Abstract
Sustained hepatic inflammation, driven by factors such as alcohol consumption, non-alcoholic fatty liver disease (NAFLD) and/or chronic viral hepatitis (Hepatitis B and C), results in damage to parenchyma, oxidative stress, and compensatory regeneration/proliferation. There is substantial evidence linking these inflammation-associated events with the increased incidence of hepatocellular carcinogenesis. While acute liver inflammation can play a vital and beneficial role in response to liver damage or acute infection, the effects of chronic liver inflammation, including liver fibrosis and cirrhosis, are sufficient in a fraction of individuals to initiate the process of transformation and the development of hepatocellular carcinoma (HCC). This review highlights immune-dependent mechanisms that may be associated with hepatocellular oncogenesis, including critical transformative events/pathways in the context of chronic inflammation and subverted tolerogenesis.
Keywords: hepatocellular, tolerance, innate, B-cells, senescence
Introduction
The liver is the largest internal organ and the primary regulator of systemic metabolism. Equally significant, the liver also functions as the primary lymphoid organ tasked with surveillance of the large and diverse antigen load inherent in the dietary intake(1). The hepatic immune system of the liver must not only be able to identify, detoxify and neutralize pathogens, it must also be able to tolerize the host to potentially damaging systemic immune responses against otherwise antigenic but beneficial nutritional components. The liver is anatomically situated to collect the blood flow directly from the gut after which it is directed through the architecturally unique, reduced-flow vasculature of the liver sinusoids, optimizing interaction with resident immune cells including lymphocytes, macrophages Kupffer cells (KC), NK/NKT and dendritic cells (2), while allowing establishment and enrichment of these otherwise mobile non-parenchymal cells (NPC). These factors combine to maintain a balance between the elimination of pathogenic components and tolerization of the local and systemic immune responses to non-pathogenic antigens. These same attributes also conspire to predispose the liver to pathologies that evolve from immune-mediated damage (hepatitis) and malignant redirection of tolerogenesis (neoplasia, persistent viral and microbial infections).
Dysregulated swelling and inflammation of the liver, defined as hepatitis, is characterized by the presence of excess inflammatory cells. When unresolved,, inflammatory components directly induce hepatic damage, often overwhelming the ability of the liver tissue to repair itself and leading to fibrosis and irreversible scar tissue formation called cirrhosis (3). Cirrhosis restructures liver tissue into nodules rich in both dying and replicating hepatocytes, compromising liver function and often leading to liver failure. This process typically occurs over decades driven by diverse etiologies including viral hepatitis, alcoholism or non-alcoholic fatty liver disease (NAFLD) and are associated with increased HCC risk (4). Intersection of hepatic immune-mediated processes including: oxidative damage (mutagenic), compensatory liver regeneration (mitogenic), and tolerogenesis to neo-antigens (tolerogenic), favors neoplastic transformation. Through the understanding of immune- dysregulation in hepatocellular carcinogenesis, key processes can be identified and beneficial interventions proposed. The following brief overview highlights some current aspects of the hepatocellular intersection of inflammation and carcinogenesis (Figure 1).
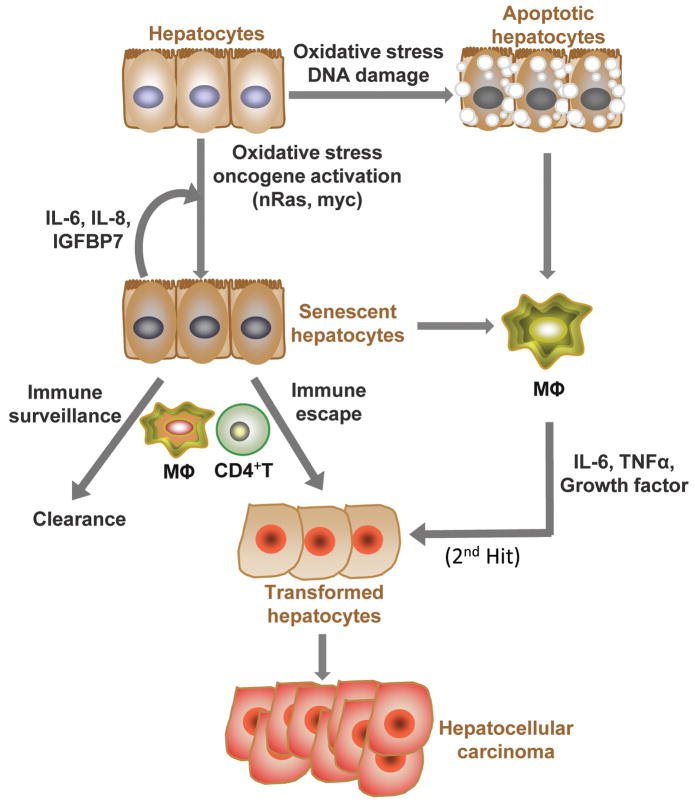
Figure 1. Avoiding hepatocellular oncogenesis through immune-mediated processes.
Failsafe mechanisms to counter malignant transformation of hepatocytes involve both cell intrinsic and extrinsic components. In response to cellular damage, cell intrinsic checkpoint pathways arrest cell growth (senescence, autophagy) and/or initiate programs that result in clearance (apoptosis). In the liver, these programs elicit specific extrinsic responses by local immune cells. During the progression to hepatocellular transformation, oncogene activation can also induce both apoptosis and senescence. Both processes result in release of cytokines and chemokines (SASP, senescence associated secretory phenotype and DAMPS, damage associated molecular patterns) into the microenvironment to recruit and instruct immune system cells to reinforce these processes. Innate immune components such as NK and KC stand watch for signs of oncogene-induced hepatocellular stress such as NKG2D and DAMPS (IL-1α, HMGb1). KC also serve as the primary source for IL-6 in the orchestration of compensatory proliferation required for both hepatic regeneration and tumor progression. CD4+ adaptive immune responses are mounted against oncogene induced senescent cells leading to clearance effected by macrophages. Oncogene-initiated cells may escape these mechanisms by acquiring additional or “2nd hits” in these pathways leading to transformation.
Oxidative stress and Kupffer cells
Within the diseased liver, free-radical production in the form of reactive oxygen and nitrogen species is initiated by cells of the immune system, including recruited neutrophils, monocytes and KC. These oxidizing components are normally balanced through redox regulation by antioxidant pathways (glutathione and superoxide dismutase) in healthy tissue. However, the robust immunologic milieu of the liver microenvironment can disrupt this balance during inflammation by deploying anti-pathogenic antioxidants. Hepatocytes sustain oxidative damage directly through chemical modification of proteins, lipids and nucleic acids. Additionally, apoptosis and necrosis programs can be triggered from alterations induced in mitochondrial permeability by the altered intracellular redox state (5). These combined processes collectively referred to as oxidative stress, further amplify the inflammatory response through the release of DAMPS (danger associated molecular patterns) and stress-related signaling molecules, culminating in chromosomal instability and oncogenic gene mutations. Furthermore, nitric oxide (NO) has been shown to protect virally- infected hepatocytes from apoptosis through activation of NF-κB and suppression of Th1 anti-tumor immune surveillance. Integration of diverse inflammatory signals initiated by oxidative stress, gut-derived microbes and endotoxins from the blood occurs through the modulation of pattern recognition receptors on resident KC. Although KCs can be involved in anti-tumor immunity, numerous human and mouse studies have recently uncovered their multi-faceted ability to contribute to promotion of liver tumorigenesis. In addition to the roles for antioxidants described above, chronic inflammation can also be mediated by KCs through the constant production of cytokines, including interleukin-6 (IL-6), TNF-α, and transforming growth factor-β (TGF-β). The contributions of these cytokines to chronic inflammation will be described below.
IL-6/STAT3 and TNF-α/NF-kB Axes
IL-6 and TNF-α, are active contributors to acute inflammatory responses(6). Expression of IL-6 and TNF-α is elevated in both liver cirrhosis and HCC (7). Although the mechanisms by which elevated IL-6 and TNF-α promote liver cancer are not clear, their signals regulate gene expression through the latent transcription factors STAT3 and NF-kB. NF-kB is the intracellular signaling effector for many pro-inflammatory cytokines including TNFα, IL-1, and TLRs. In many tumor types, NF-kB is highly activated and often addictive (inactivation leads to cell death or tumor remission) (8). In liver, NF-kB activation in non-parenchymal cells is necessary for HCC tumor promotion (9) while its function in hepatocytes is pro-survival and induction of pro-tumor cytokines including IL-6 and TNFα (10). STAT3 can function as a driver oncogene and has been shown, along with IL-6, to be part of an epigenetic switch established during transformation (11). Overlap in the transcriptional regulatory network between inflammation and transformation has been demonstrated in other tumor models suggesting inflammation-driven signaling within pre-neoplastic cells can cooperate or contribute directly to oncogenic transformation (11). With regards to hepatocellular transformation, constitutive activating mutations in both gp130 (IL-6 signal transducer) and STAT3 have been demonstrated in more than 70% of inflammatory hepatocellular adenomas (12). Furthermore, isolated human HCC stem cells are marked by high expression of IL-6 and STAT3, but often also with loss of the type 2 TGF-β receptor (TGFBR2). This suggests that IL-6 autocrine signaling may be more important than maintaining TGF-β signaling in driving transformed stem cells toward HCC (13).
Dual functions of TGFβ/Smad
TGF-β plays a complex role depending on the context and stage of the “fibrosis-cirrhosis-HCC” process (9). A recent study revealed inactivation of TGF-β signaling through deletion of TGFBR2 reduced HCC formation caused by p53 loss in Albumin-cre transgenic mice (13). In contrast, Ozturk’s group found that TGF-β treatment in vitro induced growth inhibition in well-differentiated HCC cell lines that have p53 mutations and express TGFBR2 (23). The results of their analysis of TGF-β expression in normal, cirrhotic, and HCC liver, using publicly available clinical data, showed that TGF-β was sharply increased in patients with liver cirrhosis, but was followed by a significant decrease in patients with early or advanced HCC. Furthermore, TGFBR2 is also reported to be down-regulated in 37–70% of patients with HCC (13). These paradoxical findings may be reconciled by recognizing that the roles of TGF-β in HCC tumorigenesis are inherently different at various stages of disease development. In cirrhotic liver, up-regulated TGF-β promotes the transformation and growth of neoplastic cells with existing p53 mutations while after tumor development, HCC cells may escape growth inhibition possibly by down-regulating their TGF-β receptors and “instructing” the microenvironment to shut down the expression of TGF-β. Stage-dependent TGF-β signaling is also influenced by differential cytokine-activated-kinase phosphorylation of Smad proteins. Normally TGF-β-mediated Smad3 signaling terminates hepatocyte proliferation during acute liver injury. Chronic pro-inflammatory cytokine exposure alters the phosphorylation state of Smad2/3 resulting in mitogenic (oncogenic) and fibrotic TGF-β signaling(14). The redundancy of these mechanisms in regulating TGF-β signaling underscores the necessity and importance of this pathway in hepatocellular oncogenesis.
LTβR signaling and oncogenesis
The tumor necrosis factor (TNF) super family (TNFSF) of cytokines consists of 29 members. In addition to the well documented pleiotropic roles of TNF-α in the liver, lymphotoxin (LT) α, along with LTβ and Light (TNFSF 14) have been implicated as drivers of hepatic stellate cell function/wound healing (15), liver regeneration (16) and hepatic carcinogenesis (17). These findings have evoked renewed interest in targeting LTβR in an attempt to thwart hepatocellular oncogenesis. Recent work from Haybaeck et al. has provided compelling evidence the inflammation resulting from LTαβ signaling is sufficient to drive HCC in the liver-specific AlbLTαβ murine model (17). Moreover the authors detail the increase in mRNA levels of LTβR ligands in liver samples derived from patients infected with HBV or HCV, as well as samples from patients with HCC, strengthening the link between LT signaling and HCC. While additional studies are needed to confirm the pivotal role of the LTβR in HCC, strategies designed to block signaling via LTβR might be beneficial.
Oncogene-induced senescence/apoptosis and immune regulation
Activation of individual oncogenes modeling pre-malignant initiation, elicits distinct protective programs including senescence and apoptosis. These processes are dependent upon both cell-autonomous and cell-extrinsic mechanisms that function in concert to suppress and/or eliminate cells undergoing oncogenic stress. Senescent cells display characteristic secretomes that commonly include IL-6 and IL-8 to maintain the senescent state and promote immune surveillance of senescent cells. In liver, (oncogene-induced) senescent hepatocytes also secrete CTACK, IL-1α, leptin/leptin R, MCP1 and RANTES(18). Non-initiated bystander cells including immune cells can reinforce this program by also secreting pro-senescent cytokines. Apoptotic hepatocytes also release IL-1α which triggers KCs to orchestrate compensatory proliferation, essential to development of HCC in the diethylnitrosamine (DEN) model (19). Senescence, unlike apoptosis, does not result in cell elimination. Instead cells that undergo oncogene-induced senescence constitute a quiescent population of initiated premalignancies. The presence of these senescent cells provides the opportunity for escape or progression to malignancy through accumulated “second hits”. Interestingly, a recent report described an in vivo example of immune-surveillance of such oncogene-induced senescent cells(18). Kang et al. demonstrated NrasG12V oncogene-induced senescence in liver by examining senescence marker expression in oncogenic-NrasG12V transposon- and inactivated-Ras (effector loop signaling domain deletion) transposon- transduced livers. Oncogenic NrasG12V induced markers of senescence by 12 days, but by 60 days NrasG12V-expressing cells were undetectable. Previously, an inducible p53-dependent model of HCC senescence showed the innate immune system was sufficient to mediate regression of established HCC tumors (20) suggesting a role for innate immune components in mediating surveillance of senescent cells. In Kang’s model, NrasG12V alone was not able to drive hepatic tumorigenesis in WT mice. Surprisingly NrasG12V did drive massive tumor development in CD4 -/- hosts, implicating a role for the adaptive immune system (T helper cells) in surveillance of cells undergoing oncogenic stress. Furthermore, the authors found Nras G12V peptide epitope specific Th cells indicating specificity for the driving oncogene. The authors also presented evidence that monocytes/macrophages function as effectors in clearing initiated cells but antibody-mediated depletion of neutrophils and NK cells showed only marginal or no effect. It has been noted that immunosuppressed individuals display a higher cancer rate, reviewed in (21), and in the case of hepatitis C, senescent hepatocytes build-up in infected livers of immunosuppressed patients (18). This is consistent with immune-mediated surveillance of senescence providing a major barrier to tumorigenesis by eliminating the reservoir of pre-malignant senescent cells that are primed for escape to transformation. However, additional studies, including single-cell analyses, are needed to formally exclude the possible pre-existence of senescence-resistant or otherwise transformed cells.
The Myc oncogene has been implicated in hepatocellular senescence (tumor regression), malignant progression and tumor-dependent immunoregulation. Overexpression of wild-type Myc is a feature in most HCC patients. Induction of Myc-driven genes also marks the transition from dysplastic nodules to “early” HCC (22) indicating that Myc may initiate or be an “effector” of transformation in HCC. Myc is the only oncogene (23) in its wild-type form that can induce high penetrance tumors with short latencies in most transgenic models. The Myc transgenic tumor model displays decreased latencies in response to hepatotoxins and hydrodynamic damage, implying Myc may collaborate with inflammation-driven signaling pathways (24). Interestingly, Myc is an addictive oncogene in models of lymphoma and HCC where tumor regression occurs when Myc is shut down. In lymphoma, Myc evokes apoptosis which attracts and signals macrophages to secrete TGF-β inducing senescence. Just as CD4 T cells are required for successful surveillance of NrasG12V induced senescent cells, sustained tumor regression following Myc inactivation also depends upon CD4 T cells for, induction of senescence, collapse of angiogenesis, and long term suppression of minimal residual disease (25). Myc-driven transgenic HCC models also display the ability to modulate the adaptive immune response. Specifically, when Myc-OVA transgenic mice (Tet-Myc x OVA) bearing liver tumor were challenged by transferred OVA-specific T cells, transferred T cells penetrated the tumors in high numbers but were hyporesponsive as defined by in vivo cytotoxicity and IFN-γ production(26).
The microenvironment of the liver is unique in immune function and architecture
Multiple mechanisms govern tolerance development and immune function in the liver. First, an abundance of microbial-responsive antigen presenting cells (APC) highly express inhibitory programmed death ligands-1/2 (PDL-1/2) and IL10. Second, unique reduced flow architecture facilitates tolerance through T cell “trapping” combined with suppressive cytokines IL10 and TGF-β, enhancing CD8+ T cell apoptosis. Third, active recruitment and accumulation of suppressive MDSCs and Tregs during HCC progression further tips the immune balance toward suppression (Figure 2). These factors acting in concert form a bridge between cirrhosis and HCC development.
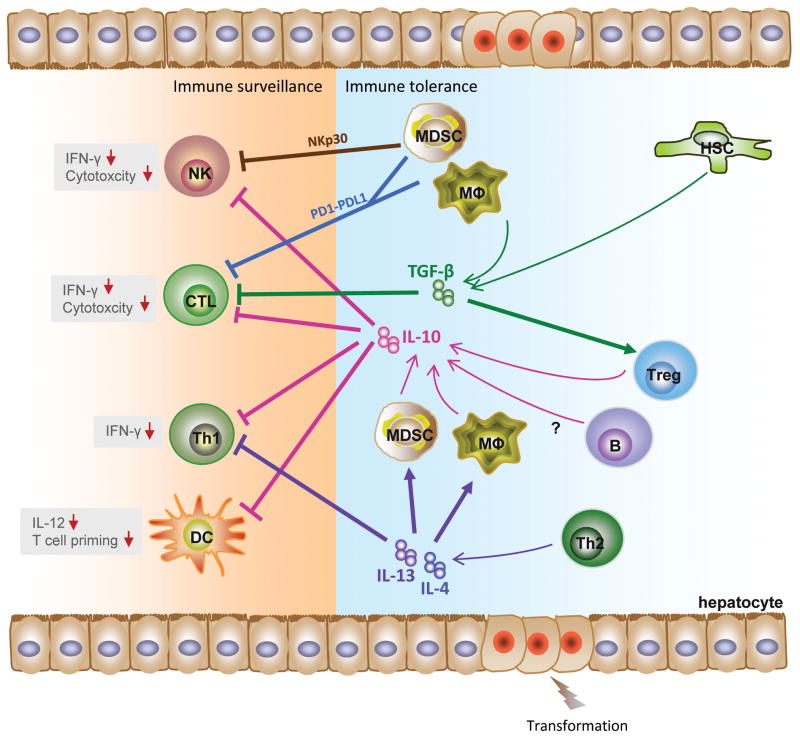
Figure 2. Malignant repurposing of immune surveillance and tolerance in HCC.
Prolonged hepatitis induces alterations in TGF-β, IL-10, IL-13, and IL-4 and leads to an immune milieu permissive for hepatocellular transformation. IL-10, produced by MDSC, KC, B and Treg cells, downregulates the anti-tumor effect of NK, CTL, Th1 and DC cells. KC and HSC activation leads to elevated TGF-β levels stimulating Tregs and inhibiting CTLs. IL-13 and IL-4 produced by Th2 further inhibit Th1 cells MDSCs inhibit NK and CTL activity through NKp30 and PD-1. The concerted effect of these activities results in reduced immune sensitivity and increased tolerance.
Chronic low level LPS drives IL10 synthesis, PDL1 and T cell Tolerance
Blood enters the liver via sinusoids supplied by the hepatic artery and portal vein. This blood from the intestine is rich in microbial-derived, TLR-activating factors that regulate innate immunity and IL10 synthesis. Lipopolysaccharide (LPS) activation of TLR4 on antigen-presenting cells, including KC, plasmacytoid DC (pDC) and myeloid DC (mDC), triggers the synthesis of multiple cytokines including TNF-α, IL-12, IL-18 and IL-10. (27) Coordinated down-regulation of this pro-inflammatory microenvironment is needed to prevent immune-mediated damage. The dynamic suppressive cytokine, IL-10, is essential for maintaining liver homoeostasis/tolerance. IL-10 modulates NK activity/function, induces T cell suppression (28), and polarizes adaptive Tregs (29) thereby suppressing the liver immune response and surveillance. Furthermore, the negative costimulatory receptor PD-1, expressed primarily on T cells and B cells, inhibits antigen-specific CD8+ T cell activation/memory and inflammatory hepatitis (30). Expression of PD-1 ligands, PD-L1 and PD-L2 is elevated in steady state liver, with highest levels of expression on KC, infiltrating macrophages, LSECs, dendritic cells, and parenchymal cells (31). Increased expression of the inhibitory receptor, PD-1, and/or interactions with its ligands, have correlated with persistence of viruses (32), HCC aggressiveness and HCC recurrence following treatment (33). Therapies targeting the interaction of PD-1 with its ligands have shown promise in fighting chronic HCV infection and enhancing anti-tumor responses. Recently, Youngblood et al., using epigenetic analysis of the Pdcd1 (PD-1 locus), suggested a unique regulatory program for PD-1 expression in antigen-specific T cells. The complete de-methylation of the Pdcd1 region coincided with sustained expression of PD-1 on exhausted CD8+ T cells in a setting of chronic viral infection (34). In contrast, acute infection was accompanied by only transient de-methylation of Pdcd1, followed by subsequent re-methylation upon differentiation into CD8+ memory cells (34). Franceschini et al. identified a role for PD-1 on T regulatory cells (Tregs) during chronic HCV infection. Infiltrating Tregs up-regulate PD-1 and upon ligation of PD-L1/2 inhibit TCR signaling and reduce IL2 signaling via inhibition of STAT5 phosphorylation, decreasing Treg proliferation (35). It is likely PD-1 can induce tumor specific CD8+ T cell apoptosis (33) and PD-1 signaling has been shown to reduce IFNγ, TNF-α, and IL2 synthesis(30). Collectively, these studies suggest a pivotal role for the PD-1-PDL1/2 axis during initial viral escape and reduced tumor surveillance.
The liver is a preferred site of T cell accumulation and activation, due in part, to unique architectural features and the abundance of APC. Increased expression of adhesion molecules facilitates the trapping of activated T cells in sinusoids, where they may undergo apoptosis induced by FasL and Trail on KC or be phagocytosed (31). Moreover, T cells that recognize antigen in the liver are typically exposed to high levels of the suppressive cytokines IL-10 and TGF-β; further modulation occurs through PD1-PDL1/2 inhibitory signaling. This T cell tolerance likely explains the evolutionary need to have a substantial innate component, potentially explaining the abundance of pDC and NK cells.
Immune suppressors
The role and types of immunosuppressive cells that accumulate in chronic infection and HCC remain incompletely understood in the liver. Myeloid derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells, which can expand and contribute to immune suppression during HCV infection and HCC (36, 37). The liver is a preferred site for homing and expansion of MDSCs (38). MDSCs can disrupt immune surveillance mechanisms including suppressing effector T cells (37), expanding Tregs (39), and impairing NK cell function (40). MDSC-derived ROS can induce oxidative stress and mediate T cell suppression during chronic HCV infection (36). The presence of intra-tumoral and circulating Tregs correlates with tumor progression (41), poor prognosis (42) and reoccurrence following surgical resection (43) of HCC. Tregs mediate immune suppression through functional modulation of tumor specific CD8+ and CD4+ T cells and by tumor specific accumulation mediated through chemokine receptor CCR4 (44). However, a recent report from Chen at el. identified a selective Treg recruitment pathway facilitated through CCR6-CCL20, selectively promoting HCC progression and predicting poor prognosis (45).
Innate surveillance
NK and NKT cells represent a major innate immune component in liver, constituting over 50% of hepatic lymphocytes in mice and humans (46). During chronic viral infection, suppression of NK cells can result in reduced surveillance. Recent evidence suggests that once HBV/HCV infections become persistent, NK cells lose their cytotoxic potential and ability to secrete IFNγ (47). NK cell function is also impaired in non-viral induced liver cirrhosis. In a carbon tetrachloride (CCl4)-induced liver fibrosis model, NK cells suppress fibrosis by killing activated HSC early, but later, NK cells are inhibited by elevated levels of TGF-β and SOCS1(39) coinciding with the critical period for HCC development. In the c-Myc/Tgfα transgenic model, MHC-1 is down-regulated and Rae1 is upregulated on dysplastic hepatocytes targeting them for NK surveillance. However, malignant progression still succeeds presumably due to insufficient NK numbers (48). NKT cells significantly increased in c-Myc/Tgfα dysplastic liver as it progressed towards malignancy. Distinct NKT cell subsets can either promote (CD4+ NKT) or inhibit (iNKT) liver cancer (49)(recently reviewed by Subleski et al. (50)) and can partially explain this observation. Bridging innate and adaptive immune systems, resident DCs are less functional in liver, but are still capable of priming anti-viral T cell responses sufficient for clearance. Upon viral escape, chronic liver inflammation renders liver DCs suppressed, as observed in chronically infected HCV patients showing a diminished ability to mature and prime T cell proliferation and induce IFN-γ (51). Interaction between HCV core protein and DCs in culture results in reduced frequency of pDC and direct inhibition of IFNα production(52). Core protein can also inhibit IL-12 production by DCs through an intracellular mechanism dependent on a combination of TLR4 signaling and cross linking of the complement receptor (53) thereby contributing to a Th2 skewed microenvironment. HBV-infected patients have diminished pDC functions resulting in part from a specific HBV antigen (HBeAg) (54). These findings suggest persistent viral infection and chronic inflammation deprive DC’s ability to prime T cell surveillance, augmenting hepatocellular carcinogenesis.
Dual role of B cells in hepatocellular carcinogenesis
Circulating B cells from cirrhotic patients have been reported to be hypo-responsive to ex vivo CD40/TLR9 stimulation, as characterized by LTα secretion, IgG production and T-cell allostimulation. A reduction in CD27+IgM+ memory B cells was also observed in cirrhotic patients (55). These changes support a reduced B cell-mediated anti-viral response allowing persistent viral infection, associated inflammation, and HCC development. In contrast, results from an inflammatory skin model of HPV16 squamous cell carcinomas (SCC) suggest a more direct, tumor promoting role for B cells, possibly via immunoglobulin accumulation. (56) Although controversial, increased levels of immunoglobulin in murine HCC models, serum from cirrhotic individuals and HCC lesions (57), all suggest a possible cause and effect linkage between the presence of immunoglobulin and HCC development. Previously, we established a murine de novo liver tumor model of adenoma and carcinoma by hydrodynamic injection of transposons containing myrAKT (AKT) and Δ90 β-catenin (β-CAT) (58). We observed hepatocellular development/progression to be largely dependent on B cells (Authors’ unpublished observation). We also found that tumor infiltrating B cells express elevated levels of TNF-α (Authors’ unpublished observation), suggesting that B cell-derived cytokines could be instrumental to tumor development/progression. Support for the role of TNF-α from B cells was recently detailed by Schioppa et al. who observed in B cell-specific TNF-α −/− mice a markedly reduced promotion of the HPV16 SCC model and a modulation of B10 cell activity (59). Collectively, these studies suggest a significant tumor-promoting role for B cells during carcinogenesis.
Perspective
Chronic liver injury, inflammatory pathway activation and oxidative stress intersect within the context of the uniquely tolerant liver microenvironment, thus facilitating hepatic oncogenesis. This review emphasizes the dynamic juncture of inflammation and oncogenesis, highlighting the critical players and immunological therapeutic targets, and suggesting areas for further research.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- HCC
hepatocellular carcinoma
- KC
Kupffer cell
- NK
natural killer
- NPC
non-parechymal cell
- NO
nitric oxide
- IL-6
interleukin-6
- TGF-β
transforming growth factor-b
- TGFBR2
type 2 TGF-β receptor
- TNF
tumor necrosis factor
- TNFSF
tumor necrosis factor super family
- LT
lymphotoxin
- DEN
diethylnitrosamine
- APC
antigen presenting cell
- PDL-1/2
programmed death ligands-1/2
- LPS
lipopolysaccharide
- pDC
plasmacytoid dendritic cell
- mDC
myeloid dendritic cell
- Treg
T regulatory cell
- MDSC
myeloid derived suppressor cell
- CCl4
carbon tetrachloride
- HBeAg
hepatitis B protein-e-antigen
- SCC
squamous cell carcinoma
- SASP
senescence-associated secretory phenotype
- DAMPS
danger-associated molecular patterns
- CTL
cytotoxic T-lymphocyte
References
- 1.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 3.Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Current Opinion in Gastroenterology. 2009;25:223–229. doi: 10.1097/mog.0b013e3283279668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber A, Boege Y, Reisinger F, Heikenwalder M. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Medical Weekly. 2011;141:w13197. doi: 10.4414/smw.2011.13197. [DOI] [PubMed] [Google Scholar]
- 5.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. Journal ofLeukocyte Biology. 2006;80:1197–1213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- 7.He G, Karin M. NF-kappaB and STAT3 -key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30:1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis- canceraxis. Hepatology. 2007;46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- 10.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harbor Perspectives in Biology. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilati C, Amessou M, Bihl MP, Balabaud C, Nhieu JT, Paradis V, Nault JC, et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. The Journal of Experimental Medicine. 2011;208:1359–1366. doi: 10.1084/jem.20110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris SM, Baek JY, Koszarek A, Kanngurn S, Knoblaugh SE, Grady WM. Transforming growth factor-beta signaling promotes hepatocarcinogenesis induced by p53 loss. Hepatology. 2012;55:121–131. doi: 10.1002/hep.24653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki K. Smad phosphoisoform signals in acute and chronic liver injury: similarities and differences between epithelial and mesenchymal cells. Cell and Tissue Research. 2012;347:225–243. doi: 10.1007/s00441-011-1178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruddell RG, Mann DA, Ramm GA. The function of serotoninwithin the liver. J Hepatol. 2008;48:666–675. doi: 10.1016/j.jhep.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, Papa S, et al. T cell-derived lymphotoxin regulates liver regeneration. Gastroenterology. 2009;136:694–704. doi: 10.1053/j.gastro.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual Review of Immunology. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 22.Kaposi-Novak P, Libbrecht L, Woo HG, Lee YH, Sears NC, Coulouarn C, Conner EA, et al. Central role of c-Myc during malignant conversion in human hepatocarcinogenesis. Cancer Research. 2009;69:2775–2782. doi: 10.1158/0008-5472.CAN-08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Tai Y, Lisanti MP, Liao DJ. c-Myc induction of programmed cell death may contribute to carcinogenesis: a perspective inspired by several concepts of chemical carcinogenesis. Cancer Biology & Therapy. 2011;11:615–626. doi: 10.4161/cbt.11.7.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beer S, Komatsubara K, Bellovin DI, Kurobe M, Sylvester K, Felsher DW. Hepatotoxin-induced changes in the adult murine liver promote MYC-induced tumorigenesis. PLoS One. 2008;3:e2493. doi: 10.1371/journal.pone.0002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, Fan AC, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ney JT, Schmidt T, Kurts C, Zhou Q, Eckert D, Felsher DW, Schorle H, et al. Autochthonous liver tumors induce systemic T cell tolerance associated with T cell receptor down-modulation. Hepatology. 2009;49:471–481. doi: 10.1002/hep.22652. [DOI] [PubMed] [Google Scholar]
- 27.Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. Eur J Immunol. 2006;36:2483–2493. doi: 10.1002/eji.200535767. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 30.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 34.Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacke R, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. Myeloid suppressor cells induced by hepatitis C virus suppress T cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343–353. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong WI, Park O, Suh YG, Byun JS, Park SY, Choi E, Kim JK, et al. Suppression of innate immunity (natural killer cell/interferon-gamma) in the advanced stages of liver fibrosis in mice. Hepatology. 2011;53:1342–1351. doi: 10.1002/hep.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 41.Lee WC, Wu TJ, Chou HS, Yu MC, Hsu PY, Hsu HY, Wang CC. The impact of CD4(+)CD25(+) T cells in the tumor microenvironment of hepatocellular carcinoma. Surgery. 2012;151:213–222. doi: 10.1016/j.surg.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Jing X, Li G, Wang T, Yang B, Zhu Z, Gao Y, et al. Foxp3(+) regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver Int. 2011 doi: 10.1111/j.1478-3231.2011.02675.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 44.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 45.Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 47.Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest. 2010;40:851–863. doi: 10.1111/j.1365-2362.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- 48.Coulouarn C, Factor VM, Conner EA, Thorgeirsson SS. Genomic modeling of tumor onset and progression in a mouse model of aggressive human liver cancer. Carcinogenesis. 2011;32:1434–1440. doi: 10.1093/carcin/bgr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. Journal of Leukocyte Biology. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subleski JJ, Jiang Q, Weiss JM, Wiltrout RH. The split personality of NKT cells in malignancy, autoimmune and allergic disorders. Immunotherapy. 2011;3:1167–1184. doi: 10.2217/imt.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 52.Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 53.Waggoner SN, Hall CH, Hahn YS. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+ T cells via suppression of dendritic cell IL-12 production. J Leukoc Biol. 2007;82:1407–1419. doi: 10.1189/jlb.0507268. [DOI] [PubMed] [Google Scholar]
- 54.Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6:e15324. doi: 10.1371/journal.pone.0015324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doi H, Iyer TK, Carpenter E, Li H, Chang KM, Vonderheide RH, Kaplan DE. Dysfunctional B-cell activation in cirrhosis due to hepatitis C infection associated with disappearance of CD27+ B-cell population. Hepatology. 2011 doi: 10.1002/hep.24689. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Massonnet B, Delwail A, Ayrault JM, Chagneau-Derrode C, Lecron JC, Silvain C. Increased immunoglobulin A in alcoholic liver cirrhosis: exploring the response of B cells to Toll-like receptor 9 activation. Clin Exp Immunol. 2009;158:115–124. doi: 10.1111/j.1365-2249.2009.04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stauffer JK, Scarzello AJ, Andersen JB, De Kluyver RL, Back TC, Weiss JM, Thorgeirsson SS, et al. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718–2727. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]