Abstract
Nephrogenic Systemic Fibrosis (NSF) is a progressive disorder occurring in some renal insufficiency patients exposed to Gd based contrast agents (GdBCA). Previous studies demonstrated that the GdBCA Omniscan® upregulated several innate immunity pathways in normal differentiated human macrophages, induced rapid nuclear localization of the transcription factor NFκB, and increased the expression and production of numerous profibrotic/proinflammatory cytokines, chemokines and growth factors. To further examine GdBCA stimulation of the innate immune system, cultured human embryonic kidney 293 (HEK293) cells expressing one of seven different human TLRs or one of two human Nucleotide Oligomerization Domain (NOD)-like receptors (NLRs) were exposed in vitro for 24 h to various GdBCA. The signaling activity of each compound was evaluated by its ability to activate an NFκB-inducible reporter gene. Omniscan® and gadodiamide induced strong TLR 4 and 7 mediated reporter gene activation. The other Gd compounds examined failed to induce reporter gene activation. TLR pathway inhibition using chloroquine or an inhibitor of IL-1 receptor associated kinase 1 (IRAK1) and IRAK4 in normal differentiated human macrophages abrogated Omniscan®-induced gene expression. Omniscan® and gadodiamide signaling via TLR 4 and 7 resulted in increased production and expression of numerous proinflammatory/profibrotic cytokines, chemokines, and growth factors including CXCL10, CCL2, CCL8, CXCL12, IL-4, IL-6, TGF-β and VEGF. These observations suggest that TLR activation by environmental stimuli may participate in the pathogenesis of NSF and of other fibrotic disorders including systemic sclerosis.
Keywords: Toll-like receptors, chemokines, cytokines, nephrogenic systemic fibrosis, Gadolinium, NFκB, fibrosing disorders, macrophages
Introduction
Nephrogenic Systemic Fibrosis (NSF) is a generalized fibrotic disease occurring in some individuals with renal insufficiency following exposure to Gd based contrast agents (GdBCA) used to enhance magnetic resonance imaging (1–4). Clinically, NSF displays many features in common with systemic sclerosis (SSc), including severe and usually progressive skin induration, progressive and eventually incapacitating joint flexion contractures, and fibrotic involvement of lungs, heart, and numerous other internal organs (4–6). Histopathologic analysis of affected NSF skin demonstrates marked dermal thickening with accumulation of thick collagen bundles extending into the subcutaneous tissue and fascia (1–6), and abundant mucin accumulation (7,8). Furthermore, several studies have demonstrated Gd deposits in affected tissues (9–12). Although some reports have characterized NSF as a non-inflammatory disorder, we and others have demonstrated increased numbers of macrophages, myofibroblasts and CD34+ fibrocytes (1–6) and described elevation of serum inflammatory markers in NSF patients with recent disease onset (3,4, and unpublished observations) indicative of the inflammatory nature of the disorder.
The mechanisms underlying GdBCA stimulation of tissue fibrosis remain largely undetermined. GdBCA contain a single Gd3+ ion complexed to either a linear or a macrocyclic chelate which increases Gd3+ solubility and decreases Gd3+ toxicity (13–15). According to Food and Drug Administration data, all NSF cases in which the GdBCA used was identified have been associated with a linear GdBCA (16), most commonly Omniscan® (62%) and Magnevist® (32%). Other published reports raised the proportion of Omniscan®-associated NSF cases to as high as 90% (17). A hypothesis to explain these observations suggested that less thermodynamically stable linear chelates undergo transmetallation, a process in which endogenous circulating ions such as Zn2+ displace Gd3+ at greater rates compared to macrocyclic chelates. It was further suggested that transmetallation is exacerbated by the markedly reduced GdBCA clearance rates in patients with renal insufficiency. However, in vitro experiments have demonstrated that exposure to both linear and macrocyclic GdBCA induces potent functional effects on cultured human normal dermal fibroblasts (8,18–26), normal human PBMC (27) and differentiated macrophages (28), and fibrocytes (29). One of these studies demonstrated strong activation of the NFκB pathway in normal human macrophages following exposure to Omniscan® and suggested that TLR activation mediated by the chelated Gd3+ compounds may be responsible for these effects (28).
Recently, the insight that TLRs are capable of recognition of a variety of endogenous damage associated molecular patterns (DAMPS) released from injured and inflamed tissues (30) has led to a proposed role of innate immunity in the development of various fibrotic disorders including SSc and pulmonary fibrosis (31–39). Indeed, SSc patients display high levels of expression of IFN-responsive genes (IRGs), markers of innate immune activation, which correlates with the modified Rodnan skin score (mRSS), a measure of the degree of SSc skin involvement, and SSc sera induce IFNα expression in normal PBMC (34,35,40). Furthermore, the TLR3 ligand poly (I:C) induces marked activation of IFNα and increased production of the potent profibrotic cytokine, TGFβ in normal and SSc fibroblasts resulting in autocrine stimulation of IFNα and TGFβ responsive gene expression and dermal inflammation and fibrosis in mice (35). Affected tissues from SSc patients often exhibit chronic inflammation suggesting that the release of endogenous TLR ligands during inflammation and TLR signaling may represent one mechanism that initiates and drives this autoimmune fibrotic disease (38). Interestingly, NSF occurs almost exclusively in patients with renal insufficiency (1–4), a clinical condition often accompanied by chronic microinflammation (41).
We have previously described changes in the transcriptome of normal differentiated human macrophages induced by exposure to the GdBCA Omniscan® (28) including the upregulated expression of IFN-responsive genes accompanied by a rapid and intense NFκB activation, as well as NFκB-dependent expression of numerous proinflammatory/profibrotic cytokines, chemokines and growth factors. The results of these studies have led to the hypothesis that GdBCA induced TLR signaling activates expression of proinflammatory/profibrotic molecules (28). To test this hypothesis, we evaluated the effect of Omniscan® on NFκB activation in cells overexpressing a single TLR or NLR. We report that cells expressing TLR4 or TLR7 were responsive to Omniscan® exposure as measured by the activation of a reporter gene under NFκB control. We then compared the ability of various GdBCA to signal through TLR4 or TLR7 and examined the effect of two TLR pathway signaling inhibitors on Omniscan®-mediated increases in proinflammatory/profibrotic cytokine, chemokine, and growth factor expression in normal human differentiated macrophages. The data presented here strongly indicate a role for TLR signaling by Omniscan® in normal differentiated human macrophages, a pathway which may play a crucial role in the pathogenesis of NSF. These results also provide support to the participation of TLR in the pathogenesis of SSc and other inflammatory fibrotic disorders.
Materials and Methods
Gd compounds
Dotarem® (Guerbet LLC, Bloomington, IL), MultiHance® (Bracco Diagnostics, Milan, IT), ProHance® (Bracco Diagnostics, Milan, IT), OptiMark® (Mallinckrodt Inc/Covidien, Hazelwood, IN), were supplied as sterile, aqueous solutions containing 500 mM of the Gd chelate. Omniscan® and gadodiamide (provided by GE Healthcare, Chalfont St. Giles, UK) were supplied as sterile, aqueous solutions containing 287 mg/ml (500 mM) gadodiamide. The Omniscan® solution contained an additional 12 mg/ml (25 mM) caldiamide sodium in water. Caldiamide (GE Healthcare) was supplied as a sterile aqueous solution containing 12 mg/ml (25 mM) caldiamide sodium in water. Gd-EDTA (GE Healthcare) was supplied as a sterile aqueous solution containing 250 mM Gd-EDTA in water. Gd-Citrate (250 μM) was prepared by mixing 1 mL of 250 μM gadolinium chloride and 1 mL of 500 μM sodium citrate solutions at pH 7.4 (42). Gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA; Sigma-Aldrich. St. Louis, MO), the Gd chelate used in Magnevist®, was dissolved in sterile PBS solution at 0.5M concentration. The reagents employed for all the studies were tested and verified by the manufacturer to be free from endotoxin contamination. The absence of endotoxin contamination was further confirmed in our laboratories utilizing the Etoxate Assay (Sigma-Aldrich) according to the manufacturer’s instructions.
TLR receptor screening
Human embryonic kidney 293 (HEK293) cells engineered to express one of seven different human TLRs (TLR2, 3, 4, 5, 7, 8 or 9) or two human NLRs (NOD1 or 2) were obtained from Invivogen (San Diego, CA). For experiments the cells were seeded into 96 well plates at a density of 20,000 cells per well in DMEM containing 10% FBS (Life Technologies Inc., Grand Island, New York, USA), 1% vitamins, 2 mM glutamine, antibiotics, and fungizone (DMEM complete medium). These cells also express a reporter construct under the control of the NFκB inducible promoter resulting in expression of secreted alkaline phosphatase (SEAP) into the culture medium reflecting the levels of NFκB activation. Cells in DMEM complete medium were exposed in duplicate for 20 h to 5 mM of one of the following Gd compounds: ProHance®, Omniscan® or gadodiamide, or to 2.7 μM Gd-EDTA or to 0.25 mM of the chelate molecule caldiamide in 200 μL medium containing Quantiblue (Invivogen) to allow colorimetric detection of SEAP. As positive controls, the cellular response to known specific TLR ligands (Invivogen) was also measured: heat-killed Listeria monocytogenes at 108 cell/well (TLR2), 1 μg/mL Poly(I:C) (TLR3), 100 ng/mL E. coli K12 LPS (TLR4), 100 ng/mL S. typhimurium flagellin (TLR5), 1 μg/mL CL097 (TLR7), 1 μg/mL CL075 (TLR8), 1 μg/mL CpG ODN 2006, 100 ng/mL C12-iEDAP (NOD1), or 10 ng/mL L18-MDP (NOD2). The absorbance at 650nm was measured on a Beckman Coulter AD 340C Absorbance Detector following the 20 h incubation.
For TLR ligand dose response experiments, the above procedure was performed using HEK293 cells expressing either human TLR4 or TLR7 cultured in DMEM complete medium at a density of 50,000 cells per well. The cells were exposed to 0.5, 1 or 5 mM of one of the following GdBCA :Gd-DTPA, Dotarem®, MultiHance®, ProHance®, OptiMark®, gadodiamide or Omniscan®, or to 2.7, 27 or 270 nM Gd-EDTA, or to 0.025, 0.05, 0.25 mM of the chelate molecule caldiamide in 200 μL medium containing Quantiblue as described above. As positive controls, the response to 1, 5 or 10 ng/mL E. coli K12 LPS (TLR4) or 1, 5 or 10 μg/mL gardiquimod (TLR7) was measured. No significant effect on cell numbers or cytotoxicity was observed as examined by the WST-1 assay (Roche Diagnostics, Indianapolis, IN).
Macrophage isolation and differentiation
Normal human peripheral blood leukoreduction filters were obtained from the Thomas Jefferson University Hospital Blood Bank following Institutional Review Board approval. Human PBMC were isolated from the leukoreduction filters by ficoll-hypaque gradient centrifugation (Amersham Pharmacia Biotech, Piscataway, NJ) and enriched for monocytes by adherence to plastic culture dishes for 2 h in RPMI containing 10% FBS (Life Technologies Inc), as described previously (27). To obtain terminally differentiated macrophages the monocytes were cultured in RPMI complete medium with 60 ng/ml M-CSF (BioVision, Mountain View, CA) and 25 ng/mL IL-10 (BioVision) for 7 days as described (28). Differentiated macrophages (5 × 105 cells/mL) were exposed for 24 h to 1 mM of one of the following GdBCA: Gd-DTPA, Dotarem®, MultiHance®, ProHance®, OptiMark®, Omniscan® or gadodiamide or to 0.05 mM caldiamide or to 0.27 mM Gd-EDTA or Gd-Citrate. No significant effect on cell numbers or cytotoxicity was observed as examined by the WST-1 assay (Roche Diagnostics).
TLR Inhibition studies
To confirm a role of TLRs in GdBCA stimulation of macrophages, normal human differentiated macrophages were washed with PBS and exposed to either 50 μM of the TLR inhibitor chloroquine (Invivogen) or 300 nM IRAK1/4 inhibitor 1-(2-(4-morpholinyl)ethyl)-2-(3-nitrobenzoylamino)benzimidazole, N-(2-morpholinylethyl)-2-(3-nitrobenzoylamido)-benzimidazole (Sigma-Aldrich) in RPMI complete medium for 1 h. This incubation was followed by addition of either PBS or PBS with 1 mM of either Omniscan®, gadodiamide or ProHance®, or 0.05 mM caldiamide or 2.7 μM Gd-EDTA for 24h. As positive controls for TLR-dependent and TLR-independent macrophage stimulation, 100 ng/mL LPS (Invivogen) or 10 μg/mL TNFα (Pierce Biotechnology, Woburn, MA), respectively, were used. Macrophage samples cultured with an equal volume of PBS served as negative controls. No significant effect on cell numbers or cytotoxicity was observed as examined by the WST-1 assay (Roche Diagnostics). Macrophage culture supernatants were isolated, filtered and maintained frozen for subsequent studies. Cells were washed twice with PBS and then processed for RNA extraction using the RNeasy kit according to the protocol recommended by the manufacturer (Qiagen, Valencia, CA) as described previously (27).
RNA interference
DharmaFECT® 1 siRNA transfection reagent, siGENOME SMARTpool small interfering RNA (siRNAs) specific for human TLR4 and TLR7 and siGENOME RISC-free control siRNA were purchased from Dharmacon (Lafayette, CO). Normal human macrophages (5 × 105) differentiated as described above were plated in six-well plates and transfected with 100 nM of siRNA for TLR4 or TLR7 or TLR4+TLR7 or with control (scrambled) siRNAs using DharmaFECT® 1 (3 μl/well) according to the manufacturer’s instructions. After 24 h, macrophages cells were treated with either 1 mM Omniscan, 1 mM gadodiamide or 1 mM ProHance for 24 h or left untreated. Total RNA was extracted and RNA levels were assessed by real time PCR as described above. No significant effect on cell numbers or cytotoxicity was observed as examined by the WST-1 assay (Roche Diagnostics).
Real-time PCR validation
Expression levels of CCL2 (MCP-1), CCL8 (MCP-2), CXCL10 (IP10) and CXCL11 (ITAC) were assayed by real-time quantitative PCR utilizing SYBR Green chemistry (Applied Biosystems, Foster City, CA) following a standard amplification protocol on an ABI Prism 7900 Sequence Detection System (Applied Biosystems). The following primers were employed: β-actin: forward 5′-TTGCCGACAGGATGCAGAA-3′, reverse 5′-GCCGATCCACACGGAGTACTT-3′; IL-4: forward 5′-TGCTGCCTCCAAGAACACAA-3′, reverse 5′-TGTAGAACTGCCGGAGCACA-3′; IL-6: forward 5′-TGAGGAGACTTGCCTGGTGAAA-3′, reverse 3′-TGGCATTTGTGGTTGGGTCA-3′; IL-13: forward 5′-AGCTGGTCAACATCACCCAGAA-3′, reverse 5′-AGCTGTCAGGTTGATGCTCCAT-3′; IFN-γ: forward 5′-TTCAGATGTAGCGGATAATGGAAC-3′, reverse 5′-TTCTGTCACTCTCCTCTTTCCA-3′ TGF-β-Transforming Growth Factor beta: forward 5′-CGAGCCTGAGGCCGACTA-3′, reverse 5′-AGATTTCGTTGTGGGTTTCCA-3′; VEGF – Vascular Endothelial Growth Factor: forward 5′-AGAAGGAGGAGGGCAGAATCAT-3′, reverse 5′-TAATCTGCATGGTGATGTTGG-3′; CCL2: forward 5′-ACCAGCAGCAAGTGTCCCAAA-3′, reverse 5′-TTTGCTTGTCCAGGTGGTCCAT-3′; CCL8: forward 5′-TCATGCTGAAGCTCACACCCTT-3′, reverse 5′-AGAATTGCCATTGCACAACTCTT-3′; CXCL10: forward 5′-ACTGCCATTCTGATTTGCTGCC-3′, reverse 5′-TGATGCAGGTACAGC GTACAGT-3′; CXCL11: forward 5′-ACTCCTTCCAAGAAGAGCAGCA-3′, reverse 5′-CCATGCCCTTCACACTCATGTT-3′; CXCL12: forward 5′-AAAGCCATGTTGCCAGAGCCAA-3′, reverse 5′ – AGCTTCGGGTCAATGCACACTT-3′; TLR4: forward 5′ – AGAACTGCAGGTGCTGGATT-3′, reverse 5′ – AGAGGTGGCTTAGGCTCTGATA-3′; TLR7: forward 5′ – CTGCTCTCTTCAACCAGACCTCTAC-3′, reverse 5′ – AGAGTGACATCACAGGGCAGAG-3′.
Relative quantification was performed by arbitrarily setting the expression level of the PBS negative control at 100 and by expressing changes in transcript levels of other samples relative to this control sample. Results obtained from 3 experiments with each of the GdBCA alone or in the presence of each inhibitor examined, utilizing triplicate samples of normal human macrophages were averaged. Relative differences in each PCR sample were corrected using human β-actin mRNA levels as an endogenous control.
Multiplex ELISA
SearchLight proteome array analyses (Aushon Biotechnology, Woburn MA) were conducted to measure the levels of IL-4, IL-6, IL-13, IFN-γ, TGF-β, VEGF, CCL2, CCL8, CXCL10, and CXCL11 in macrophage culture supernatants from the TLR inhibition studies described above following procedures described previously (43). Briefly, culture supernatant samples were diluted 1:2, 1:50, or 1:1,000 and then incubated for 1 h on array plates which had been prespotted with capture antibodies specific for each protein. Plates were decanted and washed 3 times with PBS before addition of a mixture of biotinylated detection antibodies to each well. Following incubation with detection antibodies for 30 min, plates were washed 3 times and incubated for 30 min with streptavidin horseradish peroxidase. Plates were again washed, and SuperSignal Femto chemiluminescent substrate (Pierce Biotechnology) was added. The plates were immediately imaged using the SearchLight imaging system and data were analyzed using ArrayVision software (GE Healthcare).
Statistical analysis
Real-time PCR values reflect the mean and standard deviation of three separate experiments each performed in triplicate with each of the three samples of normal human macrophages. The statistical significance of the real-time PCR data was assessed by Student’s two-tailed t test. P values less than 0.05 were considered statistically significant.
Results
Identification of TLRs involved in Omniscan® activation of NFκB in Human Embryonic Kidney 293 (HEK293) cells
To explore the ability of GdBCA to induce NFκB activation via TLR and NLR signaling, HEK293 cells expressing one of seven human TLRs (TLR2, 3, 4, 5, 7, 8 or 9) or one of two human NLRs (NOD1 or NOD2) were exposed in DMEM complete medium for 24h to one of five compounds: 5 mM of the linear GdBCA Omniscan, 5 mM gadodiamide, the linear Gd chelate component of Omniscan, 0.25 mM caldiamide, which is the amount of excess chelate present in the Omniscan formulation, 5 mM of the macrocyclic GdBCA ProHance, or to 2.7 μM of the non-chelated Gd compound Gd-EDTA. The effect of these compounds on TLR or NLR signaling was assessed colorimetrically by measuring production of secreted alkaline phosphatase resulting from the activation of NFκB in these cells. Since production of alkaline phosphatase is under the control of a stably integrated NFκB -inducible promoter the amount of alkaline phosphatase released by the cells is a direct reflection of the level of NFκB activation. All agents tested were verified to be endotoxin free by the manufacturer and confirmed using a limulus amebocyte lysate gel formation assay. No significant effect on cell numbers or cytotoxicity was observed as examined by the WST-1 assay. The specific response of the HEK293 cells expressing one specific TLR or NLR were validated following activation with the corresponding TLR or NLR specific activator.
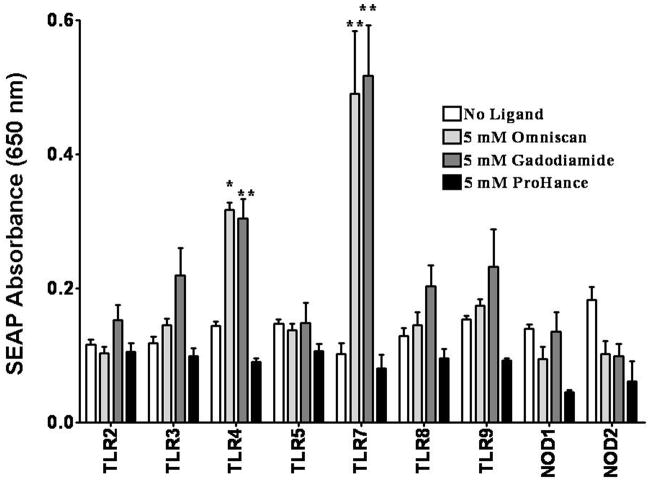
As portrayed in Figure 1, 5 mM Omniscan® induced significantly increased NFκB-dependent SEAP production in HEK293 cells expressing either TLR4 or TLR7 compared with control cells incubated with endotoxin-free PBS. Omniscan® did not cause statistically significant stimulation of reporter gene expression in the HEK293 cells expressing any of the other TLRs or NLRs. TLR7 expressing cells showed a 5-fold increase in SEAP levels compared with control cells in response to Omniscan®. These changes in SEAP expression were observed for all replicates for TLR4 and TLR7 expressing cells. Gadodiamide, the Gd chelate in Omniscan®, also induced increased NFκB-dependent SEAP production in TLR4 or TLR7 expressing HEK293 cells to levels nearly identical to those induced in response to Omniscan®. In contrast to the linear Gd chelate Omniscan®, the macrocyclic Gd chelate ProHance® did not induce increased NFκB-dependent SEAP production in HEK293 cells expressing any of the TLRs or NLRs (Figure 1). Caldiamide and the non-chelated Gd compound Gd-EDTA did not produce significant changes in any TLR or NOD expressing cell line (data not shown).
Figure 1. Assessment of GdBCA induced NFκB dependent activation in HEK293 cells expressing a single TLR or NLR.
Measurement of NFκB dependent secreted alkaline phosphatase (SEAP) levels in HEK293 cells expressing a single TLR or NLR following exposure to 5 mM Omniscan®, gadodiamide or ProHance®. Values represent the mean (+/− SD) absorbance at 650 nm of 2 replicates of 2 separate experiments on HEK293 cells in culture. Statistical significance was calculated by comparing each Gd compound to the saline control. *: p<0.05; **: p < 0.01; ***: p<0.001.
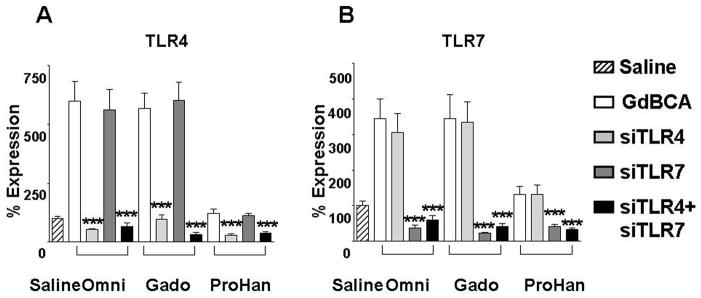
Figure 5. Effect of RNA interference of TLR expression on GdBCA induced upregulation of TLR4 and TLR7 expression in normal differentiated human macrophages.
Differentiated normal human macrophages were transfecte with 100 nM siGENOME SMARTpool small interfering RNA (siRNA) specific for TLR4 or TLR7 or both TLR4+TLR7 with DharmaFECT 4 (3 μl/well) followed by 24 h incubation with 1mM Omniscan® (Omni), gadodiamide (Gado) or ProHance® (ProHan). Expression levels of TLR4 and TLR7 were assessed by real time PCR. Values represent the mean (+/− SD) expression levels of three replicates of three separate experiments with macrophages differentiated from monocytes obtained from three different normal individuals. Note that the data are presented in a semilogarithmic scale. An siGENOME RISC-free control siRNA was used as a negative control. C(t) values for cytokines were normalized with β-actin. The saline control levels were arbitrarily set at 100% expression at each time point. Statistical significance of changes in cytokine and growth factor expression was calculated by comparing each Gd compound to the saline control. *: p<0.05; **: p < 0.01; ***: p<0.001.
Dose Response Analysis of GdBCA signaling through TLR4 and TLR7 in HEK293 cells
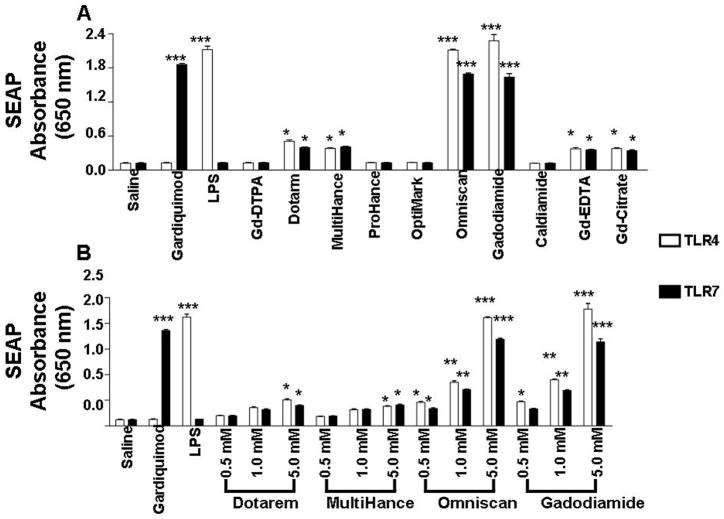
Following identification of TLR4 and TLR7 as participating in Omniscan®-induced activation of NFκB-dependent SEAP production in HEK293 cells, dose response analysis of the effects of seven Gd chelate compounds and two non-chelated Gd compounds was performed. HEK293 expressing either TLR4 or TLR7 cells were exposed in DMEM complete medium to 0.5, 1 and 5 mM concentrations of the linear GdBCAs Gd-DTPA, MultiHance®, OptiMark®, Omniscan® or gadodiamide, or of the macrocyclic GdBCAs Dotarem® or ProHance®, or to 0.27, 2.7 or 27 μM of the Gd compounds Gd-EDTA or Gd-Citrate or to 0.025, 0.05 or 0.25 mM of the non-Gd chelate molecule caldiamide. LPS (1 mg/mL) and gardiquimod (10 mg/mL) were used as positive controls for TLR4 and TLR7 activation, respectively. The concentrations of Gd compounds employed in these studies were similar to those employed in previously published in vitro studies (8,20,22–24). Following 24 h of exposure to the GdBCA, the levels of NFκB-dependent SEAP production were measured. The results showed that some of the Gd compounds induced increased SEAP production in a dose dependent manner. Figure 2A displays the results for the highest concentration of each agent tested whereas Figure 2B shows the threee concentration dose response for Dotarem®, MultiHance®, Omniscan® and gadodiamide. The linear GdBCA Omniscan® and gadodiamide induced a highly significant (p<.001) 20-fold increase in NFκB-dependent SEAP production in TLR4 and a 16-fold increase in TLR7 expressing HEK293 cells, levels similar to those induced by the LPS positive control. The linear GdBCA MultiHance®and the macrocyclic Dotarem® induced much weaker although significant (p<.05) 3-4-fold increases in SEAP production in TLR4 and TLR7 expressing cells, comparable to the 4-fold increase in SEAP production induced by the non-chelate Gd-EDTA and Gd-citrate. Figure 2B shows that the response to Dotarem®, MultiHance®, Omniscan® and gadodiamide was dose-dependent. No response was observed at any concentration examined for the linear GdBCA Gd-DTPA or OptiMark® or for the macrocyclic ProHance® or of the non-Gd chelate molecule caldiamide (Figure 2B and data not shown).
Figure 2. Comparison of NFκB dependent activation by various GdBCA in HEK293 cells expressing TLR4 or TLR7.
Measurement of NFκB dependent secreted alkaline phosphatase (SEAP) levels in HEK293 cells expressing a single TLR or NLR at 20 h following exposure in DMEM complete medium to GdBCA. Cells were exposed to 0.5, 1 and 5 mM concentrations of the linear GdBCA Gd-DTPA, MultiHance®, OptiMark®, Omniscan® or gadodiamide, or of the macrocyclic GdBCA Dotarem® or ProHance®, or to 0.27, 2.7 or 27 μM of the Gd compounds Gd-EDTA or Gd-Citrate or to 0.025, 0.05 or 0.25 mM of the non-Gd chelate molecule caldiamide. LPS (1 mg/mL) and gardiquimod (10 mg/mL) were used as positive controls for TLR4 and TLR7 activation respectively. Values represent the mean (+/− SD) absorbance at 650 nm of 3 replicate of 3 separate experiments on HEK293 cells in culture. Statistical significance was calculated by comparing each Gd compound to the saline control. Values for other samples are expressed relative to the saline control. *: p<0.05; **: p < 0.01; ***: p<0.001. A. SEAP levels secreted in response to exposure to the highest concentration of each agent tested. B. Three concentration dose response of SEAP levels secreted following exposure to Dotarem®, MultiHance®, Omniscan® or gadodiamide.
Stimulation of TLR-dependent cytokine, chemokine and growth factor expression in normal human differentiated macrophages
To determine whether TLR signaling was required for Omniscan® stimulation of macrophage cytokine, chemokine and growth factor production, normal human macrophages cultured in RPMI complete medium and differentiated in the presence of M-CSF and IL-10 were preincubated with 50 μM chloroquine or with 300 nM of the IRAK1/4 inhibitor for 1 h followed by addition of either PBS or PBS containing 1 mM of either Omniscan®, gadodiamide, or ProHance®, or 0.05 mM caldiamide or 2.7 μM Gd-EDTA for 24h. As positive controls for TLR-dependent and TLR-independent macrophage stimulation, 100 ng/mL LPS or 10 μg/mL TNFα, respectively, were used. No significant effect on cell numbers or cytotoxicity was observed as examined by the WST-1 assay.
Analysis of expression levels of profibrotic/proinflammatory cytokines by real time PCR demonstrated that Omniscan® and gadodiamide caused markedly upregulated expression of multiple chemokines (Figure 3), and cytokines and growth factors (Figure 4). Incubation of macrophages with LPS also induced increases in chemokine, cytokine and growth factor expression which were abolished as expected by both inhibitors. The inhibitors, however, failed to modify the effects induced by TNFα (data not shown). These results indicated that the stimulation of cytokines, growth factors and chemokines induced by the GdBCA was mediated by TLRs.
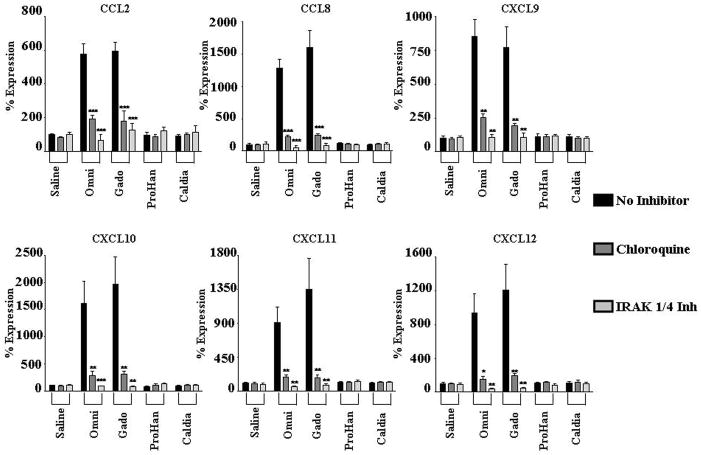
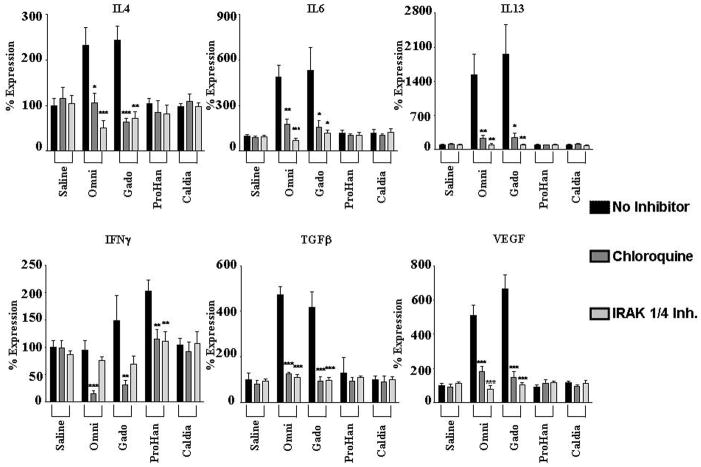
Figure 3. Effect of TLR inhibition on GdBCA induced upregulation of cytokine/growth factor expression in normal differentiated human macrophages.
Differentiated normal human macrophages were preincubated for 2h with TLR inhibitors chloroquine (300 nM) or IRAK1/4 inhibitor (10 μM) followed by 24 h incubation with 1mM Omniscan® (Omni), gadodiamide (Gado) or ProHance® (ProHan) or with 0.05 mM caldiamide (Caldia). Expression levels of various cytokine and growth factors were assessed by real time PCR. Values represent the mean (+/− SD) expression levels of three replicates of three separate experiments with macrophages differentiated from monocytes obtained from three different normal individuals. Note that the data are presented in a semilogarithmic scale. LPS (100 ng/mL) and TNFα (10 μg/mL) were used as positive controls for TLR4 and TNFα activation, respectively. C(t) values for cytokines were normalized with β-actin. The saline control levels were arbitrarily set at 100% expression at each time point. Statistical significance of changes in cytokine and growth factor expression was calculated by comparing each Gd compound to the saline control. Statistical significance of changes induced by inhibitors was calculated by comparing these levels to those induced by the GdBCA.*: p<0.05; **: p < 0.01; ***: p<0.001.
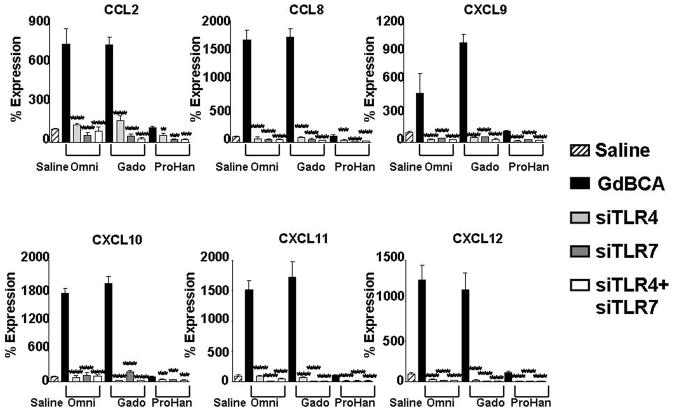
Figure 4. Effect of TLR inhibition on GdBCA induced upregulation of chemokine expression in normal differentiated human macrophages.
Differentiated normal human macrophages were preincubated for 2h with the TLR inhibitors chloroquine (300 nM) or IRAK1/4 inhibitor (10 μM) followed by 24 h incubation with 1mM Omniscan® (Omni), gadodiamide (gado) or ProHance® (ProHan) or with 0.05 mM caldiamide (Caldia). Expression levels of various chemokines were assessed by real time PCR. Values represent the mean (+/−SD) expression levels of three replicates of three separate experiments with macrophages differentiated from monocytes obtained from three different normal individuals. Note that the data are presented in a semilogarithmic scale. LPS (100 ng/mL) and TNFα (10 μg/mL) were used as positive controls for TLR4 and TNFα activation, respectively. C(t) values for cytokines were normalized with β-actin. The saline control levels were arbitrarily set at 100% expression at each time point. Statistical significance of changes in chemokine expression was calculated by comparing each Gd compound to the saline control. Statistical significance of changes induced by inhibitors was calculated by comparing these levels to those induced by the GdBCA. *: p<0.05; **: p < 0.01; ***: p<0.001.
CXCL10 displayed the greatest increase in expression of the chemokines examined, with maximal 20 fold induction by gadodiamide compared to the saline control (Figure 3). CCL8 expression was induced 16 fold, CXCL11 expression increased 13 fold, CXCL12 expression increased 12 fold, CXCL9 increased 8 fold and CCL2 showed a 6 fold increase in expression. Gadodiamide induced the strongest levels in expression. A similar pattern was observed for multiple profibrotic/proinflammatory cytokines and growth factors. IL13 displayed the greatest increase in expression, with a maximal 20 fold induction by gadodiamide at 24h (Figure 4). Marked increases in the expression of the growth factors TGF-β (6 fold) and VEGF (5 fold) and the profibrotic cytokines IL-4 (2 fold) and IL-6 (6 fold) were also observed in response to Gd compounds. ProHance had the smallest effect on cytokine and growth factor gene expression. Of interest was the observation that the GdBCA tested induced only minor expression or did not change the levels of IFN-γ with ProHance inducing only a 2 fold increase whereas gadodiamide induced a 1.4 fold increase and Omniscan did not change IFN-γ expression levels. Caldiamide did not affect expression levels of any of the genes tested (Figures 3–4). Preincubation of macrophages with either chloroquine or IRAK1/4 inhibitor abrogated gadodiamide and Omniscan® mediated increases in gene expression (Figures 3–4). These results demonstrated that Omniscan® and gadodiamide elicit stimulation of potent cytokine, chemokine and growth factor gene expression in macrophages that is dependent on TLR.
Inhibition of TLR4 and TLR7 utilizing RNA interference
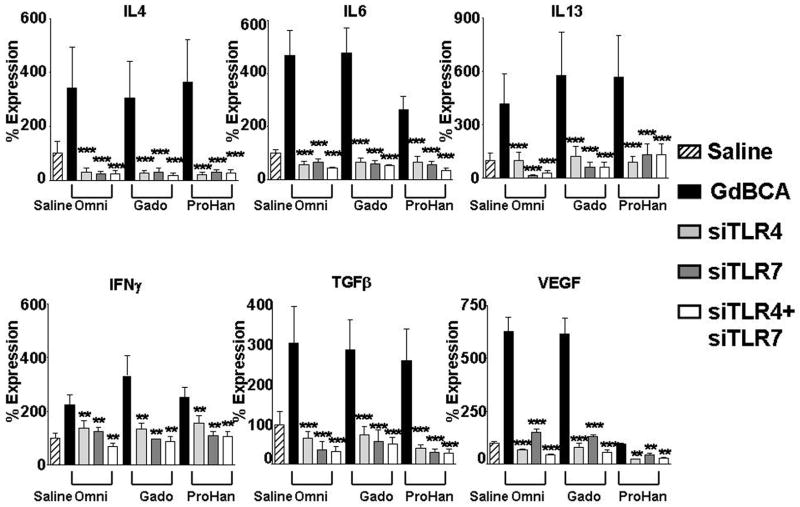
To confirm that the observed effects of chloroquine and IRAK 1/4 inhibitors were due to inhibition of the TLR pathway and were not the result of possible effects on other inflammatory pathways, TLR4 and TLR7 expression was directly targeted in normal human differentiated macrophages employing RNA interference. Normal differentiated human macrophages were transfected with control siRNA or with siRNA specific for TLR4 or TLR7 alone or in combination. RNA from these experiments was utilized in validation of gene expression by real time RT-PCR of TLR4 and TLR7 (Figure 5), chemokines (Figure 6) and proinflammatory/profibrotic cytokines and growth factors (Figure 7). The TLR4 specific siRNA induced an 84% decrease in TLR4 mRNA levels without affecting TLR7 expression levels, whereas the TLR7 specific siRNA induced an 88% decrease in TLR7 expression without affecting the RNA levels of TLR4 (data not shown). Exposure of macrophages to 1 mM Omniscan or 1 mM gadodiamide induced TLR4 (Figure 5A) and TLR7 (Figure 5B) expression and this increased expression was reduced to below the levels measured in saline control cells by the specific siRNA. The control siRNA had no appreciable effect on TLR4 or TLR7 expression compared to the saline control. Exposure of cells to either the TLR4 specific siRNA or to the TLR7 specific siRNA prior to treatment with 1 mM Omniscan or 1 mM gadodiamide resulted in abrogation of the GdBCA-induced overexpression of chemokines (Figure 6), cytokines and growth factors (Figure 7), reducing their expression to levels equivalent or lower than those measured in the saline controls.
Figure 6. Effect of RNA interference of TLR expression on GdBCA induced upregulation of chemokine expression in normal differentiated human macrophages.
Differentiated normal human macrophages were transfected with 100 nM siGENOME SMARTpool small interfering RNA (siRNA) specific for TLR4, TLR7 or TLR4+TLR7 with DharmaFECT 4 (3 μl/well) followed by 24 h incubation with 1mM Omniscan® (Omni), gadodiamide (Gado) or ProHance® (ProHan). Expression levels of various cytokine and growth factors were assessed by real time PCR. Values represent the mean (+/− SD) expression levels of three replicates of three separate experiments with macrophages differentiated from monocytes obtained from three different normal individuals. Note that the data are presented in a semilogarithmic scale. An siGENOME RISC-free control siRNA was used as a negative control. C(t) values for cytokines were normalized with β-actin. The saline control levels were arbitrarily set at 100% expression at each time point. Statistical significance of changes in cytokine and growth factor expression was calculated by comparing each Gd compound to the saline control. *: p<0.05; **: p < 0.01; ***: p<0.001.
Figure 7. Effect of RNA interference of TLR expression on GdBCA induced upregulation of cytokine/growth factor expression in normal differentiated human macrophages.
Differentiated normal human macrophages were transfected with 100 nM siGENOME SMARTpool small interfering RNA (siRNA) specific for TLR4, TLR7 or TLR4+TLR7 with DharmaFECT 4 (3 μl/well) followed by 24 h incubation with 1mM Omniscan® (Omni), gadodiamide (Gado) or ProHance® (ProHan). Expression levels of various chemokines were assessed by real time PCR. Values represent the mean (+/− SD) expression levels of three replicates of three separate experiments with macrophages differentiated from monocytes obtained from three different normal individuals. Note that the data are presented in a semilogarithmic scale. LPS (100 ng/mL) and TNFα (10 μg/mL) were used as positive controls for TLR4 and TNFα activation respectively. C(t) values for cytokines were normalized with β-actin. The saline control levels were arbitrarily set at 100% expression at each time point. Values for other samples are expressed relative to the saline control. Statistical significance of changes in chemokine expression was calculated by comparing each Gd compound to the saline control. *: p<0.05; **: p < 0.01; ***: p<0.001.
Stimulation of TLR-dependent cytokine, chemokine and growth factor production in normal human differentiated macrophages
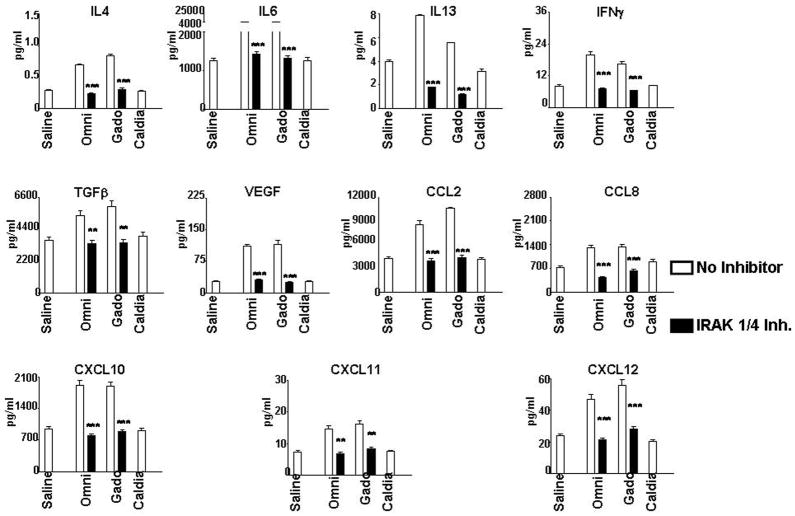
To confirm that the increased expression observed at the transcript level was reflected at the protein level, SearchLight proteome multiplex arrays were utilized to quantitate the amounts of relevant profibrotic and proinflammatory cytokines, chemokines and growth factors produced by normal human differentiated macrophages following GdBCA exposure alone or following preincubation with chloroquine or IRAK 1/4 inhibitor. The results showed that 24 h exposure to some of the Gd-containing compounds resulted in increased production and secretion of numerous chemokines, cytokines and growth factors with their significant accumulation in the culture media as shown in Figure 8. The results, expressed in pg/mL, were normalized for the value of β-actin transcripts obtained in the real-time PCR experiments in order to correct for possible variance in the number of adherent cells analyzed. All of the cytokines/growth factors that exhibited upregulated mRNA expression following exposure to Omniscan®, gadodiamide, ProHance® and Gd-EDTA also demonstrated an increase in the total amount of the corresponding secreted cytokine/growth factor with the greatest increases seen for cells exposed to Omniscan® and gadodiamide. Exposure of macrophages to caldiamide did not result in a detectable increase in the production of any of the cytokines/growth factors analyzed (Figure 8). As observed for RNA expression levels, preincubation of macrophages with either chloroquine (data not shown) or with IRAK 1/4 inhibitor abrogated the Omniscan® and gadodiamide mediated increased levels of cytokines, chemokines and growth factors to near baseline levels (Figure 8).
Figure 8. Effect of TLR inhibition on amounts of Gd compound stimulated cytokines, chemokines and growth factors in normal differentiated human macrophages.
Quantitative measurement of cytokines, chemokines and growth factors present in the culture media of Gd-compound-exposed cells was performed employing multiplex ELISA proteome array analysis. Differentiated normal human macrophages were preincubated with either 300 nM chloroquine or 10 μM IRAK1/4 inhibitor. Following preincubation, cells were incubated with either saline, 1 mM Omniscan® (Omni), 1 mM gadodiamide (Gado) or 0.05 mM caldiamide (Caldia). Values are expressed in pg/ml and represent the mean value of values obtained from duplicate results at 3 dilutions: 1:2, 1:50 and 1:1000. Statistical significance of changes in chemokine, cytokine and growth factor production was calculated by comparing each Gd compound to the saline control. Statistical significance of changes induced by inhibitors was calculated by comparing these levels to those induced by the GdBCA.*: p<0.05; **: p < 0.01; ***: p<0.001.
Discussion
While exposure of patients with renal insufficiency to GdBCA is a primary factor in NSF pathogenesis, the molecular pathways stimulated following exposure to GdBCA have not been identified. The data presented here demonstrating that Omniscan® signals via TLR4 and TLR7 provide a possible mechanism for the initiation of GdBCA-induced inflammation and fibrosis. Omniscan® and gadodiamide, the linear Gd chelate present in Omniscan®, induce potent stimulation of NFκB mediated expression of an SEAP reporter protein in HEK293 cells expressing either TLR4 or TLR7 but not in cells expressing one of the other TLR or the NOD receptors(Figures 1–2). The stimulatory effects were comparable to the levels of stimulation induced by the TLR4 ligand LPS or the TLR7 ligand gardiquimod whereas the chelate molecule caldiamide used in the Omniscan® formulation did not stimulate SEAP expression(Figure 2). One linear GdBCA, MultiHance®, showed a much weaker induction of SEAP expression (Figure 3), as did one of the macrocylic GdBCA, ProHance® in HEK293 cells. Two non-chelated Gd compounds, Gd-EDTA and Gd-Citrate stimulated SEAP expression in TLR4 or TLR7-expressing HEK293 cells at a level intermediate between the high levels induced by Omniscan® or gadodiamide and the low levels induced by MultiHance® or ProHance®.
In normal human differentiated macrophages, both Omniscan® and gadodiamide strongly induced multiple profibrotic and proinflammatory cytokines, chemokines and growth factors as measured by real time PCR of total RNA (Figures 3–4) and ELISA of culture supernatants (Figure 8), while ProHance® and Gd-EDTA induced a less pronounced increase in expression of these genes. Preincubation of differentiated macrophages with chloroquine or with the IRAK1/4 inhibitor for 1 hour abrogated the Omniscan® - or gadodiamide-induced expression and production of these proinflammatory/profibrotic molecules (Figures 3,4,8), indicating the crucial role of TLR4 and TLR7 in these effects. These observations were confirmed in experiments targeting TLR4 and TLR7 by RNA interference (Figures 6,7), thus conclusively demonstrating that the increased expression levels observed are TLR-dependent. Increased expression induced by TNFα which acts downstream of the TLR receptors was unaffected by these inhibitors.
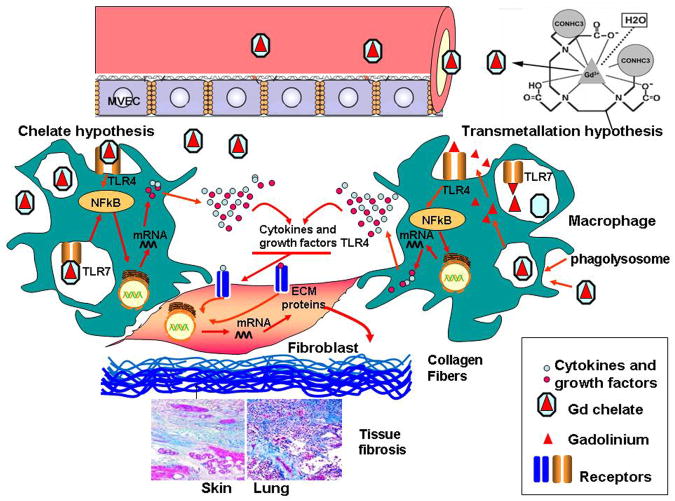
Exposure of patients with renal insufficiency to GdBCA is a primary factor in the pathogenesis of NSF (1–4) with the majority of NSF cases attributed to exposure to Omniscan® (16,17). Although the exact mechanisms responsible for the development of NSF following GdBCA exposure in patients with renal insufficiency are not known, there are two hypotheses that have been proposed as illustrated in Figure 9. In the transmetallation hypothesis, Gd-containing compounds escape into the extravascular space and transmetallation is induced by endogenous ions allowing free Gd3+ to escape. The transmetallation process is dependent on the thermodynamic stability of the GdBCA, therefore, it is more pronounced for the less stable linear chelates (44). The released Gd3+ can then from the chelate and interact with tissue macrophages resulting in the production and secretion of proinflammatory/profibrotic cytokines, chemokines and growth factors by these cells. These secreted macrophage products act on resident tissue fibroblasts, inducing their differentiation into α-SMA expressing myofibroblasts with the consequent increase in production and secretion of a variety of molecules involved in the fibrotic process including the interstitial collagens. The exact mechanisms involved in the escape of Gd-containing compounds into the extravascular space and subsequent transmetallation are not entirely known, however, it is likely that factors such as the higher GdBCA concentrations and their increased retention in the circulation owing to renal insufficiency, associated with alterations in endothelial permeability and tissue edema resulting from inflammatory or thrombotic events may all contribute to this process (44–46).
Figure 9. Diagramatic representation of two possible pathways that may participate in the induction of NSF by GdBCA.
This schematic diagram represents two possible mechanisms by which GdBCA result in activation of target cells. In the transmetallation model, free Gd3+ is displaced from the chelate complex by endogenous ions, such as Zn2+, allowing it to stimulate proinflammatory and profibrotic responses in macrophages and fibroblasts. In the chelate model, these effects are mediated by chelated GdBCA which have been retained in the body due to severely reduced clearance rates in patients with renal insufficiency. In both models the cellular recognition of either free Zn2+ (transmetallation model) or of the intact chelated GdBCA (chelate model) is mediated through TLR4 and TLR7 signaling.
In disagreement with the transmetallation hypothesis, however, multiple experimental studies have demonstrated that both linear and macrocyclic GdBCA induce potent metabolic changes in cells, including increased hyaluronan and collagen production by normal human fibroblasts (8,18–26), increased expression of proinflammatory and profibrotic cytokines, chemokines and growth factors by normal human PBMC (27) and differentiated human macrophages (28), and increased differentiation of normal human PBMC into fibrocytes (29). These experimental results have suggested an alternative hypothesis which posits that intact chelated GdBCA are responsible for GdBCA-mediated stimulation (47). In the chelate hypothesis, (Figure 9), the metabolic and molecular events described above are initiated by intact chelated GdBCA rather than by free Gd3+. The cellular pathways exploited by either chelated GdBCA or transmetallated free Gd3+ to initiate these cellular changes is largely unknown. The data presented here suggest an important role of TLR signaling in NSF pathogenesis in particular and for the development of fibrosis in general. First, the data suggest that the ability to trigger TLR signaling is more potent following exposure to Omniscan® and gadodiamide. The most obvious explanation is that these molecules possess a unique, specific molecular shape or pattern that renders them capable of signaling via TLR4 and TLR7. Caldiamide, the chelate molecule contained in Omniscan® failed to activate NFκB and Gd-EDTA activated only weakly NFκB, suggesting that the greatest effect on TLR signaling is mediated by an intact Omniscan® Gd chelate molecule. Although the molecular pattern responsible for TLR activation is not known, it is possible that the formation of coordination bonds with Gd3+ apparently alters the non-stimulatory molecular pattern of caldiamide sufficiently to render the chelate complex capable of activating TLR signaling.
Omniscan® and gadodiamide signaling via both TLR4 and TLR7 allows these compounds to exert their effects at the cell surface and in the endosome. Endosomal TLR signaling is particularly relevant for macrophages owing to the stimulatory effect of TLR signaling on phagocytosis by macrophages (48). Initial engagement of TLR4 by Omniscan® at the macrophage cell surface could induce and increase the rates of Omniscan® phagocytosis. Once phagocytosed, Omniscan® would be able to amplify its TLR signaling capacity by engaging TLR7 within the endosome. Several reports of Gd deposits found in affected tissues from NSF patients described the presence of macrophages in close proximity (9–12), suggesting that the highly acidic environment of the endosome could facilitate deposition of Gd salts, such as Gd phosphate and Gd carbonate (49). These precipitated Gd salts could then maintain a constant state of TLR activation in both macrophages and fibroblasts, producing a chronic proinflammatory/profibrotic phenotype responsible for disease persistence and progression. Gd salts could act as a secondary trigger for the innate immune system, preventing the normal resolution of the TLR response following GdBCA clearance, inducing the release of profibrotic cytokines, chemokines and growth factors.
Fibroblasts express TLR4 at the cell surface and it is possible that some of the direct effects of Gd compounds on fibroblasts such as inducing their differentiation into α-SMA expressing myofibroblasts and the increased production and secretion of molecules involved in the fibrotic process such as collagens and hyaluronan could be due to engagement of fibroblast TLRs. We are currently investigating whether Gd compounds are capable of engaging normal fibroblast TLR signaling. It is also possible that GdBCA-induced hyaluronan could also signal via TLR4 expressed on macrophages and fibroblasts, contributing to a chronic proinflammatory/profibrotic environment (39).
Taken together, the results described here demonstrate that Omniscan® and gadodiamide signal through TLR4 and TLR7 resulting in the increased expression of genes encoding several well-characterized profibrotic and proinflammatory cytokines, chemokines and growth factors in normal human differentiated macrophages. The role of TLR4 and TLR7 in this stimulation was confirmed by the abrogation of these effects by specific TLR4 and TLR7 inhibitors as well as by RNA interference studies. The present study provides strong evidence and plausible pathophysiological mechanisms for initiation and maintenance of a proinflammatory/profibrotic phenotype by Omniscan® and gadodiamide in the development of NSF. The marked ability of Omniscan® and gadodiamide to signal through TLR4 and TLR7 compared to non-chelated Gd compounds and the inability of the chelate backbone, caldiamide, to induce this response suggests that the intact Gd chelate complex is capable of initiating innate immunity activation and that the specificity of the stimulation may be dependent on an intrinsic molecular pattern present in this chelate molecule. Further study and characterization of the cellular effects of these compounds and of the mechanisms of these effects may provide valuable information regarding the early events in the pathogenesis of NSF and other fibrosing diseases such as SSc.
Acknowledgments
This work was supported by a grant from NIH (RO1 AR-019616 to SAJ).
Footnotes
Disclosures
The authors have no financial conflicts of interest.
Bibliography
- 1.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1001. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 2.Grobner T. Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1745. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of twelve cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238–249. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez SA, Artlett CM, Sandorfi N, Derk C, Latinis K, Sawaya H, Haddad R, Shanahan C. Dialysis-Associated Systemic Fibrosis (Nephrogenic Fibrosing Dermopathy) Arthritis Rheum. 2004;50:2660–2666. doi: 10.1002/art.20362. [DOI] [PubMed] [Google Scholar]
- 5.Levine JM, Taylor RA, Elman LB, Bird SJ, Lavi E, Stolzenberg ED, McGarvey ML, Asbury AK, Jimenez SA. Involvement of skeletal muscle in dialysis-associated fibrosis (Nephrogenic Fibrosing Dermopathy) Muscle & Nerve. 2004;30:569–577. doi: 10.1002/mus.20153. [DOI] [PubMed] [Google Scholar]
- 6.Gibson SE, Farver CV, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130:209–212. doi: 10.5858/2006-130-209-MIINFD. [DOI] [PubMed] [Google Scholar]
- 7.Neudecker BA, Stern R, Mark L, Steinberg S. Scleromyxedema-like lesions of patients in renal failure contain hyaluronan: a possible pathophysiological mechanism. J Cutan Pathol. 2005;9:612–615. doi: 10.1111/j.0303-6987.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 8.Edward M, Fitzgerald L, Thind C, Leman J, Burden AD. Cutaneous mucinosis associated with dermatomyositis and nephrogenic fibrosing dermopathy: fibroblast hyaluronan synthesis and the effect of patient serum. Br J Dermatol. 2007;156:473–479. doi: 10.1111/j.1365-2133.2006.07652.x. [DOI] [PubMed] [Google Scholar]
- 9.High WA, Ayers J, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56:21–26. doi: 10.1016/j.jaad.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Abraham JL, Thakral C, Skov L, Rossen K, Marckmann P. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol. 2008;158:272–280. doi: 10.1111/j.1365-2133.2007.08335.x. [DOI] [PubMed] [Google Scholar]
- 11.Thakral C, Abraham JL. Gadolinium-induced nephrogenic systemic fibrosis is associated with insoluble Gd deposits in tissues: in vivo transmetallation confirmed by microanalysis. J Cutan Pathol. 2009;36:1244–1254. doi: 10.1111/j.1600-0560.2009.01283.x. [DOI] [PubMed] [Google Scholar]
- 12.Khurana A, Greene JR, High WA. Quantification of gadolinium in nephrogenic systemic fibrosis: re-examination of a reported cohort with an analysis of clinical factors. J Am Acad Dermatol. 2008;59:218–224. doi: 10.1016/j.jaad.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Palasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000;47:1107–1114. [PubMed] [Google Scholar]
- 14.Bellin MF. MR contrast agents, the old and the new. Eur J Radiol. 2006;60:314–323. doi: 10.1016/j.ejrad.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Lorusso V, Pascolo L, Fernetti C, Anelli PL, Uggeri F, Tiribelli C. Magnetic resonance contrast agents: From the bench to the patient. Curr Pharm Des. 2005;11:4079–4098. doi: 10.2174/138161205774913336. [DOI] [PubMed] [Google Scholar]
- 16.Gadolinium-based contrast agents and nephrogenic systemic fibrosis. FDA briefing document; Joint Meeting of the Cardiovascular and Renal Drugs and Drug Safety and Risk management Advisory Committee; [Accessed December 29, 2009]. [ http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM190850.pdf.] [Google Scholar]
- 17.Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148–157. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]
- 18.Del Galdo F, Shaw MA, Jimenez SA. Proteomic analysis identification of a pattern of shared alterations in the secretome of dermal fibroblasts from systemic sclerosis and nephrogenic systemic fibrosis. Am J Pathol. 2010;177:1638–1646. doi: 10.2353/ajpath.2010.091095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piera-Velazquez S, Louneva N, Fertala J, Wermuth PJ, Del Galdo F, Jimenez SA. Persistent activation of dermal fibroblasts from patients with gadolinium-associated nephrogenic systemic fibrosis. Ann Rheum Dis. 2010;69:2017–2023. doi: 10.1136/ard.2009.127761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edward M, Quinn JA, Mukherjee S, Jensen MB, Jardine AG, Mark PB, Burden AD. Gadodiamide contrast agent ‘activates’ fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol. 2008;4:584–593. doi: 10.1002/path.2311. [DOI] [PubMed] [Google Scholar]
- 21.Edward M, Quinn JA, Burden AD, Newton BB, Jardine AG. Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology. 2010;256:735–743. doi: 10.1148/radiol.10091131. [DOI] [PubMed] [Google Scholar]
- 22.Varani J, DaSilva M, Warner RL, Deming MO, Barron AG, Johnson KJ, Swartz RD. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Invest Radiol. 2009;44:74–91. doi: 10.1097/RLI.0b013e31818f76b5. [DOI] [PubMed] [Google Scholar]
- 23.Bhagavathula N, DaSilva M, Aslam M, Dame MK, Warner RL, Xu Y, Fisher GJ, Johnson KJ, Swartz R, Varani J. Regulation of collagen turnover in human skin fibroblasts exposed to a gadolinium-based contrast agent. Invest Radiol. 2009;44:433–439. doi: 10.1097/RLI.0b013e3181a4d7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhagavathula N, Dame MK, DaSilva M, Jenkins W, Aslam MN, Perone P, Varani J. Fibroblast response to gadolinium: Role for platelet-derived growth factor receptor. Invest Radiol. 2010;45:769–777. doi: 10.1097/RLI.0b013e3181e943d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DaSilva M, O’Brien Deming M, Fligiel SE, Dame MK, Johnson KJ, Swartz RD, Varani J. Responses of human skin in organ culture and human skin fibroblasts to a gadolinium-based MRI contrast agent: comparison of skin from patients with end-stage renal disease and skin from healthy subjects. Invest Radiol. 2010;45:733–739. doi: 10.1097/RLI.0b013e3181e9436b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macneil S, Bains S, Johnson C, Idee JM, Factor C, Jestin G, Fretellier N, Morcos SK. Gadolinium contrast agent associated stimulation of human fibroblast collagen production. Invest Radiol. 2011;46:711–717. doi: 10.1097/RLI.0b013e31822b1f38. [DOI] [PubMed] [Google Scholar]
- 27.Wermuth PJ, Del Galdo F, Jimenez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60:1508–1518. doi: 10.1002/art.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Galdo F, Wermuth PJ, Addya S, Fortina P, Jimenez SA. NFκB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann Rheum Dis. 2010;69:2024–2033. doi: 10.1136/ard.2010.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vakil V, Sung JJ, Piecychna M, Crawford JR, Kuo P, Abu-Alfa AK, Cowper SE, Bucala R, Gomer RH. Gadolinium-containing magnetic resonance image contrast agent promotes fibrocyte differentiation. J Magn Reson Imaging. 2009;30:1284–1288. doi: 10.1002/jmri.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer M, Ehlers M. Toll-like receptors in autoimmunity. Ann N Y Acad Sci. 2008;1143:21–34. doi: 10.1196/annals.1443.012. [DOI] [PubMed] [Google Scholar]
- 31.York MR, Nagai T, Mangini AJ, Lemaire R, Maguire van Seventer J, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by Type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. Erratum in Arthritis Rheum. 56:1675, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Fineschi S, Goffin L, Rezzonico R, Cozzi F, Dayer JM, Meroni PL, Chizzolini C. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum. 2008;58:3913–3923. doi: 10.1002/art.24049. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Peck A, Santer D, Patole P, Schwartz SM, Molitor JA, Arnett FC, Elkon KB. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008;58:2163–2173. doi: 10.1002/art.23486. [DOI] [PubMed] [Google Scholar]
- 34.Margaritopoulos GA, Antoniou KM, Karagiannis K, Samara KD, Lasithiotaki I, Vassalou E, Lymbouridou R, Koutala H, Siafakas NM. Investigation of Toll-like receptors in the pathogenesis of fibrotic and granulomatous disorders: a bronchoalveolar lavage study. Fibrogenesis Tissue Repair. 2010;3:20. doi: 10.1186/1755-1536-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farina GA, York MR, Di Marzio M, Collins CA, Meller S, Homey B, Rifkin IR, Marshak-Rothstein A, Radstake TR, Lafyatis R. Poly(I:C) drives type I IFN-and TGFβ-mediated inflammation and dermal fibrosis stimulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;30:2583–2593. doi: 10.1038/jid.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal SK, Wu M, Livingston CK, Parks DH, Mayes MD, Arnett FC, Tan FK. Toll-like receptor 3 upregulation by type I interferon in healthy and scleroderma dermal fibroblasts. Arthritis Res Ther. 2011;13:R3. doi: 10.1186/ar3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10:276–281. doi: 10.1016/j.autrev.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Lafyatis R, York M. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol. 2009;21:617–622. doi: 10.1097/BOR.0b013e32832fd69e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.York MR. Novel insights on the role of the innate immune system in systemic sclerosis. Expert Rev Clin Immunol. 2011;7:481–489. doi: 10.1586/eci.11.40. [DOI] [PubMed] [Google Scholar]
- 40.Eloranta ML, Franck-Larsson K, Lovgren T, Kalamajski S, Ronnblom A, Rubin K, Alm GV, Ronnblom L. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis. 2010;69:1396–1402. doi: 10.1136/ard.2009.121400. [DOI] [PubMed] [Google Scholar]
- 41.Hubert FX, Voisine C, Louveet C, Heslan M, Josien R. Rat plasmacytoid dendritic cells are an abundant subset of MHC class II+ CD4+CD11b-OX62- and type I IFN-producing cells that exhibit selective expression of Toll-like receptors 7 and 9 and strong responsiveness to CpG. J Immunol. 2004;172:7485–7494. doi: 10.4049/jimmunol.172.12.7485. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y, Maozi L, Rongchang L, Wang C, Bai C, Wang K. Gadolinium induces domain and pore formation of human erythrocyte membrane: an atomic force microscopic study. Biochim Biophys Acta. 1999;1421:249–260. doi: 10.1016/s0005-2736(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 43.Powers JP, Li S, Jaen JC, Liu J, Walker NP, Wang Z, Wesche H. Discovery and initial SAR of inhibitors of interleukin-1 receptor-associated kinase-4. Bioorg Med Chem Lett. 2006;16:2842–2845. doi: 10.1016/j.bmcl.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Idee JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009;30:1249–58. doi: 10.1002/jmri.21967. [DOI] [PubMed] [Google Scholar]
- 45.Townsend RR, Cohen DL, Katholy R, Swan SK, Davies BE, Bensel K, Lambrecht L, Parker J. Safety of intravenous gadolinium (Gd-BOPTA) infusion in patients with renal insufficiency. Am J Kidney Dis. 2000;36:1207–1212. doi: 10.1053/ajkd.2000.19836. [DOI] [PubMed] [Google Scholar]
- 46.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol. 1998;5:491–502. doi: 10.1016/s1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]
- 47.Newton BB, Jimenez SA. Mechanism of NSF: New evidence challenging the prevailing theory. J Magn Reson Imaging. 2009;30:1277–1283. doi: 10.1002/jmri.21980. [DOI] [PubMed] [Google Scholar]
- 48.Ribes S, Ebert S, Czesnik D, Regen T, Zeug A, Bukowski S, Mildner A, Eiffert H, Hanisch UK, Hammerschmidt S, Nau R. Toll-like receptor prestimulation increases phagocytosis of Escherichia coli DH5alpha and Esherichia coli K1 strains by murine microglial cells. Infect Immun. 2009;77:557–564. doi: 10.1128/IAI.00903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bleavins K, Perone P, Naik M, Rehman M, Aslam MN, Dame MK, Meshinchi S, Bhagavathula N, Varani J. Stimulation of fibroblast proliferation by insoluble gadolinium salts. Biol Trace Elem Res. 2012;145:257–267. doi: 10.1007/s12011-011-9176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]