Abstract
Breastmilk chemokines have been associated with increased HIV-1 RNA levels in breastmilk and altered risk of mother-to-child HIV-1 transmission. To characterize CC and CXC chemokines in breastmilk postpartum, we collected breastmilk specimens at regular intervals for 6 months after delivery from women with and without HIV-1 infection and used commercial ELISA kits to measure breastmilk concentrations of MIP-1α, MIP-1β, RANTES, and SDF-1α. Among 54 HIV-1-infected and 26 uninfected women, mean chemokine levels were compared cross-sectionally and longitudinally at days 5 and 10, and months 1 and 3 postpartum. For both HIV-1-infected and uninfected women, breastmilk chemokine levels were highest at day 5 for MIP-1α, MIP-1β, and SDF-1α, and subsequently decreased. RANTES levels remained constant over the follow-up period among HIV-1-uninfected women, and increased moderately among HIV-1-infected women. For MIP-1β and RANTES, breastmilk levels were significantly higher among HIV-1-infected women compared to uninfected women early postpartum. In addition, HIV-1-infected women transmitting HIV-1 to their infant had consistently higher breastmilk RANTES levels than those who did not transmit, with the greatest difference observed at 1 month (2.68 vs. 2.21 log10 pg/mL, respectively; p = 0.007). In summary, all four chemokines were most elevated within the first month postpartum, a period of high transmission risk via breastmilk. MIP-1β and RANTES levels in breastmilk were higher among HIV-1-infected women than among uninfected women, and breastmilk RANTES was positively associated with vertical transmission in this study, consistent with results from our earlier cohort.
INTRODUCTION
Beastmilk accounts for 30–50% of the ~500,000 mother-to-child HIV-1 transmission events that occur annually in sub-Saharan Africa and other resource-limited settings.1,2 Understanding the dynamics of innate immune responses in breastmilk may make it possible to develop appropriate interventions or vaccination strategies to prevent breastmilk HIV-1 transmission. Chemokines and their receptors, the HIV-1 coreceptors CCR5 and CXCR4, play an important role in innate immunity against viruses and are under investigation as targets for preventive and therapeutic agents designed to protect against or contain HIV-1 infection.3–6 Both CC and CXC chemokines inhibit HIV-1 infection of cells and interrupt viral replication by blocking or downregulating surface expression of HIV-1 entry sites.3,7,8 They have been detected in high levels in HIV-1-infected long-term nonprogressors and in individuals who remain uninfected despite repeated exposure to HIV-1.9–11
In a previous study of 282 mother–infant pairs, we found that CC and CXC chemokines in breastmilk were associated with infant HIV-1 acquisition.12 Increased levels of macrophage inflammatory protein type 1 beta (MIP-1β) and stromal cell-derived factor type 1 alpha (SDF- 1α) were associated with reduced mother-to-infant HIV-1 transmission risk, whereas elevated breastmilk RANTES (regulated upon activation, normal T-cell expressed and secreted) was associated with increased risk, independent of HIV-1 RNA levels in breastmilk.12 In this study, breastmilk chemokines were also associated with HIV-1 RNA levels in maternal plasma and breastmilk. However, the study was not able to determine the causal direction, specifically, whether HIV-1 infection promotes chemokine production or whether chemokine-associated recruitment of activated cells stimulates viral replication. The study was also unable to assess whether breastmilk chemokine levels vary over time, since specimens were limited to one breastmilk sample obtained at a single time point. The question of variability is highly relevant since breastmilk composition and risk of infant HIV-1 acquisition vary during the postpartum period, with early postpartum breastmilk being more cellular and carrying a higher risk of infant infection.13
The current study was designed to address these questions by comparing breastmilk chemokines during the early postpartum period among HIV-1-infected and uninfected women. We collected breastmilk specimens from women followed over a 3-month period, and measured levels of MIP-1α, MIP-1β, RANTES, and SDF-1α, in order to assess differences associated with HIV-1 infection and patterns of breastmilk chemokine concentrations over time.
MATERIALS AND METHODS
Recruitment and clinical follow-up of study participants
Pregnant women attending three Nairobi City Council (NCC) clinics were offered counseling and HIV-1 testing at their first antenatal visit as part of routine care. Those who accepted were tested using two HIV-1 rapid tests (Determine® HIV-1/HIV-2, Abbott Laboratories, Abbott Park, IL, and Unigold®, Trinity Biotech, Bray, Ireland). After receiving the results, women who were interested in study participation were referred to the research clinic at Kenyatta National Hospital for additional information about the study. Women were eligible for the study if they were able to give voluntary informed consent, were planning to breastfeed their infants, and were planning to stay in Nairobi after delivery for at least 6 months. Human experimentation guidelines of the U.S. Department of Health and Human Services were followed, and approval for the study was obtained from the Institutional Review Boards at the University of Washington and Kenyatta National Hospital, Nairobi.
Participants were enrolled at 34 weeks gestation and seen immediately postpartum in the labor ward and subsequently at home on day 5, and in the study clinic on day 10, and monthly until 6 months postpartum. HIV-1-infected women with CD4 counts >200 received single-dose nevirapine (200 mg) at the time of delivery for prevention of mother-to-child HIV-1 transmission and their infants received prophylaxis with nevirapine according to the HIVNET 012 protocol.14 Those women who were severely immunocompromised were initiated on zidovudine, lamivudine, and nevirapine per Kenyan national guidelines as early as possible during the antenatal period.
Women were encouraged to deliver at Kenyatta National Hospital in Nairobi to facilitate collection of intrapartum and neonatal specimens for HIV-1 RNA and DNA. Breastmilk samples were obtained by manual self-expression of milk from both breasts into a single collection tube at days 5 and 10, and months 1, 3, and 6 postpartum. At these same visits a physical examination was conducted to evaluate for clinical mastitis, nipple cracking, bleeding, or ulceration, and other breast pathology. At all study visits, women received counseling on exclusive breastfeeding for 6 months and HIV-1- infected women were encouraged to stop breastfeeding as soon as was feasible. HIV-1-infected women who stopped breastfeeding before 6 months postpartum were referred to a mother-to-child HIV-1 prevention program within the hospital where free infant formula was provided. Infant blood was obtained at birth, day 10, months 1, 3, and 6 for HIV-1 DNA filter paper assays. HIV-1-infected women received HIV-1 test results for their infant at study visits, with final results provided at a nonstudy visit at 7 months.
Laboratory processes
Breastmilk was fractionated into supernatant, cellular, and lipid components after centrifugation at 710 × g for 20 minutes. Supernatant was immediately aliquoted and stored at −80°C for quantification of CC (MIP-1α, MIP-1β, RANTES) and CXC (SDF-1α) chemokines. Chemokine assays were performed using commercially available enzymelinked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, MN), and microtiter plates were read at 450 nM. Chemokine concentrations were determined using Softmax software with a four-parameter fit (Softmax; Molecular Devices Corporation, Sunnyvale, CA).
To define infant HIV-1 status, dried blood filter paper specimens were assayed for HIV-1 DNA using PCR as previously described.15 Infants were considered HIV-1 infected if they had (1) a positive filter paper DNA assay, on two consecutive dates, or (2) a single positive filter paper assay if this was the last available sample.
Statistical analysis
Maternal characteristics were compared using linear regression with robust standard errors for continuous variables and Pearson’s chi-squared tests for categorical variables. Chemokine concentrations were transformed to their base 10 logarithm (log10) for all analyses for normality and to produce equal variance across the range of concentrations. Left censoring of observations below the lower detection limit of the assays was handled by assigning levels to one-half of the minimum detection limit. The lower detection limit was 10 pg/mL for MIP-1α and MIP-1β, 2.7 pg/mL for RANTES, and 18 pg/mL for SDF-1α. The mean chemokine concentrations in breastmilk and rates of change for HIV-1-infected and uninfected women were compared at days 5 and 10, and months 1 and 3 postpartum. Comparisons were also made between month 3 and month 6 for women who were still breastfeeding at the 6-month visit.
Chemokine concentrations were compared between HIV-1-infected and uninfected women overall and at each time point. Overall concentrations were compared by calculating the mean chemokine concentration across all time points for each woman and then calculating the median of the mean concentrations for HIV-1-infected and uninfected women. The Wilcoxon rank sum test was used to test the difference of medians between the two groups. HIV-1-infected and uninfected women were compared at each time point both in terms of the likelihood of having a chemokine concentration greater than the lower limit of detection and their mean chemokine concentration. Logistic regression was used to compare the likelihood of a chemokine concentration above the lower limit of detection, and linear regression with robust standard error estimates was used to compare chemokine concentrations between HIV-1-infected and uninfected women as well as to assess potential correlates of chemokine concentrations cross-sectionally at each time point.
Generalized estimating equation (GEE) models with exchangeable correlation structure were used to compare rates of change in chemokine concentrations in breastmilk over time between HIV-1-infected and uninfected women.16,17 In addition, among HIV-1-infected women GEE was used to assess the association between CD4 count (<500 cells/µL, ≥500 cells/µL) and chemokine concentrations and to compare mean breastmilk chemokine concentrations between women who did and did not transmit HIV-1 to their infant. All analyses were conducted using Stata statistical software (Stata Corp., College Station, TX).
RESULTS
Cohort characteristics at baseline and during follow-up
From October 2003 to February 2005, 55 HIV-1-infected and 30 HIV-1-uninfected women were enrolled. Among these, 5 (6%) women (1 HIV-1- infected and 4 HIV-1-uninfected) were lost to follow-up prior to delivery or elected not to breastfeed and were no longer eligible for the study (Fig. 1). Median age was 25 years (Interquartile range [IQR] = 22–29) and the majority (86%) of women were married with a history of two lifetime sexual partners (IQR = 2–3) and one previous pregnancy (IQR = 0–2). Most women reported living in a single room house with a median monthly rent of $19.80 (IQR = $13.20–$26.40). HIV-1-infected and uninfected women were similar in age, parity, and socioeconomic status; however, HIV-1-infected women had significantly fewer years of education, an earlier age of sexual debut, and more lifetime sexual partners than HIV-1-uninfected women (Table 1). Median CD4 count was 447 cells/µL at 36 weeks gestation and 475 cells/µL at 1 month postpartum for the 54 HIV-1-infected women.
FIG. 1.
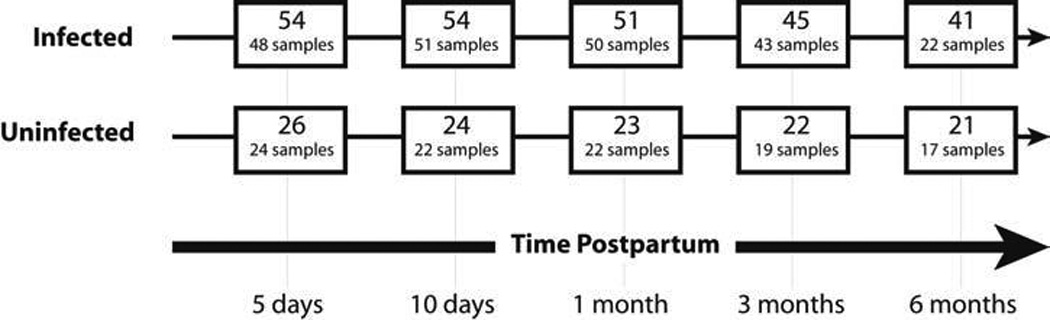
Flowchart depicting the number of women in follow-up and the number of specimens collected at each time point. Discrepancies between number in follow-up and number of samples collected are due to missed visits, inability to express breastmilk, or discontinuation of breast feeding. Kenyan national guidelines encourage HIV-1-infected women to discontinue breastfeeding prior to 6 months postpartum, which accounts for drop-off in sample collection.
Table 1.
Cohort Characteristics
| Characteristic | HIV-1-infected (n = 54) |
HIV-1-uninfected (n = 26) |
p value |
|---|---|---|---|
| Median (IQRa) or Number (%) | |||
| Age (years) | 25 (22, 28) | 25 (23, 29) | 0.52 |
| Education (years) | 0.03 | ||
| None | 5 (9) | 2 (7) | |
| Primary | 33 (60) | 9 (30) | |
| Secondary | 12 (22) | 15 (50) | |
| College | 5 (9) | 4 (13) | |
| Prior pregnancies | 1 (1,2) | 1 (0,2) | 0.44 |
| Persons per room | 2.5 (2, 3) | 2 (1.5, 3) | 0.23 |
| Monthly rent (U.S. dollars) | 18.48 (13.20, 24.60) | 20.50 (17.76, 35.53) | 0.18 |
| Age at sexual debut (years) | 18 (16, 19) | 19 (18, 19) | 0.01 |
| Sexual partners in lifetime | 3 (2, 4) | 2 (1, 2) | <0.001 |
| CD4 T-cell count (cells/µL) | 446.5 (293, 609) | NA | — |
| CD4 T-cell percent | 24 (18, 32) | NA | — |
| Vaginal delivery | 38 (76) | 24 (92) | 0.08 |
| Mastitisb | 3 (10) | 6 (11) | 0.90 |
| Infant birthweight (g) | 3100 (2900, 3400) | 3100 (2900, 3500) | 0.97 |
IQR, interquartile range.
Any time during 6 months of postpartum follow-up.
Among the 80 women in follow-up, 66 (83%) had vaginal deliveries. Forty (74%) of 54 HIV-1-infected women and 19 (73%) of 26 HIV-1-uninfected women were breastfeeding at 3 months postpartum (p = 0.90). Clinical mastitis was diagnosed in nine (11%) women, six of whom were HIV-1-infected and three HIV-1-uninfected (p = 0.92). Other breast pathology, including breast abscess, local swelling of the breast, nipple cracking, or bleeding, was identified in nine (17%) HIV-1-infected women and three (12%) uninfected women (p = 0.55).
Of the 54 HIV-1-infected women, nine (17%) transmitted HIV-1 to their infants. Among these, three (33%) infants were infected at birth, one (11%) was found to be infected at day 10, three (33%) at month 1, and two (22%) at month 3. None of the infants acquired HIV-1 between 3 and 6 months of age. During the 6-month postpartum period, four (5%) infants died, of whom three were born to an HIV-1-infected mother and one to an uninfected mother (p = 0.66).
Specimen collection
Fifty-five (69%) of the 80 women provided samples at all four primary time points (day 5, day 10, month 1, and month 3), with breastmilk collection at each of these time points from 38 (70%) HIV-1-infected women and 17 (65%) HIV-1-uninfected women (p = 0.65). Over the follow-up period, 13 (16%) mother–infant pairs stopped breastfeeding or dropped out of the study prior to the 3-month visit; of these, two stopped after the day 5 visit, four after the day 10 visit, and seven after the 1-month visit. Among the 39 (45%) women who were still breastfeeding at month 6, all provided samples at this time point.
Breastmilk chemokines, HIV-1 status, and other correlates
Chemokine concentrations were generally above the minimum detectable level (Table 2a). Overall, the median of the mean chemokine concentrations for all women was 1.37 log10 pg/mL for MIP-1α, 1.87 log10 pg/mL for MIP-1β, 2.08 log10 pg/mL for RANTES, and 2.07 for SDF-1α. Correlates of breastmilk chemokine concentrations at individual visits, as well as over time, were assessed for each of the four chemokines. We found no association between breastmilk levels of MIP-1α, MIP-1β, RANTES, and SDF-1α and women’s age, socioeconomic status, or parity. There was also no evidence of an association between chemokine concentrations in breastmilk and breast pathology during the postpartum period. When we restricted the analysis to HIV-1-infected women with a baseline CD4 T-cell count ≥200 cells/µL, higher baseline CD4 count was significantly associated with lower levels of breastmilk RANTES and SDF-1α. Relative to women with a CD4 cell count <500 cells/µL, those with a CD4 cell count ≥500 cells/µL had 0.28 log10 pg/mL lower RANTES (95% CI 0.06, 0.50; p = 0.01) and 0.37 log10 pg/mL lower SDF-1α (95% CI 0.06, 0.67; p = 0.02).
Table 2.
(a) Number and Percent of Specimens with Chemokine Concentration above the Lower Limit of Detection, and (b) Mean Chemokine Concentrations by HIV-1 Status
| MIP-1α HIV-1 status |
MIP-1β HIV-1 status |
RANTES HIV-1 status |
SDF-1α HIV-1 status |
|||||
|---|---|---|---|---|---|---|---|---|
| Time point | + | − | + | − | + | − | + | − |
| 2a | n (%) | n (%) | n (%) | n (%) | ||||
| 5 days | 47 (98) | 23 (96) | 48 (100) | 23 (96) | 48 (100) | 22 (92) | 44 (92) | 21 (91) |
| 10 days | 43 (86) | 21 (95) | 50 (100) | 20 (91) | 49 (98) | 22 (100) | 38 (76) | 18 (82) |
| 1 month | 42 (84) | 21 (95) | 49 (98) | 18 (82) | 49 (98) | 21 (95) | 32 (64) | 14 (64) |
| 3 months | 36 (84) | 19 (100) | 39 (91) | 16 (84) | 39 (91) | 17 (89) | 28 (65) | 12 (63) |
| 6 months | 18 (82) | 17 (100) | 21 (95) | 14 (82) | 21 (95) | 15 (88) | 14 (64) | 10 (59) |
| HIV-1 status | HIV-1 status | HIV-1 status | HIV-1 status | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | + | − | |||||
| 2b | mean | p | mean | p | mean | p | mean | p | ||||
| 5 days | 1.95 | 2.07 | 0.51 | 2.45 | 2.42 | 0.85 | 2.15 | 1.88 | 0.08 | 2.40 | 2.35 | 0.76 |
| 10 days | 1.35 | 1.27 | 0.49 | 1.96 | 1.57 | 0.005 | 2.13 | 1.84 | 0.057 | 2.05 | 1.95 | 0.59 |
| 1 month | 1.22 | 1.21 | 0.90 | 1.73 | 1.51 | 0.10 | 2.30 | 1.86 | 0.003 | 1.84 | 1.77 | 0.72 |
| 3 months | 1.13 | 1.23 | 0.26 | 1.49 | 1.41 | 0.53 | 1.81 | 1.79 | 0.90 | 1.83 | 1.74 | 0.67 |
| 6 months | 1.01 | 1.10 | 0.29 | 1.46 | 1.23 | 0.07 | 1.80 | 1.56 | 0.28 | 1.76 | 1.75 | 0.95 |
Maternal HIV-1 infection status was significantly associated with breastmilk MIP-1β and RANTES levels, but not MIP-1α, and SDF-1α. At 1 month, HIV-1-infected women were significantly more likely to have a MIP-1β level above the lower limit of detection (p = 0.04) (Table 2a), and when we compared log10 concentrations between HIV-1-infected and uninfected women at each time point, HIV-1-infected women had significantly higher MIP-1β concentration at day 10 postpartum than HIV-1-uninfected women (difference: 0.39 log10 pg/mL; 95% CI 0.12, 0.66; p = 0.005) (Table 2b). Breastmilk RANTES was also higher among HIV-1-infected women compared to uninfected women at 10 days (0.29 log10 pg/mL; 95% CI −0.008, 0.58; p = 0.06) and 1-month postpartum (0.44 log10 pg/mL; 95% CI 0.15, 0.72; p = 0.003). The difference approached statistical significance at 5 days postpartum (0.27 log10 pg/mL, 95% CI: −0.04, 0.58; p = 0.08). There were no significant differences in the proportion of samples above the detection limit for either MIP-1α or SDF-1α at any time point, and levels of MIP-1α and SDF-1α were not statistically different between the two groups of women at any time point. Among the 54 HIV-1-infected women, a total of 4 (7%) women were on highly active antiretroviral therapy (HAART) at day 10, and 2 (4%) women were taking HAART at months 1 and 3. The chemokine levels of these women were comparable to women not on HAART.
Longitudinal evaluation of breastmilk chemokines
The overall median concentration including all time points was not significantly different between HIV-1-infected and uninfected women for either MIP-1β or RANTES. When all time points were included, the median of the mean MIP-1β concentration in breastmilk was 1.87 log10 pg/mL among the HIV-1-infected and 1.86 log10 pg/mL among the HIV-1-uninfected women (p = 0.47). For RANTES, levels were 2.16 log10 pg/mL and 2.05 log10 pg/mL among HIV-1-infected and uninfected women, respectively (p = 0.06).
However, there was appreciable variation in concentrations between the two groups over time (Fig. 2). The highest breastmilk concentrations of MIP-1α, MIP-1β, and SDF-1α were observed on day 5 and the highest concentration of RANTES was observed on day 10, after which concentrations declined significantly from peak levels. From day 5 to day 10, the rate of decline in MIP-1β concentration was significantly faster among HIV-1-uninfected women decreasing by 0.17 log10 pg/mL per day (95% CI 0.12, 0.22; p < 0.001) compared to 0.11 log10 pg/mL per day (95% CI 0.08, 0.14; p < 0.001) among infected women (p = 0.04). There was no difference between HIV-1-infected and uninfected women in the rate of change in concentration of MIP-1α or SDF-1α during this period (p = 0.19 and p = 0.69, respectively). Concentrations of MIP-1α decreased by 0.13 log10 pg/mL per day (95% CI 0.09, 0.16; p < 0.001) among infected women compared to 0.17 log10 pg/mL per day (95% CI 0.12, 0.22; p < 0.001) among uninfected women. SDF-1α decreased by 0.08 log10 pg/mL per day (95% CI 0.04, 0.12; p < 0.001) among infected women and 0.10 log10 pg/mL per day (95% CI 0.04, 0.15; p = 0.001) among uninfected women during the same period. After day 10 postpartum, MIP-1β concentrations continued to decline at a slow rate among HIV-1-infected women (p < 0.01), but there was no significant change in concentration in HIV-1-uninfected women. MIP-1α and SDF-1α concentrations did not change significantly after day 10 in either of the two groups of women.
FIG. 2.
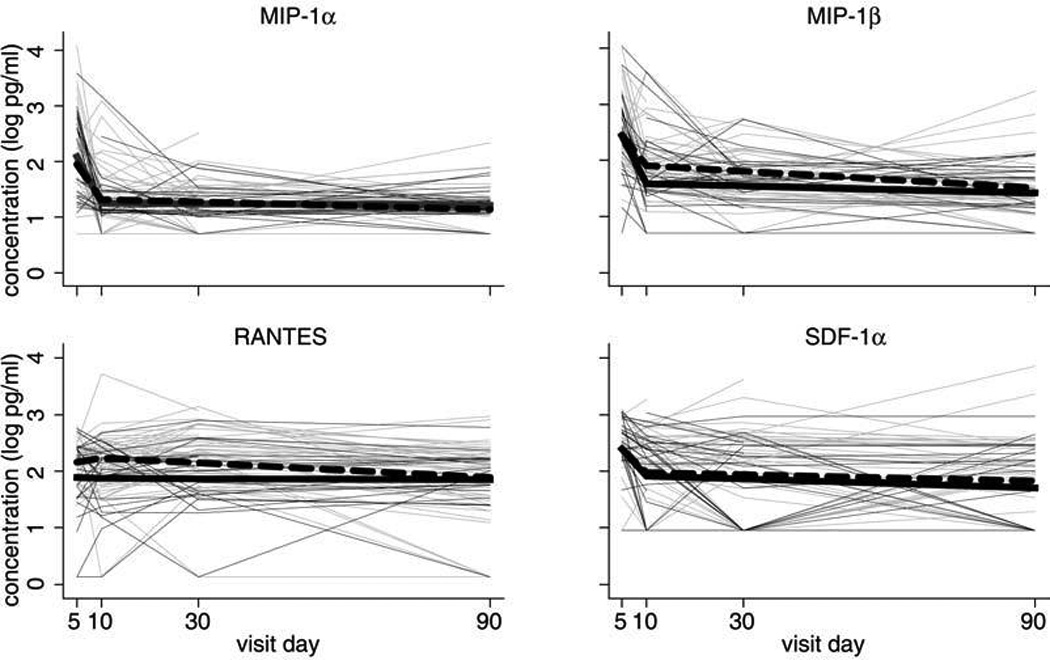
Chemokine concentrations (log10 pg/mL) at 5 days, 10 days, 1 month, and 3 months postpartum among HIV-1-infected ( ) and HIV-1-uninfected women (
) and HIV-1-uninfected women ( ). Thicker lines indicate generalized estimating equations regression lines for HIV-1-infected women (
). Thicker lines indicate generalized estimating equations regression lines for HIV-1-infected women ( ) and HIV-1-uninfected women (
) and HIV-1-uninfected women ( ).
).
RANTES levels peaked at day 5 postpartum in HIV-1-uninfected women and at 1 month postpartum in HIV-1-infected women. There was no significant change in RANTES concentration between day 5 and day 10 for either HIV-1-infected or uninfected women (p = 0.73 and p = 0.84, respectively), and after day 10, there was no change in the concentration of RANTES in uninfected women (p = 0.84). However, there were changes in RANTES levels in infected women, among whom there was a trend for an increase of 0.008 log10 pg/mL in RANTES concentration from day 10 to month 1 (p = 0.06), and a significant decline of 0.007 log10 pg/mL from month 1 to month 3 (p < 0.01).
Among the 20 HIV-1-infected and 14 uninfected women who provided breastmilk samples at month 3 and month 6, there was no significant change in the concentration of any of the four chemokines between the two time points for either of the two groups of women (data not shown).
Risk of mother-to-child HIV-1 transmission
Nine infants acquired HIV-1 from their mothers during the follow-up period. Breastmilk RANTES levels were consistently higher among transmitters than nontransmitters (Fig. 3). The greatest difference was observed at 1-month postpartum when RANTES concentration was 2.68 log10 pg/mL among women who transmitted HIV-1 to their infants compared to 2.21 log10 pg/mL among HIV-1-infected women who did not transmit (p = 0.007).
FIG. 3.

RANTES concentrations (log10 pg/mL) at 5 days, 10 days, 1 month, and 3 months postpartum among HIV-1 transmitting ( ) and nontransmitting women (
) and nontransmitting women ( ). Thicker lines indicate generalized estimating equations regression lines for HIV-1 transmitting women (
). Thicker lines indicate generalized estimating equations regression lines for HIV-1 transmitting women ( ), nontransmitting women (
), nontransmitting women ( ), and HIV-1-uninfected women (
), and HIV-1-uninfected women ( ).
).
DISCUSSION
In this study we sought to understand the dynamics of chemokine concentrations in breastmilk and to establish whether there were differences in breastmilk chemokines between HIV-1-infected and uninfected women. We performed serial assays for MIP-1α, MIP-1β, RANTES, and SDF-1α on breastmilk from HIV-1-infected and uninfected women, and found the highest concentrations of these chemokines occurred within the first 2 weeks postpartum for both groups. This is similar to what has been observed for other cytokines.18–22 Interleukin (IL)-1β, IL-2, IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), monokine induced by interferon-gamma (MID), and interferon-gamma inducible protein of 10 kDa (IP-10) also have their highest levels early postpartum, suggesting a physiologic basis for observed elevations in chemokine levels.18–22 Early breastmilk is more cellular, and the presence of increased inflammatory cytokines and chemokines would be consistent with this increased cellularity. It may also contribute to increased rates of transmission during early breastfeeding, the period when a large proportion of transmission events via breastmilk occur.1,13,23,24
When we compared HIV-1-infected women and uninfected women, we found HIV-1-infected women to have higher levels of both MIP-1β and RANTES. HIV-1-infected women were not different from HIV-1-uninfected women in age, parity, socioeconomic status, or other measured characteristics. In addition, adjustment for these variables did not influence results. HIV-1 infection may be responsible for elevation of these chemokines in breastmilk. It has been shown that HIV-1-infected cells produce RANTES and other chemokines.25 This supports the finding in our earlier study that HIV-1 RNA levels in both plasma and breastmilk correlated positively with breastmilk chemokines,12 and leads us to postulate that HIV-1 may be the driving force behind the higher MIP-1β and RANTES levels among HIV-1-infected women. It is unclear why this was not the case for MIP-1α and SDF-1α, and a better understanding of underlying mechanisms may elucidate this.
We also found RANTES levels in breastmilk to be significantly associated with HIV-1 acquisition in the infant. In our previous cohort, higher breastmilk RANTES also correlated with increased transmission risk, and we were able to adjust for plasma and breastmilk viral load in that study, demonstrating that the positive association was independent of virus levels in these compartments.12 Several in vitro studies have demonstrated that elevated RANTES may enhance HIV-1 replication in macrophages, the predominant cell type in breastmilk, despite binding and potentially blocking or downregulating CCR5, the HIV-1 coreceptor.26,27 One hypothesis for its effect on vertical transmission is that RANTES acts in the infant to augment HIV-1 infection of infant cells directly or through local recruitment of susceptible infant target cells. Alternatively, breastmilk RANTES may be a marker for other factors that are associated with both RANTES and HIV-1 transmission, such as inflammatory breast diseases. We did not observe an association between mastitis and elevated chemokine levels in this study, as we had in our previous cohort of 282 lactating HIV-1-infected women,12 which may be due to the fact that our sample size was small for assessing characteristics with low prevalence such as clinical mastitis. The relatively small sample size may also explain why we did not observe an effect on transmission risk for other chemokines evaluated in this study. In addition, we were not able to test for subclinical mastitis in this cohort of women. In our previous study, women with subclinical mastitis had elevated levels of MIP-1α, MIP-1β, and RANTES.12
In summary, we found that breastmilk concentrations of CC and CXC chemokines were highest early postpartum and that breastmilk MIP-1β and RANTES were both elevated in the setting of HIV-1 infection. Higher RANTES levels were associated with increased risk of HIV-1 transmission to the infant. There are potential implications of these findings, since agents that block CCR5 and CXCR4 coreceptors are being explored for therapeutic and preventive measures, including new medications and microbicides. We anticipate that knowledge of associations with HIV-1 transmission and concentrations of chemokines in breastmilk over time will help guide research, development, and testing of such agents.
ACKNOWLEDGMENTS
Research was funded by the U.S. National Institute of Dental and Craniofacial Research (NIDCR) Grant DE14826. R. Bosire, J. Mabuka, and M. Majiwa were scholars in the AIDS International Training and Research Program supported by the National Institutes of Health/Fogarty International Center D43 TW000007. C. Farquhar was supported by the National Institute of Child Health and Development (NICHD) Grant HD41879.
REFERENCES
- 1.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Geneva: UNAIDS; 2006. AIDS epidemic update: December 2006; p. 98. [Google Scholar]
- 3.Amara A, Gall SL, Schwartz O, et al. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi WT, Tian S, Dong CZ, et al. Unique ligand binding sites on CXCR4 probed by a chemical biology approach: Implications for the design of selective human immunodeficiency virus type 1 inhibitors. J Virol. 2005;79:15398–15404. doi: 10.1128/JVI.79.24.15398-15404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Choi WT, Dong CZ, et al. SMM-chemokines: A class of unnatural synthetic molecules as chemical probes of chemokine receptor biology and leads for therapeutic development. Chem Biol. 2006;13:69–79. doi: 10.1016/j.chembiol.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Lusso P. HIV and chemokines: Implications for therapy and vaccine. Vaccine. 2002;20:1964–1967. doi: 10.1016/s0264-410x(02)00079-8. [DOI] [PubMed] [Google Scholar]
- 7.Garzino-Demo A, DeVico AL, Cocchi F, Gallo RC. Beta-chemokines and protection from HIV type 1 disease. AIDS Res Hum Retroviruses. 1998;14(Suppl 2):S177–S184. [PubMed] [Google Scholar]
- 8.Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: Independence from G protein signaling and importance of coreceptor down-modulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 9.Ullum H, Cozzi Lepri A, Victor J, et al. Production of beta-chemokines in human immunodeficiency virus (HIV) infection: Evidence that high levels of macrophage inflammatory protein-1beta are associated with a decreased risk of HIV disease progression. J Infect Dis. 1998;177:331–336. doi: 10.1086/514192. [DOI] [PubMed] [Google Scholar]
- 10.Scala E, D’Offizi G, Rosso R, et al. C-C chemokines, IL-16, and soluble antiviral factor activity are increased in cloned T cells from subjects with long-term nonprogressive HIV infection. J Immunol. 1997;158:4485–4492. [PubMed] [Google Scholar]
- 11.Saha K, Bentsman G, Chess L, Volsky DJ. Endogenous production of beta-chemokines by CD4+, but not CD8+, T-cell clones correlates with the clinical state of human immunodeficiency virus type 1 (HIV-1)-infected individuals and may be responsible for blocking infection with non-syncytium-inducing HIV-1 in vitro. J Virol. 1998;72:876–881. doi: 10.1128/jvi.72.1.876-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farquhar C, Mbori-Ngacha DA, Redman MW, et al. CC and CXC chemokines in breastmilk are associated with mother to child HIV-1 transmission. Curr HIV Res. 2005;3:361–369. doi: 10.2174/157016205774370393. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau CM, Nduati RW, Richardson BA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 15.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 18.Michie CA, Tantscher E, Schall T, Rot A. Physiological secretion of chemokines in human breast milk. Eur Cytokine Netw. 1998;9:123–129. [PubMed] [Google Scholar]
- 19.Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004;25:297–304. [PubMed] [Google Scholar]
- 20.Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005;135:1–4. doi: 10.1093/jn/135.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Takahata Y, Takada H, Nomura A, Nakayama H, Ohshima K, Hara T. Detection of interferon-gamma-inducible chemokines in human milk. Acta Paediatr. 2003;92:659–665. doi: 10.1080/08035250310002614. [DOI] [PubMed] [Google Scholar]
- 22.Ustundag B, Yilmaz E, Dogan Y, et al. Levels of cytokines (IL-1beta, IL-2, IL-6, IL-8, TNF-alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediators Inflamm. 2005;2005:331–336. doi: 10.1155/MI.2005.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson BA, John-Stewart GC, Hughes JP, et al. Breast-milk infectivity in human immunodeficiency virus type 1-infected mothers. J Infect Dis. 2003;187:736–740. doi: 10.1086/374272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 25.Wetzel MA, Steele AD, Henderson EE, Rogers TJ. The effect of X4 and R5 HIV-1 on C, C-C, and C-X-C chemokines during the early stages of infection in human PBMCs. Virology. 2002;292:6–15. doi: 10.1006/viro.2001.1249. [DOI] [PubMed] [Google Scholar]
- 26.Gross E, Amella CA, Pompucci L, Franchin G, Sherry B, Schmidtmayerova H. Macrophages and lymphocytes differentially modulate the ability of RANTES to inhibit HIV-1 infection. J Leuk Biol. 2003;74:781–790. doi: 10.1189/jlb.0403187. [DOI] [PubMed] [Google Scholar]
- 27.Kelly MD, Naif HM, Adams SL, Cunningham AL, Lloyd AR. Dichotomous effects of CC-chemokines on HIV replication in monocytes and monocyte-derived macrophages. J Immunol. 1998;160:3091–3095. [PubMed] [Google Scholar]


