Abstract
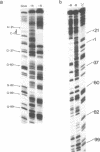
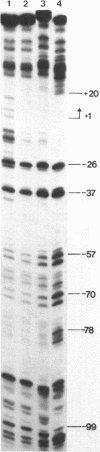
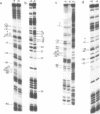
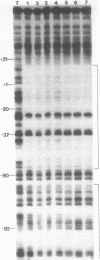
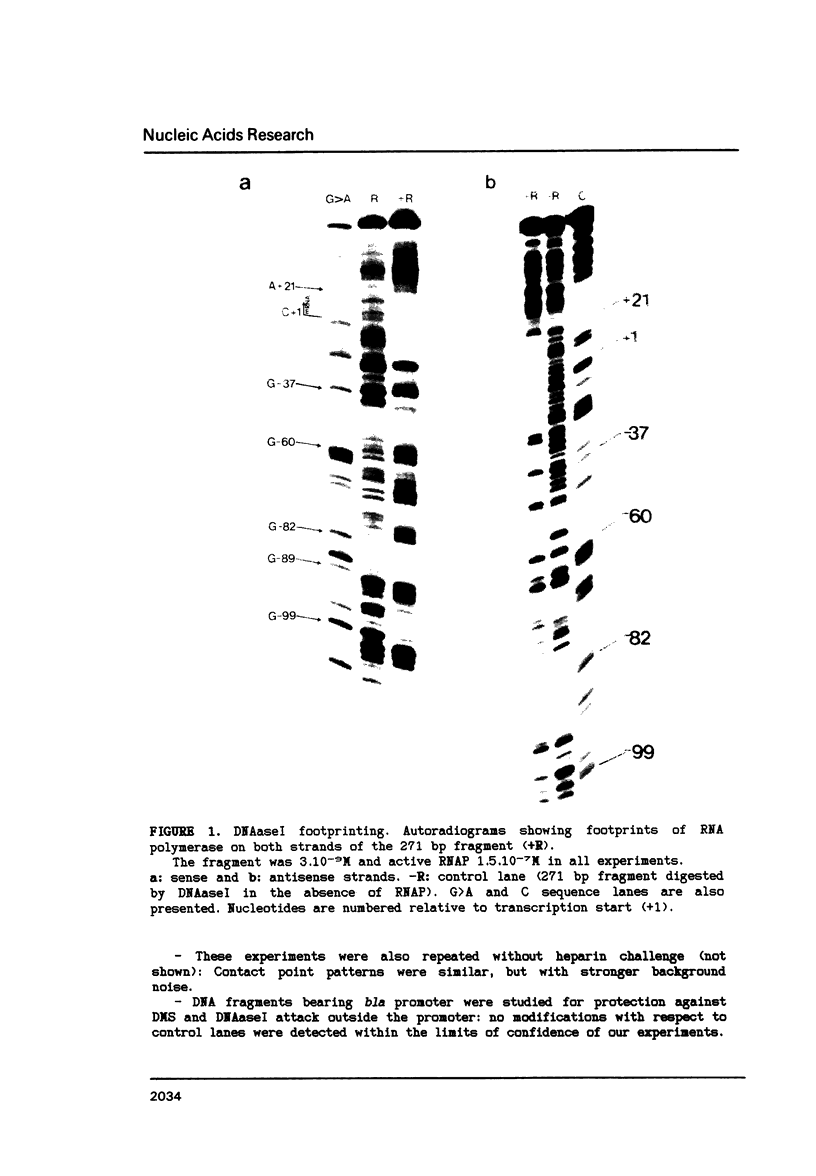
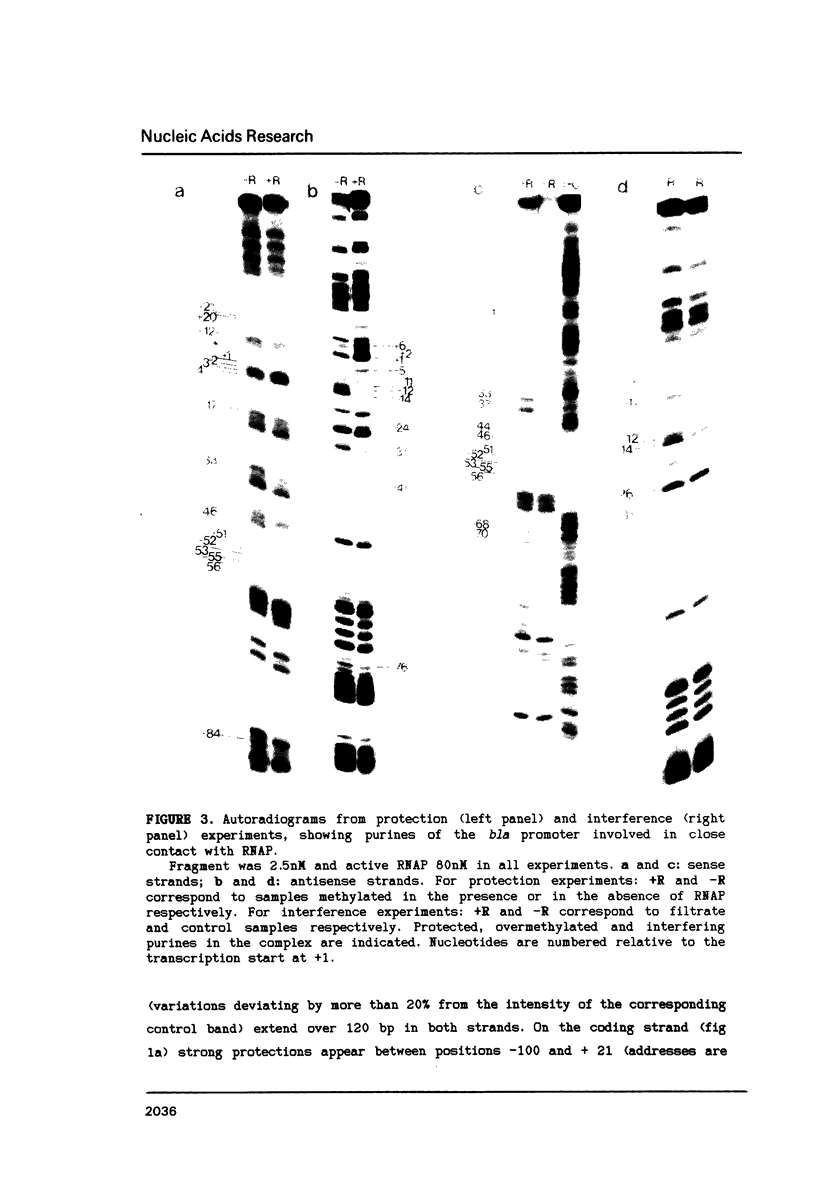
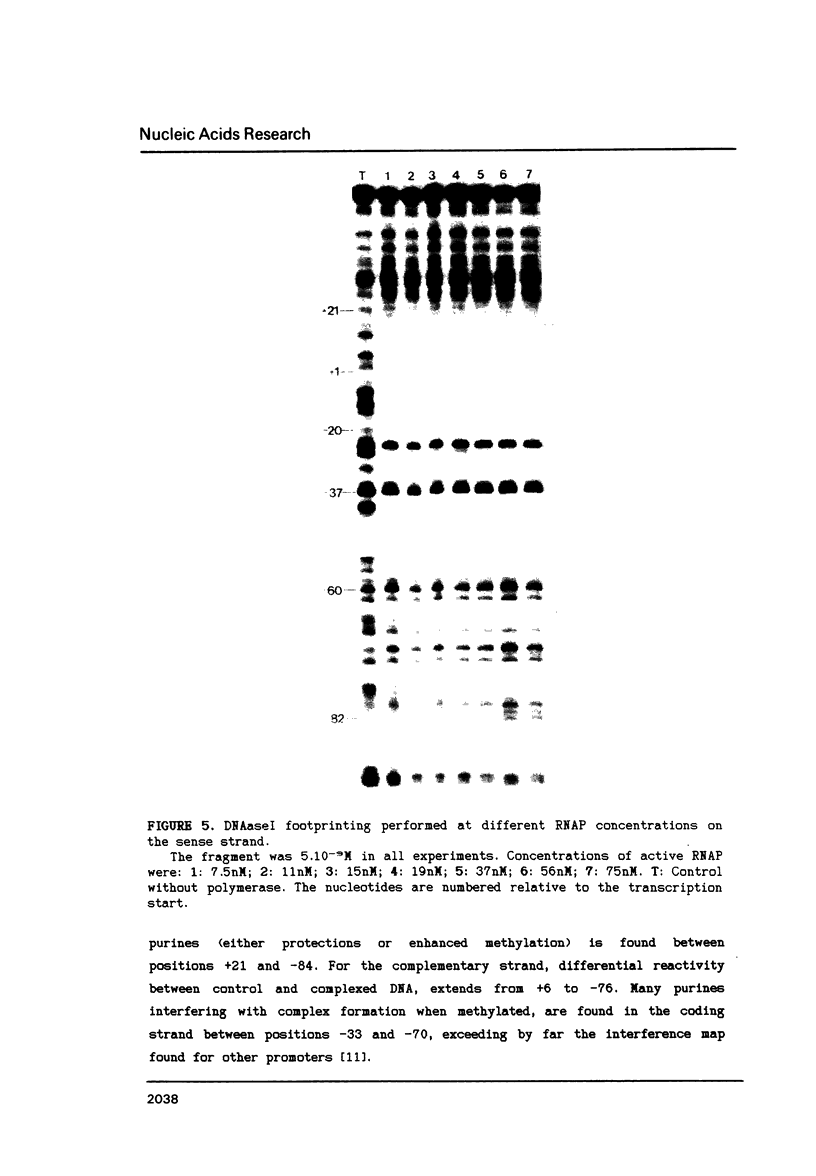
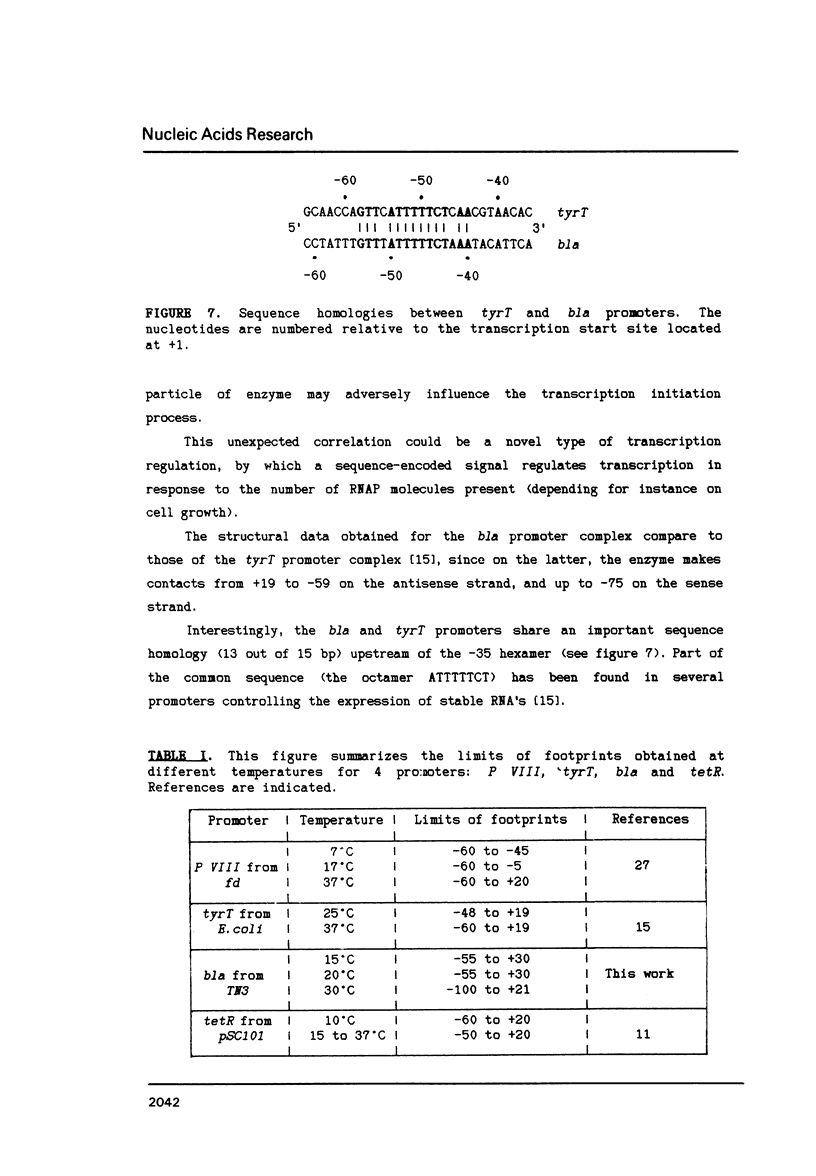
The structure of the final initiation complex between E. coli RNA polymerase (RNAP) and the bla promoter from the transposon TN3 has been probed by footprinting experiments and base accessibility to dimethyl sulfate at 37 degrees C. At RNAP/promoter molar ratios "standard" for these experiments (greater than or equal to 10), the contacts on bla extend from -100 to +20, i.e. a length exceeding twice the dimension of the RNAP major axis [33]. Since footprinting at about equimolar amounts of RNAP and bla extends to the usual (-55 to +20) promoter domain, it is very likely that at least two RNAP's participate in the complex observed at tenfold higher RNAP/bla ratios. Under the latter conditions, the extended footprint (-100 to +20) is observed above 30 degrees C, whereas at 15 degrees C, only the -55 to +20 promoter area is contacted. Furthermore, gel retardation experiments show the presence of two complexes of different migration rates. We have reported earlier [21] that at the "standard" RNAP/bla ratio, transcription initiation from the bla promoter is inhibited. The correlation of this inhibition with the postulated two RNAP/bla complex suggests a regulation of bla gene expression by RNAP availability controlled for instance by growth rate. These results can be correlated with those reported in [14, 15] for the tyrT promoter. Interestingly, both promoter share significant sequence homologies.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auble D. T., Allen T. L., deHaseth P. L. Promoter recognition by Escherichia coli RNA polymerase. Effects of substitutions in the spacer DNA separating the -10 and -35 regions. J Biol Chem. 1986 Aug 25;261(24):11202–11206. [PubMed] [Google Scholar]
- Buc H., McClure W. R. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985 May 21;24(11):2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Busby S., Spassky A., Chan B. RNA polymerase makes important contacts upstream from base pair -49 at the Escherichia coli galactose operon P1 promoter. Gene. 1987;53(2-3):145–152. doi: 10.1016/0378-1119(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Deuschle U., Kammerer W., Gentz R., Bujard H. Promoters of Escherichia coli: a hierarchy of in vivo strength indicates alternate structures. EMBO J. 1986 Nov;5(11):2987–2994. doi: 10.1002/j.1460-2075.1986.tb04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Valentin G., Ehrlich R. Dynamic and structural characterisation of multiple steps during complex formation between E. coli RNA polymerase and the tetR promoter from pSC101. Nucleic Acids Res. 1987 Jan 26;15(2):575–594. doi: 10.1093/nar/15.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Valentin G., Ehrlich R. Interaction between E. coli RNA polymerase and the tetR promoter from pSC101: homologies and differences with other E. coli promoter systems from close contact point studies. Nucleic Acids Res. 1986 Mar 11;14(5):1967–1983. doi: 10.1093/nar/14.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R., Larousse A., Jacquet M. A., Marin M., Reiss C. In vitro transcription initiation from three different Escherichia coli promoters. Effect of supercoiling. Eur J Biochem. 1985 Apr 15;148(2):293–298. doi: 10.1111/j.1432-1033.1985.tb08838.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich R., Marin M., Larousse A., Gabarro-Arpa J., Schmitt B., Reiss C. Promoter recognition and transcription initiation in E. coli. Folia Biol (Praha) 1984;30(Spec No):105–118. [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Gómez-Eichelmann M. C. Effect of nalidixic acid and novobiocin on pBR322 genetic expression in Escherichia coli minicells. J Bacteriol. 1981 Dec;148(3):745–752. doi: 10.1128/jb.148.3.745-752.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer B., Müller D., Köster H. The pathway of E. coli RNA polymerase-promoter complex formation as visualized by footprinting. Nucleic Acids Res. 1985 Aug 26;13(16):5995–6013. doi: 10.1093/nar/13.16.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet M. A., Ehrlich R. In vivo and in vitro effect of mutations in tetA promoter from pSC101: insertion of poly(dA.dT) stretch in the spacer region does not inactivate the promoter. Biochimie. 1985 Sep;67(9):987–997. doi: 10.1016/s0300-9084(85)80293-5. [DOI] [PubMed] [Google Scholar]
- Kammerer W., Deuschle U., Gentz R., Bujard H. Functional dissection of Escherichia coli promoters: information in the transcribed region is involved in late steps of the overall process. EMBO J. 1986 Nov;5(11):2995–3000. doi: 10.1002/j.1460-2075.1986.tb04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Requirement for an upstream element for optimal transcription of a bacterial tRNA gene. Nature. 1983 Sep 15;305(5931):248–250. doi: 10.1038/305248a0. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Prosen D. E., Cech C. L. An Escherichia coli RNA polymerase tight-binding site on T7 DNA is a weak promoter subject to substrate inhibition. Biochemistry. 1986 Sep 23;25(19):5378–5387. doi: 10.1021/bi00367a005. [DOI] [PubMed] [Google Scholar]
- Shanblatt S. H., Revzin A. Kinetics of RNA polymerase-promoter complex formation: effects of nonspecific DNA-protein interactions. Nucleic Acids Res. 1984 Jul 11;12(13):5287–5306. doi: 10.1093/nar/12.13.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Spassky A., Kirkegaard K., Buc H. Changes in the DNA structure of the lac UV5 promoter during formation of an open complex with Escherichia coli RNA polymerase. Biochemistry. 1985 May 21;24(11):2723–2731. doi: 10.1021/bi00332a019. [DOI] [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. D. Spacer mutations in the lac ps promoter. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1069–1072. doi: 10.1073/pnas.79.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelaar W., Schutter W. G., Arnberg A. C., van Bruggen E. F., Stender W. The quaternary structure of Escherichia coli RNA polymerase studied with (scanning) transmission (immuno)electron microscopy. Eur J Biochem. 1983 Sep 15;135(2):263–269. doi: 10.1111/j.1432-1033.1983.tb07647.x. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Mace H. A., Berman M. L. RNA polymerase interactions with the upstream region of the E. coli tyrT promoter. Cell. 1983 Nov;35(1):265–273. doi: 10.1016/0092-8674(83)90229-5. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- deHaseth P. L., Goldman R. A., Cech C. L., Caruthers M. H. Chemical synthesis and biochemical reactivity of bacteriophage lambda PR promoter. Nucleic Acids Res. 1983 Feb 11;11(3):773–787. doi: 10.1093/nar/11.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]