (Table 1). Acetaminophen, shock, and hepatitis A have favorable outcomes, with spontaneous recovery rates of 58% to 64%, compared to drug-induced, autoimmune, and indeterminate ALF (≈20% to 25%). The median time from listing to transplant was typically one day (range <1–4 days) compared to a time from listing to death without transplantation of 3 days (range <1–6 days), suggesting that organ shortages limit transplantation of ALF patients. While overall outcomes have improved since the era before transplants, ALF remains a challenging syndrome with high mortality and a major burden to the medical care system.
Table 1.
Etiology and Clinical Characteristics of 1,147 Cases of ALF
| Feature | Acetaminophen (n = 532) |
Drugs (n = 133) |
Indeterminate (n = 161) |
Hepatitis A (n = 31) |
Hepatitis B (n = 83) |
All Others (n = 207) |
|---|---|---|---|---|---|---|
| Age (years)* | 37 (28–45) | 46 (33–56) | 38 (26–50) | 47 (40–57) | 42 (29–54) | 42 (29–56) |
| Female Sex | 76% | 67% | 58% | 45% | 42% | 76% |
| Jaundice to Coma (days)* | 0 (0–1) | 9 (3–20) | 9 (2–20) | 3 (1–8) | 7 (2–14) | 7 (1–17) |
| Coma grade ≥ 3 | 52% | 38% | 50% | 55% | 54% | 41% |
| ALT (U/L)* | 4067 (2138–6731) | 600 (260–1537) | 847 (396–2111) | 2404 (1367–3333) | 1707 (745–2815) | 650 (172–1867) |
| Bilirubin (mg/dL)* | 4.5 (2.9–6.6) | 20.2 (12.1–28.3) | 23.0 (9.2–29.7) | 11.9 (9.7–27.5) | 19.7 (12.4–25.6) | 15.3 (6.3–26.7) |
| Spontaneous Survival | 65% | 29% | 25% | 58% | 25% | 34% |
| Transplantation | 9% | 41% | 43% | 29% | 47% | 33% |
| Death Without Transplantation | 26% | 31% | 32% | 13% | 28% | 33% |
ALF in Children
The Pediatric ALF Study Group5 developed in 2000 comprises 19 clinical centers in the United States (n = 16), Canada (n = 1), and the United Kingdom (n = 2); it maintains a similar registry of ALF in children and is conducting a randomized controlled trial of N-acetylcysteine (NAC) for non–acetaminophen-related ALF.
In children, signs of hepatic encephalopathy can be subtle and appear late in the course of injury.5,8 Accordingly, modified criteria for ALF are used: either encephalopathy with coagulopathy (INR > 1.5) or more severe coagulopathy alone (INR > 2.0) in a child with evidence of acute liver injury, thus allowing enrollment to occur at an earlier point in the illness.
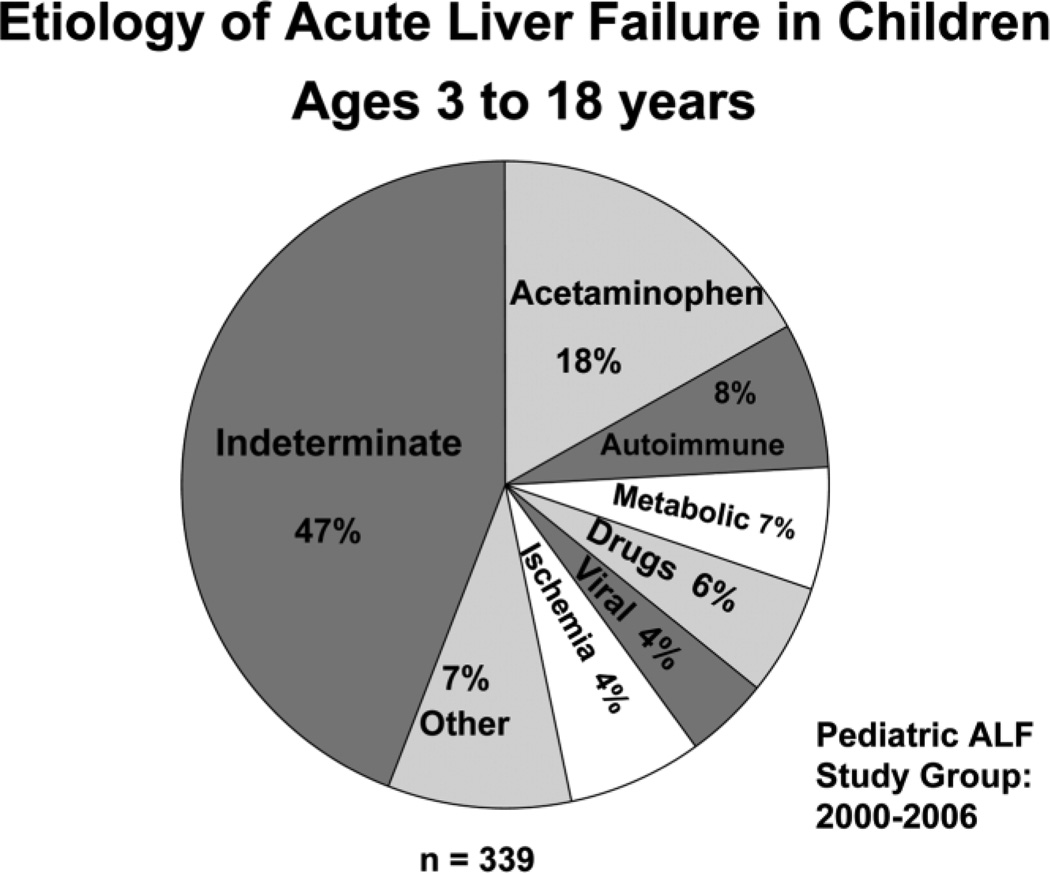
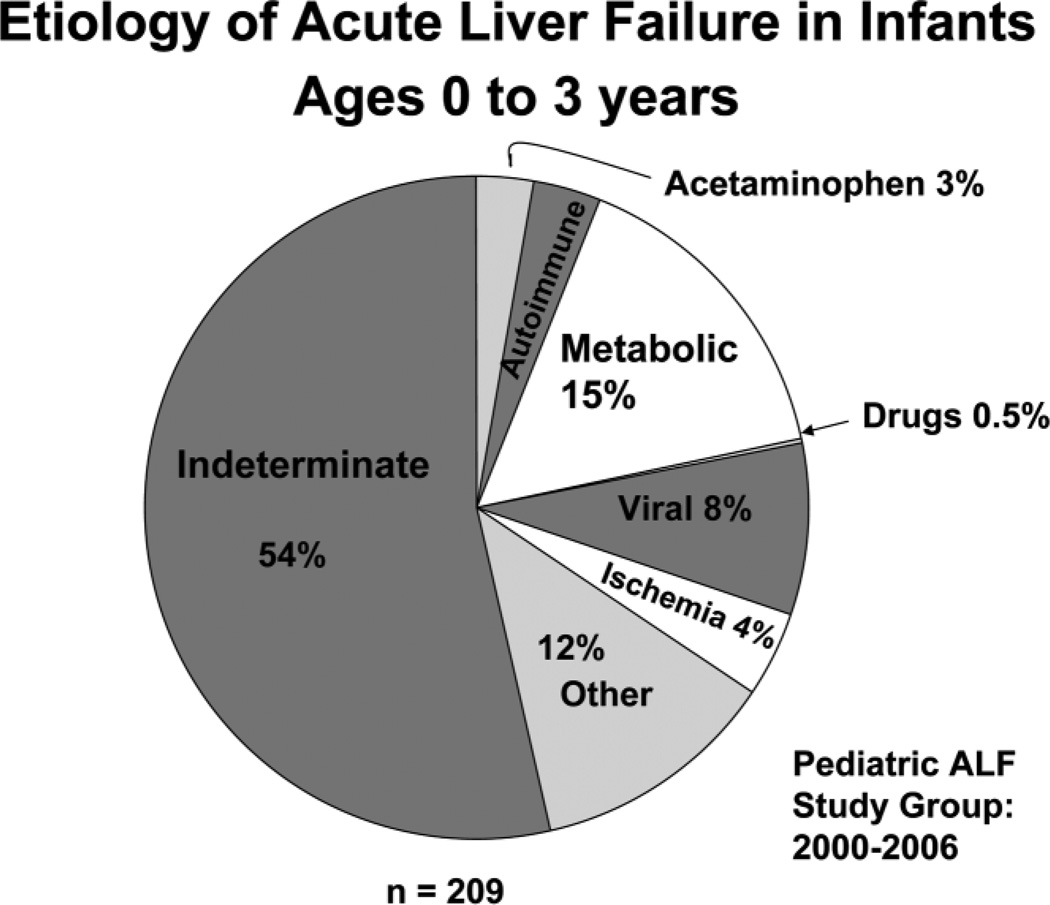
Currently, the Pediatric ALF database has enrolled 548 cases, 38% of which occurred in children less than 3 years of age. Etiologies were: acetaminophen overdose in 12% of cases, metabolic causes in 10% of cases, viral hepatitis in 6% of cases, ischemia in 4% of cases, non-acetaminophen drug-induced liver injury in 4% of cases, and other causes in 15% of cases; 49% were considered indeterminate. The distribution of etiologies differed from adults and varied by age (Figs. 2, 3).5,8,9 In older children, acetaminophen toxicity and Wilson disease were more frequent. Unusual viral causes predominated. There was no hepatitis B, 1 hepatitis C, and 5 hepatitis A cases, but 11 herpes simplex virus, 7 Epstein-Barr virus, and 1 instance each of adenovirus, enterovirus, paramyxovirus, and influenza A infections. There were 21 cases of idiosyncratic drug-induced liver disease.
Fig. 2.
Etiology of ALF in the 339 pediatric patients over the age of 3 years who were enrolled in the U.S. Pediatric ALF Study Group database between January 2000 and December 2006.
Fig. 3.
Etiology of ALF in the 339 pediatric patients under the age of 3 years who were enrolled in the U.S. Pediatric ALF Study Group database between January 2000 and December 2006.
Outcomes of ALF were more favorable in children than in adults.10 Overall, 56% survived short-term without transplantation, 31% received a graft, and 13% died. Importantly, 16% of children with ALF who never developed hepatic encephalopathy either died or required liver transplantation, providing further support to use of the modified ALF criteria. Diagnoses associated with high rates of survival included acetaminophen toxicity (93%)5,11 and hepatitis A (100%).
The pediatric trial of NAC for non–acetaminophen-induced ALF is ongoing. Other areas of research focus include investigation of the cause of indeterminate cases of ALF5 and better delineation of the metabolic causes of liver injury in children.12
Natural History
The leading causes of death in ALF are cerebral edema and sepsis.4,13 Respiratory distress requiring mechanical ventilation and/or acute renal failure are also common as are bacterial and fungal infections.14 Coagulopathy itself rarely is life threatening and can be corrected.
In the ALF Study Group patients, 44% were listed for transplantation, but only 25% of the entire group received a transplant. The short-term posttransplant outcome was good, with 90% survival at 1 month and 70% survival at 1 year.4,13 Long-term outcome after ALF is not well defined.15,16 Concerns include neurological damage, complications of intensive care, and persistent or recurrent liver disease following spontaneous recovery or liver transplantation. Early detection of cerebral edema is difficult, because clinical signs such as posturing and pupillary dilatation are signs of advanced cerebral edema. Long-term neurological impairment due to cerebral edema is well recognized and includes developmental delays and impaired cognition of varying degrees. If evidence of cerebral edema is present in children or adults at the time of transplant, developmental delay is frequent but probably not inevitable.15–18
Recurrence of ALF after spontaneous recovery occurs with repeated acetaminophen overdoses.19,20 Other forms of liver disease rarely recur, except for posttransplant autoimmune hepatitis.21 More rigorous long-term follow up studies are needed to better guide management of cerebral edema and the timing of liver transplantation in ALF.22
Evaluation and Management
ALF requires an expert, multidisciplinary, collaborative effort among hepatologists, transplant surgeons, intensive care physicians and nurses, nephrologists, neurosurgeons, and transplant coordinators.23 Patients should be rapidly evaluated for etiology and severity of liver injury and an urgent assessment made regarding suitability for liver transplantation.
Specific therapies for unique causes of ALF are few: NAC for acetaminophen overdose and prompt delivery for pregnancy-related ALF. Children with inborn errors of metabolism appear to benefit by removal of galactose-containing formula in children with galactosemia, intravenous glucose and avoidance of fasting for children with inherited defects in fatty acid oxidation, and prompt administration of 2-(2-nitro-4-3 trifluoro-methylbenzoyl)-1,3-cyclohexanedione (NTBC) in children with hereditary tyrosinemia type 1 24. Other therapies in use but of unproven benefit include activated charcoal and high-dose intravenous penicillin for mushroom poisoning;25 corticosteroids for autoimmune hepatitis;2 copper chelation, plasmapheresis, and antioxidant therapy for Wilson disease;27 lamivudine or entecavir for acute hepatitis B;28 acyclovir for herpes simplex virus infection;29 hemodynamic support for shock or ischemic liver injury; and decompressive surgery or transjugular intrahepatic portosystemic shunts (TIPS) for acute Budd-Chiari syndrome.30 Currently, the only effective therapy of ALF is liver transplantation. Transfer to a transplant center is appropriate for any patient with severe coagulopathy during an acute hepatic illness, regardless of whether hepatic encephalopathy is present. Although a number of prognostic scores have been developed to predict outcome without transplantation, none is considered standard at present. Current United Network for Organ Sharing (UNOS) criteria for Status 1 listing are (1) onset of any degree of hepatic encephalopathy within 8 weeks of onset of acute liver injury; (2) absence of pre-existing liver disease; (3) life expectancy of less than 7 days; and (4) in the intensive care unit (ICU) requiring either mechanical ventilation, renal dialysis, or with severe coagulopathy (INR > 2.0). When an organ becomes available, the difficult but critical decision is made. Bad outcomes are possible: performing transplantation on someone who might have survived, failing to perform transplantation on someone who ultimately succumbs, or worse, performing transplantation on someone too late and losing both the patient and the donor organ.
Special dilemmas related to ALF management are many: when liver ultrasound suggests cirrhosis, this may be false positive due to a nodular necrotic liver. Likewise, patients who are known hepatitis B carriers are sometimes inappropriately excluded from listing because of pre-existing liver disease.31 Intracranial pressure monitoring remains controversial, because it is invasive and complications occur.22 Evidence for efficacy of NAC outside of early acetaminophen poisoning awaits results from the ALF Study Group trial.32 Nonspecific therapies of promise that deserve future evaluation include inhibitors of liver injury and apoptotic pathways (caspase or specific kinase inhibitors) and promoters of liver regeneration (growth factors).33
Liver Transplantation for ALF
Overview and Outcomes
Transplant candidacy must be determined rapidly once the diagnosis of ALF is made. Underlying medical or psychosocial conditions that may preclude consideration include alcohol or drug abuse, repeated suicide attempts, or inadequate family support. Because of the rapid progression of ALF and persistent organ shortages, improved predictive models and more donor organs are sorely needed.34 Outcome data from the European experience have been presented recently.35 Between 1995 and 2005, 90,445 candidates for liver transplantation were listed in the United States. During this time, ALF accounted for 3.9% of overall listings.36
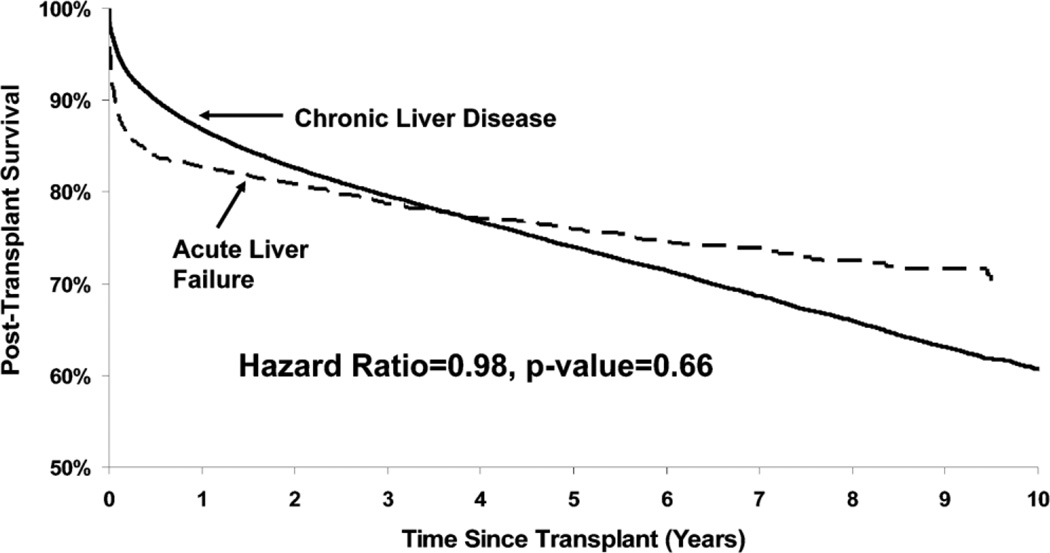
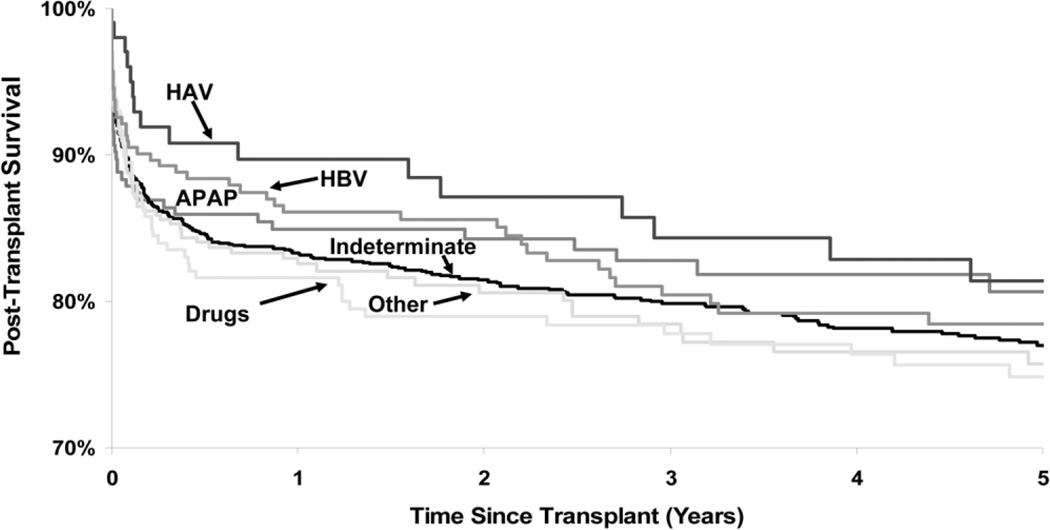
Rates of survival after liver transplantation were similar between patients with ALF or end-stage liver disease (Fig. 4). Although survival was decreased during the first year after transplantation, at 4 years, the ALF cohort had improved survival compared to patients with chronic liver disease. Among transplant recipients, those with acetaminophen and viral hepatitis-related ALF had better post-transplant survival than patients with ALF of other viral causes as well as autoimmune and drug-induced liver disease (Fig. 5).
Fig. 4.
Adjusted survival after liver transplantation for ALF versus chronic end-stage liver disease. Data adjusted for recipient age, gender, race, body mass index, medical condition, dialysis, diabetes, life-support, previous abdominal surgery, HCV-positivity, portal vein thrombosis, as well as donor factors including age, race, cause of death, DCD, cold ischemia time, partial or split liver and living donor. From the Scientific Registry of Transplant Recipients; December 2006.
Fig. 5.
Adjusted survival after liver transplantation according to cause of ALF. Data adjusted for recipient age, gender, race, body mass index, medical condition, dialysis, diabetes, life-support, previous abdominal surgery, HCV-positivity, portal vein thrombosis, as well as donor factors including age, race, cause of death, DCD, cold ischemia time, partial or split liver, and living donor. Abbreviations: HAV, hepatitis A virus; HBV, hepatitis B virus; APAP, acetaminophen. From the Scientific Registry of Transplant Recipients; December 2006.
Use of Living Donors
There is relatively meager experience worldwide with living donation for ALF.37–39 Although well accepted for children where left lobes can be used, its use in adults, which requires right-lobe donation, remains controversial. The decision to embark on living donor liver transplantation for ALF needs to take into account the time involved evaluating the donor candidate versus the time spent waiting for a deceased donor organ. Hastening the donor evaluation raises many issues, including whether the necessary elements in donor candidate evaluation have been adequately completed, ethical considerations, and the eventual outcome for both recipient and donor.
Preliminary results on outcome of transplantation for ALF are available from the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL), a NIH-supported consortium of 9 liver transplant centers that collect data on both recipients and donors. From 1998 to 2006, 1,079 potential living donor recipients were enrolled in the cohort, among whom 11 had ALF. Eight received a living donor liver transplant; 2 received a deceased donor transplant, and 1 recovered spontaneously. The average length of stay after donor surgery was 5 days, and 4 donors suffered complications including bile leak, abscess, pleural effusion, bacterial infection, and neuropraxia. Eight of 10 patients who underwent transplantation and all donors survived. Thus, living donor liver transplantation is a viable but challenging approach to management of ALF in adults.
Liver Transplantation for Children
The decision to transplant is more difficult in children than in adults because they will face an entire lifetime needing immunosuppression. Likewise, children with ALF fare poorly compared to those with chronic end-stage liver disease,8,40 with an average 6-month survival from the time of listing of 60% versus 90% for children with chronic liver disease. Even after liver grafting, differences persist, with ALF carrying a 6-month posttransplant survival rate of 80%, versus 92% among children with chronic liver disease. Improving prognostic accuracy in children who present with ALF is an important goal.41,42
Acetaminophen-Related ALF
Acetaminophen poisoning currently represents the most common cause of ALF in North America and Europe.43 Patterns of presentation differ between countries, with more unintentional cases reported from the United States than Europe. Between 400 and 500 deaths occur annually in the United States due to acetaminophen-related liver failure.44
Pathophysiology
Acetaminophen is a dose-related toxin, with higher dosing generating the highly reactive intermediate, N-para-aminoquinonimine (NAPQI). When glutathione is depleted,45 NAPQI binds to cell proteins to yield cysteine adducts.46 A recently developed high-pressure liquid chromatography with electrochemical detection (HPLC-EC) assay has allowed the detection of adducts in tissue and serum samples.47 In patients with acetaminophen toxicity enrolled in the ALF Study Group registry, acetaminophen-cysteine adduct levels were present in virtually all patients and persisted for an average of 7 days, declining in parallel with aminotransferase levels.32 Importantly, significant levels were detected in 7 of 36 (19%) indeterminate cases of ALF,32 suggesting that these cases represented unrecognized instances of acetaminophen toxicity. Similar results were seen in children.11 Future studies are needed to define the absolute level of adducts associated with significant hepatic toxicity.
Intentional Versus Unintentional Overdose
A suicidal overdose involves a single time-point ingestion of a large amount of acetaminophen with suicidal intent, while an unintentional case involves ingestion of lesser multiple doses over several days usually for pain control and without suicidal intent, hence the term “therapeutic misadventure”.48,49 Among 275 acetaminophen-related cases reviewed, intentional and unintentional cases were similar in number, whereas 8% could not be categorized.50
Acetaminophen liver injury is characterized by a “hyperacute” biochemical pattern, with markedly high levels of alanine aminotransferase (ALT; median 4,149 U/L and as high as 26,600 U/L) and only modest elevations in bilirubin (median 4.5 mg/dL). Women predominate (74%), with a median age of 36 years. Despite better survival (Table 1), acetaminophen-related cases make up the largest number of deaths. Comparing intentional and unintentional cases revealed few differences (Table 2). Total dosages taken and severity of illness were similar. The unintentional cases were more likely to consume narcotics and to have used multiple acetaminophen-containing preparations, possibly reflecting tolerance to acetaminophen in patients addicted to the narcotic-acetaminophen combinations.51 Alcohol use was more commonly associated with unintentional cases, but its role in worsening the liver injury is unclear.
Table 2.
Comparison of Intentional and Unintentional Acetaminophen Overdose
| Feature | Intentional (n = 122) |
Unintentional (n = 131) |
P Value |
|---|---|---|---|
| Age (years)* | 34 (17–68) | 38 (18–76) | NS |
| Female Sex | 74% | 73% | NS |
| Total Dose (grams)* | 25 (1.2–90) | 20 (2.5–180) | NS |
| Dose per day (grams)* | 25 (1.2–90) | 7.5 (1.0–7.8) | NS |
| Coma Score ≥ 3 | 39% | 55% | |
| Maximum ALT (U/L)* | 5326 (179–19,826) | 3319 (176–18,079) | NS |
| History of depression | 45% | 24% | |
| Antidepressant use | 38% | 37% | NS |
| Narcotic compound | 18% | 63% | |
| Multiple preparations | 5% | 38% | |
| Spontaneous survival | 66% | 64% | NS |
| Transplantation | 7% | 9% | NS |
| Death without transplantation | 27% | 27% | NS |
Summarized from reference 50 on a prospective consecutive series of 275 cases of ALF due to acetaminophen overdose, in 22 of whom the intent could not be determined.
Median values (range).
How often ALF develops with therapeutic doses of acetaminophen is uncertain.50 However, patients reporting ingestion of lower doses were more often alcohol abusers. Patients with acute viral hepatitis who ingest acetaminophen appear more likely to develop ALF.52,53 Low levels of acetaminophen adducts have been identified in some cases of ALF due to viral hepatitis, but the effect on outcome is uncertain.54
Treatment of Acetaminophen Overdose
A recent Cochrane Systematic Review concluded that data supporting most established interventions for acetaminophen overdose except for NAC are weak.55 Although efforts to limit gastrointestinal absorption or to dialyze off the parent compound may be used, detoxification of the reactive intermediate is the most effective and accepted therapy.57,58
NAC is a specific antidote that supplies glutathione, a sulfhydryl donor, to limit NAPQI formation yielding instead the readily excreted, nontoxic mercapturic acid. When given within 24 hours of overdose, NAC clearly decreases the risk of liver injury.59–61 Administration of NAC more than 24 hours after overdose is not supported by prospective studies.59 Because side effects are minimal, NAC is often given up to 72 hours after ingestion.
Plasma drug levels can be helpful in determining severity of injury if the time of ingestion is known.57 Intravenous NAC is used if encephalopathy or nausea are present.63 Allergic reactions (hives, bronchospasm), seen particularly with the intravenous form, are generally mild, responding to antihistamines and/or cessation of the infusion. NAC has been associated with exacerbation of asthma, and one case of fatal bronchospasm has been reported. Recommendations as to duration of NAC therapy vary. Plasma acetaminophen levels are not useful for this purpose.
Acetaminophen Toxicity: the United Kingdom Experience
Paracetamol, as acetaminophen is known in Europe, accounts for 200–500 deaths and 20–40 liver transplants annually in the United Kingdom alone.19, 65 Only 25% of cases are thought to be unintentional overdoses. Deaths from acetaminophen overdose have been associated with older age, male sex, and alcohol or drug use. A surge in the incidence of cases of ALF due to acetaminophen occurred in the mid-1990s with 37,000 hospitalizations and 562 deaths in England and Wales in 1997.66 Acetaminophen overdoses accounted for 37% of emergency liver transplants.
Legislation was adopted in the United Kingdom in 1998 aimed at restricting the quantities found in the home, limiting package size to 16 tablets for general sales outlets and 24 tablets for pharmacies. As a result, the number of hospital admissions related to acetaminophen overdoses, number of transplant listings, and deaths all decreased by varying amounts in the range of 27%-33% between 1995–1998 and 2001–2004. 67–70
Other Causes of ALF
Autoimmune Hepatitis
Although autoimmune hepatitis (AIH) is a chronic disease, an acute presentation occurs in approximately 22% of patients,71 an even smaller number presenting with ALF. The diagnosis generally relies on clinical suspicion, the presence of serum autoantibodies, liver histology (if available), and response to corticosteroid therapy. Of 1,147 patients entered into the ALF Study Group registry between 1998–2007, 52 (~5%) were attributed to AIH.4 Histologic features of AIH-related ALF may differ from those features typically associated with a more chronic presentation.72
Randomized controlled trials have shown that corticosteroids provide no benefit overall in ALF.73,74 However, cases of ALF due to AIH might be an exception. In the ALF Study Group data, patients treated with corticosteroids had a higher rate of spontaneous recovery than patients with AIH who were untreated. Better criteria for diagnosis and prospective studies are needed before any conclusions about the role of immunosuppression in ALF associated with AIH can be made.
Drug-Induced Liver Injury
Idiosyncratic drug-induced liver disease accounted for 11% of cases in the ALF Study Group registry.75,76 The establishment of causality in these cases is challenging,77,78 Among 119 drug-induced cases from the ALF Study Group, women predominated (67%) with a median age of 43 years, peak ALT of 571 U/L, and peak bilirubin of 21.6 mg/dL. Implicated medications included antituberculous drugs in 19 cases, sulfonamides in 10 cases, phenytoin in 7 cases, disulfiram in 4 cases, troglitazone in 4 cases, propylthiouracil in 4 cases, bromfenac in 4 cases, and herbals and dietary supplements in 11 cases. Outcome of drug-induced ALF was poor (Table 1); of those patients who underwent transplantation, however, 96% survived in the short-term, giving an overall survival of 64%.
Viral Hepatitis–Induced ALF
Although acute hepatitis A and B have declined over the last decade, they accounted for 7.3% and 3% of US ALF cases, respectively.79 Hepatitis C and E accounted for only 1 case each and Epstein-Barr virus only 4 cases of more than 1000 cases.80,81 Other previously unidentified viruses have been suspected of causing indeterminate ALF.7,82 However, searches for viral agents using sera from indeterminate patients found no cases of parvovirus B19, hepatitis E or SEN virus.83,84 Other rare causes of ALF include Dengue virus infection85 and herpes zoster and simplex. Finally, reactivation of hepatitis B or infection with hepatitis B virus (HBV) harboring precore and core-promoter gene mutations or with genotype Bj or D appear to be important causes of ALF in some areas of the world or in special situations.86,87
Patients with hepatitis A virus (HAV) ALF have a better spontaneous (58% versus 24%) and overall survival rate (87% versus 61%) than those with ALF due to HBV. Although it has been reported that patients with chronic hepatitis C who are superinfected with HAV have a high risk for ALF,88 no such cases were seen in the ALF Study Group database nor by others.89,90 Superinfection with hepatitis D in carriers of HBV is another risk factor for ALF but was uncommon in the ALF Study Group patients.91
Antiviral treatment may be considered for HBV ALF based on a retrospective German study.27 Other results are conflicting. Herpes simplex virus–related ALF is difficult to diagnose and has a poor prognosis unless treatment is initiated early.92–94 Although declining in incidence, viral infections remain important causes of ALF.
Nonspecific Therapies for ALF
N-acetylcysteine
Since the 1950s, a variety of therapies have been evaluated. Typically, small controlled trials failed to show evidence of benefit with most of these approaches, including corticosteroids, heparin, insulin and glucagon, and prostaglandin E1.22,73,95–98 N-acetylcysteine, the standard antidote for acetaminophen overdose, has recently been suggested to be beneficial in other forms of ALF,18 possibly through its effects on hemodynamics in multiorgan failure and its renal protective actions.99–101 A prospective, randomized, double-blind, placebo-controlled trial of NAC for cases of ALF not due to acetaminophen has been completed with 173 patients enrolled at 25 study sites, who received placebo or intravenous NAC (Acetadote) over 3 days. Trial results will be available in Spring 2008.
Use of Hypothermia
Animal and human studies have shown that reduction of body temperature between 32°C and 35°C results in a consistent lowering of intracranial pressure and thereby decreased likelihood of cerebral edema.102,103 The possible mechanisms responsible for this include normalization of cerebral blood flow, reduction in delivery of ammonia to the brain, amelioration of anaerobic glycolysis and oxidative stress in astrocytes, and decrease in brain extracellular glutamate.104,105
The role of hypothermia in clinical practice remains unclear. Hypothermia has been recommended for use in patients after cardiac arrest or after traumatic brain injury based on controlled trials but has been applied with varying frequency.106,107 Technical aspects are not complex: the patient with ALF shows remarkable vasodilatation so that an external cooling blanket is usually adequate. Intracaval cooling balloons usually are needed in settings after trauma or cardiac arrest where vasoconstriction predominates.
Complications of hypothermia remain a concern; systemic vascular resistance increases and cardiac output decreases. Patients shiver, requiring paralytics and intubation, thus limiting its use in subjects with early coma grades. Pancreatitis, hyperglycemia, increased risk of infection, and lowering of platelet counts have been reported.105,107 Although hypothermia might theoretically limit hepatic regeneration, a mouse model of hypothermia showed no decrease in this parameter.108 It remains unclear whether therapeutic or prophylactic cooling can improve outcomes and/or protect brain function in these critically ill patients.
Plasmapheresis
Over the years, means of exchanging elements in plasma with normal components have been streamlined so that current systems are fully automated and can rapidly exchange 10 L of plasma, consistent with full replacement of the extracellular fluid compartment. The benefits of such a system include removal of toxins, including protein-bound substances, and repletion of factors synthesized by the liver. In small, somewhat heterogeneous studies, plasmapheresis decreased plasma ammonia levels and improved hepatic encephalopathy, generally within hours.109,110 Cerebral blood flow increased as well as cerebral perfusion pressure.111 Mean arterial pressure was improved, cardiac index decreased, and systemic vascular resistance increased during plasmapheresis.112 The effect of plasmapheresis on survival from ALF has been more difficult to determine.109,113 However, an interim analysis of the large, controlled study currently underway in Europe has shown promise of benefit in the plasmapheresis group.
Hepatic Assist Devices as Therapy of ALF
Hepatic assist devices generally fall into two categories: (1) non– cell-based detoxification systems and (2) cell-based systems to provide metabolic support as well as detoxification (bioartificial liver).114 Non– cell-based systems include open-loop and closed-loop approaches. Open-loop approaches include the single-pass albumin dialysis system and plasma exchange.115 Closed-loop systems include the Prometheus albumin dialysis system116 and the molecular absorbents recirculation system (MARS).117 Both approaches have limitations including thermodynamic limitation on removal rate,118 nonselective losses with open-loop systems, and equilibrium limitations on total removal and effective perfusion time with closed-loop systems.119
Five cell-based systems have been assessed clinically, including the ELAD system (Vital Therapies),120 HepatAssist (Arbios, formerly Circe),121 MELS (Charite Medical Center),122 BLSS (Excorp Medical),123 and AMC BAL (Amsterdam Medical Center).124 The systems differ in cell source and mass, use of plasma or whole blood, the rate of perfusion, and duration of treatment (continuous versus intermittent). Cell masses vary from 100–500 g/cartridge and flow rates from 20–200 mL/ minute. Advantages and disadvantages exist for each cell-based therapy. All appear to be safe and have at least some biologic effect, but none has received FDA approval for use in the United States. A Cochrane review of artificial and bioartificial support systems concluded that results have yet to demonstrate evidence that these systems affect survival in ALF.125
An important issue in design of bioartificial liver systems is whether plasma or whole blood is used. Because of the separation process, the effective treatment rate for plasma perfusion is one-third to one-half that for whole blood. A further issue in device design is the interaction between perfusion rate, cell mass, and bioreactor efficacy. No current bioartificial liver device even approaches the perfusion rate or extraction efficiency of the native liver. Improvement in function of bioartificial livers should aim to increase efficiency of the hepatocytes within the system rather than adding more (relatively inefficient) hepatocytes. All three non–cell-based albumin-recirculating systems have proven to be safe and to efficiently remove suspected toxins. None have yet to be shown to improve survival in ALF.
Cell-Based Liver Support
Bioartificial liver devices are meant to allow time for spontaneous recovery or for the retrieval of a liver graft (bridge to transplantation) and to manage the systemic manifestations of ALF. Surrogate, short-term endpoints include decreases in serum ammonia, bilirubin, and amino acid abnormalities as well as improvements in hepatic encephalopathy, cerebral blood flow, and renal and pulmonary function. In judging the efficacy of bioartifi-cial liver support, however, the critical endpoint is increased survival with or without transplantation.
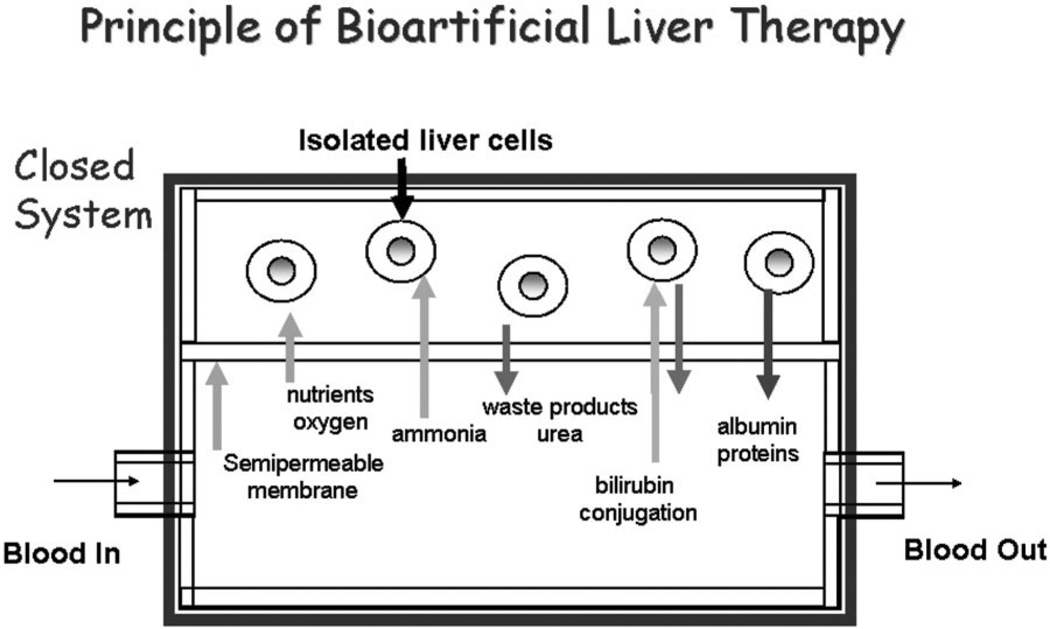
The general principles of bioartificial liver therapy are shown in Fig. 6. With a closed loop design, the patient’s circulation is separated from isolated liver cells by a semi-permeable membrane, protecting the cells from damage by recipient immune cells and eliminating the need for immunosuppression. The membrane also protects the patient from exposure to the allogeneic or xenogeneic cells used in the device. Besides oxygen and nutrients, albumin-bound nonpolar waste molecules are also permeable to the membrane, allowing conjugation of bilirubin and other solutes and further detoxification by the liver cells. The membrane is also permeable to proteins synthesized by hepatocytes in the device.
Fig. 6.
Schematic representation of a closed bioartificial liver assist device. Isolated allogeneic or xenogeneic hepatocytes are separated from the patient’s circulation by a semipermeable membrane which avoids the need for immunosuppression. The membrane must be permeable from the patient’s circulation to the perfusate of the hepatocytes to allow passage of necessary nutrients as well as to the metabolic products (bilirubin, ammonia) that need to be detoxified by the hepatocytes. In the opposite direction, the membrane must be permeable to the proteins synthesized by the isolated liver cells as well as the detoxified products. Some nutrients, such as oxygen, are supplied directly to the hepatocytes particularly when high cell densities are used (not shown).
Design and results of more than 30 bioartificial liver systems for treatment of ALF have been reported since the first publication in 1987.126 The ELAD uses hepatocytes from a hepatoblastoma cell line (C3A) attached to hollow fibers within the bioartificial liver and has been extensively studied.127 The HepatAssist 2000 system was studied in a phase 1 clinical trial in 1997128 followed by a large randomized trial of 170 patients enrolled at 20 sites.121 Though survival was improved with HepatAssist therapy, the differences did not achieve statistical significance.
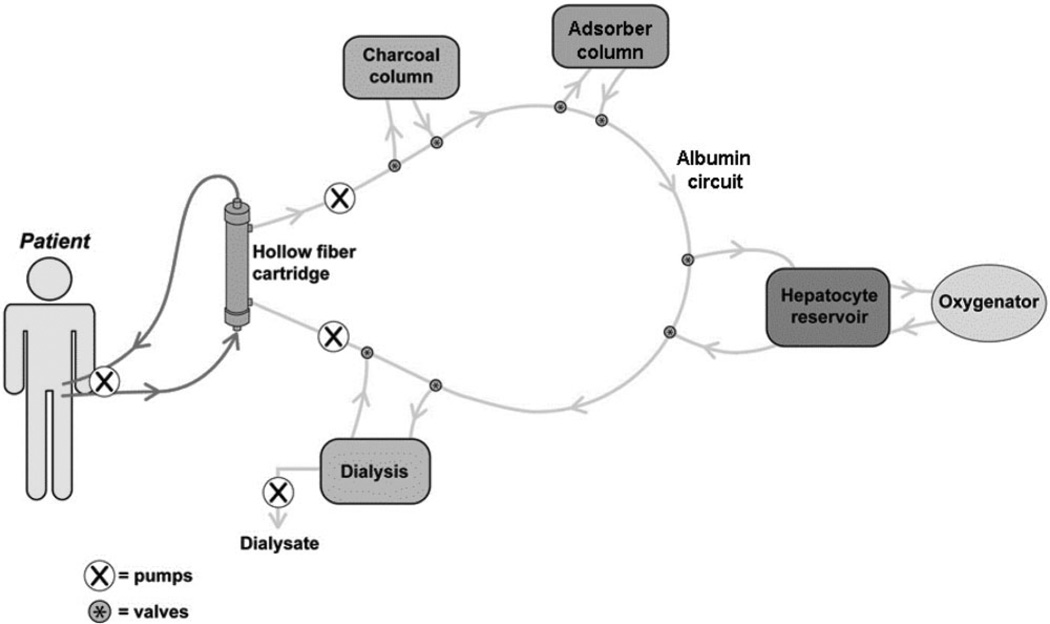
The next generation of bioartificial liver systems employ a “hybrid” or multimodal approach, combining albumin dialysis, charcoal therapy, adsorbent therapy, and dialysis with hepatocyte cell-based therapy (Fig. 7). The extracorporeal circuit consists of the patient’s whole blood whereas the perfusate circuit is an albumin-based solution. In one system, porcine hepatocyte spheroids are used along with albumin dialysis and absorbent therapy.129 In preliminary studies using 200 g of hepatocytes, the porcine hepatocyte spheroid system provided cerebral protection not evident in the control group.
Fig. 7.
Schematic representation of a hybrid liver assist device which employs both cellular and noncellular components for detoxification in an albumin-based extracorporeal perfusate circuit.
Bioreactor Environments
Bioreactors may be used for human liver progenitor cell expansion. Simple hepatocyte suspension cultures are limited by the anchorage-dependence of hepatocytes for long-term function and survival. More complex systems rely on anchorage-dependence, but require a support biomatrix to prevent apoptosis-mediated cell death (anoikis).130 Hepatocytes may grow in 2-dimensional (2-D) “sandwiches” or as aggregates where they provide their own extracellular matrix and supporting cellular structures.131–133 The macroenvironment offered by the culture system appears to be critical. A variety of biomatrix signals have been evaluated including fibronectin, vitronectin, laminin, collagen, fibrinogen, and proteoglycans. Physical signals that influence cell differentiation include temperature, pH, chemical gradients, pressure forces, and media perfusion.134–135
Currently, a 4-compartment hepatocyte bioreactor demonstrating spontaneous aggregation of hepatocytes and nonparenchymal cells into 3-dimensional sinusoids is used.136–140 The ultimate goal is to construct liver tissue in vitro for implantation.
Hepatocyte Transplantation
After success in animal models, human hepatocyte transplantation for ALF was attempted in the late 1990s.141 Studies used cryopreserved human hepatocytes infused into a small number of children with limited success.142–143 These studies provided a proof-of-principle for cell transplantation therapy, but further progress in technical issues is needed before prospective trials can be initiated. Important issues include the optimal dose of hepatocytes, preferred site of transplantation (liver, spleen, elsewhere), better cryopreservation techniques, use of apoptosis inhibitors,144 optimal dose and duration of immunosuppression, improved means of monitoring presence and function of transplanted cells, and development of alternative sources for hepatocytes for transplantation such as from stem cells of hepatic, bone marrow, or fetal origin.145
Tissue Engineering
Stem Cells
Stem cells are defined by their capacity for self-renewal and differentiation into multiple cell types.146–147 Human embryonic stem cells have great potential and a remarkable ability to expand as well as a unique “cryopreservability”. Disadvantages are the limited number of approved human cell lines and the incomplete understanding of how to control the sequence of activation of genes that leads from undifferentiated stem cells to mature, functional hepatocytes. Undifferentiated embryonic cells themselves cannot be used, because they form tumors if transplanted into any site other than in utero.
To date, maturation of fully functional mature hepatocytes from embryonic stem cells has yet to be achieved efficiently using human embryonic stem cell lines. 146–150 Another source of hepatocytes, however, are pluripotent cells that have the capacity to proliferate and differentiate into hepatocytes and cholangiocytes.151–155 Methods for the identification, isolation, expansion, and cryopreservation of hepatic stem cells have been developed.156–158 Hepatic stem cells are found in the ductal plates of fetal and neonatal tissues159 as well as in the Canals of Hering in pediatric and adult liver.160–162 Growth of hepatic stem cells as hepatocytes into cords can be promoted by use of defined matrices and growth factors, making them a potential source of mature liver cells for cell-culture studies, cell transplantation, and bioartificial liver assist devices.
Liver Tissue Engineering
Most in vitro studies of liver function and hepatocyte pathways have used cells cultured in single (2-D) cell layers that do not recapitulate the unique structure of liver with its apical and basal lateral membranes, separate directions of plasma and bile flow, and complex interactions with nonparenchymal cells. Using perfusion microreactors that are seeded with primary rat hepatocytes,163 these cells can be maintained demonstrating greater functional activity than cells in conventional 2-D culture.164 When liver-derived endothelial cells are added, they proliferate and form microvessel-like networks within 3-D reactor cultures, maintaining sinusoidal endothelial cell-specific markers and typical fenestrations.165 Morphogenesis and proliferation of endothelial and stellate cells appear to be governed by perfusion rates.
Synthetic Extracellular Matrices for 3-D Liver Regeneration
For any complex liver culture system, a biodegradable scaffold is needed which provides microvascular and macrovascular networks and supports the function of cells.166 A covalently cross-linked synthetic extracellular matrix material, made of a complex of proteins, was developed for 3-D culture of cells in vitro and in vivo.162,167,168
Rat hepatocytes can be cultured in 3-D hydrogels for more than 3 weeks without loss of drug metabolizing activity, providing a tool for the in vitro evaluation of medications for their effects on drug metabolism and potential for hepatic injury.166,168–170
Human hepatocytes can also be cultured in hydrogel systems, which have been shown to be capable of rescuing nude mice after 90% liver resection by using the synthetic extracellular matrix material to implant either mature human hepatocytes or a mixture of human liver progenitor cells on the residual liver tissue.
Liver Organ Engineering
Classical approaches to tissue engineering have used cells seeded onto resorbable matrices and have had modest success in replicating simple, relatively avascular structures, such as cartilage or bone, but have been unable to create more complex parenchymal organs, such as liver. Because of inadequate vascularization, the largest composite graft that will survive must be less than 1.5 cm3. A potential solution to this shortcoming is the use of a preexisting, microcirculatory bed as a scaffold to engineer a vascularized neo-organ.171–173 In this approach, entire vascular beds (such as omentum) are removed, cultivated in a bioreactor system where they are seeded with progenitor cells, and then reimplanted using microsurgical techniques.174 Recently, vascularized beds have been successfully maintained ex vivo for several days while they were seeded with multipotent adult progenitor cells, such as liver-derived oval cells.175 Ultimately, vascular beds may provide a means of organ engineering that might be applied to hepatic support in humans.
Recommendations for Future Research.
There have been many advances in understanding and management of ALF, but the syndrome remains challenging and a still-too-common cause of mortality. Important areas for research include:
Continued prospective monitoring to provide information on secular trends in numbers and causes of ALF.
Studies aimed at identifying the cause of “indeterminate” ALF, using newer methodologies such as genomics, gene expression and protein arrays, proteomics, and metabolomics.
Basic research into the nature and pathogenesis of massive tissue injury that leads to multiorgan failure as exemplified by ALF.
Initiation of prospective, well-designed clinical trials of potential therapies for ALF; possibilities include corticosteroids for autoimmunity-related ALF, hypothermia for cerebral edema, and albumin dialysis systems for temporary support.
Improvement in predictive markers and modeling of markers for spontaneous survival (and alternatively death) using standard as well as innovative biomarkers.
Further basic and applied research on growth and proliferation of hepatocytes as well as hepatic and embryonic stem cells for potential use in hepatocyte transplantation as well as in bioartificial liver devices.
Continued experimental animal and in vitro studies aimed at improving the efficacy and safety of bioartificial liver support systems, which may ultimately qualify for clinical trials in humans.
Expanded research on liver tissue engineering with the long-term aim of developing a bioartificial liver that might be used in humans.
Acknowledgment
We wish to acknowledge the sponsorship of the Acute Liver Failure Workshop by the National Institute of Diabetes and Digestive and Kidney Diseases with the support of the National Institute of Biomedical Imaging and Bioengineering and the Office of Rare Diseases of the National Institutes of Health. We also thank Drs. Fei Wang, Patricia Robuck, and Linda Griffith for the efforts in organizing meeting. The speakers at the workshop included William M. Lee, Robert H Squires, Jr, Robert J. Fontana, Timothy Davern, II, Lorenzo Rossaro, Robert Merion, James Trotter, John Bucuvalas, Michael Narkewicz, Laura James, Julie Polson, Anne Larson, William Bernal, R. Todd Stravitz, Adrian Reuben, Anna S.F. Lok, Andres T. Blei, Lars E. Schmidt, John F. Patzer, II, Tarek Hassanein, Scott Nybert, Jorg C. Gerlach, Humberto Soriano, Lola Reid, Linda G. Griffith, Glenn D. Prestwich, and Geoffrey Gurtner.
The Acute Liver Failure Study Group and the Pediatric Acute Liver Failure Study Group are supported by NIH grants U01-DK58639 and U01-DK-072146.
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study
- ALF
acute liver failure
- ALT
alanine aminotransferase
- ANA
antinuclear antibody
- CYP
cytochrome P450
- FDA
Food and Drug Administration
- FPSA
fractionated plasma separation and adsorption
- HAV
hepatitis A virus
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HELLP
hemolysis, elevated liver enzymes, low platelets
- HPLC-EC
high-pressure liquid chromatography with electrochemical detection
- ICU
intensive care unit
- IL
interleukin
- MELD
model of end-stage liver disease
- NAC
N-acetylcysteine
- NAPQI
N-para-aminoquinonimine
- NIBIB
National Institute of Biomedical Imaging and Bio-engineering
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NIH
National Institutes of Health
- INR
international normalized ratio
- SMA
smooth muscle antibody
- TGFβ1
transforming growth factor beta-1
- TIPS
transjugular intrahepatic portosystemic shunt
- TNFα
tumor necrosis factor alpha
- UNOS
United Network for Organ Sharing
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Trey C, Davidson CS. The management of fulminant hepatic failure. In: Popper H, Schaffner F, editors. Progress in Liver Disease. New York, NY: Grune & Stratton; 1970. pp. 282–298. [PubMed] [Google Scholar]
- 2.Ritt DJ, Whelan G, Werner DJ, Eigenbrodt EH, Schenker S, Combes B. A cutehepatic necrosis with stupor orcomaAn analysis of thirty-one patients. Medicine. 1969;48:151–172. doi: 10.1097/00005792-196903000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Schiødt FV, Atillasoy E, Shakil O, Schiff ER, Caldwell C, Kowdley KV, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 4.Ostapowicz GA, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SBH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:945–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 5.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol R, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoofnagle JH, Carithers RL, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 7.Acharya SK, Dasarathy S, Kumer TL, Sushman S, Prasanna KS, Tandon A, et al. Fulminant hepatitis in a tropical population: clinical course, cause, and early predictors of outcome. Hepatology. 1996;23:1448–1455. doi: 10.1002/hep.510230622. [DOI] [PubMed] [Google Scholar]
- 8.Lee WS, McKiernan P, Kelly DA. Etiology, outcome and prognostic indicators of childhood fulminant hepatic failure inthe United Kingdom. J Pediatr Gastroenterol Nutr. 2005;40:575–581. doi: 10.1097/01.mpg.0000158524.30294.e2. [DOI] [PubMed] [Google Scholar]
- 9.Durand P, Debray D, Mandel R, Baujard C, Branchereau S, Gauthier F, et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139:871–876. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan A, Cheeseman P, Mieli-Vergani G. Approaches to acute liver failure in children. Pediatr Transplant. 2004;8:584–588. doi: 10.1111/j.1399-3046.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 11.James LP, Alonso EM, Hynan LS, Hinson JA, Davern TJ, Lee WM, et al. the Pediatric Acute Liver Failure Study GroupDetection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:676–681. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 12.Shneider BL, Rinaldo P, Emre S, Bucuvalas J, Squires R, Narkewicz M, et al. Abnormal concentrations of esterified carnitine in bile: a feature of pediatric acute liver failure with poor prognosis. Hepatology. 2005;41:717–721. doi: 10.1002/hep.20631. [DOI] [PubMed] [Google Scholar]
- 13.Higgins PDR, Fontana RJ. Liver transplantation in acute liver failure. Panminerva Med. 2002;52:93–97. [PubMed] [Google Scholar]
- 14.Wade J, Rolando N, Philpott-Howard J, Wendon J. Timing and etiology of bacterial infections in a liver intensive care unit. J Hosp Infect. 2003;53:144–146. doi: 10.1053/jhin.2002.1363. [DOI] [PubMed] [Google Scholar]
- 15.Alper G, Jarjour IT, Reyes JD, Towbin RB, Hirsch WL, Bergman I. Outcome of children with cerebral edema caused by fulminant hepatic failure. Pediatr Neurol. 1998;18:299–304. doi: 10.1016/s0887-8994(97)00218-x. [DOI] [PubMed] [Google Scholar]
- 16.Jackson EW, Zacks S, Zinn S, Ryan J, Johnson MW, Gerber DA, et al. Delayed neuropsychological dysfunction after liver transplantation for acute liver failure. A matched, case-controlled study. Liver Transpl. 2002;8:932–936. doi: 10.1053/jlts.2002.35550. [DOI] [PubMed] [Google Scholar]
- 17.Jepsen P, Qin P, Norgard B, Agerbo E, Mortensen PB, Vilstrup H, et al. The association between admission for poisoning with paracetamol or other weak analgesics and subsequent admission for psychiatric disorder: A Danish nationwide case-control study. Aliment Pharmacol Ther. 2005;22:645–651. doi: 10.1111/j.1365-2036.2005.02638.x. [DOI] [PubMed] [Google Scholar]
- 18.Hattori H, Higuchi Y, Tsuji M, Inomata Y, Uemoto S, Asonuma K, et al. Living-related liver transplantation and neurological outcome in children with fulminant hepatic failure. Transplantation. 1998;65:686–692. doi: 10.1097/00007890-199803150-00015. [DOI] [PubMed] [Google Scholar]
- 19.Makin AJ, Wendon J, Williams R. A 7-year experience of severe acet-aminophen-induced hepatotoxicity (1987–1993) Gastroenterology. 1995;109:1907–1916. doi: 10.1016/0016-5085(95)90758-0. [DOI] [PubMed] [Google Scholar]
- 20.Bernal W, Wendon J, Rela M, Heaton N, Williams R. Use and outcome of liver transplantation in acetaminophen-induced acute liver failure. Hepatology. 1998;27:1050–1055. doi: 10.1002/hep.510270421. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed R, Hubscher SG, Mirza DF, Gunson BK, Mutimer DJ. Post-transplantation chronic hepatitis in fulminant hepatic failure. Hepatol-ogy. 1997;25:1003–1007. doi: 10.1002/hep.510250435. [DOI] [PubMed] [Google Scholar]
- 22.Vaquero J, Fontana RJ, Larson AM, Bass NM, Davern TJ, Shakil AO, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–1589. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 23.Polson J, Lee WM. American Association for the Study of Liver DiseaseAASLD Position Paper: the management of acute liver failure. Hepatol-ogy. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 24.Grompe M. The pathophysiology and treatment of hereditary tyrosine-mia type 1. Semin Liv Dis. 2001;21:563–572. doi: 10.1055/s-2001-19035. [DOI] [PubMed] [Google Scholar]
- 25.Broussard CN, Aggarwal A, Lacey SR, Post AB, Gramlich T, Henderson JM, et al. Mushroom poisoning-from diarrhea to liver transplantation. Am J Gastroenterol. 2001;96:3195–3198. doi: 10.1111/j.1572-0241.2001.05283.x. [DOI] [PubMed] [Google Scholar]
- 26.Viruet EJ, Torres EA. Steroid therapy in fulminant hepatic failure secondary to autoimmune hepatitis. P R Health Sci J. 1998;17:297–300. [PubMed] [Google Scholar]
- 27.Rodriguez Farina E, Tremosa Llurba G, Xiol Quingles X, Lores Obradors A, Castellote Alonso J, Gornals Soler JB, et al. D-penicillamine and plasmapheresis in acute liver failure secondary to Wilson’s disease. Rev Esp Enferm Dig. 2003;95:60–65. [PubMed] [Google Scholar]
- 28.Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat. 2006;13:256–263. doi: 10.1111/j.1365-2893.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 29.Peters DJ, Greene WH, Ruggiero F, McGarrity TJ. Herpes simplex-induced fulminant hepatitis in adults: a call for empiric therapy. Dig Dis Sci. 2000;45:2399–2404. doi: 10.1023/a:1005699210816. [DOI] [PubMed] [Google Scholar]
- 30.Kori I, Bar-Zohar D, Carmiel-Haggai M, Samuels D, Nakache R, Oren R, et al. Budd-Chiari syndrome and acute portal vein thrombosis: management by a transjugular intrahepatic portosystemic shunt (TIPS) and portal vein interventions via a TIPS. J Gastrointest Surg. 2006;10:417–421. doi: 10.1016/j.gassur.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Flowers MA, Heathcote J, Wanless IR, Sherman M, Reynolds WJ, Cameron RG, et al. Fulminant hepatitis as a consequence of reactivation of hepatitis B virus infection after discontinuation of low-dose methotrexate therapy. Ann Intern Med. 1990;112:381–382. doi: 10.7326/0003-4819-112-5-381. [DOI] [PubMed] [Google Scholar]
- 32.Davern TJ2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 34.Baley B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299–305. doi: 10.1097/00003246-200301000-00048. [DOI] [PubMed] [Google Scholar]
- 35.Burroughs AK, Sabin CA, Rolles K, Delvart V, Karam V, Buckels J, et al. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367:225–232. doi: 10.1016/S0140-6736(06)68033-1. [DOI] [PubMed] [Google Scholar]
- 36.2005 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry for Transplant Recipients: Transplant Data 1995–2004: Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation, Rockville, MD: United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association, Ann Arbor, MI; 2006. [Google Scholar]
- 37.Liu CL, Fan ST, Lo CM, Yong BH, Fung AS, Wong J. Right-lobe live donor liver transplantation improves survival of patients with acute liver failure. Br J Surg. 2002;89:317–322. doi: 10.1046/j.0007-1323.2001.02035.x. [DOI] [PubMed] [Google Scholar]
- 38.Uemoto S, Inomata Y, Sakurai T, Egawa H, Fujita S, Kiuchi T, et al. Living donor liver transplantation for fulminant hepatic failure. Transplantation. 2000;70:152–157. [PubMed] [Google Scholar]
- 39.Nishizaki T, Hiroshige S, Ikegami T, Uchiyama H, Hashimoto K, Soe-jima Y, et al. Living-donor liver transplantation for fulminant hepatic failure in adult patients with a left lobe. Surgery. 2002;131:S182–S189. doi: 10.1067/msy.2002.119574. [DOI] [PubMed] [Google Scholar]
- 40.Baliga P, Alvarez S, Lindblad A, Zeng L. Studies of Pediatric Liver Transplantation Research Group Posttransplant survival in pediatric fulminant hepatic failure: the SPLIT experience. Liver Transpl. 2004;10:1364–1371. doi: 10.1002/lt.20252. [DOI] [PubMed] [Google Scholar]
- 41.Liu E, MacKenzie T, Dobyns EL, Parikh CR, Karrer RM, Narkewicz MR, et al. Characterization of acute liver failure and development of a continuous risk of death staging system in children. J Hepatol. 2006;44:134–141. doi: 10.1016/j.jhep.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Miyake Y, Sakaguchi K, Iwasaki Y, Ikeda H, Makino Y, Kobashi H, et al. New prognostic scoring model for liver transplantation in patients with non-acetaminophen-related fulminant hepatic failure. Transplantation. 2005;80:930–936. doi: 10.1097/01.tp.0000173651.39645.35. [DOI] [PubMed] [Google Scholar]
- 43.Larsen FS, Kirkegaard P, Rasmussen A, Hansen BA. The Danish liver transplantation program and patients with serious acetaminophen intoxication. Transplant Proc. 1995;27:3519–3520. [PubMed] [Google Scholar]
- 44.Nourjah P, Willy M. Epidemiology of acetaminophen-related overdose. Department of Health and Human Services, Center for Drug Evaluation and Research, Food and Drug Administration; 2002. Available at: www.fda.gov/ohrms/dockets/ac/02/briefing/3882b1.htm. [Google Scholar]
- 45.Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. Acet-aminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther. 1974;16:676–684. doi: 10.1002/cpt1974164676. [DOI] [PubMed] [Google Scholar]
- 46.Pumford NR, Hinson JA, Benson RW, Roberts DW. Immunoblot analysis of protein containing 3-(cystein-S-yl) acetaminophen adducts in serum and subcellular liver fractions from acetaminophen- treated mice. Toxicol Appl Pharmacol. 1990;104:521–532. doi: 10.1016/0041-008x(90)90174-s. [DOI] [PubMed] [Google Scholar]
- 47.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, et al. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high- performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepato-toxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767–773. [PubMed] [Google Scholar]
- 49.Schiødt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–1117. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- 50.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani EK, Hynan LS, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1367–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 51.Shayiq RM, Roberts DW, Rothstein K, Snawder JE, Benson W, Ma X, et al. Repeat exposure to incremental doses of acetaminophen provides protection against acetaminophen-induced lethality in mice: an explanation for high acetaminophen dosage in humans without hepatic injury. Hepa-tology. 1999;29:451–463. doi: 10.1002/hep.510290241. [DOI] [PubMed] [Google Scholar]
- 52.Rezende G, Roque-Afonso AM, Samuel D, Gigou M, Nicand E, Ferre V, et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38:613–618. doi: 10.1053/jhep.2003.50366. [DOI] [PubMed] [Google Scholar]
- 53.Hutin YJ, Pool V, Cramer EH, Nainan OV, Weth J, Williams IT, et al. A multi-state, food-borne outbreak of hepatitis A. New Engl J Med. 1999;340:595–602. doi: 10.1056/NEJM199902253400802. [DOI] [PubMed] [Google Scholar]
- 54.Polson J, Ocama P, Larson AM, Hynan L, Lalani E, Harrison M, et al. U.S. ALF Study Group. Role of acetaminophen in acute liver failure due to viral hepatitis [abstract] Hepatology. 2003;34:544A. [Google Scholar]
- 55.Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2006;26:CD003328. doi: 10.1002/14651858.CD003328.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Spiller HA, Winter ML, Klein-Schwartz W, Bangh SA. Efficacy of activated charcoal administered more than four hours after acetaminophen overdose. J Emerg Med. 2006;30:1–5. doi: 10.1016/j.jemermed.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 57.Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20:1058–1063. doi: 10.1016/s0196-0644(05)81352-6. [DOI] [PubMed] [Google Scholar]
- 58.Burkhart KK, Janco N, Kulig KW, Rumack BH. Cimetidine as adjunc-tive treatment for acetaminophen overdose. Hum Exp Toxicol. 1995;14:299–304. doi: 10.1177/096032719501400311. [DOI] [PubMed] [Google Scholar]
- 59.Keays R, Harrison PM, Wendon JA, Forbes A, Gove C, Alexander GJ, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303:1026–1029. doi: 10.1136/bmj.303.6809.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones AL. Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. J Toxicol Clin Toxicol. 1998;36:277–285. doi: 10.3109/15563659809028022. [DOI] [PubMed] [Google Scholar]
- 61.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdoseAnalysis of the national multi-center study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 62.O’Grady JG, Gimson AE, O’Brien CJ, Pucknell A, Hughes RD, Williams R. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology. 1988;94:1186–1192. doi: 10.1016/0016-5085(88)90011-x. [DOI] [PubMed] [Google Scholar]
- 63.Kanter MZ. Comparisonof oral and i.v. acetylcysteine inthe treatment of acetaminophen poisoning. Am J Health Syst Pharm. 2006;63:1821–1827. doi: 10.2146/ajhp060050. [DOI] [PubMed] [Google Scholar]
- 64.Acetadote Package Insert. Nashville, TN: Cumberland Pharmaceuticals; [Google Scholar]
- 65.Hawton K, Ware C, Mistry H, Hewitt J, Kingsbury S, Roberts D, et al. Why patients choose paracetamol for self poisoning and their knowledge of its dangers. BMJ. 1995;310:164. doi: 10.1136/bmj.310.6973.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawton K, Simkin S, Deeks J, Cooper J, Johnston A, Waters K, et al. UK Legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ. 2004;329:1076–1081. doi: 10.1136/bmj.38253.572581.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilkinson S, Taylor G, Templeton L, Mistral W, Salter E, Bennett P. Admissions to Hospital for deliberate self-harm in England 1995–2000: an analysis of Hospital Episode Statistics. J Public Health Med. 2002;24:179–183. doi: 10.1093/pubmed/24.3.179. [DOI] [PubMed] [Google Scholar]
- 68.Hughes B, Durran A, Langford N, Mutimer D. Paracetamol Poisoning—impact of pack size restrictions. J Clin Pharm Ther. 2003;28:307–310. doi: 10.1046/j.1365-2710.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 69.Atcha Z, Majeed A. Paracetamol related deaths in England and Wales, 1993–97. Health Stat Q. 2000;7:5–9. [Google Scholar]
- 70.Greene S, Dargan P, Leman P, Jones A. Paracetamol availability and recent changes in paracetamol poisoning: is the 1998 legislation limiting availability of paracetamol being followed? Postgrad Med J. 2006;82:520–523. doi: 10.1136/pgmj.2005.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrari R, Pappas G, Agostinelli D, Muratori P, Muratori L, Lenzi M, et al. Type 1 autoimmune hepatitis: patterns of clinical presentation and differential diagnosis of the ‘acute’ type. Q J Med. 2004;97:407–412. doi: 10.1093/qjmed/hch072. [DOI] [PubMed] [Google Scholar]
- 72.Kessler WR, Cummings OW, Eckert G, Chalasani N, Lumeng L, Kwo PY. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol. 2004;2:625–631. doi: 10.1016/s1542-3565(04)00246-0. [DOI] [PubMed] [Google Scholar]
- 73.Rakela J, Mosley JW, Edwards VM, Govindarajan S. Alpertmes EAdouble-blinded randomized trial hydrocortisone in acute hepatic failureThe Acute Hepatic Failure Study Group. Dig Dis Sci. 1991;36:1223–1228. doi: 10.1007/BF01307513. [DOI] [PubMed] [Google Scholar]
- 74.Harry R, Auzinger G, Wendon J. The effects of supraphysiological doses of corticosteroids in hypotensive liver failure. Liver Int. 2003;23:71–77. doi: 10.1034/j.1600-0676.2003.00813.x. [DOI] [PubMed] [Google Scholar]
- 75.Zimmerman HJ. Drug-induced liver disease. Drugs. 1978;16:25–45. doi: 10.2165/00003495-197816010-00002. [DOI] [PubMed] [Google Scholar]
- 76.Lee WMAcetaminophen, the US. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 77.Standardization of definitions criteria of causality assessment of adverse drug reactions. Drug-induced liver disorders: report of an international consensus meeting. Int J Clin Pharmacol Ther Toxicol. 1990;28:317–322. [PubMed] [Google Scholar]
- 78.Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618–631. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 79.Schiødt FV, Davern TJ, Shakil OA, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003;98:448–453. doi: 10.1111/j.1572-0241.2003.t01-1-07223.x. [DOI] [PubMed] [Google Scholar]
- 80.Chu CM, Yeh CT, Liaw YF. Fulminant hepatic failure in acute hepatitis C: increased risk in chronic carriers of hepatitis B virus. Gut. 1999;45:613–617. doi: 10.1136/gut.45.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palanduz A, Yildirmak Y, Telhan L, Arapoglu M, Urganci N, Tufekci S, et al. Fulminant hepatic failure and autoimmune hemolytic anemia associated with Epstein-Barr virus infection. J Infect Dis. 2002;45:96–98. doi: 10.1053/jinf.2002.0993. [DOI] [PubMed] [Google Scholar]
- 82.Fonseca JC, Souza RA, Brasil LM, Araujo JR, Ferreira LC. Fulminant hepatic failure in children and adolescents in Northern Brazil. Rev Soc Bras Med Trop. 2004;37:67–69. doi: 10.1590/s0037-86822004000100019. [DOI] [PubMed] [Google Scholar]
- 83.Umemura T, Tanaka E, Ostapowicz G, Lee WM, Heringlake S, Manns MP, et al. Investigation of SEN virus infection in patients with crypto-genic acute liver failure, aplastic anemia and acute and chronic non-A-E hepatitis. J Infect Dis. 2003;188:1545–1552. doi: 10.1086/379216. [DOI] [PubMed] [Google Scholar]
- 84.Lee WM, Brown KE, Young NS, Dawson GJ, Schlauder GG, Gutierrez RA, et al. No evidence for hepatitis E or parvovirus B19 infection in patients with acute liver failure. Dig Dis Sci. 2006;51:1712–1715. doi: 10.1007/s10620-005-9061-5. [DOI] [PubMed] [Google Scholar]
- 85.Poovorawan Y, Hutagalung Y, Chongsrisawat V, Boudville I, Bock HL. Dengue virus infection: a major cause of acute hepatic failure in Thai children. Ann Trop Paediatr. 2006;26:17–23. doi: 10.1179/146532806X90565. [DOI] [PubMed] [Google Scholar]
- 86.Ozasa A, Tanaka Y, Orito E, Sugiyama M, Kang JH, Hige S, et al. Influence of genotypes and precore mutations on fulminant or chronic outcome of acute hepatitis B virus infection. Hepatology. 2006;44:326–334. doi: 10.1002/hep.21249. [DOI] [PubMed] [Google Scholar]
- 87.Wai CT, Fontana RJ, Polson J, Hussain M, Shakil AO, Han SH, et al. Clinical outcome and virological characteristics of hepatitis B-related acute liver failure in the United States. J Viral Hepat. 2005;12:192–198. doi: 10.1111/j.1365-2893.2005.00581.x. [DOI] [PubMed] [Google Scholar]
- 88.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 89.Bianco E, Stroffolini T, Spada E, Szkio A, Marzolini F, Ragni P, et al. Case fatality rate of acute viral hepatitis in Italy: 1995–2000. An updateDig Liver Dis. 2003;35:404–408. doi: 10.1016/s1590-8658(03)00157-9. [DOI] [PubMed] [Google Scholar]
- 90.Sagnelli E, Stroffolini T, Almasio P, Mele A, Coppola N, Ferrigno L, et al. Exposure to HAV infection in patients with chronic liver disease in Italy, a multicentre study. J Viral Hepat. 2006;13:67–71. doi: 10.1111/j.1365-2893.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 91.Casey JL, Brown TL, Colan EJ, Wignall FS, Gerin JL. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci U S A. 1993;90:9016–9020. doi: 10.1073/pnas.90.19.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harma M, Hockerstedt K, Krogerus L, Lautenschlager I. Pretransplant human herpesvirus 6 infection of patients with acute liver failure is a risk factor for posttransplant human herpesvirus 6 infection of the liver. Transplantation. 2006;81:367–372. doi: 10.1097/01.tp.0000195771.83614.0b. [DOI] [PubMed] [Google Scholar]
- 93.Verma A, Dhawan A, Zuckerman M, Hadzic N, Baker AJ, Mieli-Vergani G. Neonatal herpes simplex virus infection presenting as acute liver failure: prevalent role of herpes simplex virus type I. J Pediatr Gastroenterol Nutr. 2006;42:282–286. doi: 10.1097/01.mpg.0000214156.58659.4c. [DOI] [PubMed] [Google Scholar]
- 94.Ichai P, Afonso AM, Sebagh M, Gonzalez ME, Codes L, Azoulay D, et al. Herpes simplex virus-associated acute liver failure: a difficult diagnosis with a poor prognosis. Liver Transpl. 2005;11:1550–1555. doi: 10.1002/lt.20545. [DOI] [PubMed] [Google Scholar]
- 95.Berman DH, Leventhal RI, Gavaler JS, Cadoff EM, Van Thiel DH. EASL Study Group Randomised trial of steroid therapy in acute liver failure. Gut. 1979;20:620–623. doi: 10.1136/gut.20.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rake MO, Flute PT, Shilkin KB, Lewis ML, Winch J, Williams R. Early intensive therapy of intravascular coagulation in acute liver failure. Lancet. 1971;vii:1215–1218. doi: 10.1016/s0140-6736(71)90540-x. [DOI] [PubMed] [Google Scholar]
- 97.Harrison PM, Hughes RD, Forbes A, Portmann B, Alexander GJM, Williams R. Failure of insulin and glucagon infusions to stimulate liver regeneration in fulminant hepatic failure. J Hepatol. 1990;10:332–336. doi: 10.1016/0168-8278(90)90141-d. [DOI] [PubMed] [Google Scholar]
- 98.Sinclair SB, Levy GA Treatment of fulminant viral hepatic failure with prostaglandin EA preliminary report. Dig Dis Sci. 1991;36:791–800. doi: 10.1007/BF01311239. [DOI] [PubMed] [Google Scholar]
- 99.Harrison PM, Keays R, Bray GP, Alexander GJM, Williams R. Improved outcomeof paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335:1572–1573. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 100.Schneider J, Lutun P, Boudjema K, Wolf P, Tempe´ J-D. In vivo evidence of enhanced guanylyl cyclase activation during the hyperdynamic circulation of acute liver failure. Hepatology. 1994;19:38–44. [PubMed] [Google Scholar]
- 101.Alonso A, Lau J, Jaber BL, Weintraub A, Sarnak MJ. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1–9. doi: 10.1053/j.ajkd.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Jalan R, Damink SW, Deutz NE, Lee A, Hayes PC. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet. 1999;354:1164–1168. doi: 10.1016/s0140-6736(98)12440-6. [DOI] [PubMed] [Google Scholar]
- 103.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intra-cranial hypertension. Gastroenterology. 2004;127:1338–1346. doi: 10.1053/j.gastro.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 104.Vaquero J, Blei AT. Mild hypothermia for acute liver failure: a review of mechanisms of action. J Clin Gastroenterol. 2005;39(Suppl 2):147–S157. doi: 10.1097/01.mcg.0000155515.94843.55. [DOI] [PubMed] [Google Scholar]
- 105.Vaquero J, Rose C, Butterworth RF. Keeping cool in acute liver failure: rationale for the useof mild hypothermia. J Hepatol. 2005;43:1067–1077. doi: 10.1016/j.jhep.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 106.Polderman KH. Application of therapeutic hypothermia inthe ICU: opportunities, pitfallsofapromising treatment modalityPart I: Indications and evidence. Intensive Care Med. 2004;30:556–575. doi: 10.1007/s00134-003-2152-x. [DOI] [PubMed] [Google Scholar]
- 107.Polderman KH. Applicationof therapeutic hypothermia inthe ICU: opportunities, pitfallsofa promising treatment modality. Part 2Practical aspects and side effects. Intensive Care Med. 2004;30:757–769. doi: 10.1007/s00134-003-2151-y. [DOI] [PubMed] [Google Scholar]
- 108.Vaquero J, Belanger M, James L, Herrero R, Desjardins P, Cote J, et al. Mild hypothermia attenuates liver injury and improves survival in mice with acetaminophen toxicity. Gastroenterology. 2007;132:372–383. doi: 10.1053/j.gastro.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 109.Kondrup J, Almdal T, Vilstrup H, Tygstrup N. High volume plasma exchange in fulminant hepatic failure. Int J Artif Organs. 1002;11:669–676. [PubMed] [Google Scholar]
- 110.Clemmesen JO, Kondrup J, Nielsen LB, Larsen FS, Ott P. Effects of high-volume plasmapheresis on ammonia, urea, and amino acids in patients with acute liver failure. Am J Gastroenterol. 2001;96:1217–1223. doi: 10.1111/j.1572-0241.2001.03706.x. [DOI] [PubMed] [Google Scholar]
- 111.Larsen FS, Hansen BA, Ejlersen E, Secher NH, Clemmesen JO, Tygstrup N, et al. Cerebral blood flow, oxygen metabolism and transcranial Dopp-ler sonography during high-volume plasmapheresis in fulminant hepatic failure. Eur J Gastroenterol. 1996;8:261–265. doi: 10.1097/00042737-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 112.Clemmesen JO, Larsen FS, Ejlersen E, Schiødt FV, Ott P, Hansen BA. Haemodynamic changes after high-volume plasmapheresis in patients with chronic and acute liver failure. Eur J Gastroenterol Hepatol. 1997;9:55–60. doi: 10.1097/00042737-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 113.Larsen FS, Hansen BA, Jorgensen LG, Secher NH, Kirkegaard P, Tyg-strup N. High-volume plasmapheresis and acute liver transplantation in fulminant hepatic failure. Transplant Proc. 1994;26:1788. [PubMed] [Google Scholar]
- 114.Patzer JFII. Advances in bioartificial liver devices. Ann NY Acad Sci. 2001;944:320–332. doi: 10.1111/j.1749-6632.2001.tb03844.x. [DOI] [PubMed] [Google Scholar]
- 115.Krisper P, Haditsch B, Stauber R. In vivo quantification of liver dialysis: comparison of albumin dialysis and fractionated plasma separation. J Hepatol. 2005;43:451–457. doi: 10.1016/j.jhep.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 116.Rifai K, Manns M. Clinical experience with Prometheus . Ther Apher Dial. 2006;10:132–137. doi: 10.1111/j.1744-9987.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 117.Stange J, Hassanein T, Mehta R, Mitzner S, Bartlett R. The molecular absorbents recycling system as a liver support system based on albumin dialysis: a summary of preclinical investigations, prospective, randomized, controlled clinical trial, and clinical experience from 19 centers. Artif Organs. 2002;26:103–110. doi: 10.1046/j.1525-1594.2002.06822.x. [DOI] [PubMed] [Google Scholar]
- 118.Patzer J. Principles of bound solute dialysis. Ther Apher Dial. 2006;10:118–124. doi: 10.1111/j.1744-9987.2006.00352.x. [DOI] [PubMed] [Google Scholar]
- 119.Patzer J, Safta S, Miller R. Slow continuous ultrafiltration with bound solute dialysis. ASAIO J. 2006;52:47–58. doi: 10.1097/01.mat.0000196524.36394.0d. [DOI] [PubMed] [Google Scholar]
- 120.Millis J, Losanoff J. Technology insight: liver support systems. Nat Clin Pract Gastroenterol Hepatol. 2005;2:398–405. doi: 10.1038/ncpgasthep0254. [DOI] [PubMed] [Google Scholar]
- 121.Demetriou A, Brown R, Busuttil R, Fair J, McGuire B, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–670. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sauer I, Kardassis D, Zeillinger D, Pascher A, Gruenwald A, Pless G, et al. Clinical extracorporeal hybrid liver support - phase 1 study with primary porcine liver cells. Xenotransplantation. 2003;10:460–469. doi: 10.1034/j.1399-3089.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 123.Patzer J, Kramer D, Mazariegos G, Lopez-Solis R, Giraldo M, Grogan T, et al. Clinical safety evaluation of Excorp Medical Inc. bioartificial liver support system (BLSS). [abstract] ASAIO J. 2000;46:241A. doi: 10.1097/00002480-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 124.Sosef MN, Abrahamse LSL, Van de Kerkhove M-P, Hartman R, Chamu-leau RAFM, Van Gulik TM. Assessment of the AMC-Bioartificial liver in the anhepatic pig. Transplantation. 2002;73:204–209. doi: 10.1097/00007890-200201270-00009. [DOI] [PubMed] [Google Scholar]
- 125.Kjaergard L, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure. JAMA. 2003;289:217–222. doi: 10.1001/jama.289.2.217. [DOI] [PubMed] [Google Scholar]
- 126.Matsumura K, Guevara G, Huston H, Hamilton W, Rikimaru M, Ya-masaki G, et al. Hybrid bioartificial liver in hepatic failure: preliminary clinical report. Surgery. 1987;101:99–103. [PubMed] [Google Scholar]
- 127.Millis L, Kramer D, O’Grady J, Heffron T, Caldwell S, Hart M, et al. Results of phase I trial of the extracorporeal liver assist device for patients with fulminant hepatic failure. [abstract] Am J Transplant. 2001;1(Suppl 1):391. [Google Scholar]
- 128.Watanabe F, Mullon CJ-P, Hewitt W, Arkadopoulos N, Kahaku E, Eguchi S, et al. Clinical experience with a bioartifical liver (BAL) in the treatment of severe liver failure: a phase I clinical trial. Ann Surg. 1997;225:484–94. doi: 10.1097/00000658-199705000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nyberg S, Hardin J, Amiot B, Argikar U, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transpl. 2005;11:901–910. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 130.Nyberg S, Cerra F, Gruetter R. Brain lactate by magnetic resonance spectroscopy during fulminant hepatic failure in the dog. Liver Transplant Surg. 1997;4:158–165. doi: 10.1002/lt.500040203. [DOI] [PubMed] [Google Scholar]
- 131.Nyberg S. Galactosamine-induced fulminant hepatic failure [letter] Hepatology. 1997;26:1367–1368. doi: 10.1002/hep.510260544. [DOI] [PubMed] [Google Scholar]
- 132.Frisch S, Screaton R. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 133.Gerlach JC, Kerim M, Sauer IM, Schrade P, Efimova E, Mieder T, et al. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: A morphologic study. Transplant. 2003;76:781–786. doi: 10.1097/01.TP.0000083319.36931.32. [DOI] [PubMed] [Google Scholar]
- 134.Monga PS, Hout MS, Baun MJ, Micsenyi A, Muller P, Tummalapalli L, et al. Mouse Fetal Liver Cells in Artificial Capillary Beds in Three-Dimensional Four-Compartment Bioreactors. Am J Pathol. 2005;167:1279–1292. doi: 10.1016/S0002-9440(10)61215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmidt-Heck W, Zeilinger K, Pfaff M, Toepfer S, Driesch D, Pless G, et al. Network analysis of the kinetics of amino acid metabolism in a liver cell bioreactor. In: Barreiro JM, Martin-Sanchez F, Maojo V, Sanz F, editors. International Symposium on Biological and Medical Data Analysis 2004; 5th International Symposium (Lecture Notes in Computer Science, Vol. 3337); Heidelberg, Germany: Springer; 2004. pp. 427–438. [Google Scholar]
- 136.Gerlach J, Zeilinger K, Holland G, Mu¨ller C, Neuhaus P. Recovery of primary human hepatocytes and nonparenchymal cells from preservation injury to tissue-like structures in large-scale bioreactors for liver support: An initial TEM study. J Invest Surg. 2003;16:83–92. doi: 10.1080/08941930390194370. [DOI] [PubMed] [Google Scholar]
- 137.Zeilinger K, Holland G, Sauer IM, Efimova E, Kardassis D, Obermayer N, et al. Time course of primary liver cell reorganization in three-dimensional high-density bioreactors for extracorporeal liver support: an immunohisto-chemical and ultrastructural study. Tissue Eng. 2004;10:1113–1124. doi: 10.1089/ten.2004.10.1113. [DOI] [PubMed] [Google Scholar]
- 138.Sauer IM, Zeilinger K, Pless G, Kardassis D, Theruvath T, Pascher A, et al. Extracorporeal Liver Support based on primary human liver cells and albumin dialysis - treatment of a patient with primary graft non-function. J Hepatol. 2003;39:649–653. doi: 10.1016/s0168-8278(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 139.Sauer IM, Kardassis D, Zeilinger K, Pascher A, Gruenwald A, Pless G, et al. Clinical extracorporeal hybrid liver support - phase I study with primary porcine liver cells. Xenotransplantation. 2003;10:460–469. doi: 10.1034/j.1399-3089.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 140.Sauer I, Gerlach J. Modular extracorporeal liver support (MELS) Artif Organs. 2002;26:703–706. doi: 10.1046/j.1525-1594.2002.06931_1.x. [DOI] [PubMed] [Google Scholar]
- 141.Strom S, Fisher R, Thompson M, Sanyal A, Cole P, Ham J, et al. Hepa-tocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplant. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 142.Strom S, Bruzzone P, Cai E, Lehmann T, Mitamura K, Miki T. Hepa-tocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006;15:S105–S110. doi: 10.3727/000000006783982395. [DOI] [PubMed] [Google Scholar]
- 143.Soriano HE, Superina R, Ferry GD, Wang LJ, Alonso E, Almond S, et al. Hepatocyte transplantation (HTx) in children with liver failure [abstract] Hepatology. 2001;34(Suppl 2):205A. [Google Scholar]
- 144.Zvibel I, Smets F, Soriano H. Anoikis: roadblock to cell transplantation. Cell Transplant. 2002;11:621–630. doi: 10.3727/000000002783985404. [DOI] [PubMed] [Google Scholar]
- 145.Strom S, Cai H, Ellis E, Mitamura M, Miki T. Bigger may not be better when it comes to hepatocytes. Liver Transplant. 2006;12:16–18. doi: 10.1002/lt.20593. [DOI] [PubMed] [Google Scholar]
- 146.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 147.Schmelzer E, Zhang L, Melhem A, Yao H, Turner R, McClelland R, et al. Hepatic stem cells and the liver’s maturational lineages. In: Potten CS, Clarke RB, Wilson J, Renehan AG, editors. Tissue Stem Cells: Biology and Applications. London, UK: Dekker Publishers; 2006. [Google Scholar]
- 148.Odorico JS, Kaufman DS, Thomson J. Multi-lineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 149.Rambhatia L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- 150.Lavon N, Yanaka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 151.Thiese ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 152.Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepa-tocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci USA. 2000;97:12132–12137. doi: 10.1073/pnas.97.22.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679–2688. doi: 10.1242/jcs.115.13.2679. [DOI] [PubMed] [Google Scholar]
- 154.Kubota H, Storm R, Reid LM. Variant forms of α-fetoprotein transcripts expressed in human hemopoietic and hepatic progenitors. J Biol Chem. 2002;277:27629–27635. doi: 10.1074/jbc.M202117200. [DOI] [PubMed] [Google Scholar]
- 155.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastroinest Liver Physiol. 2006;290:G859–G870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 156.Schmelzer E, Reid LM. Phenotype of pluripotent hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 157.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao H, et al. Human hepatic stem cells in livers from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Susick R, Moss N, Kubota H, Lecluyse E, Hamilton G, Luntz T, et al. Hepatic progenitors and strategies for liver cell therapies. Ann N YAcad Sci. 2001;944:398–419. doi: 10.1111/j.1749-6632.2001.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 159.Gupta S, Chowdhury JR. Therapeutic potential of hepatocyte transplantation. Semin Cell DevBiol. 2002;13:439–446. doi: 10.1016/s1084952102001325. [DOI] [PubMed] [Google Scholar]
- 160.Turner WS, Schmelzer E, McClelland R, Wauthier E, Chen W, Reid LM. Human hepatoblast phenotype maintained by hyaluronan hydro-gels. J Biomed Mater Res B Appl Biomater. 2007;82:156–168. doi: 10.1002/jbm.b.30717. [DOI] [PubMed] [Google Scholar]
- 161.Riley CM, Fuegy PW, Firpo MA, Shu XZ, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials. 2006;27:5935–5943. doi: 10.1016/j.biomaterials.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices (sECMs) for tissue engineering. J Biomed Mater Res. 2006;79A:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 163.Griffith LG, Naughton G. Tissue engineering-current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 164.Sivaraman A, Leach JK, Townsend S, Iida T, Hogan BJ, Stolz DB, et al. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab. 2005;6:569–591. doi: 10.2174/138920005774832632. [DOI] [PubMed] [Google Scholar]
- 165.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 166.Prestwich G, Liu Y, Yu B, Shu XZ, Scott A. 3-D culture in synthetic extracellular matrices: new tissue models for drug toxicology and cancer drug discovery. Adv Enzyme Regul. 2007;47:196–207. doi: 10.1016/j.advenzreg.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 167.Prestwich GD, Shu XZ, Liu Y, Cai S, Walsh JF, Hughes CW, et al. Injectable synthetic extracellular matrices for tissue engineering and repair. Adv Exp Med Biol. 2006;585:125–133. doi: 10.1007/978-0-387-34133-0_9. [DOI] [PubMed] [Google Scholar]
- 168.Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ cross-linkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 169.Cai S, Liu Y, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 170.Liu Y, Ahmad S, Shu XZ, Sanders RK, Kopesec SA, Prestwich GD. Accelerated repair of cortical bone defects using a synthetic extracellular matrix to deliver human demineralized bone matrix. J Orthoped Res. 2006;24:1454–1462. doi: 10.1002/jor.20148. [DOI] [PubMed] [Google Scholar]
- 171.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 172.Gurtner GC, Callaghan MJ, Longaker MT. Progress and potential for regenerative medicine. Annu Rev Med. 2007;58:299–312. doi: 10.1146/annurev.med.58.082405.095329. [DOI] [PubMed] [Google Scholar]
- 173.Chung S, Hazen A, Levine JP, Baux G, Olivier WA, Yee HT, et al. Vascularized acellular dermal matrix island flaps for the repair of abdominal muscle defects. Plast Reconstr Surg. 2003;111:225–232. doi: 10.1097/01.PRS.0000034934.05304.ED. [DOI] [PubMed] [Google Scholar]
- 174.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Klein-man ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 175.Tepper OM, Galiano RD, Kalka C, Gurtner GC. Endothelial progenitor cells: the promise of vascular stem cells for plastic surgery. Plast Reconstr Surg. 2003;111:846–854. doi: 10.1097/01.PRS.0000039576.63523.08. [DOI] [PubMed] [Google Scholar]