Abstract
The inverse-electron-demand Diels-Alder cycloaddition between trans-cyclooctenes and tetrazines is biocompatible and exceptionally fast. We utilized this chemistry for site-specific fluorescence labeling of proteins on the cell surface and inside living mammalian cells by a two-step protocol. E. coli lipoic acid ligase site-specifically ligates a trans-cyclooctene derivative onto a protein of interest in the first step, followed by chemoselective derivatization with a tetrazinefluorophore conjugate in the second step. On the cell surface, this labeling was fluorogenic and highly sensitive. Inside the cell, we achieved specific labeling of cytoskeletal proteins with green and red fluorophores. By incorporating the Diels-Alder cycloaddition, we have broadened the panel of fluorophores that can be targeted by lipoic acid ligase.
Due to their small size and advantageous photophysical properties relative to Green Fluorescent Protein, chemical fluorophores have the potential to be very useful for live cell imaging. An impediment to their widespread use, however, is the difficulty of targeting them to specific cellular proteins. Our lab has developed an approach to the targeting problem called PRIME, for Probe Incorporation Mediated by Enzymes1-3. In PRIME, a mutant of the E. coli enzyme lipoic acid ligase (LplA) is used to catalyze the covalent conjugation of chemical probes to recombinant proteins fused to a 13-amino acid LplA acceptor peptide, or LAP, inside living cells. The naturally high sequence-specificity of LplA makes this targeting method highly specific. In comparison to other intracellular post-translational protein labeling methods – SNAP4, HaloTag5, and FlAsH6 – PRIME uniquely offers the combination of a small peptide-directing sequence and high labeling specificity.
PRIME, however, has so far only been demonstrated inside cells with small blue fluorophores based on coumarin1;7;8. To be generally useful, PRIME must be extended to other fluorophores, particularly red-shifted ones with excellent photophysical properties such as tetramethylrhodamine (TMR) and Alexa Fluors. Our experience with engineering the LplA active site to accommodate unnatural substrates1;2;7 suggests that major structural redesign would be required for LplA to directly ligate these much larger dyes. An alternative strategy is to use LplA to ligate a functional group handle, which can then be chemoselectively derivatized in the cellular context with fluorophores of interest.
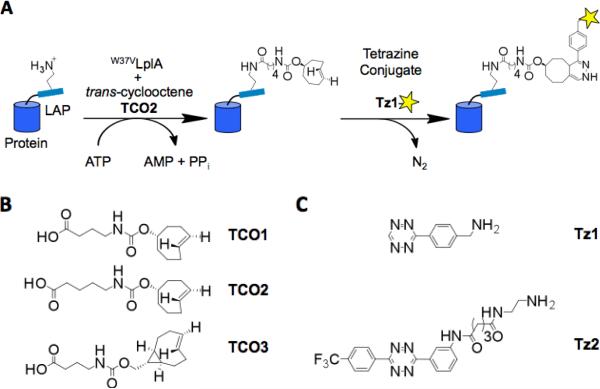
We considered three types of reactions for the chemoselective derivatization: copper-catalyzed azidealkyne cycloadditions (CuAAC)9, strain-promoted azidecycloalkyne cycloadditions10, and inverse-electron-demand Diels-Alder cycloadditions of tetrazines and trans-cyclooctenes11. CuAAC is restricted to the cell surface due to its dependence on toxic Cu(I)12. We previously used PRIME in conjunction with strain-promoted cycloaddition for fluorescent labeling of cell surface proteins2. The slow kinetics of this reaction (k = 10-3 to 1 M-1s-1)13, however, limited our overall labeling yield and hence the achievable signal-to-noise ratio for imaging. Both CuAAC (k up to 104 M-1s-1/M copper)14 and the Diels-Alder cycloaddition (k up to 104 M-1s-1)15 are much faster. The Diels-Alder reaction is also compatible in principle with the cell interior, although the only previous demonstration was intracellular labeling of a taxol derivative16. Due to both its speed and potential for intracellular compatibility, we selected the Diels-Alder cycloaddition for this study (Figure 1A).
Figure 1.
Two-step, site-specific fluorescence labeling of proteins using lipoic acid ligase (LplA) and Diels-Alder cycloaddition. (A) Optimized labeling scheme. In the first step, the Trp37→Val mutant of LplA ligates transcyclooctene TCO2 onto LplA acceptor peptide (LAP), which is fused to the protein of interest. In the second step, ligated trans-cyclooctene is chemoselectively derivatized with a fluorophore conjugated to Tz1 tetrazine. (B) Three transcyclooctenes synthesized and evaluated in this study. (C) Two tetrazines used in this study.
To utilize this chemistry, we first needed to choose between having LplA ligate the tetrazine or the transcyclooctene. We noted that the trans-cyclooctene moiety would be less bulky and therefore require less re-engineering of LplA. This is because tetrazine itself is unstable in aqueous solution, and must be stabilized by conjugation to one or more aromatic rings17, making the overall moiety quite large. Additionally, tetrazines quench the fluorescence of some covalently attached fluorophores, until reaction with trans-cyclooctene16. To allow for the possibility of fluorogenic labeling, we opted to conjugate the fluorophore to tetrazine.
Based on our experience, LplA prefers substrates with 3 – 4 linear methylenes linking the carboxylate and the bulky feature1. We therefore synthesized three transcyclooctene substrates for LplA: TCO1, TCO2, and TCO3, with structures shown in Figure 1B and syntheses enabled by our photochemical flow method (Scheme 1)18. TCO1 and TCO2 differ only in the length of their aliphatic linkers, while TCO3 has a cyclopropane ring fusion, which adds strain and accelerates the cycloaddition up to 160-fold19. We prepared a panel of LplA mutants and screened for their ability to ligate these three TCOs onto LAP using an HPLC assay (Table 1). Not surprisingly, wild-type LplA was unable to ligate any of the three substrates efficiently. Our other LplAs each harbored a single mutation at Trp37, a gate-keeper residue that has given us access to various unnatural substrates in the past1;20. We tested the Trp37→Gly mutant, with the active site maximally enlarged, as well as Trp37→Ile and →Val mutants that carve out a smaller, hydrophobic hole. We found that TCO2 scored significantly better than the other probes, and was best paired with the Val mutant (designated W37VLplA, Table 1).
Table 1.
Ligation efficiencies of lipoic acid ligase variants with the three frans-cyclooctene substrates (TCO1-3). The relative abilities of wild-type and mutant ligases to ligate TCO1-3 onto LplA acceptor peptide (LAP) were measured by an HPLC assay after 30 min. reaction time. Average, normalized product percentages from triplicate measuremeasurements are shown. (--) indicates no detectable product. Errors, ± 1 s. d.
| Ligase | Wi Id-type | Trp37→Gly | Trp37→lie | Trp37→Val |
|---|---|---|---|---|
| Probe | ||||
| TC01 | 0.5 ±0.0 | -- | 0.5 ±0.4 | -- |
| TC02 | 2.6 ±0.9 | 52.6 ± 1.1 | 77.8 ± 1.6 | 100.0 ±8.3 |
| TC03 | 6.5 ± 1.0 | 22.6 ± 2.0 | 6.0 ± 0.6 | 6.6 ± 0.6 |
Enzyme-dependent ligation was confirmed by negative controls omitting ATP or W37VLplA, and by mass spec-trometry (Supporting Figure 1A and 1B). We estimated the Michaelis-Menten kcat of TCO2 ligation onto LAP to be 0.34 ± 0.02 s-1, comparable to the fastest unnatural probe that we have reported to date (aryl azide: 0.31 ± 0.04 s-1)20, and only 2-fold slower than ligation of the natural substrate lipoic acid by the same enzyme (Supporting Figure 2).
We next focused on the design and syntheses of the tetrazine-fluorophore conjugates. The previously reported tetrazine structure Tz1 (Figure 1C) reacts rapidly with trans-cyclooctenes, and has been used for small-molecule labeling in the cellular context16. However, the lack of a second aryl substitution on Tz1 leaves it susceptible to non-specific reactions with cellular nucleophiles and dienophiles. Following the design of Blackman21, we synthesized an alternative 3,6-diaryl-s-tetrazine, Tz2 (Figure 1C; synthesis shown in Supporting Figure 3). Structures closely related to Tz2 had been shown to be unusually stable toward amines and thiols21. The electron withdrawing p-trifluoromethyl substituent of Tz2 augments the reactivity toward trans-cyclooctenes.
Both Tz1 and Tz2 were conjugated to fluorescein. Upon reaction with excess trans-cyclooctene, we measured 13.4- and 16.7-fold increases in fluorescein emission, respectively, in agreement with previous reports describing similar dyes16 (Supporting Figure 4). We also measured second order rate constants for reaction with TCO2-ligated LAP, and found values of 5000 ± 700 and 380 ± 40 M-1 s-1 for Tz1-fluorescein and Tz2-fluorescein, respectively (Supporting Figure 5).
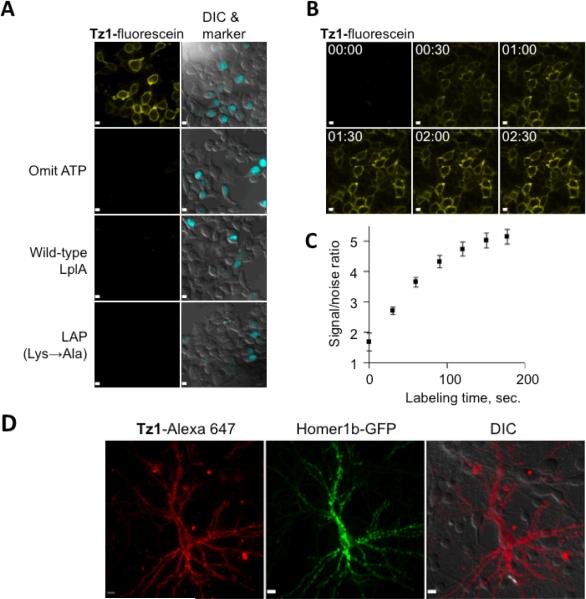
We proceeded to test cell surface fluorescence labeling. Here, nucleophiles are less abundant than inside cells, so we utilized only the faster tetrazine probe, Tz1-fluorescein. LAP-tagged low density lipoprotein receptor (LAP-LDL receptor) was expressed in human embryonic kidney 293T cells (HEK cells). We externally supplied 5 μM W37VLplA, 100 μM TCO2 and ATP for 15 minutes. We rinsed off the excess reagents, then stained the cells with 100 nM Tz1-fluorescein for 5 minutes and observed specific labeling on transfected cells after further brief rinsing (Figure 2A). Negative controls with ATP omitted, wild-type LplA, or inactive LAP mutant all eliminated the labeling signal.
Figure 2.
Cell surface fluorescence labeling using LplA and Diels-Alder cycloaddition. (A) HEK 293T cells expressing LAP-tagged low density lipoprotein receptor were treated with purified W37VLplA and TCO2, followed by Tz1-fluorescein. Fluorescein images (yellow) are shown beside overlaid images of DIC and the cyan fluorescent protein transfection marker. Negative controls with ATP omitted, wild-type ligase, and inactive LAP are shown. (B) Time-lapse imaging of the same experiment without rinsing away 50 nM Tz1-fluorescein, captured every 30 seconds. (C) Signal-to-noise quantification of (B). Noise is defined as fluorescence signal on untransfected cells. Error bars, 2 SEM. (D) Rat neurons expressing LAP-tagged neuroligin-1 and green fluorescent protein-tagged Homer1b (Homer1b-GFP, green) were treated with purified W37VLplA and TCO2, followed by Tz1-Alexa 647 (red), then imaged live after brief rinsing. All scale bars, 10 μm.
Furthermore, we found it possible to perform fluorogenic labeling of LAP-LDL receptor, using 50 nM Tz1-fluorescein and without rinsing. Fluorescence signal accumulated specifically on transfected cells, with signal-to-noise ratios saturating after approximately 3 minutes, and with minimal background signal from the surrounding excess Tz1-fluorescein (Figure 2B and 2C).
To extend cell surface labeling to other colors and cell types, we conjugated Tz1 to Alexa 647, a brighter fluorophore suitable for single molecule fluorescence detection and super-resolution imaging22. Tz1-Alexa 647 was used to label LAP-tagged neuroligin-1 on the surface of rat neurons with high specificity and minimal apparent toxicity (Figure 2D).
We directly compared Diels-Alder cycloaddition with two other bioorthogonal labeling chemistries that are compatible with the cell surface: CuAAC and strain-promoted azide-alkyne cycloaddition. We found that under otherwise identical conditions, Diels-Alder cycloaddition gave specific signal at 10 nM of Tz1-Alexa 647, while other methods required at least 1 μM of dye to achieve a similar signal-to-noise ratio. These results demonstrate that the Diels-Alder cycloaddition is much more sensitive while retaining similar specificity (Supporting Figure 6A). Additionally, using an assay of cellular ATP content, we found that labeling by the Diels-Alder cycloaddition was not toxic, in contrast to CuAAC with TBTA ligand23 (although CuAAC with a new generation ligand, THPTA24, was considerably less toxic), (Supporting Figure 6B).
Negative charges on fluorescein and Alexa 647 prevent their tetrazine conjugates from crossing cell membranes. To label intracellular proteins, we first prepared cell-permeable derivatives, Tz1- and Tz2-fluorescein diacetate. Upon entering the cell interior, endogenous esterases hydrolyze the acetyl groups and release the intact tetrazine-fluorescein conjugate. For initial experiments, we expressed nuclear-localized, LAP-tagged blue fluorescent protein (nuclear LAP-BFP), as well as cytoplasmic W37VLplA inside HEK cells. The whole-cell localization of W37VLplA versus the nuclear localization of its LAP substrate imposes an additional test of labeling specificity.
We first compared Tz1- and Tz2-fluorescein diacetate at 10 μM, 1 μM, and 100 nM loading concentrations. Tz1 was expected to generate higher signal because of its higher Diels-Alder reactivity, but higher background was also expected due to its increased lability. Inside cells, we observed that Tz1 indeed generated more background fluorescence than Tz2 at high loading concentrations, but due to its much higher labeling signal, the overall signal-to-noise ratio (Supporting Figure 7). We therefore chose Tz1 over Tz2 for subsequent intracellular labeling experiments.
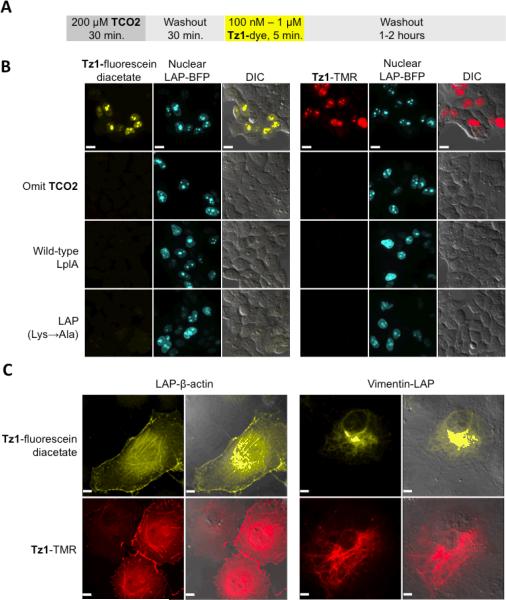
We synthesized another cell-permeable, red-shifted fluorophore conjugate, Tz1-tetramethylrhodamine (Tz1-TMR), and proceeded to optimize the cellular labeling conditions for both this conjugate and Tz1-fluorescein diacetate. Following the optimized protocol shown in Figure 3A, we observed labeling signal specific to the nuclei of transfected cells, despite the presence of LplA in both the cytosol and the nucleus. Negative controls with TCO2 omitted, wild-type LplA, or an inactive LAP mutant abolished labeling signals (Figure 3B). We also examined the labeling specificity by lysing cells after Tz1-fluorescein diacetate treatment, and imaging the fluorescence of the lysate after gel separation. Supporting Figure 8 shows that a single protein corresponding to the size of LAP-BFP was selectively labeled over the endogenous proteome.
Figure 3.
Intracellular fluorescence labeling with LplA and Diels-Alder cycloaddition. (A) General protocol. (B) HEK 293T cells expressing nuclear-localized, LAP-tagged blue fluorescent protein (LAP-BFP) and cytosolic W37VLplA were labeled as in (A), with 500 nM Tz1-fluorescein diacetate (yellow, left panels) or 1 μM Tz1-tetramethylrhodamine (Tz1-TMR, red, right panels), followed by 2-hour dye wash-out and imaged live. Negative controls with TCO2 omitted, wild-type LplA, or inactive LAP also shown. (C) COS-7 cells expressing W37VLplA and LAP-tagged β-actin (left panels), or LAP-tagged vimentin (right panels) were treated as in (A), with 100 nM Tz1-fluorescein diacetate (yellow) or 1 μM Tz1-tetramethylrhodamine (red), followed by 1-hour dye washout and imaged live. Scale bars, 10 μm.
We were unable to achieve fluorogenic labeling inside cells because high fluorescence signal was observed inside untransfected cells as well as cells free of TCO2 treatment, immediately upon loading of both Tz1-fluorophore conjugates (data not shown). We were, however, able to wash away off-target dyes after 2 hours. In COS-7 cells, where the required dye washout time was shorter, we also successfully labeled actin filaments (LAP-β-actin) and intermediate filaments (vimentin-LAP) with high specificity (Figure 3C). Supporting Figure 9 shows that actin and vimentin filaments labeled by Tz1-TMR co-localized perfectly with filaments detected by immunofluorescence staining in the same cells, indicating that the labeling was specific.
In summary, we have found that the tetrazine-transcyclooctene Diels-Alder cycloaddition is highly efficient for the fluorescence labeling of cell surface proteins and sufficiently bioorthogonal for labeling of intracellular proteins. We utilized this fast chemistry for the extension of PRIME to a panel of useful fluorophores, including tetramethylrhodamine and Alexa 647, while retaining a level of specificity comparable to direct fluorophore ligation by PRIME1. This method is generally applicable to different proteins in various cell types.
On the cell surface, we achieved fluorogenic labeling using tetrazine-fluorescein, but failed to accomplish fluorogenic labeling with Alexa 647 because its red-shifted fluorescence emission was not significantly quenched when conjugated to Tz1 (Supporting Figure 4B). Inside the cell, we observed a tradeoff between the reactivity and stability of two different tetrazine structures. We surmise that, while monoaryl-substituted Tz1 is significantly more reactive than diaryl Tz2 toward trans-cyclooctene, the former is also more prone to cross-reactivity with endogenous nucleophiles or dienophiles. Our study therefore illustrates the need for next-generation tetrazines that are less kinetically hindered by protective substitutions, and more able to quench the fluorescence of red dyes.
Supplementary Material
ACKNOWLEDGMENT
Funding was provided by the National Institutes of Health (R01 GM072670 and P20 RR017716), the American Chemical Society, the Dreyfus Foundation, and MIT. Spectra were obtained with instrumentation supported by NSF grants: CHE-0840401 and CHE-0541775. We thank Chayasith Uttamapinant and Jennifer Yao (MIT) for plasmids, reagents, and helpful discussions, as well as Carolyn Kwa (MIT) for assistance with neuron cultures. Masahito Yamagata (Harvard) provided the neuroligin-1 plasmid. A. Tangpeerachaikul was funded by a Novartis undergraduate fellowship.
Footnotes
Supporting Information. Synthesis and characterization of trans-cyclooctenes and tetrazine-fluorophore conjugates. Validation of trans-cyclooctene ligation by LplA. Kinetic characterizations. Additional cell imaging experiments and experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Uttamapinant C, White KA, Baruah H, Thompson S, Fernandez-Suarez M, Puthenveetil S, Ting AY. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10914–10919. doi: 10.1073/pnas.0914067107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Suarez M, Baruah H, Martinez-Hernandez L, Xie KT, Baskin JM, Bertozzi CR, Ting AY. Nat. Biotechnol. 2007;25:1483–1487. doi: 10.1038/nbt1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puthenveetil S, Liu DS, White KA, Thompson S, Ting AY. J. Am. Chem. Soc. 2009;131:16430–16438. doi: 10.1021/ja904596f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. Nat. Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 5.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohane RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. ACS Chem. Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 6.Griffin BA, Adams SR, Tsien RY. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JD, Thompson S, Ting AY. Biochemistry. 2011 Aug 31; doi: 10.1021/bi201037r. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin X, Uttamapinant C, Ting AY. ChemBioChem. 2011;12:65–70. doi: 10.1002/cbic.201000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 10.Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2005;127:11196. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 11.Blackman ML, Royzen M, Fox JM. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem., Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem. Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 14.Presolski SI, Hong V, Cho SH, Finn MG. J. Am. Chem. Soc. 2010;132:14570–14576. doi: 10.1021/ja105743g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaraj NK, Upadhyay R, Hatin JB, Hilderbrand SA, Weissleder R. Angew. Chem., Int. Ed. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Angew. Chem., Int. Ed. 2010;49:2869–2872. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balcar J, Chrisam G, Huber FX, Sauer J. Tetrahedron Lett. 1983;24:1481–1484. [Google Scholar]
- 18.Royzen M, Yap GPA, Fox JM. J. Am. Chem. Soc. 2008;130:3760–3761. doi: 10.1021/ja8001919. [DOI] [PubMed] [Google Scholar]
- 19.Taylor MT, Blackman ML, Dmitrenko O, Fox JM. J. Am. Chem. Soc. 2011;133:9646–9649. doi: 10.1021/ja201844c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baruah H, Puthenveetil S, Choi YA, Shah S, Ting AY. Angew. Chem., Int. Ed. 2008;47:7018–7021. doi: 10.1002/anie.200802088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackman ML. Thesis. University of Delaware; Newark, DE: 2011. [Google Scholar]
- 22.Jones SA, Shim SH, He J, Zhuang XW. Nat. Methods. 2011;8:499–505. doi: 10.1038/nmeth.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 24.Hong V, Steinmetz NF, Manchester M, Finn MG. Bioconjugate Chem. 2010;21:1912–1916. doi: 10.1021/bc100272z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.